Abstract
Objective
PD-1 inhibitors have improved efficacy in many cancers. There are currently no reports of the use of PD-1 inhibitors, such as nivolumab, for metastatic biliary tract cancer (mBTC). This study reviewed the efficacy and safety of nivolumab for mBTC with the aim of exploring ways to improve efficacy and survival.
Methods
Thirty patients with mBTC were voluntarily treated with nivolumab at the PLA General Hospital. Nivolumab 3 mg/kg was administered. Progression-free survival (PFS) and overall survival were evaluated by Kaplan–Meier and univariate and multivariate analyses were carried out for clinical characteristics. Objective response rate (ORR), disease control rate (DCR), and treatment-related adverse events (AEs) were also evaluated.
Results
The median treatment cycle is four cycles. One case was complete response, 5 cases partial response, 12 cases stable, and 12 cases progression. ORR was 20%, DCR was 60%, and PFS was 3.1 months (95% CI: 2.13–4.06). The AEs of nivolumab monotherapy were fatigue (three cases), fever (two cases), hypothyroidism (one case), skin reaction (one case), and liver injury (one case). Nivolumab combined with chemotherapy related grade 1–2 hematologic toxicity were leukopenia (five cases) and thrombocytopenia (two cases), and grade 3–4 were leukopenia (three cases). Non-hematologic toxicity grade 1–2 were nausea and vomiting (four cases), fatigue (four cases), fever (three cases), peripheral neurotoxicity (three cases), and hypothyroidism (one case). Univariate analysis showed that PFS of nivolumab combined with chemotherapy was statistically significant compared with that of nivolumab monotherapy (4.1 vs 2.3 months, P=0.031). Programmed death-ligand 1 (PD-L1) expression positively has no relationship with better PFS in contrast with PD-L1 negatively (3.6 vs 3.0 months P>0.05). Multivariate analysis show nivolumab combined with chemotherapy was only the independent factor for longer PFS (HR: 0.432, P<0.05).
Conclusion
The safety of nivolumab in mBTC is controllable. Further selection of superior populations is needed to improve the efficacy of nivolumab in mBTC.
Keywords: metastatic biliary tract cancer, nivolumab, PD-L1, PD-1
Introduction
Biliary tract cancers (BTCs) include intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), and gallbladder cancer (GBC). According to data from Cancer Statistics in China 2015, the incidence of GBC was 52.8 per 100,000 and the mortality was 40.7 per 100,000.1 Using the Surveillance, Epidemiology and End Results (SEER) database, patients with localized CCA who are selected for cancer-directed surgery are strongly associated with improved survival.2 Unfortunately, most patients barely have opportunity for surgery when diagnosed. SEER data revealed that only 12% patients with iCCA underwent hepatic resection. Even after resection, the rate of prolonged survival is low.3 Metastatic biliary tract cancers (mBTCs) have dismal life survivals of <1 year.4 Cisplatin plus gemcitabine is recommended as first-line therapy for mBTCs.5 In a Phase II study, the median overall survival (OS) was 11.7 months in the cisplatin–gemcitabine group and 8.1 months among the 206 patients in the capecitabine– cisplatin group (HR, 0.64; 95% CI: 0.52–0.80; P<0.001).4 Beyond these treatments, there are limited effective systemic therapy options. So more options are needed to prolong survival time for mBTCs patients.
