Abstract
Ca2+ channel β-subunit interactions with pore-forming α-subunits are long-thought to be obligatory for channel trafficking to the cell surface and for tuning of basal biophysical properties in many tissues. Unexpectedly, we demonstrate that transgenic expression of mutant α1C subunits lacking capacity to bind CaVβ can traffic to the sarcolemma in adult cardiomyocytes in vivo and sustain normal excitation-contraction coupling. However, these β-less Ca2+ channels cannot be stimulated by β-adrenergic pathway agonists, and thus adrenergic augmentation of contractility is markedly impaired in isolated cardiomyocytes and in hearts. Similarly, viral-mediated expression of a β-subunit–sequestering peptide sharply curtailed β-adrenergic stimulation of WT Ca2+ channels, identifying an approach to specifically modulate β-adrenergic regulation of cardiac contractility. Our data demonstrate that β subunits are required for β-adrenergic regulation of CaV1.2 channels and positive inotropy in the heart, but are dispensable for CaV1.2 trafficking to the adult cardiomyocyte cell surface, and for basal function and excitation-contraction coupling.
Keywords: Cardiology, Muscle Biology
Keywords: Calcium, Calcium channels, Excitation contraction coupling
Introduction
In heart cells, Ca2+ influx via CaV1.2 channels mediates excitation-contraction (E-C) coupling, controls action potential duration, and regulates gene expression. CaV1.2 channels are multi-subunit proteins composed minimally of a pore-forming α1C and regulatory β and α2δ subunits (1–4). In adult ventricular cardiomyocytes, most CaV1.2 channels localize to transverse tubules where they lie in close proximity (~12 nm) and apposed to ryanodine receptors (RyR2) at dyadic junctions (5). Dysregulation of CaV1.2 activity, surface density, or subcellular localization in cardiomyocytes can result in cardiac arrhythmias, heart failure, and sudden death.
Reconstitution experiments concluded that binding to β subunits is indispensable for α1C trafficking to the cell surface (6–14). The physiological relevance of this finding was initially supported by β2 knockout mice, which were embryonic lethal, likely secondary to a decreased L-type Ca2+ current (15). An initial idea that β binding to the α-interaction domain (AID) of the α1-subunit I-II loop shielded an ER retention signal in the I-II loop to allow forward trafficking of the channel proved inadequate in subsequent experiments (9, 16–18). Surprisingly, cardiomyocyte-specific, conditional deletion of the Cacnb2 gene in adult mice reduced β2 protein by 96% but caused only a modest 29% reduction in Ca2+ current, with no obvious cardiac impairment (19). Interpretation of this result is ambiguous, however, as it is complicated by the remnant (~4%) β2 expression as well as the presence other CaVβ isoforms expressed in adult cardiomyocytes (13). Moreover, a contrasting viewpoint was provided by a study in which shRNA-mediated knockdown of β2 in adult rat myocytes substantially diminished Ca2+ current (20).
To definitively address the controversies regarding the role of β subunits in mediating trafficking and regulation of Ca2+ channels in the heart, we created transgenic mice lines with 3 mutations in the AID, which renders the pore-forming α1C subunit incapable of binding β subunits. With this new model, we definitively demonstrate in vivo that β subunit binding to α1C is not required for trafficking and that the basal function of β-less Ca2+ channels is only minimally altered.
Instead, we found that the β subunit is obligatory for transducing β-adrenergic signals to cardiac CaV1.2 channels. Cardiac CaV1.2 channels are prominently upregulated by β-adrenergic agonists via activation of protein kinase A (PKA) (21, 22) as part of the fundamental flight-or-fight response, yet the detailed mechanisms by which PKA activates CaV1.2 remain unknown despite several decades of investigation. Recently, we reported that alanine substitution of all consensus, conserved PKA phosphorylation sites (> 22 serines/ threonines) in the α1C subunit did not affect adrenergic regulation of CaV1.2 in vivo (23). Prior studies also ruled out a contribution for the β subunit, as substitution or elimination of potential PKA phosphorylation sites did not perturb β-adrenergic regulation (24–27), although other consensus PKA sites are present in the N-terminal regions of the protein. We found that β subunit binding to α1C, but not PKA phosphorylation of β, is absolutely essential for the augmentation of Ca2+ current and cardiac contractile response to β-adrenergic PKA stimulation. These findings identify the key regulatory mechanisms impacting β-adrenergic regulation of Ca2+ influx and contractility in the heart.
Results
β-less CaV1.2 channels traffic to membrane in adult cardiomyocytes.
