Abstract
Synapse formation is comprised of target cell recognition, synapse assembly, and synapse maintenance. Maintaining established synaptic connections is essential for generating functional circuitry and synapse instability is a hallmark of neurodegenerative disease. While many molecules impact synapse formation generally, we know little about molecules that affect synapse maintenance in vivo. Using genetics and developmental time course analysis in C.elegans, we show that the α-tubulin acetyltransferase ATAT-2 and the signaling hub RPM-1 are required presynaptically to maintain stable synapses. Importantly, the enzymatic acetyltransferase activity of ATAT-2 is required for synapse maintenance. Our analysis revealed that RPM-1 is a hub in a genetic network composed of ATAT-2, PTRN-1 and DLK-1. In this network, ATAT-2 functions independent of the DLK-1 MAPK and likely acts downstream of RPM-1. Thus, our study reveals an important role for tubulin acetyltransferase activity in presynaptic maintenance, which occurs via the RPM-1/ATAT-2 pathway.
Research organism: C. elegans
Introduction
Synapse formation is comprised of several steps including target recognition, synapse assembly and synapse maintenance (Chia et al., 2013; Jin and Garner, 2008). Synapse maintenance, also referred to as synapse stability, is required to complete the synapse formation process, and is also important for maintaining circuitry and allowing plasticity throughout an animal’s life (Lin and Koleske, 2010). Indeed, increasing evidence indicates synapse instability is a hallmark of many neurodegenerative diseases, including Alzheimer’s disease (Lin and Koleske, 2010; Selkoe, 2002; Spires-Jones and Hyman, 2014). Understanding how synapse maintenance influences nervous system development, plasticity and disease will require far greater knowledge of the molecules and signaling networks that regulate this process.
Previous genetic studies have been invaluable for informing our understanding of how synapse maintenance is regulated in vivo. At the fly neuromuscular junction (NMJ), genes encoding regulators of the microtubule cytoskeleton, such as Dynactin and Ankyrin, are crucial for maintaining the presynaptic terminal (Eaton et al., 2002; Pielage et al., 2008). Spectrin, a scaffold that links cell adhesion with the microtubule cytoskeleton, is also important for NMJ synapse maintenance (Massaro et al., 2009; Pielage et al., 2005).
In C. elegans, mechanosensory neurons that form glutamatergic neuron-neuron synapses (reminiscent of mammalian central synapses) have proven particularly valuable for understanding the molecular and genetic underpinnings of synapse maintenance. For instance, pharmacological and genetic perturbation of microtubules impairs presynaptic bouton maintenance in these cells (Chen et al., 2014). Genetic screens using mechanosensory neurons revealed that the microtubule minus-end binding protein PTRN-1/CAMSAP and the actin binding protein ZYX-1 are required for synapse maintenance (Luo et al., 2014; Marcette et al., 2014; Richardson et al., 2014). Thus, studies from both flies and worms emphasize the power genetic model systems wield in identifying molecules, and potentially unraveling entire signaling networks, that are required for synapse maintenance.
While increasing evidence has linked genetic perturbation of the microtubule cytoskeleton with synapse deterioration, it remains unknown whether mutants that affect post-translational modification of microtubules, such as acetylation, affect synapse maintenance. Two α-tubulin acetyltransferases, MEC-17 and ATAT-2, were identified in C. elegans. MEC-17 and ATAT-2 function via enzymatic acetyltransferase activity and non-enzymatic mechanisms to regulate microtubule structure, touch sensation, axon polarity and axon degeneration in mechanosensory neurons (Akella et al., 2010; Neumann and Hilliard, 2014; Shida et al., 2010; Topalidou et al., 2012). Despite this prior work, it remains unknown if α-tubulin acetyltransferases affect the synapse, and in particular synapse maintenance, in any system. The importance of addressing this question is highlighted by evidence that altered α-tubulin acetylation is associated with neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease (Godena et al., 2014; Govindarajan et al., 2013; Hempen and Brion, 1996; Pellegrini et al., 2017).
The Pam/Highwire/RPM-1 (PHR) proteins, including C. elegans RPM-1, are enormous signaling hubs that also have ubiquitin ligase activity (Grill et al., 2016). PHR proteins are important regulators of neuronal development with conserved roles in synapse formation (Bloom et al., 2007; Schaefer et al., 2000; Wan et al., 2000; Zhen et al., 2000), axon guidance (Bloom et al., 2007; Lewcock et al., 2007; Park and Rongo, 2018) and axon termination (Borgen et al., 2017b; Feoktistov and Herman, 2016; Schaefer et al., 2000). PHR proteins regulate synapse formation at NMJs (Bloom et al., 2007; Wan et al., 2000; Zhen et al., 2000) and glutamatergic neuron-neuron synapses formed by C. elegans mechanosensory neurons (Schaefer et al., 2000). At present, it is unclear whether PHR proteins impact synapse formation by regulating synapse assembly or maintenance. Furthermore, PHR protein signaling can influence microtubules in the context of axon guidance and termination (Borgen et al., 2017b; Hendricks and Jesuthasan, 2009; Lewcock et al., 2007). However, whether there is a functional genetic relationship between PHR proteins and tubulin acetyltransferases and, if so, how this influences synapse formation and maintenance remains unknown.
Here, we use developmental time course analysis, genetics and pharmacology to show that the α-tubulin acetyltransferase ATAT-2 regulates synapse maintenance in mechanosensory neurons, and does so via its enzymatic acetyltransferase activity. Moreover, ATAT-2 functions in a pathway with RPM-1 to regulate presynaptic maintenance in mechanosensory neurons, as well as behavioral habituation to repeated gentle touch. Genetic analysis indicates that RPM-1 is a hub in a network containing ATAT-2, PTRN-1 and DLK-1. Importantly, the RPM-1/ATAT-2 pathway represents a mechanism that functions independent of DLK-1 to regulate synapse maintenance. Overall, our findings not only reveal a novel, functional role for ATAT-2, but place it within an RPM-1 signaling network that is required for synapse maintenance.
Results
RPM-1 regulates presynaptic bouton maintenance during development
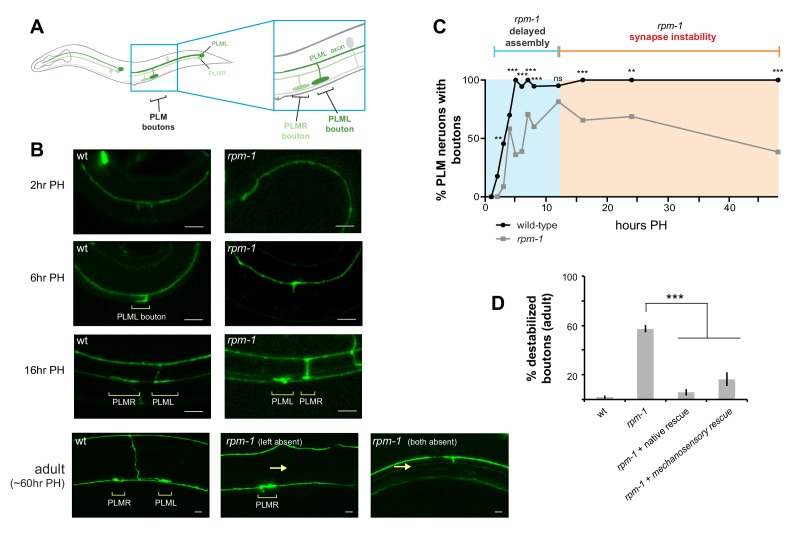
C. elegans has two PLM mechanosensory neurons, each of which has a single primary axon that extends a collateral branch to form chemical synapses (Figure 1A,B). Similar to neurons in the mammalian central nervous system, C. elegans mechanosensory neurons form glutamatergic, neuron-neuron connections (Chalfie et al., 1985; Lee et al., 1999). Previous work showed RPM-1, the C. elegans PHR protein, is an important regulator of PLM neuron synapse formation (Grill et al., 2016; Schaefer et al., 2000). In adult animals, the PLM neurons of rpm-1 mutants lack both the collateral branch and chemical synapses (Figure 1B). At present, it is uncertain whether this defect arises from failed synapse assembly or impaired synapse maintenance. To address this, we began by revisiting time-course analysis of PLM presynaptic bouton development in wild-type (wt) animals and rpm-1 mutants. When L1 larvae hatch, the primary PLM axon has extended, but the synaptic branch is absent (Figure 1B,C). By 6 hr post-hatch (PH), wt animals have formed morphological presynaptic boutons (Figure 1B,C). In contrast, synaptic bouton development is delayed in rpm-1 mutants at 6 hours PH. Despite this initial delay, rpm-1 mutants eventually form synaptic boutons, which are readily observable at 12 hr PH (Figure 1B,C). Between 12 and 48 hr PH, boutons destabilize and the synaptic branch retracts in rpm-1 mutants to phenotypic levels characteristic of adult rpm-1 mutants (Figure 1B,C). Transgenic rescue showed expression of RPM-1 using the native promoter or a mechanosensory neuron promoter rescued these defects (Figure 1D). These results indicate that RPM-1 primarily regulates presynaptic bouton maintenance by functioning cell autonomously in mechanosensory neurons. Notably, our results differ from a prior study that suggested synaptic branch defects in rpm-1 mutants arise primarily from impaired initial bouton formation with a relatively small frequency of bouton loss over time (Schaefer et al., 2000).