Programmed death 1 (PD-1) inhibitor shows promising results in controlling kinds of tumors. Since Freeman confirmed PD-1/programmed death-ligand 1 (PD-L1) pathway, it definitely gives out an immune way to control cancer.6 In the multicenter Phase I trial published in 2012, the results showed that antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate [ORR] of 6%–17%) and prolonged stabilization of disease (rates of 12%–41% at 24 weeks) in patients with advanced cancers, including non–small-cell lung cancer, melanoma, and renal cell cancer.7 The earliest study mentioned PD-1 inhibitor with BTC showed that mismatch-repair status predicted clinical benefit of pembrolizumab.8 Patients with deficiency- mismatch repair (MMR) treated with pembrolizumab had an ORR of 71% in noncolorectal cancer patients, including four cases who had BTCs. Later KEYNOTE-028 studied the use of pembrolizumab in patients with BTCs.9 All patients were required to show more than 1% tumor PD-L1 expression. In this study, the ORR was 17% and the disease control rate (DCR) was 34%. However, there are few studies about nivolumab and BTCs, although nivolumab and pembrolizumab are the same type of PD-1 inhibitor. With the aim of exploring novel therapy, our center evaluated the clinical efficacy and safety of nivolumab for patients with mBTCs in this retrospective study and found the population that could potentially benefit from this treatment.
Patients and methods
Patients
From May 2016 to September 2018, 60 patients with mBTCs were voluntarily treated with nivolumab in non-clinical setting at the People’s Liberation Army General Hospital. A total of 30 patients were included in this review. This treatment was approved by our research ethics committee, and all necessary regulatory approvals were obtained. All patients signed the informed consent for immunotherapy. The inclusion criteria were as follows: 1) patients were eligible for the study if they had received a histopathological or cytologic diagnosis of BTC (iCCA or eCCA, or GBC); 2) patients with mBTCs had at least one measurable lesion, which was defined by Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1) and had done at least one measurement; 3) routine blood tests were done with tumor biomarker index and liver and kidney functions; and 4) signed informed consent before treatment. Data were retrospectively obtained from patients’ medical history. Patient consent to review their medical records had been included in the informed consent for immunotherapy. This retrospective study was approved by the local ethics committee of Chinese PLA General Hospital.
Treatment and dose modification
Nivolumab 3 mg/kg was given every 2 or 3 weeks until confirmed progression or unacceptable toxicity. Treatment was delayed because of severe toxic effects or patient’s intolerance. Treatment could be recommenced after further biliary stenting if patients were found to have obstructive jaundice. Combining nivolumab with other agents was left to the doctors’ choice as well as the general health status.
Efficacy and safety assessments
Patients were followed up till progression, death due to any reason, treatment interruption due to intolerable toxicity, or till the cut-off date of September 1, 2018. Disease progression and new lesion development were evaluated every two/three cycles by means of CT. Tumor responses were assessed according to the RECIST v1.1. Tumor responses included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR was defined as the addition of CR and PR (CR + PR). The DCR was defined as the addition of objective response and stabilization rates (CR + PR + SD). Toxicities or adverse events (AEs) were evaluated according to the National Cancer Institute Common Toxicity Criteria version 4.0 at every cycle. Blood samples were collected from all patients on the day before treatment during each therapy cycle. Primary analysis was progression-free survival (PFS), which was defined as the time period from initiating nivolumab treatment to disease progression or death, whichever occurred first. Secondary analysis was OS, tumor response, and AEs. OS was defined as the time period from initiating nivolumab treatment to the date of death due to any cause or last follow-up visit.
Statistical analysis
Quantitative data are presented as median (range) or number of patients (percentage). Survival analysis was conducted by the Kaplan–Meier analysis and comparison by the log-rank test. Exploratory univariate analyses were performed with the log-rank test using the following variables: age, gender, location, Eastern Cooperative Oncology Group (ECOG) score, number of metastases, PD-L1 expression status, and nivolumab treatment line, whether combined with chemotherapy or not. PD-L1 positivity was defined as staining in 1% of cells in tumor nests or PD-L1-positive band as assessed at a central laboratory by prototype immunohistochemical assay. Cox multivariate models were performed based on the univariate analyses results. The HR was calculated using Cox proportional hazard regression modeling. AEs were summarized using frequency counts and percentages. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was regarded as statistically significant.