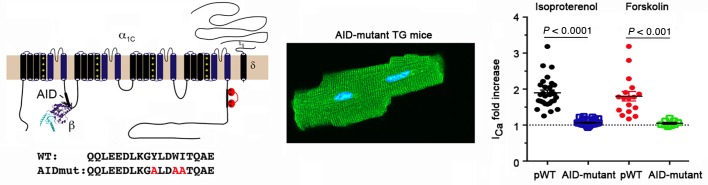
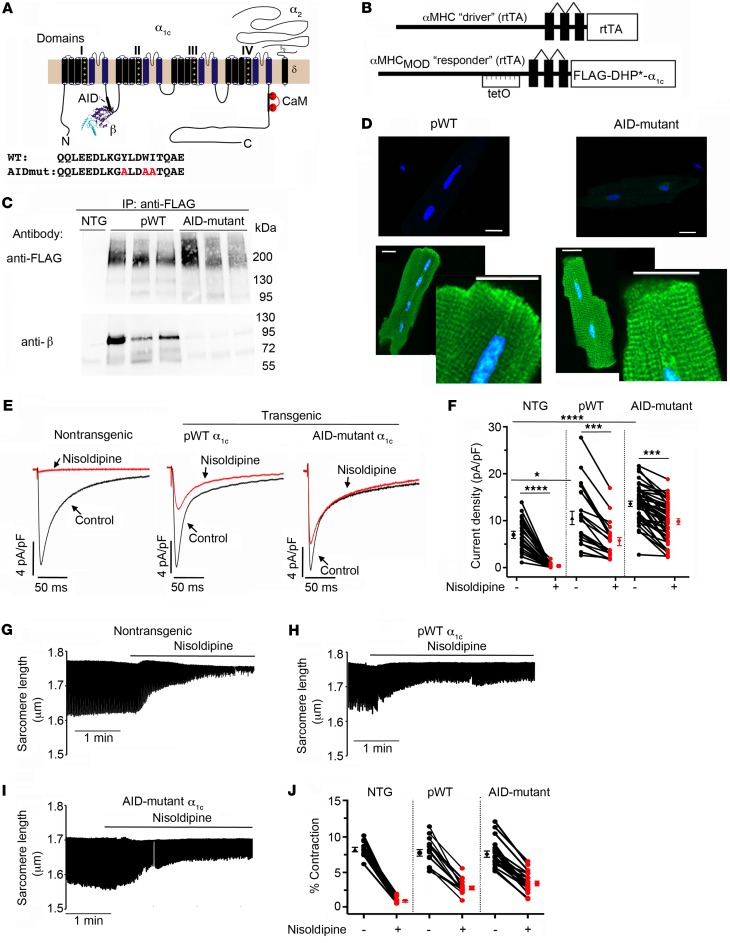
Alanine substitutions of 3 conserved residues—Y467, W470, and I471—in rabbit α1C AID (Figure 1A) increases the KD of β subunit binding from 5 nM to greater than 6 M (28–31). β2 subunits failed to coprecipitate with the AID-mutant α1C when coexpressed with AID-mutant α1C in tsA201 cells (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/JCI123878DS1) confirming the critical importance of this region for β binding. We then created transgenic mice with cardiac-specific and doxycycline-inducible expression of N-terminal 3X-FLAG–tagged dihydropyridine-resistant (DHP-resistant) (T1066Y/Q1070M) (32, 33) AID-mutant rabbit α1C (Figure 1B). Controls were provided by transgenic FLAG-tagged DHP-resistant α1C subunits with WT AIDs, termed pseudo-WT (pWT) α1C. Coimmunoprecipitation experiments from transgenic mice hearts confirmed that pWT α1C associates with endogenous β subunit, but AID-mutant α1C does not (Figure 1C). The anti–β antibody recognizes all CaVβ subunits, thus ruling out compensation from other β subunits in heart and thus confirming that the AID motif is essential to mediate the high-affinity binding between α1C and β2 in cardiomyocytes.
Figure 1. AID-mutant α1C channels trafficking and function in cardiomyocytes.
(A) Schematic of rabbit cardiac α1C subunit topology showing β-subunit binding to α-interacting domain (AID) motif in I-II loop. WT and mutant-AID motif in the I-II loop of α1C. (B) Schematic representation of the binary transgene system. The αMHCMOD construct is a modified αMHC promoter containing the tet-operon for regulated expression of FLAG-tagged DHP-resistant (DHP*) α1C. (C) Anti-FLAG (upper) and anti-β immunoblots (lower) of anti–FLAG antibody immunoprecipitation of cardiac homogenates of nontransgenic (NTG), pWT α1C, and AID-mutant α1C mice. Representative of 3 experiments. (D) Immunostaining of pWT and AID-mutant α1C cardiomyocytes. Anti-FLAG and FITC-conjugated secondary antibodies, and nuclear labeling with Hoechst stain. Negative control omitted anti–FLAG antibody. Images obtained with confocal microscopy at ×40. Scale bars: 20 μm. (E) Exemplar whole-cell CaV1.2 currents recorded from freshly dissociated cardiomyocytes of NTG, pWT, and AID-mutant α1C transgenic mice. Pulses from –60 mV to 0 mV before (black traces) and 3 minutes after (red traces) administration of 300 nM nisoldipine. (F) Scatter plot showing current densities before and after administration of 300 nM nisoldipine. Mean ± SEM. *P < 0.05 NTG versus transgenic pWT α1C, ****P < 0.0001 NTG versus transgenic AID-mutant α1C and also NTG pre- versus post-nisoldipine, ***P < 0.001 pWT or AID-mutant α1C pre- versus post-nisoldipine. One-way ANOVA and Dunnett’s multiple comparison test. NTG, n = 8 cardiomyocytes from 5 mice; pWT, n = 21 cardiomyocytes from 7 mice; AID-mutant, n = 45 cardiomyocytes from 9 mice. (G–I) Representative time courses of changes in sarcomere length after superfusion of 300 nM nisoldipine-containing solution for cardiomyocytes isolated from NTG mice (G) and pWT (H) and AID-mutant transgenic α1C mice. Cardiomyocytes were field-stimulated at 1 Hz. (J) Scatter plot showing percentage of contraction of sarcomere length in the absence and presence of nisoldipine for cardiomyocytes isolated from NTG mice and pWT and AID-mutant α1C transgenic mice. NTG, n = 12 cells from 3 mice; pWT, n = 16 cells from 3 mice; AID-mutant, n = 18 cells from 3 mice.
We assessed the impact of loss of β binding on AID-mutant α1C subcellular localization and functional expression in cardiomyocytes using 3 complementary approaches. First, immunofluorescence experiments using anti–FLAG antibody on fixed cardiomyocytes indicated that both transgenic pWT α1C and AID-mutant α1C channels displayed a similar striated pattern consistent with surface membrane distribution and localization in transverse tubules (Figure 1D). Second, we exploited the T1066Y/Q1070M mutations that impart relative DHP-resistance (32, 33) to block Ca2+ currents from endogenous DHP-sensitive CaV1.2 with nisoldipine and isolate Ca2+ current from transgenic pWT α1C or AID-mutant α1C channels. Compared with cardiomyoctes isolated from NTG control mice, cardiomyocytes isolated from both pWT and AID-mutant α1C transgenic mice had increased peak Ca2+ currents, and substantial peak Ca2+ currents remaining after exposure to nisoldipine (Figure 1, E and F). Third, field-stimulated contraction of cardiomyocytes isolated from transgenic AID-mutant α1C mice persisted in the presence of 300 nM nisoldipine (Figure 1, I and J), similar to the contraction of cardiomyocytes isolated from transgenic pWT α1C mice in the presence of nisoldipine. Contraction of cardiomyoyctes isolated from NTG was markedly inhibited by nisoldipine (Figure 1, G and H). Overall, these results demonstrate that transgenic β-less AID-mutant α1C channels traffic to the sarcolemma and trigger E-C coupling in cardiomyocytes. This is in stark contrast to the necessary role of β binding for surface trafficking and function of CaV1.2 channels reconstituted in heterologous cells (Supplemental Figure 1, A and B), or expressed in hippocampal neurons (34).