Figure 1. RPM-1 functions cell autonomously in mechanosensory neurons to regulate presynaptic bouton maintenance.
(a) Schematic highlighting location of collateral synaptic branch and chemical synapses in PLM mechanosensory neurons. (b) Confocal images at different developmental time points showing presynaptic boutons of PLM neurons are delayed in formation and destabilize in rpm-1 mutants. Note brackets denote PLM presynaptic boutons (PLML and PLMR) and arrows highlight synaptic branch retraction. Note that in confocal images at 16 hr and 60 hr PH, PLML synaptic branch is out of focal plane but bouton is visible. (c) Developmental time course showing synaptic boutons in PLM neurons of rpm-1 mutants are delayed in formation but reach normal levels by 16 hr PH (blue). Subsequently, rpm-1 mutant boutons are progressively lost over time (orange). (d) Quantitation showing bouton maintenance defects in adult rpm-1 mutants, and rescue with transgenic RPM-1 expressed using native rpm-1 promoter or mechanosensory neuron promoter. Significance tested using Fisher’s exact test for c, and Student’s t-test with Bonferroni correction for d. ***p<0.001, **p<0.01 and ns = not significant (p>0.05).
RPM-1 is localized to presynaptic terminals during development
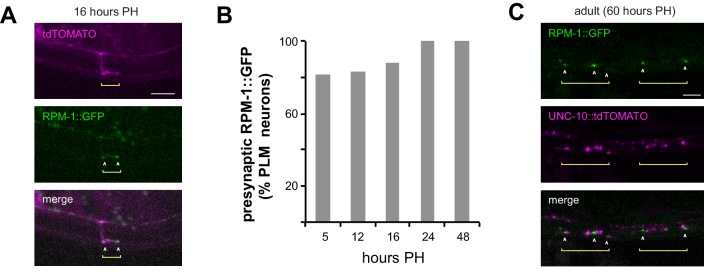
To assess RPM-1 localization during development, we transgenically expressed RPM-1::GFP in mechanosensory neurons along with tdTOMATO as a cell fill (Figure 2). RPM-1 was observed at presynaptic boutons during synapse development (Figure 2A). RPM-1 localization to presynaptic boutons occurred as early as 5 hr PH, when boutons are first consistently present, and was observed through adulthood (Figure 2B,C).
Figure 2. RPM-1 localizes to presynaptic terminals of developing and adult mechanosensory neurons.
(a) Confocal images showing RPM-1::GFP localized at presynaptic boutons of PLM neuron at 16 hr PH. tdTOMATO shows PLM axon and presynaptic terminal morphology. (b) Quantitation of RPM-1::GFP presynaptic localization in PLM neurons at different times in development. (c) Confocal image showing RPM-1 localized to periactive zones adjacent to active zone marker UNC-10::tdTOMATO in adults.
To confirm RPM-1 is localized at presynaptic terminals, we used confocal microscopy to evaluate localization of RPM-1 and the active zone component UNC-10/RIM (Koushika et al., 2001). In adult animals, RPM-1 localized directly adjacent to UNC-10 at presynaptic terminals (Figure 2C). This indicates RPM-1 localizes to the periactive zone of presynaptic terminals in mechanosensory neurons. Our observation is consistent with prior studies that examined RPM-1 localization in motor neurons (Abrams et al., 2008).
Collectively, these results support several conclusions. 1) RPM-1 is localized to presynaptic terminals, which is consistent with its role in presynaptic maintenance. 2) Our observation that RPM-1 is present early in the synapse formation process and persists into adulthood suggests signaling that affects presynaptic maintenance could be initiated relatively early in the synapse formation process. 3) Localization of RPM-1 to presynaptic terminals early in development is consistent with delayed presynaptic bouton formation in rpm-1 mutants.
rpm-1 mutants assemble synapses prior to synapse destabilization
Defects in presynaptic bouton morphology and retraction of the synaptic branch suggested rpm-1 mutants have synapse maintenance defects (Figure 1). Failed synapse maintenance could arise because synapses deteriorate or because the synaptic branch successfully extends to postsynaptic target neurons, but fails to properly assemble presynaptic components.
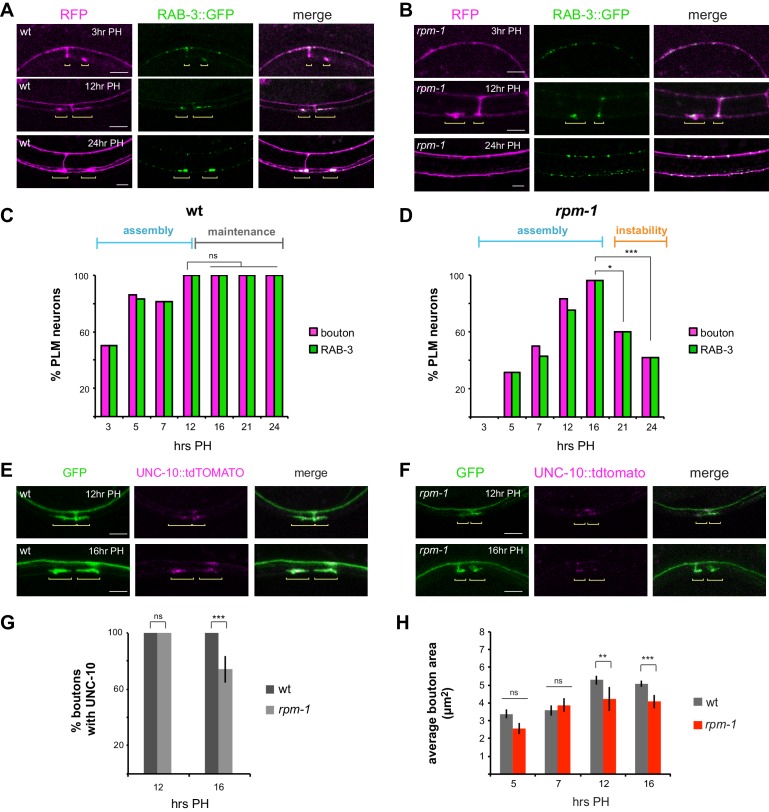
To initially test this, we evaluated a synaptic vesicle marker, RAB-3 (Luo et al., 2014; Nonet et al., 1997). Using integrated transgenes expressing RAB-3::GFP and RFP cell fill in mechanosensory neurons, we evaluated several developmental time points. In wt animals, synaptic boutons began to form by 3 hr PH, and we observed RAB-3::GFP accumulation at presynaptic terminals even at this early time point in synapse assembly (Figure 3A). We also noted that RAB-3 accumulated at the axon point where the synaptic branch initially descends (Figure 3A). At 12 and 24 hr PH, RAB-3 was enriched at presynaptic terminals (Figure 3A). In rpm-1 mutants, we observed accumulation of RAB-3 at presynaptic terminals by 12 hours PH (Figure 3B). In contrast to wt animals, synapses destabilize in rpm-1 mutants with synaptic branches retracting by 24 hours PH and RAB-3 present only in the primary axon (Figure 3B).
Figure 3. RPM-1 regulates synapse maintenance.
(a) Confocal images showing synaptic vesicle marker RAB-3 (green) at presynaptic terminals of PLM mechanosensory neurons during development. RFP shows PLM morphology (magenta). Yellow brackets highlight presynaptic terminals of PLML and PLMR neurons. Note one synaptic branch is out of focal plane. (b) rpm-1 mutants accumulate RAB-3 (green) at presynaptic terminals by 12 hours PH, but presynaptic terminals fail to be maintained leading to synaptic branch retraction by 24 hr PH. (c) Developmental time course of presynaptic boutons and RAB-3::GFP accumulation in wt animals. Full assembly of presynaptic terminals with RAB-3 occurs by 12 hr PH. (d) Developmental time course of presynaptic boutons and RAB-3::GFP in rpm-1 mutants. Presynaptic assembly with RAB-3 is complete by 16 hours PH, but is not maintained and presynaptic terminals are lost at later time points. (e, f) UNC-10::tdTOMATO marks the active zone and assembles at presynaptic terminals in e) wt and (f) rpm-1 mutants at critical synapse assembly time points of 12 and 16 hr PH. (g) Quantitation of boutons containing UNC-10. At 12 hr PH, all boutons contain UNC-10 in wt and rpm-1 mutants. At 16 hr PH, there is a small defect in UNC-10 accumulation at presynaptic terminals of rpm-1 mutants. (h) Quantitation of bouton area. rpm-1 boutons are initially the same size as wt boutons (5 and 7 hours PH). rpm-1 mutants show small decreases in bouton size just prior to synapse loss (12 and 16 hr PH). Significance tested using Fisher’s exact test. ***p<0.001, *p<0.05 and ns = not significant.