Result
Between May 2016 and September 2018, a total of 30 patients were considered eligible for the final analysis. 30 out of 60 patients were excluded from the analysis because they had done no measurement after treatment or failed to follow-up. Clinicopathological characteristics at the initiation of nivolumab are shown in Table 1. Eighteen patients (60%) were male and 12 (40%) were female. The median age was 53 years (range among 36–80 years old). The histological type was moderately differentiated in 16 patients (53.3%) and low differentiated in nine patients (6.7%). ECOG performance status (PS) score of 0–1 was 10 (33.3%) and of 2–3 was 20 (66.7%). Primary tumors located in eCCA, including GBC were 15 (50%) and 15 (50%) in iCCA. Of the 30 patients with BTC who were screened for PD-L1 expression, 11 (36.6%) had PD-L1-positive tumors. Thirteen patients received nivolumab in first-line therapy and 17 (56.7%) received in second- or further-line therapy. Nivolumab monotherapy was administered in 13 patients (46.6%); in nine patients, nivolumab was combined with gemcitabine; and in eight patients, it was combined with oxaliplatin or Nab-Paclitaxel (nab-PTX). The median treatment cycle was four cycles. Seventeen patients had ≥2 metastases and 13 had <2. Twenty-two patients had liver metastasis, 14 had peritoneal lymph node metastasis, 8 had lung metastasis, 6 had bone metastasis, and 7 had peritoneal metastasis (including overlap). Five patients received percutaneous transhepatic cholangial drainage treatment.
Table 1.
Clinical characteristics of the study population (n=30)
| Characteristics | No (%) |
|---|---|
|
| |
| Age, years (median) | 53 (36–80) |
| <53 | 15 (50) |
| ≥53 | 15 (50) |
| Gender | |
| Male | 18 (60) |
| Female | 12 (40) |
| Location* | |
| Extrahepatic | 15 (50) |
| Intrahepatic | 15 (50) |
| ECOG PS* | |
| 0–1 | 10 (33.3) |
| 2–3 | 20 (66.7) |
| Line | |
| 1 | 13 (43.3) |
| 2 | 14 (46.7) |
| 3–4 | 3 (10) |
| Nivolumab combined | |
| No | 13 (46.6) |
| Gemcitabine and others | 17 (53.3) |
| Histological type | |
| Low | 9 (6.7) |
| Median | 16 (53.3) |
| High | 5 (16.7) |
| Cycle of treatment (range) | Median 4 (1–11) |
| <4 | 24 (80) |
| ≥4 | 6 (20) |
| PD-L1 expression^ | |
| Negative | 19 (63.3) |
| Positive | 11 (36.6) |
| Number of metastasis organs | |
| ≥2 | 17 (56.7) |
| <2 | 13 (43.3) |
| Organ of metastasis | |
| Liver | 22 (73.3) |
| Peritoneal lymph node | 14 (46.7) |
| Lung | 8 (26.7) |
| Bone | 6 (20.0) |
| Post-PTCD treatment | 5 (16.7) |
| Peritoneal metastasis | 7 (23.3) |
Notes:
Extrahepatic biliary tract cancers include eCCA and (GBC).
PD-L1 positive was defined as staining in 1% of cells in tumor nests or PD-L1-positive band as assessed at a central laboratory by prototype IHC assay.
Abbreviations: eCCA, extrahepatic cholangiocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; GBC, gallbladder cancers; PD-L1, programmed death-ligand 1; PTCD, percutaneous transhepatic cholangial drainage.
The median PFS was 3.1 months (95% CI: 2.13–4.06) (Figure 1). One patient (3.3%) achieved CR, five (16.7%) achieved PR, twelve (40%) were SD, and twelve (40%) were PD. The ORR and DCR were 20% and 60%, respectively (Table 2).
Figure 1.