We also considered that endogenous WT α1C channels could couple with AID-mutant α1C channels to facilitate trafficking of β-less channels to the surface membranes in cardiomyocytes, which could be the basis for the observed differences between cardiomyocytes and heterologous expression systems. To determine whether coupling-induced trafficking could occur, we coexpressed either DHP-resistant pWT α1C or DHP-resistant AID-mutant α1C with both WT α1C and β2 subunits in tsA201. In the presence of nisoldipine, which inhibits the WT α1C channels, tsA201 cells expressing the AID-mutant α1C channels had no remaining Ca2+ current (Supplemental Figure 1C, right), whereas cells expressing the DHP-resistant pWT α1C had remaining current (Supplemental Figure 1C, left), implying that at least in tsA201 cells, β-less channels were unable to “hitchhike” to the membrane with WT channels.
PKA modulation of CaV1.2 channels is dependent on α1C-β interactions.
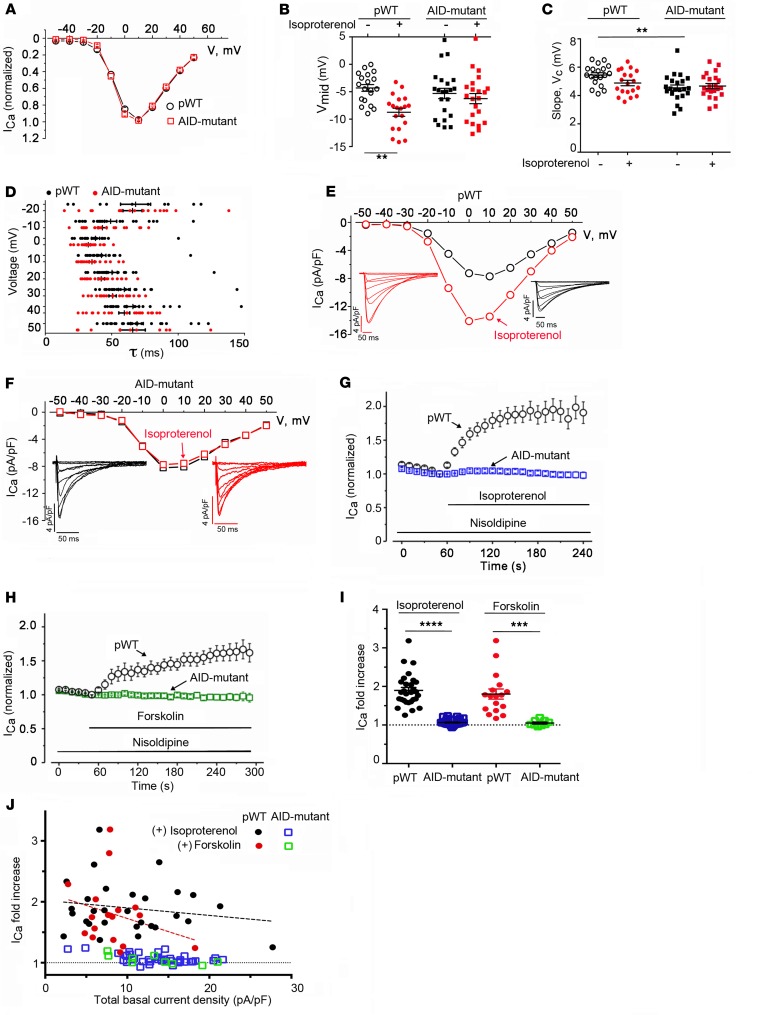
In heterologous expression studies, β subunits not only enable α1C surface trafficking, but also can differentially induce, depending on β subunit isoform, a hyperpolarizing shift in the voltage dependence of CaV1.2 activation and increase the channel open probability (Po) (12, 27). We assessed the biophysical properties of the transgenic β-less AID-mutant α1C channels compared with transgenic pWT Ca2+ channels. Surprisingly, normalized current-voltage (I-V) relationships of nisoldipine-resistant transgenic pWT and AID-mutant α1C channels were remarkably similar (Figure 2A). The midpoint potentials, derived from a Boltzmann function, for steady-state activation demonstrated a small, nonsignificant 1-mV hyperpolarizing shift for the AID-mutant channels compared with control pWT channels (Figure 2B), whereas the slope factors for the 2 channel types were not different (Figure 2C). Furthermore, the inactivation kinetics of nisoldipine-resistant Ca2+ currents were not significantly different at any test potential between cardiomyocytes isolated from pWT and AID-mutant α1C, respectively (Figure 2D). Therefore, in adult cardiomyocytes, CaV1.2 channels comprised of transgenic β-less α1C have similar voltage dependence of activation and inactivation kinetics as transgenic pWT CaV1.2 channels.
Figure 2. AID-mutant CaV1.2 channels lack β-adrenergic regulation.