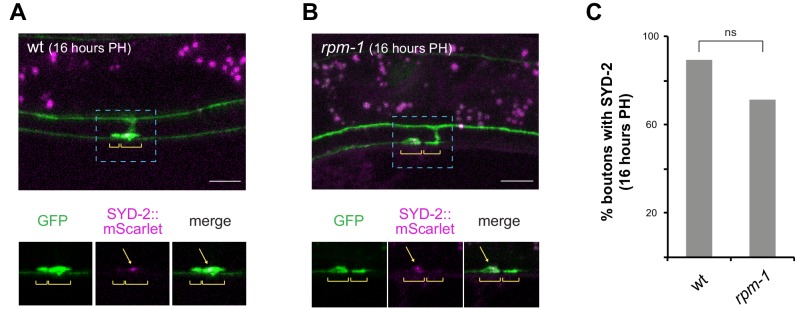
Figure 3—figure supplement 1. SYD-2 active zone marker accumulates in rpm-1 mutants at 16 hr PH.
Quantitation across a wider range of developmental time points showed that in wt animals presynaptic bouton frequency is maximal at 12 hr PH, and RAB-3 accumulates in every bouton (Figure 3C). RAB-3 accumulation at presynaptic terminals was maintained across all time points examined (Figure 3C). For rpm-1 mutants, quantitation indicated presynaptic bouton formation is slightly delayed, but all PLM neurons in rpm-1 mutants have presynaptic boutons containing RAB-3 by 16 hr PH (Figure 3D). These results are consistent with rpm-1 mutants having largely normal, although slightly delayed, synapse assembly. In contrast, presynaptic maintenance is strongly impaired in rpm-1 mutants with significant, rapid loss of presynaptic boutons and RAB-3 by 21 hr PH, and further reductions by 24 hours PH (Figure 3D). Notably, presynaptic terminals that do not destabilize in rpm-1 mutants retain RAB-3 (Figure 3D).
Next, we tested two presynaptic active zone markers, UNC-10/RIM and SYD-2/Liprin. We focused on 12 and 16 hr PH, as RAB-3 analysis demonstrated these are key time points for assessing completion of synapse assembly (Figure 3A,C). In wt animals, UNC-10::tdTOMATO labeled presynaptic terminals at both 12 and 16 hr PH (Figure 3E). Likewise, presynaptic terminals of rpm-1 mutants contained UNC-10 at 12 and 16 hr PH (Figure 3F). Quantitation indicated that UNC-10 is at all presynaptic terminals in rpm-1 mutants at 12 hours PH, and the majority of terminals at 16 hours PH (Figure 3G). Similar results occurred with mScarlet::SYD-2 (Figure 3—figure supplement 1). Interestingly, a small but significant decrease in the number of terminals with UNC-10 occurred in rpm-1 mutants at 16 hr PH compared to wt animals (Figure 3G). While a subtle observation, 16 hr PH is a critical time point just prior to synapse destabilization and branch retraction in rpm-1 mutants. This observation prompted us to also assess presynaptic bouton size over development in rpm-1 mutants. Bouton size was normal in rpm-1 mutants at 5 and 7 hr PH (Figure 3H). A small, but significant, decrease in presynaptic bouton size emerged in rpm-1 mutants at 12 and 16 hr PH (Figure 3H).
These results with multiple presynaptic markers support several important points. First, our results indicate that synapses assemble in rpm-1 mutants, and while delayed, this process is largely normal in rpm-1 mutants. Second, analysis of presynaptic bouton morphology, RAB-3 and UNC-10 indicate synapses rapidly destabilize in the absence of RPM-1. Finally, we observed subtle changes in bouton size and UNC-10 accumulation just prior to synapse destabilization, which suggests these mild presynaptic changes are likely to signal the onset of failed synapse maintenance. Taken as a whole, these results indicate RPM-1 is an important regulator of synapse maintenance in mechanosensory neurons.
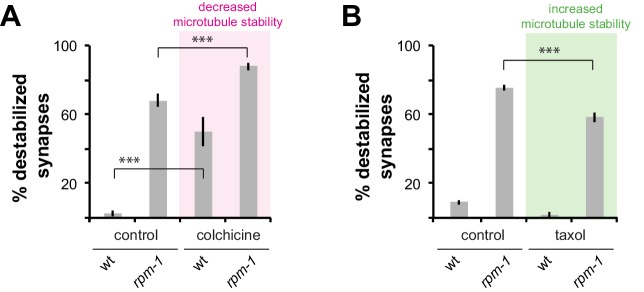
Loss of RPM-1 in combination with pharmacological manipulation of microtubule stability enhances synapse destabilization
We previously showed that RPM-1 signaling affects microtubule stability during growth cone collapse and axon termination (Borgen et al., 2017b). Therefore, we wanted to assess how synapse maintenance defects in rpm-1 mutants are affected by pharmacological manipulation of microtubule stability. Consistent with prior work (Chen et al., 2014; Richardson et al., 2014), treating wt animals with colchicine, a microtubule-destabilizing drug, resulted in loss of PLM synapses (Figure 4A). Treating rpm-1 mutants with colchicine significantly enhanced synapse maintenance defects (Figure 4A). Conversely, treatment of rpm-1 mutants with the microtubule-stabilizing drug taxol suppressed synapse maintenance defects (Figure 4B). These results are consistent with destabilized synapses in rpm-1 mutants resulting from less stable microtubules.
Figure 4. Drugs that alter microtubule stability affect synapse maintenance defects in rpm-1 mutants.
(a) Decreasing microtubule stability with colchicine enhances synapse maintenance defects in rpm-1 mutants. (b) Increasing microtubule stability with taxol suppresses synapse maintenance defects in rpm-1 mutants. Significance tested using Student’s t-test with Bonferroni correction. ***p<0.001.
ATAT-2 and RPM-1 function in a pathway to regulate synapse maintenance
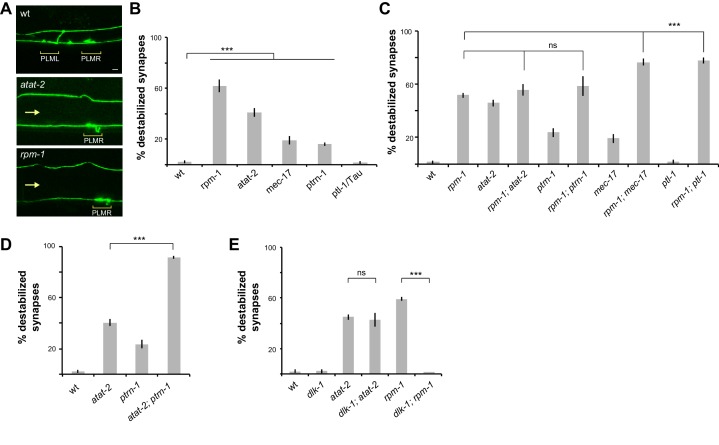
Next, we wanted to test the genetic relationship between RPM-1 and molecules that affect microtubules. We started by evaluating how null mutants for different microtubule binding proteins and tubulin acetyltransferases affect synapse maintenance. Interestingly, loss of function in atat-2, an α-tubulin acetyltransferase, resulted in synapse maintenance defects similar to rpm-1 mutants in which presynaptic boutons are lost and the synaptic branch is absent (Figure 5A). Quantitation indicated theses defects occurred at a moderate but significant frequency in atat-2 mutants (Figure 5B). This observation was confirmed using a second transgenic background (Figure 5—figure supplement 1). Destabilized synapses were also observed in mutants for mec-17, another α-tubulin acetyltransferase isoform, and the minus-end binding protein ptrn-1 (Figure 5B). However, defects in these mutants occurred at lower frequency than in atat-2 mutants. Our observation that ptrn-1 affects PLM synapse maintenance is consistent with a prior study (Marcette et al., 2014). Synapse maintenance defects were not observed in ptl-1/Tau mutants (Figure 5B).
Figure 5. Several mutants that affect microtubules interact with rpm-1 to affect synapse maintenance.