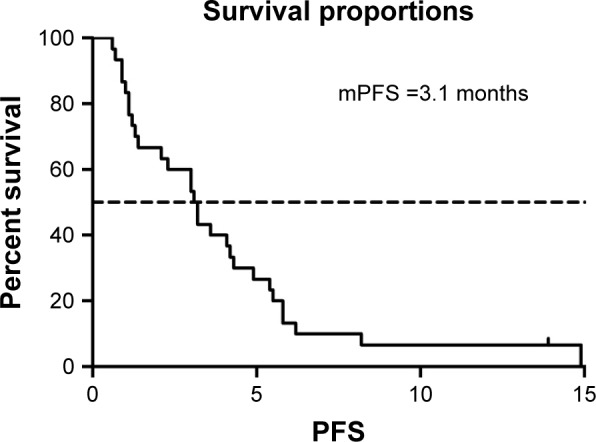
mPFS of patients (3.1 months, 95% CI: 2.13–4.06).
Abbreviation: mPFS, median progression-free survival.
Table 2.
Tumor response
| Response (n=30) | |
|---|---|
|
| |
| CR | 1 (3.3%) |
| PR | 5 (16.7%) |
| SD | 12 (40%) |
| PD | 12 (40%) |
| ORR | 20% |
| DCR | 60% |
| Median progression-free survival | 3.1 months |
Notes: DCR, CR + PR + SD; ORR, CR + PR.
Abbreviations: CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progression disease; PR, partial response; SD, stable disease.
We selected the eight variables for univariate analysis. The results are shown in Table 3. There was no statistical difference among patients with different gender, intrahepatic or extrahepatic tumor, ECOG PS score, and treatment line. PFS was 4.2 months in patients aged >53 years compared with 3.0 months in those <53 years (P<0.05) (Figure 2). More than two organ metastases seemed to be associated with shorter PFS (P<0.05) (Figure 3). Study also showed that nivolumab combined with chemotherapy had longer PFS compared with nivolumab only (4.3 vs 2.1, P<0.05) (Figure 4). With regard to the most concerned PD-L1 expression status, positive PD-L1 had no relationship with better PFS in contrast with negative PD-L1 (3.6 vs 3.0 P>0.05) (Figure 5). In multivariate Cox regression analysis, the PFS of nivolumab combined with chemotherapy was the only one predictor of longer PFS compared with nivolumab monotherapy (HR =0.43, 95% CI: 0.194–0.953, P<0.05) (Table 3).
Table 3.
Univariate and multivariate analyses
| Characteristics | PFS (months) | Univariate analyses P-value | Multivariate analyses HR (95% CI), P-value |
|---|---|---|---|
|
| |||
| Age (median 53 years) | 0.047* | ||
| <53 years | 3.0 | ||
| ≥53 years | 4.2 | ||
| Gender | 0.101 | ||
| Male | 3.0 | ||
| Female | 3.6 | ||
| Location | 0.808 | ||
| Extrahepatic | 3.2 | ||
| Intrahepatic | 3.0 | ||
| ECOG PS | 0.943 | ||
| 0–1 | 3.2 | ||
| 2–3 | 3.0 | ||
| Line | 0.093 | ||
| 1 | 3.2 | ||
| >1 | 3.0 | ||
| PD-L1 expression | 0.801 | ||
| Negative | 3.0 | ||
| Positive | 3.6 | ||
| Nivolumab combined | 0.031* | HR 0.43, 95% | |
| No | 2.3 | CI: 0.194–0.953 | |
| Gemcitabine or others | 4.1 | P<0.05 | |
| Number of metastasis | 0.049* | ||
| organs | |||
| ≥2 | 1.4 | ||
| <2 | 4.1 | ||
Note:
P<0.05.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death-ligand 1; PFS, progression-free survival.
Figure 2.
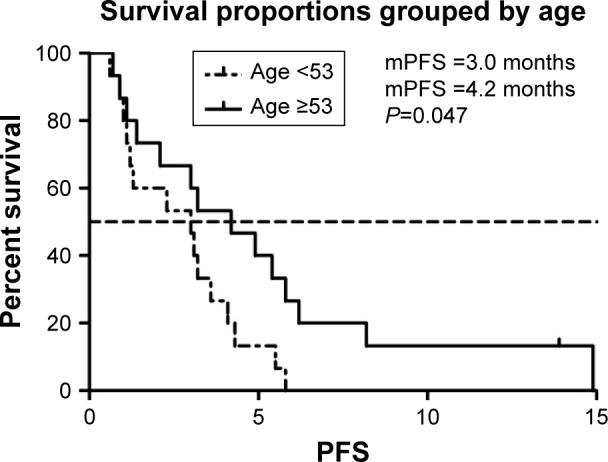
mPFS of patients of different age groups.