(A) Normalized CaV1.2 current-voltage relationships for transgenic pWT and AID-mutant α1C cardiomyocytes in the presence of nisoldipine (n = 19 cardiomyocytes from 3 pWT α1C transgenic mice; n = 18 cardiomocytes from 6 AID-mutant α1C transgenic mice). (B and C) Bar graphs of Boltzmann function parameters Vmid and slope (Vc). **P < 0.01, ANOVA and Sidak’s multiple comparison test; n = 19 cardiomyocytes from 3 pWT α1C transgenic mice; n = 18 cardiomocytes from 6 AID-mutant α1C transgenic mice. (D) Summary of time constants of inactivation at the indicated potentials obtained from a single exponential fit (n = 24 pWT α1C cardiomyocytes from 4 mice and n = 24 AID-mutant α1C cardiomyocytes from 4 mice). P > 0.05 pWT versus AID-mutant for all voltages using Sidak’s multiple comparison test. (E and F) Exemplar nisoldipine-resistant current-voltage relationships of transgenic pWT α1C (E) and AID-mutant α1C (F) acquired in the absence (black trace) and presence of 200 nM isoproterenol (red trace). (G) Diary plot of normalized nisoldipine-resistant ICa amplitude at 0 mV (normalized to 1 at 50 seconds prior to isoproterenol) of pWT and AID-mutant α1C cardiomyocytes. Cells exposed to 300 nM nisoldipine followed by 200 nM isoproterenol in the continued presence of nisoldipine. pWT, n = 30 cardiomyocytes from 5 mice; AID-mutant, n = 45 cardiomyocytes from 7 mice. P < 0.0001 by 1-way ANOVA/multiple comparison at all time points 30 seconds after isoproterenol. (H) Diary plot of normalized nisoldipine-resistant ICa amplitude at +10 mV (normalized to 1 at 50 seconds, prior to forskolin) of pWT and AID-mutant α1C cardiomyocytes. Cells exposed to 300 nM nisoldipine followed by 10 μM forskolin in the continued presence of nisoldipine. pWT: n = 15 cardiomyocytes from 2 mice; AID-mutant: n = 20 cardiomyocytes from 6 mice. P < 0.0001 by 1-way ANOVA/multiple comparison at all time points 30 seconds after forskolin. (I) Bar graph of isoproterenol- or forskolin-induced fold increase in nisoldipine-resistant ICa. Mean ± SEM. ***P < 0.001; ****P < 0.0001 by t test. (J) Graph of isoproterenol- and forskolin-induced increase in nisoldipine-resistant current stratified by total basal current density before nisoldipine for pWT α1C and AID-mutant α1C transgenic mice. Lines fitted by linear regression for pWT cells for isoproterenol (black) and forskolin (red). For isoproterenol, pWT α1C, n = 29 cardiomyocytes; AID-mutant α1C, n = 45 cardiomyocytes. For forskolin, pWT α1C, n = 17 cardiomyocytes; AID-mutant α1C, n = 9 cardiomyocytes.
We next determined the sensitivity of CaV1.2 channels containing either transgenic pWT α1C or AID-mutant α1C to PKA modulation. In cardiomyocytes isolated from mice expressing transgenic pWT α1C, 200 nM isoproterenol increased the nisoldipine-insensitive current by a mean of 1.9-fold ± 0.1-fold (Figure 2, E–J), and shifted the Vmid in the hyperpolarizing direction by a mean of 4.4 mV (Figure 2B). Similarly, forskolin, which directly activates adenylyl cyclase, thereby bypassing β-adrenergic receptors, increased transgenic pWT α1C Ca2+ currents by 1.8-fold ± 0.1-fold (Figure 2, H–J). In sharp contrast, Ca2+ currents through transgenic AID-mutant α1C CaV1.2 channels were insensitive to either isoproterenol (Figure 2, B, F, G, I, and J) or forskolin (Figure 2, H–J). In cardiomyocytes, there is an inverse relationship between total peak current and isoproterenol-induced or forskolin-induced fold increase in Ca2+ current (27). In cardiomyocytes isolated from transgenic pWT α1C mice, we observed an inverse relationship between basal current density and isoproterenol- or forskolin-induced increase in Ca2+ current (Figure 2J). For the transgenic AID-mutant β-less channels, however, activation of PKA by either forskolin or isoproterenol had no effect on Ca2+ current, regardless of basal Ca2+ current density (Figure 2J).
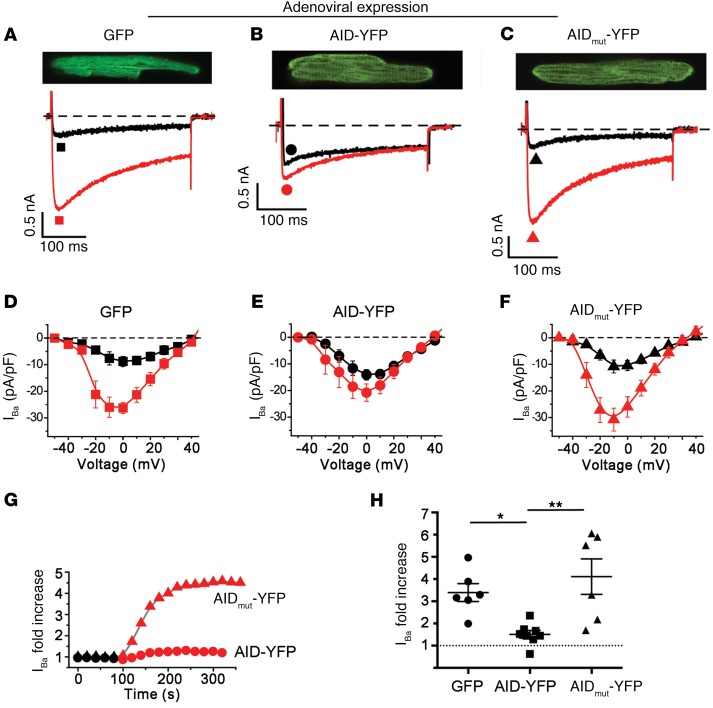
To address whether the YWI/AAA mutations themselves produced an intrinsic insensitivity of the channel to PKA modulation, we sought to engender conditions under which there would be a predominance of β-less endogenous CaV1.2 channels in isolated cardiomyocytes. We achieved this by using adenovirus to overexpress a YFP-tagged 18-residue AID peptide derived from α1C I-II loop (or a mutant YWI/AAA peptide as a control) in cultured adult guinea pig ventricular cardiomyocytes. We reasoned that this intervention would serve as a sponge for endogenous β subunits, leaving a majority of endogenous CaV1.2 channels devoid of β. In control cells expressing either GFP or YFP-tagged mutant (YWI/AAA) AID peptide incapable of binding β, 1 μM forskolin resulted in a robust 4- to 5-fold increase in whole-cell current amplitude (Figure 3, A, C, D, and F–H). By contrast, this response was sharply curtailed in cardiomyocytes overexpressing YFP-AID peptide (Figure 3, B, E, G, and H). Hence, β-less WT α1C channels also demonstrate a marked insensitivity to PKA modulation.
Figure 3. β-less WT endogenous CaV1.2 channels are not stimulated by PKA.