(a) Confocal images of presynaptic boutons and synaptic branches in adult PLM neurons. In wt animal, presynaptic boutons from PLML and PLMR are visible. Note one synaptic branch is shown and the other is out of the focal plane. atat-2 and rpm-1 mutants lack a synaptic branch and only show PLMR bouton (note loss of PLML, arrow). (b) Quantitation of synapse maintenance defects for indicated genotypes. Note atat-2 shows higher frequency defects than ptrn-1 or mec-17. (c) Quantitation showing synapse maintenance defects are similar in rpm-1; atat-2 and rpm-1; ptrn-1 double mutants compared to rpm-1 single mutants. In contrast, rpm-1; mec-17 and rpm-1; ptl-1 double mutants show enhanced defects compared to rpm-1 single mutants. (d) Quantitation indicates synapse maintenance defects are enhanced in atat-2; ptrn-1 double mutants compared to atat-2 single mutants. (e) Quantitation showing synapse maintenance defects are suppressed in rpm-1; dlk-1 double mutants, but not atat-2; dlk-1 double mutants. Significance tested using Student’s t-test with Bonferroni correction. ***p<0.001 and ns = not significant.
Figure 5—figure supplement 1. Analysis with a second transgenic background indicates atat-2 regulates synapse maintenance.
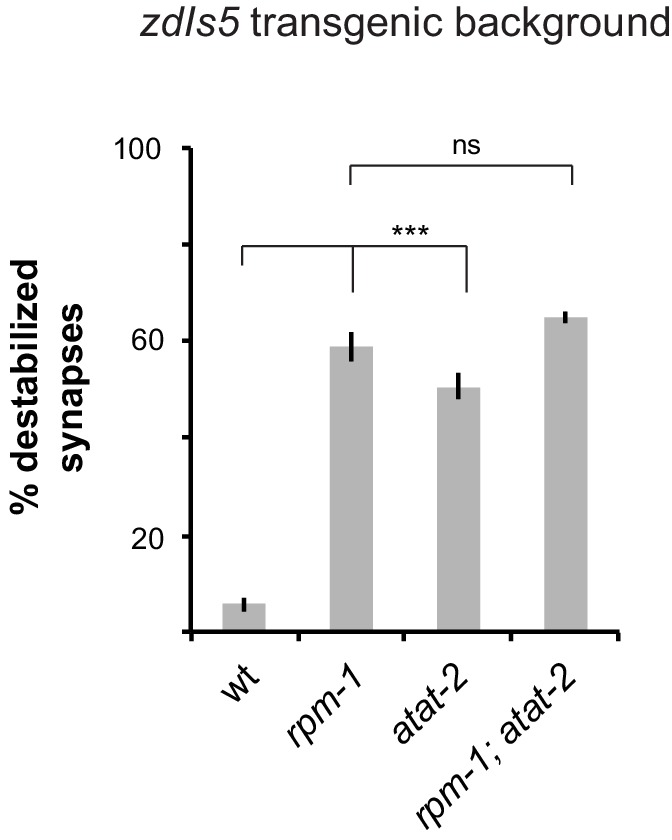
Having tested how different mutants for microtubule binding proteins and tubulin acetyltransferases impact synapse maintenance in PLM mechanosensory neurons, we constructed double mutants with rpm-1. Interestingly, rpm-1; atat-2 double mutants showed a similar frequency of synapse maintenance defects as rpm-1 single mutants (Figure 5C). We validated this result using a second transgenic background (Figure 5—figure supplement 1). These results demonstrate that ATAT-2 functions in the same pathway as RPM-1. Similarly, the frequency of synapse maintenance defects was not increased in rpm-1; ptrn-1 double mutants compared to single mutants, which suggests PTRN-1 and RPM-1 function in the same pathway (Figure 5C). We note that this result differs with a prior study that suggested RPM-1 and PTRN-1 function in parallel pathways to regulate synapse formation (Marcette et al., 2014). In contrast to outcomes with atat-2 and ptrn-1, the frequency of destabilized synapses was enhanced in rpm-1; mec-17 and rpm-1; ptl-1 double mutants (Figure 5C). These results indicate that MEC-17 and PTL-1/Tau function in parallel pathways with RPM-1 to regulate synapse maintenance.
Given our observations indicating that RPM-1 functions in the same pathway as both ATAT-2 and PTRN-1, we tested the genetic relationship between ptrn-1 and atat-2. To do so, we evaluated atat-2; ptrn-1 double mutants. These animals showed strong, significant enhancement of synaptic maintenance defects compared to single mutants (Figure 5D). Thus, ptrn-1 and atat-2 function in parallel genetic pathways to regulate synapse maintenance.
Previous studies showed one mechanism by which RPM-1 regulates synapse formation is ubiquitination and inhibition of the DLK-1 MAP kinase (Grill et al., 2007; Nakata et al., 2005; Yan et al., 2009). Therefore, we tested the relationship between dlk-1 and atat-2. Consistent with this prior work, synapse maintenance defects were strongly suppressed in dlk-1; rpm-1 double mutants compared to rpm-1 single mutants (Figure 5E). In contrast, we did not observe suppression of synapse maintenance defects in dlk-1; atat-2 double mutants compared to atat-2 single mutants (Figure 5E).
To our knowledge, these results show for the first time that the tubulin acetyltransferases ATAT-2 and MEC-17 are required for presynaptic bouton maintenance, with ATAT-2 playing a particularly prominent role. Furthermore, our results demonstrate that ATAT-2 and RPM-1 function in a novel pathway to regulate synapse maintenance, while ATAT-2 functions in parallel to PTRN-1 and independently of DLK-1. The simplest model that explains our findings is that ATAT-2 and DLK-1 are part of a signaling network that is differentially regulated downstream of RPM-1. Because dlk-1 suppresses rpm-1 but not atat-2, it is particularly likely that ATAT-2 functions downstream of RPM-1. If ATAT-2 functioned upstream of RPM-1, one would expect suppression of both rpm-1 and atat-2 by dlk-1, which did not occur.
ATAT-2 acetyltransferase activity is required for synapse maintenance
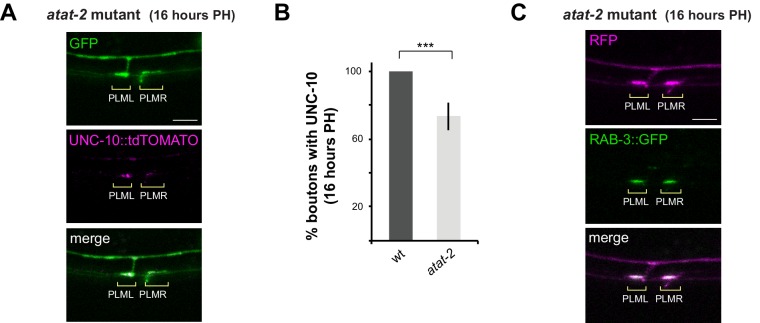
Genetic interactions with rpm-1, and the loss of presynaptic boutons and synaptic branches in PLM neurons of atat-2 mutants prompted us to further evaluate if synapse maintenance was impaired in these animals. Indeed, we observed that presynaptic terminals form and accumulate UNC-10/RIM and RAB-3 in atat-2 mutants at 16 hr PH (Figure 6—figure supplement 1A,C). Quantitation indicated that a small defect in UNC-10/RIM accumulation occurred at 16 hours PH in atat-2 mutants (Figure 6—figure supplement 1B). Thus, similar to rpm-1 mutants, presynaptic assembly is largely normal in atat-2 mutants with subtle defects in accumulation of UNC-10 occurring at the critical 16-hr PH developmental time point. These results indicate ATAT-2 is affecting synapse maintenance, which is consistent with ATAT-2 functioning in the same pathway as RPM-1.
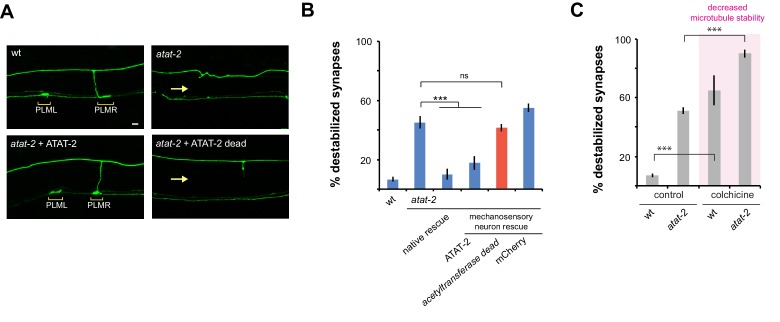
To further validate synapse maintenance defects in atat-2 mutants, we performed transgenic rescue experiments. Synapse destabilization in atat-2 mutants was rescued by expression of ATAT-2 using either the native atat-2 promoter or a mechanosensory neuron promoter (Figure 6A,B). These results demonstrate that ATAT-2 functions cell autonomously in mechanosensory neurons to regulate presynaptic maintenance.