Abbreviation: mPFS, median progression-free survival.
Figure 3.
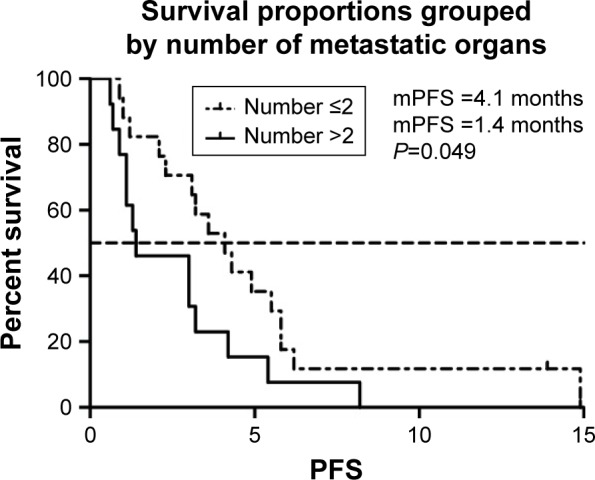
mPFS of patients of organ metastases.
Abbreviation: mPFS, median progression-free survival.
Figure 4.
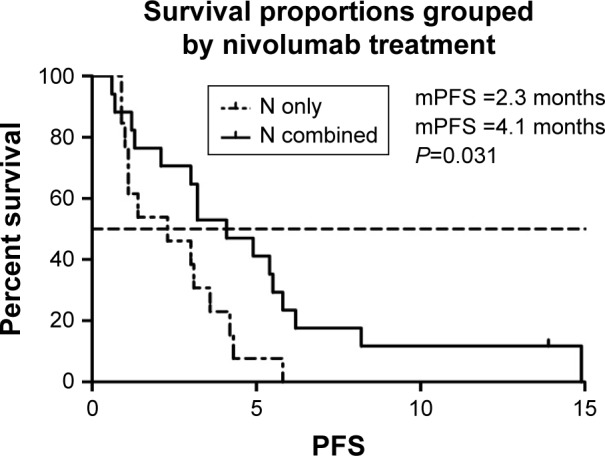
mPFS of patients of nivolumab treatment.
Abbreviation: mPFS, median progression-free survival.
Figure 5.
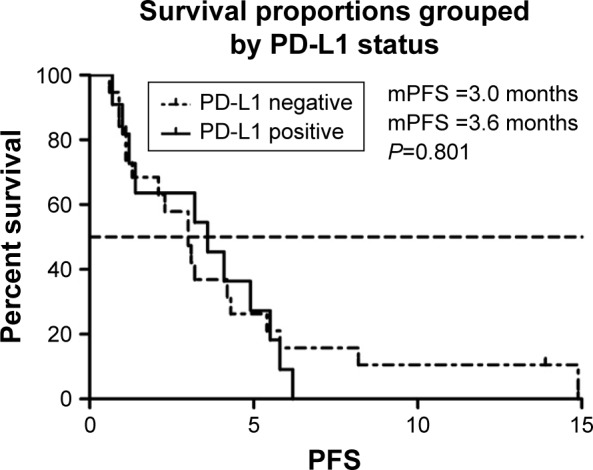
mPFS of patients of PD-L1 expression.
Abbreviations: mPFS, median progression-free survival; PD-L1, programmed death-ligand 1.