(A–C) Adenovirus-induced GFP, AID-YFP, and AID-mutant YFP expression in cultured guinea pig ventricular myocytes. Top: exemplar confocal images from guinea pig cardiomyocytes expressing GFP, AID-YFP peptide, or AID-mutant YFP peptide. Bottom: exemplar whole-cell Ba2+ currents from GFP and YFP-expressing guinea pig ventricular cardiomyocytes before (black trace) and after (red trace) application of 1 μM forskolin. (D–F) Current-voltage relationships from GFP, AID-YFP, and AID-mutant YFP–expressing cardiomyocytes before (black) and after (red) superfusion of 1 μM forskolin. (G) Representative diary plot showing time course of forskolin-induced increase in CaV1.2 current. (H) Forskolin-induced increase in CaV1.2 current. *P < 0.05, **P < 0.01 by 1-way ANOVA and Tukey’s multiple comparison test.
We also considered 2 trivial explanations that could potentially account for the insensitivity of AID-mutant α1C to PKA stimulation: (a) these channels were already phosphorylated by PKA under basal conditions, or (b) the β-adrenergic signaling pathway was compromised in cardiomyocytes from AID-mutant α1C transgenic mice. To address whether transgenic AID-mutant α1C channels were basally PKA phosphorylated, we used a cell-permeable cAMP-PKA inhibitor (Rp-8-Br-cAMPS), which functions by occupying cAMP binding sites thereby preventing activation of PKA holoenzyme. Rp-8-Br-cAMPS reverses isoproterenol-mediated upregulation of endogenous CaV1.2 by approximately 96% (23). In transgenic AID-mutant mice cardiomyocytes, Rp-8-Br-cAMPS did not inhibit nisoldipine-resistant basal current (Supplemental Figure 2A), ruling out the idea that AID-mutant α1C channels were basally PKA phosphorylated. The integrity of the β-adrenergic pathway in transgenic AID-mutant mice cardiomyocytes was assessed by probing whether isoproterenol application led to phosphorylation of phospholamban, a well-known PKA target in the heart (35). Western blotting indicated that phospholamban was appropriately phosphorylated at Ser16 in response to isoproterenol (Supplemental Figure 2B), confirming that the β-adrenergic signaling pathway was intact in AID-mutant transgenic mice cardiomyocytes.
β-adrenergic regulation of CaV1.2 does not require PKA phosphorylation of β subunits.
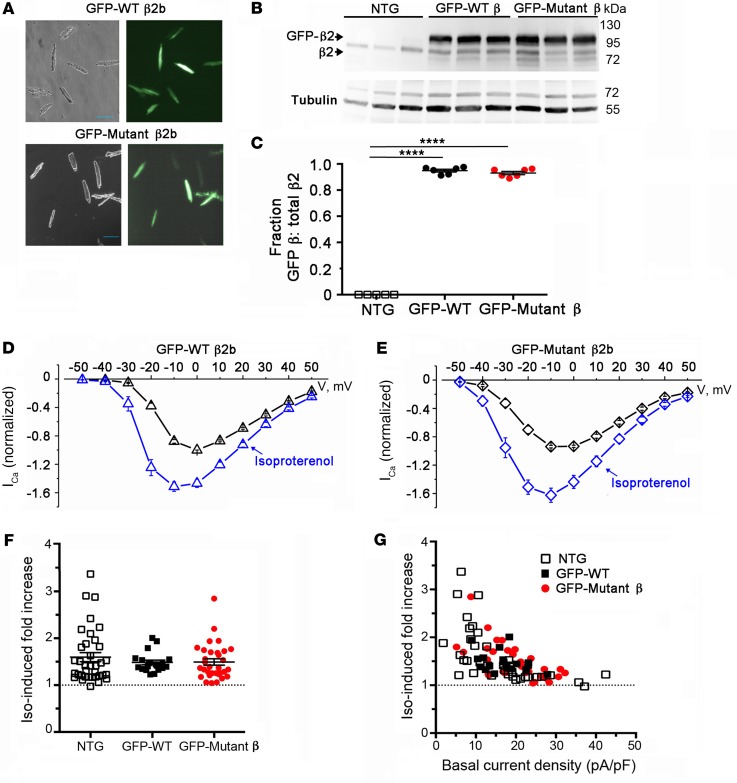
The simplest explanation for the necessary role of α1C-β interaction in PKA modulation of CaV1.2 is that the β-subunit contains phosphorylation site(s) that are vital to this regulation. Indeed, 2 phosphorylation sites on β2 C-terminus (Ser512 and Ser570) were previously identified and proposed to play a role in PKA modulation of CaV1.2 (36). However, a knockin mouse expressing a β2 subunit truncated after Pro501 displayed normal PKA modulation of CaV1.2, thus ruling out involvement of any putative C-terminal phosphorylation sites (24). Nevertheless, it remained possible that previously unappreciated phosphorylation sites N-terminal to Pro501 could mediate the increased CaV1.2 channel activity in response to activated PKA. Using both manual sequence analyses and several web-based PKA phosphorylation prediction tools (37–41), we identified 18 conserved consensus PKA phosphorylation sites in the N-terminus, SH3, and GK domains of human β2b (residues labeled red in Supplemental Figure 3). We mutated all 18 Ser/Thr residues to Ala in human β2b, and generated transgenic mice with inducible cardiomyocyte-specific expression of either GFP-tagged WT or 18-mutant β2b subunits using the same bitransgenic system as in Figure 1B. The WT and mutant β2b transgenic mice were fed doxycycline for 1 week, thus ensuring high levels of expression of the GFP-tagged β2 subunits (Figure 4A). We exploited the larger size of GFP-tagged β2 subunits compared with endogenous β to determine relative expression of transgenic and native β2 subunits (Figure 4B). Western blot indicated that in cardiomyocytes from transgenic mice, both GFP β2 and GFP-mutant β2 were markedly overexpressed (~9:1) compared with endogenous β2 (Figure 4C). Isoproterenol increased peak CaV1.2 current by a mean of 1.5-fold ± 0.1-fold in GFP-WT β2-expressing cells and 1.6-fold ± 0.1-fold in GFP-mutant β2-expressing cells, respectively, similar to nontransgenic mice (Figure 4, D–G). For both GFP-WT and GFP-mutant β2b Ca2+ channels, isoproterenol shifted the Vmid of steady-state activation by –7.0 mV and –7.5 mV, respectively. These data indicate that, although the α1C-β2 interaction is necessary for β-adrenergic regulation of CaV1.2, direct PKA phosphorylation of β2 is not involved.