Figure 6. ATAT-2 tubulin acetyltransferase activity functions in mechanosensory neurons to regulate presynaptic maintenance.
(a) Confocal images of presynaptic boutons and synaptic branches in adult PLM neurons. In the wt animal, presynaptic boutons are shown for both PLML and PLMR neurons (brackets). Note one synaptic branch is shown and other is out of focal plane. atat-2 mutant lacks presynaptic boutons from both PLML and PLMR, and synaptic branch absent (arrow). Expression of ATAT-2 in mechanosensory neurons rescues defects. ATAT-2 lacking acetyltransferase activity (ATAT-2 dead) fails to rescue (b) Quantitation of synapse maintenance defects for indicated genotypes. Defects in atat-2 mutants are rescued by using native atat-2 or mechanosensory neuron promoters to transgenically express ATAT-2. No significant rescue occurs with ATAT-2 lacking acetyltransferase activity. (c) Microtubule destabilizing drug colchicine enhances synapse maintenance defects in atat-2 mutants. Significance tested using Student’s t-test with Bonferroni correction. ***p<0.001 and ns = not significant.
Figure 6—figure supplement 1. UNC-10/RIM and RAB-3 accumulate at presynaptic terminals of atat-2 mutants early in development.
Importantly, rescue experiments allowed us to test whether the enzymatic acetyltransferase activity of ATAT-2, or non-enzymatic mechanisms affect synapse maintenance. To do so, we performed rescue with ATAT-2 (G125W, G127W) which lacks acetyltransferase activity (Topalidou et al., 2012). Unlike wt ATAT-2, acetyltransferase dead ATAT-2 failed to rescue synapse maintenance defects (Figure 6A,B). This indicates that ATAT-2 functions via its acetyltransferase activity to regulate synapse maintenance. This finding is consistent with the nature of the atat-2 allele we used, ok2415, which contains a large deletion in the acetyltransferase domain (Shida et al., 2010).
To provide further evidence that ATAT-2 influences microtubule stability to affect synapse maintenance, we evaluated how the microtubule destabilizing drug, colchicine, affects defects in atat-2 mutants. Consistent with ATAT-2 function increasing microtubule stability, synapse maintenance defects caused by atat-2 (lf) were enhanced by colchicine (Figure 6C).
Taken together, these results demonstrate that ATAT-2 α-tubulin acetyltransferase activity regulates synapse maintenance by functioning in mechanosensory neurons. Importantly, this is the first evidence in any system that tubulin acetyltransferase activity is required to maintain synapse stability.
RPM-1 and ATAT-2 function in a pathway to regulate short-term learning
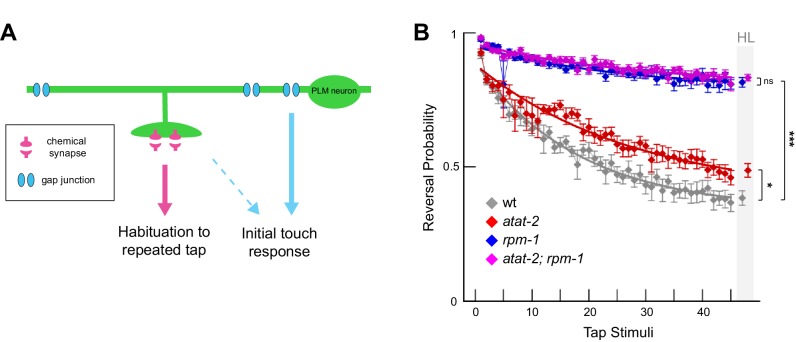
Our results showed that ATAT-2 and RPM-1 function in a linear pathway to regulate maintenance of chemical synapses in PLM mechanosensory neurons. To test the genetic relationship between rpm-1 and atat-2 in a behavioral context, we evaluated habituation to repeated tap stimuli, a form of gentle touch, that is sensed by the mechanosensory neurons.
C. elegans respond to tapping the plate they are grown on by reversing their direction of movement. Initial tap sensation is thought to be primarily mediated by electrical gap junction synapses along the primary axon (Figure 7A) (Chalfie et al., 1985; Wicks and Rankin, 1995). Repeated tap stimulus leads to habituation, a simple form of short-term learning in which responses progressively decrease. Tap habituation is influenced by the glutamatergic chemical synapses of mechanosensory neurons (Crawley et al., 2017; Giles et al., 2015; Rankin and Wicks, 2000). RPM-1 functions in mechanosensory neurons to regulate habituation, and habituation defects in rpm-1 mutants likely result, at least in part, from defects in chemical synapse formation (Crawley et al., 2017; Giles et al., 2015). Because electrical synapse formation is not impaired in rpm-1 mutants, they respond normally to initial tap (Borgen et al., 2017b; Giles et al., 2015; Meng et al., 2016).
Figure 7. Habituation to repeated mechanical stimulation is affected by ATAT-2 and RPM-1.
(a) Chemical and electrical synapses in PLM mechanosensory neurons primarily affect habituation to repeated tap stimuli and initial tap sensation, respectively (adapted from Crawley et al., 2017). (b) Multi-worm tracker was used to quantitate tap habituation for indicated adult genotypes. Habituation is defective in atat-2 mutants (red) and rpm-1 mutants (blue) compared to wt animals. rpm-1; atat-2 double mutants (magenta) are not significantly different than rpm-1 single mutants indicating atat-2 and rpm-1 function in a linear pathway to regulate habituation. Habituation level (HL) is shaded in grey. Significance assessed by Student’s t-test with Bonferroni correction. *p<0.05, ***p<0.001 and ns = not significant.
Consistent with prior studies, wt adult animals habituated to repeated tap with decreased responses over time, while habituation was strongly impaired in rpm-1 mutants (Figure 7B). Consistent with defects in PLM synapse maintenance, habituation was also defective in atat-2 animals, although defects were less severe than those observed in rpm-1 animals (Figure 7B). rpm-1; atat-2 double mutants phenocopied rpm-1 single mutants (Figure 7B). These results with whole animal behavior provide further support that RPM-1 and ATAT-2 function in the same pathway.
Prior studies (Crawley et al., 2017; Giles et al., 2015; Rankin and Wicks, 2000) and our results here are all consistent with impaired chemical synapses in mechanosensory neurons affecting habituation to repeated tap, a simple form of short-term learning. However, it is notable that the frequency of synapse maintenance defects in rpm-1 mutants is only slightly stronger than atat-2 mutants (Figure 5B), while habituation defects are much stronger in rpm-1 mutants (Figure 7B). This might occur because RPM-1 is a signaling hub that regulates several downstream pathways (Grill et al., 2016). In contrast, atat-2 is only known to affect microtubules. Because synapses in rpm-1 mutants face several insults to signaling compared to atat-2 mutants, it is possible remaining synapses that do not destabilize could be functionally weaker in rpm-1 mutants than atat-2 mutants. This idea is supported by previous studies in Drosophila which have shown that loss of function in the RPM-1 ortholog Highwire results in synapse formation defects and synaptic transmission defects that are mediated by distinct molecular mechanisms (Borgen et al., 2017a; Collins et al., 2006).
Discussion
This study breaks new ground on several fronts regarding the molecular and genetic mechanisms that regulate synapse maintenance (Figure 8). 1) We provide new evidence that PHR proteins, such as RPM-1, impact synapse formation during development primarily via effects on synapse maintenance. 2) We show for the first time that ATAT-2 regulates presynaptic maintenance, and does so via its α-tubulin acetyltransferase activity. 3) ATAT-2 acts in a novel pathway with RPM-1 that functions cell autonomously to regulate presynaptic maintenance. 4) Extensive genetic analysis revealed RPM-1 is a hub in a signaling network consisting of ATAT-2, PTRN-1 and DLK-1. 5) Finally, our results indicate that ATAT-2 functions independently of DLK-1, and is therefore likely to function downstream of RPM-1 to affect synapse maintenance.
Figure 8. ATAT-2 tubulin acetyltransferase activity functions in a pathway with RPM-1 to regulate synapse maintenance.