The AEs of nivolumab monotherapy were fatigue (three cases, 10%), fever (two cases, 6.7%), hypothyroidism (one case, 3.3%), skin reaction (one case, 3.3%), and liver injury (one case, 3.3%). In patients treated with nivolumab plus chemotherapy, the most common grade 1–2 AEs were leu-kopenia (five cases, 16.6%), thrombocytopenia (two cases, 6.7%), nausea and vomiting (four cases, 13.3%), fatigue (four cases, 13.3%), fever (three cases, 13.3%), peripheral neurotoxicity (three cases, 13.3%), and hypothyroidism (one case, 3.3%), while the most common grade 3–4 hematologic AEs were leukopenia (three cases, 10%). Most of these AEs, except peripheral sensory neuropathy caused by oxaliplatin and nab-PTX, can be managed. No serious acute hypersensitivity occurred during the study (Table 4).
Table 4.
Analysis of adverse events
| Adverse events | No (%)
|
||
|---|---|---|---|
| Nivolumab | Nivolumab+chemotherapy | ||
|
| |||
| 1–2 grade | 1–2 grade | 3–4 grade | |
|
| |||
| Non-hematologic | |||
| Rash | 1 (3.3) | 0 | 0 |
| Nausea/vomiting | 1 (3.3) | 4 (13.3) | 0 |
| Diarrhea | 0 | 2 (6.7) | 0 |
| Liver damage | 1 (3.3) | 0 | 0 |
| Fatigue | 3 (10) | 4 (13.3) | 0 |
| Peripheral sensory neuropathy | 0 | 3 (10) | 0 |
| Fever | 2 (6.7) | 3 (10) | 0 |
| Hypothyroidism | 1 (3.3) | 1 (3.3) | 0 |
| Alopecia | 0 | 0 | 0 |
| Hematologic | |||
| Anemia | 0 | 1 (3.3) | 0 |
| Leukopenia | 0 | 5 (16.7) | 3 (10) |
| Thrombocytopenia | 0 | 2 (16.7) | 0 |
Discussion
BTC is a kind of highly aggressive neoplasm characterized by the lack of effective therapy and dismal prognosis.10 Continued advances in treatment offer hope for improving the prognosis of this group of highly lethal cancers.11 National Comprehensive Cancer Network Guidelines recommend combination therapy of gemcitabine plus cisplatin as category 1 therapy. In BTCs, novel therapeutic approaches include targeting fibroblast growth factor receptor 2, isocitrate dehydrogenase 1/2, PD-L1/PD-1, tumor mutational burden, cyclin-dependent kinase inhibitor 2A, mesenchymal–epithelial transition, BRAF, BAP1, EGFR, MMR deficiency, and ERBB2 (HER2).12 This study is the first retrospective study of an immune checkpoint inhibitor in patients with mBTCs.
The median PFS was 3.1 months (95% CI: 2.13–4.06). Compared with previous data from Phase III trials that support the current standard therapy for patients with BTC, cisplatin–gemcitabine group had PFS with a median of 8.0 months (95% CI: 6.6–8.6).4 It appears as a large difference between the two therapies. With regard to baseline characteristics, this trial had included 23.8% locally advanced patients and 76.2% metastatic patients and nearly 90% patients had good condition to receive first-line chemotherapy. Instead, 66.7% patients in our study had poor PS and only 43.3% patients received first-line therapy; 46.5% were given nivolumab in second-line therapy, and 3 in 30 patients were in third- or fourth-line therapy. All patients harbor at least one metastasis organ.
In recent intern results of KEYNOTE-028 about safety and efficacy of pembrolizumab (MK-3475) in patients with advanced BTC, ORR was 17% (95% CI: 5%–39%).9 Four (17%) patients had PR, 4 (17%) had SD, and 12 (52%) had PD as their best response. The result is numerically similar to our study with ORR of 20% and DCR of 60%. Interestingly, one patient had CR with a PFS of 14.9 months administered with nivolumab plus cisplatin and gemcitabine in our study. Another patient remains on treatment (duration of treatment, 13.9 months). The result shows that PD-1 inhibitor had better disease control.