Figure 4. PKA phosphorylation of CaV β is not required for β-adrenergic regulation of CaV1.2. (A) Bright-field and GFP image of WT and mutant β2b-expressing cardiomyocytes.
Scale bars: 100 μm. (B) Immunoblots using anti–β2 antibody (upper) and anti–tubulin antibody of homogenates from the hearts of nontransgenic (NTG) and doxycycline-fed GFP-WT β2 and GFP-mutant β2-expressing mice. (C) Graph of densitometry of fraction of GFP-β/total β. Mean ± SEM; n = 6 mice for NTG, WT, and mutant β2. ****P < 0.0001 compared with nontransgenic by 1-way ANOVA and Dunnett’s multiple comparison test. (D and E) Normalized current-voltage relationships of GFP-WT β2 and GFP-mutant β2 cardiomyocytes acquired before and after superfusion of 200 nM isoproterenol. Isoproterenol shifted the Vmid of steady-state activation of GFP-WT β2 and GFP-mutant β2 cardiomyocytes by –7.0 mV (P < 0.0001, t test, n = 15) and –7.5 mV (P < 0.001, t test, n = 30), respectively. (F) Column scatter plot depicting the fold increase in peak current caused by isoproterenol. Mean ± SEM; n = 36 cardiomyocytes from 5 NTG mice; n = 19 cardiomyocytes from 4 GFP-WT β2b mice; n = 32 cardiomyocytes from 5 mutant β2b mice. P = 0.55 by 1-way ANOVA. (G) Graphs of isoproterenol-induced increase in current stratified by total basal current density for cardiomyocytes isolated from NTG mice, GFP-WT β2b mice, and GFP-mutant β2b transgenic mice.
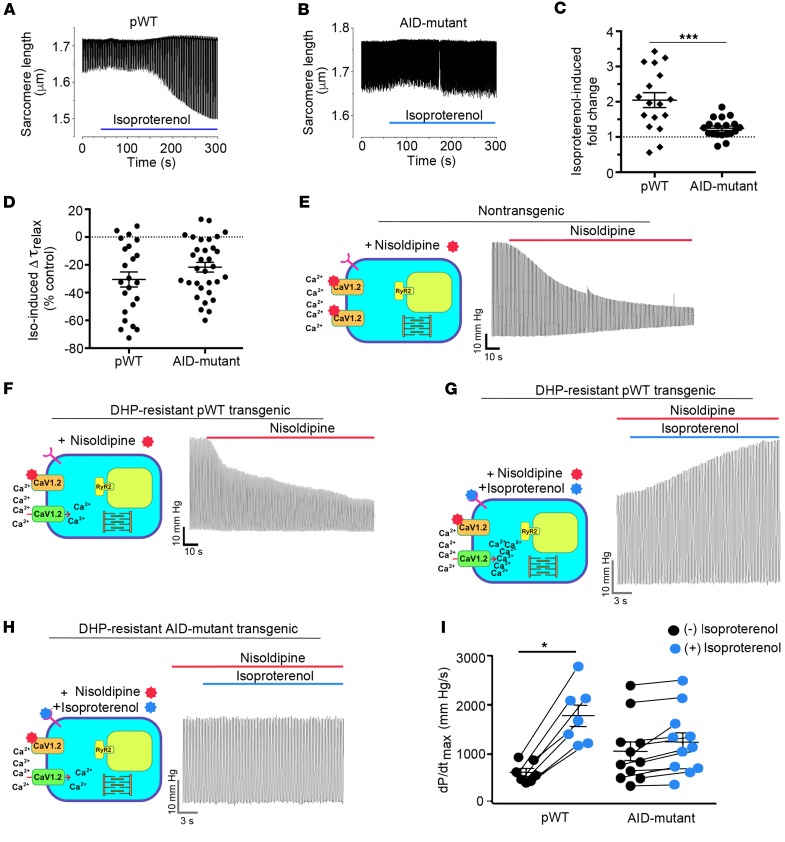
β-adrenergic regulation of cardiac contractility requires PKA regulation of CaV1.2. We next exploited the findings that transgenic β-less AID-mutant α1C channels are insensitive to PKA modulation to probe the specific role of CaV1.2 modulation in the positive inotropic effect of β-adrenergic agonists in both isolated cardiomyocytes and in the whole heart. In transgenic pWT α1C cardiomyocytes, with endogenous CaV1.2 channels silenced with nisoldipine, isoproterenol produced a robust 100% increase in fractional shortening (Figure 5, A and C). By contrast, this response was severely diminished in cardiomyocytes expressing transgenic β-less AID-mutant α1C channels in which isoproterenol produced a relatively meager 25% increase in fractional shortening (Figure 5, B and C). Consistent with the effects of isoproterenol on phospholamban phosphorylation (Supplemental Figure 2B), isoproterenol enhanced relaxation in cardiomyocytes isolated from both pWT and AID-mutant α1C transgenic mice (Figure 5D).
Figure 5. Attenuated β-adrenergic–stimulated inotropy in AID-mutant α1C transgenic mice.
(A and B) Cells with robust shortening induced by 1 Hz electrical stimulation in the presence of 300 nM nisoldipine were used. Isoproterenol (200 nM) was superfused with 300 nM nisoldipine. (C) Plot of isoproterenol-induced fold change in sarcomere length compared with before isoproterenol. Mean ± SEM; n = 17 for pWT α1C cardiomyocytes and n = 19 cardiomyocytes for AID-mutant α1C. ***P < 0.001 by t test. (D) Plot of isoproterenol-induced percentage of change in τrelaxation of sarcomere length compared with before isoproterenol. Mean ± SEM; n = 23 cardiomyocytes from 3 mice and n = 32 cardiomyocytes from 3 mice. P = 0.16 by t test. (E and F) Representative traces depicted effect of perfusion of 300 nM nisoldipine on left ventricular contraction in isolated Langendorff-perfused hearts resected from NTG mice and pWT α1C transgenic mice. (G and H) Representative traces of nisoldipine-resistant LV pressure before and during isoproterenol infusion, in hearts resected from pWT α1C and AID-mutant α1C transgenic mice. (I) Quantitative summary of dP/dtmax before and during isoproterenol infusion. n = 7 pWT α1C transgenic mice; n = 11 AID-mutant α1C transgenic mice. *P < 0.05 by t test.