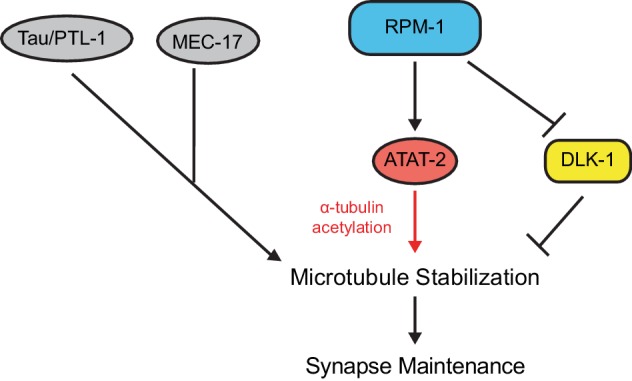
Model summarizing genetic and pharmacological results suggesting RPM-1 functions upstream of ATAT-2 acetyltransferase activity to regulate microtubule stability and synapse maintenance. Outcomes indicate the RPM-1/ATAT-2 pathway functions independently of DLK-1 to regulate synapse maintenance.
RPM-1 regulates synapse maintenance during development
Experiments in worms, flies and mice showed that PHR proteins, such as RPM-1, regulate synapse formation (Bloom et al., 2007; Grill et al., 2016; Schaefer et al., 2000; Wan et al., 2000; Zhen et al., 2000). Important progress has been made in understanding signaling networks regulated by RPM-1 and PHR proteins, which act as both signaling hubs and ubiquitin ligases (Grill et al., 2016). Nonetheless, we still lack a clear cellular explanation for why synapse formation is abnormal in the absence of PHR proteins. One prior study examined the presence of presynaptic boutons, and hinted that synapse assembly might be the principal defect in rpm-1 mutants with possible minor defects in synapse maintenance (Schaefer et al., 2000). We now expand significantly on this prior work with more extensive developmental time course analysis of bouton morphology, and several presynaptic markers. Our results indicate that synapse formation defects in rpm-1 mutants result primarily from a failure to maintain synapses (Figures 1, 3 and Figures 3—figure supplement 1). Consistent with RPM-1 regulating synapse maintenance during development, we observed RPM-1 accumulation at presynaptic terminals during synapse formation through adulthood (Figure 2).
In mice, the RPM-1 ortholog Phr1 regulates synapse formation at the NMJ. While it remains unknown if this is due to defects in synapse assembly or maintenance, the Diantonio group observed orphan presynaptic terminals in Phr1 knockout mice (Bloom et al., 2007). Our results suggest these orphan presynaptic terminals could reflect failed synapse maintenance. Consistent with this, studies in flies and mice indicate orphan terminals are a hallmark of destabilizing synapses (Bhattacharya et al., 2016; Eaton et al., 2002; Graf et al., 2011; Pielage et al., 2008).
Our findings demonstrate that RPM-1 regulates developmental synapse maintenance, which we consider the final step in synapse formation. This differs from long-term synapse maintenance, which facilitates synapse integrity for months and is regulated by molecules like αLaminin and p190Rho at central synapses, and LRP4 at NMJs (Barik et al., 2014; Kerrisk et al., 2013; Lin and Koleske, 2010; Omar et al., 2017). The relationship between regulators of developmental synapse maintenance and long-term synapse maintenance remains unclear and awaits future studies.
ATAT-2 functions via acetyltransferase activity to regulate synapse maintenance
In C. elegans, there are two α-tubulin acetyltransferases, ATAT-2 and MEC-17 (Akella et al., 2010; Shida et al., 2010). We show here that both molecules affect synapse formation in mechanosensory neurons (Figure 5). ATAT-2 is a more prominent player and functions via enzymatic acetyltransferase activity to regulate synapse maintenance (Figures 5 and 6). The implications of these findings potentially extend beyond C. elegans, as the mammalian acetyltransferase ortholog called Atat1 affects hippocampal development and touch sensation (Kalebic et al., 2013; Kim et al., 2013; Morley et al., 2016). Whether these phenotypes arise from defects in synapse maintenance now becomes an intriguing question.
Our genetic analysis also revealed that ATAT-2 functions in a linear pathway with RPM-1 (Figure 8). This was observed in two functional contexts: synapse maintenance in mechanosensory neurons (Figure 5) and behavioral habituation mediated by these neurons (Figure 7). Consistent with RPM-1 and ATAT-2 acting in a linear pathway, both RPM-1 and ATAT-2 function cell autonomously in mechanosensory neurons to regulate presynaptic maintenance (Figures 1D and 6A,B).
Several observations indicate the RPM-1/ATAT-2 pathway influences microtubule stability to impact synapse maintenance. Treating either rpm-1 or atat-2 mutants with colchicine enhanced synapse destabilization defects (Figures 4A and 6C). RPM-1 functions in parallel to other molecules that can stabilize microtubules, such as PLT-1/Tau and MEC-17 (Figure 5). Finally, the enzymatic α-tubulin acetyltransferase activity of ATAT-2 was necessary for synapse maintenance (Figure 6B).
Our results do not provide definitive evidence for the order of ATAT-2 and RPM-1 within this novel pathway. We attempted to address this with transgenic bypass experiments, but results were inconclusive. Nonetheless, there are several reasons why RPM-1 most likely functions upstream of ATAT-2 (Figure 8). First, RPM-1 is a signaling hub and ubiquitin ligase that positively and negatively regulates at least six different downstream signaling pathways (Grill et al., 2016). In contrast, ATAT-2 regulates microtubules directly and is not known to regulate signaling events (Akella et al., 2010; Shida et al., 2010; Topalidou et al., 2012). Second, RPM-1 functions in parallel to other molecules that can stabilize microtubules, including MEC-17 and PTL-1/Tau. If RPM-1 were to function downstream of microtubule stability in general, we might expect the same genetic relationship between RPM-1 and all mutants that affect microtubule stability, which did not occur. The final argument is perhaps the most convincing. Our results indicate that ATAT-2, PTRN-1 and DLK-1 function within the RPM-1 signaling network (Figure 5). While dlk-1 can suppress rpm-1, it failed to suppress atat-2. The simplest explanation for this is a model in which ATAT-2 and DLK-1 have opposing functions with both molecules acting downstream of RPM-1 (Figure 8). If ATAT-2 were to function upstream of RPM-1, we would expect dlk-1 to suppress both atat-2 and rpm-1, which did not occur. Despite cumulative reasons for favoring the model that RPM-1 functions upstream of ATAT-2, we cannot entirely rule out the alternative possibility.
It is intriguing that the functional genetic relationship between RPM-1 and molecules that affect microtubule stability differs between synapse maintenance in mechanosensory neurons described here (Figure 8), and axon termination of the same neurons that occurs in a different anatomical location (Borgen et al., 2017b). For example, RPM-1 functions in the same pathway as both ATAT-2 and PTRN-1 to regulate synapse maintenance (Figure 5C), but acts in parallel opposing pathways to these molecules to regulate axon termination (Borgen et al., 2017b). Further, RPM-1 functions in parallel to PTL-1/Tau to regulate synapse maintenance (Figure 5C), but Tau likely inhibits RPM-1 during axon termination (Borgen et al., 2017b). Thus, the functional genetic relationship between RPM-1 and regulators of microtubule stability varies with subcellular location and the developmental process in question. These findings in C. elegans might explain why studies in fish and mice that analyzed different types of neurons arrived at opposing conclusions about how PHR protein signaling influences microtubule stability (Hendricks and Jesuthasan, 2009; Lewcock et al., 2007).
Another worthwhile consideration emerges from our findings here, and prior observations about axon termination (Borgen et al., 2017b). We now uncover a second example of a functional genetic relationship between RPM-1 and PTL-1/Tau, which has interesting implications given the prominence of Tau in neurodegenerative disease (Brunden et al., 2009; Wang and Mandelkow, 2016). Likewise, our discovery that RPM-1 functions in a pathway with ATAT-2 acetyltransferase activity to regulate synapse maintenance could have important disease implications, as alterations in α-tubulin acetylation and synapse instability are associated with neurodegenerative diseases (Godena et al., 2014; Govindarajan et al., 2013; Hempen and Brion, 1996; Lin and Koleske, 2010; Pellegrini et al., 2017). Thus, converging themes from several studies, including this one, suggest it could be informative to test whether the RPM-1/ATAT-2 pathway impacts neurodegenerative disease models.
Materials and methods
Genetics and transgenics
C. elegans strains were maintained using standard procedures. Alleles used included: rpm-1 (ju44), mec-17 (ok2109), ptrn-1 (tm5597), ptl-1 (ok621), dlk-1 (ju476), and atat-2 (ok2415). Integrated transgenes and extrachromosomal arrays used in this study are as follows: muIs32 (Pmec-7GFP), zdIs5 (Pmec-4GFP), bggEx8 (Prpm-1RPM-1), bggEx127 (Pmec-3RPM-1::GFP), bggEx141 (Pmec-7SYD-2::mScarlet), jsIs973 (Pmec-7mRFP), jsIs821 (Pmec-7RAB-3::GFP), bggIs28 (Pmec-7UNC-10::tdTOMATO), and bggIs34 (Pmec-3RPM-1::GFP). jsIs973 and jsIs821 were kind gifts from Dr. Michael Nonet (Washington University). All alleles were outcrossed a minimum of four times. All double mutants were constructed following standard mating procedures. Genotypes were confirmed by PCR, or sequencing as needed. Primers and PCR conditions are available upon request.