When treated with nivolumab, patients of different genders, different tumor locations, different ECOG PS scores, and different treatment lines showed no significant difference in PFS. Our results showed a significant survival benefit in older patients than in younger ones, which might be ascribed to tumor’s more aggressive biological behavior in young patients than in old ones. Age itself was a high-risk factor in many cancers.13 Furthermore, when patients had more than two organ metastases, they had shorter PFS (P<0.05). Similar relationship between organ metastases and patients’ survival has been reported in many studies.14,15 Technically, the more tumor burden, the more the patients suffer.
Our study also showed that patients treated with nivolumab plus chemotherapy had a significant longer PFS than those treated with only nivolumab. (HR =0.43, 95% CI: 0.194–0.953, P<0.05). Early studies of PD-1 inhibitor involved PD-1 inhibitor comparing with chemotherapy in first- or second-line in advanced Non-small-cell lung cancer.16–18 Recent studies began to explore PD-1/PD-L1 inhibitor plus different options, such as chemotherapy and radiotherapy.19,20 In KEYNOTE 189 study, estimated rate of OS at 12 months was 69.2% (95% CI: 64.1–73.8) in the pembrolizumab-combination group vs 49.4% (95% CI: 42.1–56.2) in the placebo-combination group (HR =0.49, 95% CI: 0.38–0.64, P<0.001).19 The addition of pembrolizumab to standard chemotherapy of pemetrexed and a platinum-based drug resulted in significantly longer OS and PFS than chemotherapy alone. Data from IMpower150 reported in American Association for Cancer Research 2018 show that, regardless of PD-L1 expression level, atezolizumab (anti-PD-L1) plus bevacizumab plus chemotherapy can prolong PFS compared with only bevacizumab plus chemotherapy.21 Our study showed that nivolumab with chemotherapy had longer PFS than nivolumab only. This may suggest that nivolumab combined therapies are promising treatment regimens.
Recent clinical trials using PDL1/PD-1 immune checkpoint blockade agents have shown fantastic efficacy in various malignancies, with responses strongly associated with PD-L1 expression, as assessed by immunochemistry staining.22 However, in our study, baseline tumor cell PD-L1 status seems not to have an apparent effect on PFS. Patients with positive expression of PD-L1 had median PFS (mPFS) of 3.6 months and patients with negative PD-L1 had mPFS of 3.0 months in our study. Data from KEYNOTE-028 also showed no survival benefit.9 Although there was no statistical difference, we can see a trend that positive PD-L1 expression seemed to be associated with better PFS. Tumor mutation burden (TMB) is an emerging biomarker with utility in predicting response to immunotherapy.23,24 Increased TMB is thought to lead to increased neo-epitope production, which attracts tumor lymphocyte infiltration.24 However, we hadn’t done TMB in this study due to the potential heavy burden for patients.
AEs are of concern in most studies. In general, immune-related AEs occur quite early, mostly within weeks to 3 months after initiation of immune checkpoint blockers. In our study, there were no drug-related serious AEs reported during treatment, neither hematologic nor non-hematologic toxic events. The AEs of nivolumab were only fatigue (10%), fever (6.7%), hypothyroidism (3.3%), skin reaction (3.3%), and liver injury (3.3%). Rash is the most common AE in the ipilimumab combined with nivolumab in the European Society For Medical Oncology clinical guideline for management of toxicities from immunotherapy.25 The reported thyroid dysfunction rate varies from 5% to 10%, irrespective of tumor types.26 Hepatitis occurs in 5%–10% (of which 1%–2% is grade 3) of patients during therapy with ipilimumab, nivolumab, and pembrolizumab at the approved doses as single agents.27 There was no pneumonitis associated with checkpoint blockade onset reported in our study. The AEs of chemotherapy plus PD-1 inhibitor were mainly hematologic as expected. The incidence of neuro-related AEs is reported as 1%.25 Chemotherapy may induce peripheral neurotoxicity and enhance the incidence of PD-1 inhibitor related neuro-related AE.