We then assessed the role of CaV1.2 modulation in β-adrenergic agonist-induced positive inotropy at the whole-organ level by inserting a pressure-transduced balloon into the left ventricle of Langendorff-perfused transgenic mice hearts. This approach enabled measurement of cardiac contractility independent of vascular or systemic effects. Hearts were paced at 400 beats per minute to remove the potentially confounding effect of heart rate variability on contractility (42). After baseline measurements, 300 nM nisoldipine was infused into the coronary arteries via the aorta to suppress endogenous CaV1.2 channel currents. In hearts from nontransgenic mice, nisoldipine markedly reduced basal cardiac contractility due to the block of endogenous CaV1.2 channels (Figure 5E). In pWT α1C hearts, infusion of nisoldipine yielded a comparatively weaker effect on basal contractility owing to the expression of DHP-resistant Ca2+ channels (Figure 5F); a further infusion of 200 nM isoproterenol strongly increased cardiac contractility by 3.3-fold (Figure 5, G and I). By contrast, using the same experimental paradigm in hearts from β-less AID-mutant transgenic mice, the response to isoproterenol was nearly abolished, yielding an average increase in cardiac contractility of only 1.2-fold (Figure 5, H and I).
Discussion
Much of our current understanding regarding mechanisms underlying CaV1.2 trafficking and modulation derives from studies on recombinant channels reconstituted in heterologous cells. These cells lack the complex cytoarchitecture and intracellular milieu of adult cardiomyocytes. Recently, we developed an approach that utilizes transgenic mice expressing doxycycline-inducible, cardiac-specific, DHP-resistant α1C. Compared with knockin mice models (43, 44), this approach is both cost-effective and rapid, and perhaps more importantly, enables us to induce brief expression of mutant channels in adults, permitting the comparison of WT and mutant α1C structure-function mechanisms in the absence of developmental abnormalities and heart failure. The titration of the level of CaV1.2 expression is important, as the magnitude of β-adrenergic stimulation of CaV1.2 is reduced with increased basal current density (27, 45–49). Stratifying the magnitude of β-adrenergic–mediated upregulation of CaV1.2 current by total basal current density attenuates this confounding variable (Figure 2J and Figure 4G).
Overall, we show that in cardiomyocytes, the AID motif is required for the high-affinity interaction between α1C and β subunits, and that β-less CaV1.2 channels traffic to the dyad and produce currents that mediate normal E-C coupling. The AID-mutant β-less Ca2+ currents were completely refractory to PKA activation. These findings, combined with our recent studies (23), fundamentally recast our views on mechanisms underlying CaV1.2 trafficking and PKA modulation in cardiomyocytes as they show that (a) it is possible for β-less channels to traffic to the cell surface, (b) β2 binding to α1C is indispensable for PKA modulation of CaV1.2, and that β-adrenergic regulation of CaV1.2 can be specifically attenuated by sequestering β subunits, and (c) conserved consensus PKA phosphorylation sites in α1C (23) and β2b are not required for β-adrenergic regulation of CaV1.2 in the heart. Further, we directly show that β-adrenergic modulation of CaV1.2 is critical for sympathetic augmentation of cardiac inotropy, which is essential for the fight-or-flight response.
When coexpressed with α1 subunits in heterologous expression systems such as Xenopus oocytes or human embryonic kidney (HEK) cells, β subunits markedly augment current density by increasing membrane targeting and altering electrophysiological properties (6–8). In the adult heart, however, Ca2+ channels can traffic to the surface membrane without binding to β. How β-less α1C channels traffic to the dyad in cardiomyocytes but not in a less complex system such as HEK cells is not yet clear. Although low-affinity interactions between heterologously expressed β subunit GK and SH3 domains and the CaV2.1 α subunit in oocytes have been described (50), these potential interactions do not appear to be sufficient to rescue the trafficking of AID-mutant CaV1.2 channels in tsA-201 cells. Moreover, conditional knockout of Cacnb2 in adult cardiomyocytes caused only a 29% reduction in current density (19).
Regardless of the mechanisms enabling trafficking to the cell surface, β-less CaV1.2 channels are functionally normal under basal conditions in adult cardiomyocytes. However, the β-less channels cannot be regulated by adrenergic-PKA stimulation, although the β subunit does not appear to be the functional target of PKA. To differentiate between the lack of β binding as opposed to the mutations in the AID as causative of the defect in β-adrenergic regulation of CaV1.2, we used the complementary approach of expressing using adenovirus, YFP-AID– and YFP-mutant AID–containing peptides in cultured adult guinea pig ventricular myocytes. The response to forskolin was markedly reduced by preventing β subunits from interacting with endogenous WT α1C, implying that lack of β binding to α1C is sufficient to prevent β-adrenergic regulation of CaV1.2 in the heart. Our studies cannot address where and when β subunits first interact with α1C subunits in the heart.
Identifying the functional PKA target is more complicated. It is likely not solely α1C, based on our prior studies eliminating all conserved consensus PKA phosphorylation sites in the α1C subunit (23). Likewise, it is not solely β, based on eliminating all conserved PKA phosphorylation sites in β2 (Figure 4). Thus, our findings suggest that either there is redundancy between α1C and β subunits, such that PKA phosphorylation of either subunit is sufficient to mediate adrenergic regulation of Ca2+ channels in the heart, or that PKA phosphorylation of the core CaV1.2 subunits, α1C and β, are not necessary for β-adrenergic regulation of the Ca2+ influx in the heart. This can be addressed by cross-breeding the transgenic mice harboring Ala substitutions of all PKA consensus sites in α1C and β2b. Although PKA phosphorylation of β is not required, β subunits, via binding to the I-II loop, could regulate pore opening and voltage-sensor movement. The domain I S6-AID linker forms a continuous helix that may act as a rigid rod through which β subunits modulate channel gating (51).