For rescue experiments, transgenic extrachromosomal arrays were constructed by injecting DNA of interest with a coinjection marker, Pmyo-2RFP (2 ng/µl) or Pttx-3RFP (50 ng/µL) and pBluescript to reach a total DNA concentration of 100 ng/µL. For all rescue experiments, two or more independently derived transgenic lines were analyzed for a given genotype. Supplementary file 1 details transgenic extrachromosomal arrays and injection conditions.
Cloning
For atat-2 rescues with the native promoter, the atat-2 locus (including promoter, open reading frame, and 3’ UTR) was PCR amplified from N2 genomic DNA. Primer sequences used for cloning are available upon request. For atat-2 rescue with mechanosensory neuron promoters, atat-2 cDNA was amplified from C. elegans RNA and TOPO cloned into pCR8 Gateway entry vector (Invitrogen) to generate pBG-GY896. pBG-GY896 was recombined into a destination vector containing the mec-7 promoter, pBG-GY119, to generate pBG-GY897 (Pmec-7ATAT-2). Site-directed mutagenesis was performed on pBG-GY896 to change two glycine residues into tryptophan (G125W and G127W) resulting in pBG-GY898. Mutation of these conserved glycine residues in the ATAT-2 paralog MEC-17 (G121W and G123W) was previously shown to render MEC-17 catalytically inactive (Topalidou et al., 2012). After mutagenesis, pBG-GY898 was recombined with the Pmec-7 destination vector to yield pBG-GY899 (Pmec-7ATAT-2 dead). For expression of SYD-2::mScarlet, syd-2 genomic DNA was cloned from C. elegans and TOPO cloned into pCR8 Gateway entry vector to generate pBG-GY699. pBG-GY699 was recombined into a destination vector containing the mec-7 promoter and a C-terminal mScarlet tag, pBG-GY880, to generate pBG-GY936 (Pmec-7SYD-2::mScarlet).
Developmental analysis of synapses
The transgenic strain muIs32 (Pmec-7GFP) was used to label PLM neurons for analysis of synaptic branch and boutons during development. Where indicated, bggIs28 was used to coexpress the active zone marker UNC-10::tdTOMATO. Transgenic arrays were used to express SYD-2::mScarlet in muIs32. For RAB-3::GFP analysis (jsIs821), jsIs973 (Pmec-7RFP) was used as a cell fill to visualize PLM axon and presynaptic bouton morphology. Synchronized developmental time course analysis was done by collecting freshly hatched L1 larvae and aging animals in hour-long intervals at room temperature (22°C). At indicated time points between 1 and 48 hr post-hatch (PH), animals were mounted in 5 mM levamisole (M9 buffer) on agar pads to score phenotypes. A minimum of 18 PLM neurons were scored at each time point for all markers with the exception of SYD-2::mScarlet which was scored in at least 14 PLM neurons. Phenotypes were scored using epifluorescent microscopy, and all images were acquired using confocal microscopy. Epifluorescent microscopy was done using 100x magnification on a Leica CTR6500 microscope with Leica Application Suite software. Confocal microscopy was done under 63x magnification and 2x zoom factor on a Leica SP8 confocal microscope. Z-stacks were collected (0.5 µm slices for larvae and 1 µm for adults) and maximum intensity projections are shown for each genotype. Image analysis was done using ImageJ software from NIH image (http://rsb.info.nih.gov/ij/).
Synaptic bouton defects were scored by tracking the PLM synaptic branch to the ventral nerve cord. Branches with presynaptic boutons/varicosities were scored as synapses. If no bouton was discernable, even if the branch was present, it was scored as a destabilized synapse. Similar logic was used to evaluate RAB-3::GFP, UNC-10::tdTOMATO and SYD-2::mScarlet at presynaptic terminals.
RPM-1 localization in PLM neurons
Transgenic ju44 mutants expressing RPM-1::GFP using the mec-3 promoter (Pmec-3RPM-1::GFP) and tdTOMATO using the mec-7 promoter (Pmec-7tdTOMATO) were anesthetized with levamisole. Freshly hatched L1 larvae were allowed to age for 5–6 hr at 22°C before imaging. RPM-1::GFP and tdTOMATO were assessed using confocal microscopy and 63x magnification with 2x zoom factor. Adult animals were imaged using 40x magnification and 2x zoom. Increased fluorescence of tdTOMATO was essential for visualizing PLM morphology in L1 larvae because of their small size, and relatively weak expression of the mec-7 promoter at this age (data not shown). Periactive localization of RPM-1 at presynaptic terminals was assessed using both RPM-1::GFP and UNC-10::tdTOMATO as an active zone marker. Because UNC-10::tdTOMATO is difficult to observe in early L1 animals due to low expression and small boutons, we performed this analysis using young adult PLM synapses.
Microtubule pharmacology
For pharmacological manipulation of microtubule stability, taxol (2 µm), colchicine (0.25 mM) or the vehicle DMSO were spread on NGM plates and left overnight. Plates were seeded with OP50 E. coli, and 16 hr later 3–5 P0 adults were placed on plates with drugs. F1 progeny developed normally on colchicine or taxol, and synapse maintenance was scored in young adults. Opposing effects of taxol and colchicine on rpm-1 mutants indicates these drugs are at appropriate concentrations for use in C. elegans, which was also shown previously (Borgen et al., 2017b).
Habituation
Tap habituation experiments were performed as described previously with minor modifications (Giles et al., 2015). Briefly, age-synchronized animals (~50–100) were cultivated from egg until gravid adult (72–75 hours PH) at 23°C, and assayed on 5 cm NGM plates with 50 μL of E. coli. (OP50). Using Multi-Worm Tracker (Swierczek et al., 2011), animal behavior was recorded for 550 s. After the first 100 s, 45 tap stimuli were given with a 10 s inter-stimulus interval. Response to tap was measured by reversal probability (the fraction of animals that reversed their locomotion within 2 s of the tap). For each plate, exponential curves were fit to responses across stimuli, and habituation level (HL) was measured as the value of the fit at the final stimulus. All strains analyzed contained the muIs32 transgene.
Statistics
For developmental time course analysis, we used the Fisher’s exact test to compare the percentage of PLM neurons lacking synapses in single populations of rpm-1 and wt at each time point. Data points presented represent the mean of the population. A minimum of 18 PLM neurons were scored at each time point for all markers, except SYD-2::mScarlet which was scored in at least 14 PLM neurons.
For analysis of synapse maintenance defects (% destabilized synapses), we scored a minimum of five independent sets of 20–50 PLM neurons from adult animals for each genotype. For rescue experiments, data was acquired from a minimum of two independent transgenic lines for each genotype. Data shown represents the mean and error bars represent the SEM. Comparisons between genotypes were done using the Student’s t-test, with Bonferroni correction for the number of comparisons in each experiment.
For habituation, data presented represents the mean and error bars represent the SEM across 12 plates (50–100 animals per plate) tested on three independent days per genotype. Differences were assessed by comparing habituation level using Student’s t-tests with Bonferroni correction for multiple comparisons.
Acknowledgements
We thank the C elegans knockout consortium for several alleles, and the C elegans Genetics Center (NIH Office of Research Infrastructure Programs, P40 OD010440) for providing strains. BG was supported by a grant from the NIH (R01 NS072129). MB is a Neuroscience Scholar of the Esther B O’Keeffe Charitable Foundation.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Brock Grill, Email: bgrill@scripps.edu.
Graeme W Davis, University of California, San Francisco, United States.
K VijayRaghavan, National Centre for Biological Sciences, Tata Institute of Fundamental Research, India.
Funding Information
This paper was supported by the following grant:
National Institute of Neurological Disorders and Stroke R01 NS072129 to Brock Grill.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Methodology, Writing—original draft.
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft.
Formal analysis, Validation, Investigation, Methodology, Writing—original draft.