Limitations of our study included small sample size and the absence of data about patient quality of life and overall survival.
In this single-center retrospective study, the safety of nivolumab in advanced/metastatic BTCs is controllable. Further selection of superior populations is needed to improve the efficacy of nivolumab in mBTC.
Acknowledgments
This paper is supported by the National Natural Science Foundation of China (31671298).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma – a population-based study. Ann Surg Oncol. 2008;15(2):600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22(7):1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 4.Park K, Kim KP, Park S, Chang HM. Comparison of gemcitabine plus cisplatin versus capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer. Asia Pac J Clin Oncol. 2017;13(1):13–20. doi: 10.1111/ajco.12592. [DOI] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 6.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asaoka Y, Ijichi H, Koike K. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;373(20):1979. doi: 10.1056/NEJMc1510353. [DOI] [PubMed] [Google Scholar]
- 9.Bang YJ, Doi T, Braud FD, et al. 525 safety and efficacy of pembrolizumab (MK-3475) in patients (PTS) with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur J Cancer. 2015;51:S112. [Google Scholar]
- 10.Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol. 2015;33(16):1845–1848. doi: 10.1200/JCO.2014.59.7591. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Yu SE, Kim KH, et al. Individualized metabolic profiling stratifies pancreatic and biliary tract cancer: a useful tool for innovative screening programs and predictive strategies in healthcare. EPMA J. 2018;9(3):287–297. doi: 10.1007/s13167-018-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deleon TT, Ahn DH, Bogenberger JM, et al. Novel targeted therapy strategies for biliary tract cancers and hepatocellular carcinoma. Future Oncol. 2018;14(6):553–566. doi: 10.2217/fon-2017-0451. [DOI] [PubMed] [Google Scholar]
- 13.Chong VH, Telisinghe PU, Bickle I, Abdullah MS, Lim E, Chong CF. Increasing incidence of colorectal cancer, starting at a younger age for rectal compared to colon cancer in Brunei Darussalam. Asian Pac J Cancer Prev. 2015;16(12):5063–5067. doi: 10.7314/apjcp.2015.16.12.5063. [DOI] [PubMed] [Google Scholar]
- 14.Hendriks LE, Derks JL, Postmus PE, et al. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: results from a population-based study. Eur J Cancer. 2015;51(17):2534–2544. doi: 10.1016/j.ejca.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto Y, Hayashi N, Sakamoto Y, et al. Predictors of long-term survival in patients with stage IV colorectal cancer with multi-organ metastases: a single-center retrospective analysis. Int J Clin Oncol. 2015;20(6):1140–1146. doi: 10.1007/s10147-015-0835-2. [DOI] [PubMed] [Google Scholar]
- 16.Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 17.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matter-Walstra K, Schwenkglenks M, Aebi S, et al. A cost-effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol. 2016;11(11):1846–1855. doi: 10.1016/j.jtho.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 20.Sagiv-Barfi I, Rajapaksa A, Czerwinski D, et al. Abstract 2941: local tumor irradiation combined with α-PDL-1 immune checkpoint inhibition results in local and systemic anti-tumor responses: successful translation of a mouse model to a human case series. Cancer Research. 2014;74(19 Supplement):2941. [Google Scholar]
- 21.Reck M, Socinski MA, Cappuzzo F, et al. 134PD primary PFS and safety analyses of a randomized phase III study of carboplatin + paclitaxel +/− bevacizumab, with or without atezolizumab in 1L non-squamous metastatic NSCLC (IMpower150) J Thorac Oncol. 2018;13(4):S77–S78. [Google Scholar]
- 22.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan TA, Wolchok JD, Snyder A. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2015;373(20):1984. doi: 10.1056/NEJMc1508163. [DOI] [PubMed] [Google Scholar]
- 24.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 26.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 27.Valsecchi ME. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]