Conceptualization, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Abrams B, Grill B, Huang X, Jin Y. Cellular and molecular determinants targeting the Caenorhabditis Elegans PHR protein RPM-1 to perisynaptic regions. Developmental Dynamics. 2008;237:630–639. doi: 10.1002/dvdy.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik A, Lu Y, Sathyamurthy A, Bowman A, Shen C, Li L, Xiong WC, Mei L. LRP4 is critical for neuromuscular junction maintenance. Journal of Neuroscience. 2014;34:13892–13905. doi: 10.1523/JNEUROSCI.1733-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya MR, Geisler S, Pittman SK, Doan RA, Weihl CC, Milbrandt J, DiAntonio A. TMEM184b promotes axon degeneration and neuromuscular junction maintenance. Journal of Neuroscience. 2016;36:4681–4689. doi: 10.1523/JNEUROSCI.2893-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Miller BR, Sanes JR, DiAntonio A. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes & Development. 2007;21:2593–2606. doi: 10.1101/gad.1592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgen M, Rowland K, Boerner J, Lloyd B, Khan A, Murphey R. Axon termination, pruning, and synaptogenesis in the giant fiber system of Drosophila melanogaster is promoted by highwire. Genetics. 2017a;205:1229–1245. doi: 10.1534/genetics.116.197343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgen MA, Wang D, Grill B. RPM-1 regulates axon termination by affecting growth cone collapse and microtubule stability. Development. 2017b;144:4658–4672. doi: 10.1242/dev.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for alzheimer's disease and related tauopathies. Nature Reviews Drug Discovery. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis Elegans. The Journal of Neuroscience. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Lee A, Liao CP, Liu YW, Pan CL. RHGF-1/PDZ-RhoGEF and retrograde DLK-1 signaling drive neuronal remodeling on microtubule disassembly. PNAS. 2014;111:16568–16573. doi: 10.1073/pnas.1410263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PH, Li P, Shen K. Cell biology in neuroscience: cellular and molecular mechanisms underlying presynapse formation. The Journal of Cell Biology. 2013;203:11–22. doi: 10.1083/jcb.201307020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Crawley O, Giles AC, Desbois M, Kashyap S, Birnbaum R, Grill B. A MIG-15/JNK-1 MAP kinase cascade opposes RPM-1 signaling in synapse formation and learning. PLOS Genetics. 2017;13:e1007095. doi: 10.1371/journal.pgen.1007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/S0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Feoktistov AI, Herman TG. Wallenda/DLK protein levels are temporally downregulated by Tramtrack69 to allow R7 growth cones to become stationary boutons. Development. 2016;143:2983–2993. doi: 10.1242/dev.134403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles AC, Opperman KJ, Rankin CH, Grill B. Developmental Function of the PHR Protein RPM-1 Is Required for Learning in Caenorhabditis elegans. G3: Genes|Genomes|Genetics. 2015;5:2745–2757. doi: 10.1534/g3.115.021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ, De Vos KJ. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nature Communications. 2014;5:5245. doi: 10.1038/ncomms6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schlüter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for alzheimer's disease. EMBO Molecular Medicine. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Heerssen HM, Wright CM, Davis GW, DiAntonio A. Stathmin is required for stability of the Drosophila neuromuscular junction. Journal of Neuroscience. 2011;31:15026–15034. doi: 10.1523/JNEUROSCI.2024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill B, Bienvenut WV, Brown HM, Ackley BD, Quadroni M, Jin Y. C. elegans RPM-1 regulates axon termination and synaptogenesis through the rab GEF GLO-4 and the rab GTPase GLO-1. Neuron. 2007;55:587–601. doi: 10.1016/j.neuron.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Grill B, Murphey RK, Borgen MA. The PHR proteins: intracellular signaling hubs in neuronal development and axon degeneration. Neural Development. 2016;11:8. doi: 10.1186/s13064-016-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempen B, Brion JP. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer's disease. Journal of Neuropathology and Experimental Neurology. 1996;55:964–972. doi: 10.1097/00005072-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Hendricks M, Jesuthasan S. PHR regulates growth cone pausing at intermediate targets through microtubule disassembly. Journal of Neuroscience. 2009;29:6593–6598. doi: 10.1523/JNEUROSCI.1115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annual Review of Cell and Developmental Biology. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- Kalebic N, Martinez C, Perlas E, Hublitz P, Bilbao-Cortes D, Fiedorczuk K, Andolfo A, Heppenstall PA. Tubulin acetyltransferase αtat1 destabilizes microtubules independently of its acetylation activity. Molecular and Cellular Biology. 2013;33:1114–1123. doi: 10.1128/MCB.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrisk ME, Greer CA, Koleske AJ. Integrin α3 is required for late postnatal stability of dendrite arbors, dendritic spines and synapses, and mouse behavior. Journal of Neuroscience. 2013;33:6742–6752. doi: 10.1523/JNEUROSCI.0528-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Li L, Ghorbani M, You L, Yang XJ. Mice lacking α-tubulin acetyltransferase 1 are viable but display α-tubulin acetylation deficiency and dentate gyrus distortion. Journal of Biological Chemistry. 2013;288:20334–20350. doi: 10.1074/jbc.M113.464792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. A post-docking role for active zone protein rim. Nature Neuroscience. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis Elegans. The Journal of Neuroscience. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock JW, Genoud N, Lettieri K, Pfaff SL. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron. 2007;56:604–620. doi: 10.1016/j.neuron.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annual Review of Neuroscience. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Schaefer AM, Dour S, Nonet ML. The conserved LIM domain-containing focal adhesion protein ZYX-1 regulates synapse maintenance in Caenorhabditis elegans. Development. 2014;141:3922–3933. doi: 10.1242/dev.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcette JD, Chen JJ, Nonet ML. The Caenorhabditis elegans microtubule minus-end binding homolog PTRN-1 stabilizes synapses and neurites. eLife. 2014;3:e01637. doi: 10.7554/eLife.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro CM, Pielage J, Davis GW. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. The Journal of Cell Biology. 2009;187:101–117. doi: 10.1083/jcb.200903166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Chen CH, Yan D. Regulation of gap junction dynamics by UNC-44/ankyrin and UNC-33/CRMP through VAB-8 in C. elegans neurons. PLOS Genetics. 2016;12:e1005948. doi: 10.1371/journal.pgen.1005948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley SJ, Qi Y, Iovino L, Andolfi L, Guo D, Kalebic N, Castaldi L, Tischer C, Portulano C, Bolasco G, Shirlekar K, Fusco CM, Asaro A, Fermani F, Sundukova M, Matti U, Reymond L, De Ninno A, Businaro L, Johnsson K, Lazzarino M, Ries J, Schwab Y, Hu J, Heppenstall PA. Acetylated tubulin is essential for touch sensation in mice. eLife. 2016;5:e20813. doi: 10.7554/eLife.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Neumann B, Hilliard MA. Loss of MEC-17 leads to microtubule instability and axonal degeneration. Cell Reports. 2014;6:93–103. doi: 10.1016/j.celrep.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis Elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. The Journal of Neuroscience. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar MH, Kerrisk Campbell M, Xiao X, Zhong Q, Brunken WJ, Miner JH, Greer CA, Koleske AJ. CNS neurons deposit laminin α5 to stabilize synapses. Cell Reports. 2017;21:1281–1292. doi: 10.1016/j.celrep.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Rongo C. RPM-1 and DLK-1 regulate pioneer axon outgrowth by controlling wnt signaling. Development. 2018;145:dev164897. doi: 10.1242/dev.164897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Wetzel A, Grannó S, Heaton G, Harvey K. Back to the tubule: microtubule dynamics in Parkinson's disease. Cellular and Molecular Life Sciences. 2017;74:409–434. doi: 10.1007/s00018-016-2351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Current Biology. 2005;15:918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Wicks SR. Mutations of the Caenorhabditis Elegans brain-specific inorganic phosphate transporter eat-4 affect habituation of the tap-withdrawal response without affecting the response itself. The Journal of Neuroscience. 2000;20:4337–4344. doi: 10.1523/JNEUROSCI.20-11-04337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CE, Spilker KA, Cueva JG, Perrino J, Goodman MB, Shen K. PTRN-1, a microtubule minus end-binding CAMSAP homolog, promotes microtubule function in Caenorhabditis elegans neurons. eLife. 2014;3:e01498. doi: 10.7554/eLife.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. 2000;26:345–356. doi: 10.1016/S0896-6273(00)81168-X. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. PNAS. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierczek NA, Giles AC, Rankin CH, Kerr RA. High-throughput behavioral analysis in C. elegans. Nature Methods. 2011;8:592–598. doi: 10.1038/nmeth.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalidou I, Keller C, Kalebic N, Nguyen KC, Somhegyi H, Politi KA, Heppenstall P, Hall DH, Chalfie M. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Current Biology. 2012;22:1057–1065. doi: 10.1016/j.cub.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/S0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nature Reviews Neuroscience. 2016;17:22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Wicks SR, Rankin CH. Integration of mechanosensory stimuli in caenorhabditis elegans. The Journal of Neuroscience. 1995;15:2434–2444. doi: 10.1523/JNEUROSCI.15-03-02434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron. 2000;26:331–343. doi: 10.1016/S0896-6273(00)81167-8. [DOI] [PubMed] [Google Scholar]