The loss of β-adrenergic activation of CaV1.2 correlated with a markedly attenuated β-adrenergic contractile response. Originally proposed by Fabiato, CaV1.2 current has 2 distinct roles in E-C coupling: triggering the release of Ca2+ from the sarcoplasmic reticulum (SR) and loading the cell (and SR) with Ca2+ (52). The loss of adrenergic regulation of CaV1.2 could affect both triggering of RyR2 and the loading of SR with Ca2+, thereby attenuating the adrenergically driven inotropic response. We believe that our findings are the first to demonstrate experimentally the vital role of β-adrenergic stimulation of CaV1.2 in shaping the flight-or-fight response in the heart, and validate a recently proposed mathematical model predicting that the loss of β-adrenergic stimulation of CaV1.2 would markedly limit Ca2+ transients and contraction (53). PKA and Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation of RyR2 also enhances the open probability of the RyR2 Ca2+ release channels in the SR by enhancing their sensitivity to cytosolic (54) and synchronizing SR Ca2+ release (55–57). It remains controversial, however, as to whether increasing the open probability of RyR2 is critically important for inotropic responses in the heart (58–60). We demonstrate that without augmented CaV1.2 current to load the cell with additional Ca2+ and/or enhance RyR opening via Ca2+-induced Ca2+ release, β-adrenergic agonist-induced phosphorylation of RyR2 and phospholamban does not result in substantial β-adrenergic augmentation of cardiac contractility.
In summary, we have found that Ca2+ channel β-subunit binding to the pore-forming α1C subunit is not required for trafficking and function of the Ca2+ channel in the heart. The loss of α1C-β2 binding causes marked attenuation of β-adrenergic–induced stimulation of CaV1.2 and inotropy. Thus, we identify a new function for β subunits in the heart: as an essential component of the PKA-mediated augmentation of CaV1.2 and increased cardiac contractility that occurs during the physiological fight-or-flight response.
Methods
Reagents.
Nisoldipine and Rp-8-Br-cAMPS were purchased from Santa Cruz Biotechnology. All other chemicals were acquired from MilliporeSigma.
Animals.
The α1C transgenic constructs were generated by fusing rabbit Cacna1c cDNA (accession X15539) to the modified murine α-myosin heavy chain (MHC) tetracycline-inducible promoter (“responder” line) vector (gift of Jeffrey Robbins and Jeffrey Molkentin, University of Cincinnati, Cincinnati, OH) (61, 62). The α1C subunit was engineered to be both DHP insensitive with the substitutions T1066Y and Q1070M (32, 33) and tagged with a 3X-FLAG epitope. We made alanine substitutions of 3 conserved residues, Y467, W470, and I471, in the AID domain of rabbit α1C (Figure 1A). Two distinct AID-mutant α1C were created and studied. The results obtained from each of these lines were equivalent and therefore the data were pooled. The β2b transgenic constructs were generated by ligating a N-terminal GFP-tagged human CACNB2b cDNA (accession AAG01473) to the tetracycline-inducible vector. These mice were bred with cardiac-specific (αMHC), doxycycline-regulated, codon-optimized reverse transcriptional transactivator (rtTA) mice (obtained via the Mutant Mouse Resource and Research Center [MMRRC]) (63) to generate double-transgenic mice. The α1C transgenic animals received 0.2 g/kg doxycycline-impregnated food (Bio Serv catalog S3888) for 1–2 days and the GFP-β2b transgenic mice received the doxycycline-impregnated food for 1 week to maximize expression.
Generation of adenoviral vectors and infection of guinea pig ventricular cardiomyocytes.
Replication-deficient adenoviral vectors expressing AID-YFP and AID-mutant YFP were generated using the AdEasy Adenoviral Vector System (Agilent Technologies) according to the manufacturer’s instructions. Briefly, sequences for AID-YFP and AID-mutant YFP were PCR-amplified and cloned into pShuttle-CMV vector. After linearization with PmeI, shuttle vectors were electroporated into BJ5183 cells containing pAdEasy-1 viral plasmid. Positive recombinants were amplified, linearized with Pac I, and transfected into AD-293 cells using the calcium phosphate precipitation method. Transfected cells were monitored for development of adenoviral plaques, after which the cells were freeze-thawed and the lysate used to infect a 10-cm dish of 90% confluent HEK293 cells. Viral expansion and purification were carried out as previously described (64).
Adult guinea pig ventricular myocytes were isolated by enzymatic digestion using a Langendorff perfusion apparatus, and cultured as previously described (27). Animal treatment and use were in accordance with a protocol approved by the Columbia University Institutional Animal Care and Use Committee. Heart cells were infected 2–3 hours after plating with 5–20 μl adenoviral vector stock (≈1011–1012 viral particles/ml).
Immunoprecipitation, immunoblots, and immunofluorescence.
Cardiac lysates from 6- to 12-week-old doxycycline-fed transgenic mice were prepared from either whole hearts or isolated ventricular cardiomyocytes (65). Immunoprecipitations were performed in modified RIPA buffer consisting of 50 mM Tris HCl; pH 7.4, 150 mM NaCl, Triton X-100 (0.25%), 10 mM EDTA, 10 mM EGTA, 10 μM Calpain inhibitor I, 10 μM Calpain inhibitor II, and Complete Mini tablets (1 per 7 ml), using anti–FLAG antibody (MilliporeSigma) overnight. Immune complexes were collected using protein A (Amersham) for 2 hours, followed by extensive washing. Proteins were size-fractionated, transferred to nitrocellulose membranes, and probed with anti–FLAG antibody (MilliporeSigma), anti–tubulin antibody (Santa Cruz Biotechnology), and custom anti–α1C and anti–β2 antibodies (65). Detection was performed with a charge-coupled device camera (Carestream Imaging), and ImageQuant software was used for quantification. Isolated cardiomyocytes were fixed for 15 minutes in 4% paraformaldehyde, and indirect immunofluorescence was performed using a 1:200 rabbit anti–FLAG antibody and 1:200 FITC-labeled goat–anti-rabbit antibody (MilliporeSigma). Images were acquired using a confocal microscope.
Cellular electrophysiology.
Membrane currents from isolated mouse ventricular cardiomyocytes (66) were measured by the whole-cell patch-clamp method using a MultiClamp 700B amplifier and pCLAMP 10 software (Molecular Devices) as described (65). The pipette solution contained 40 mM CsCI, 90 mM Cs gluconate, 10 mM BAPTA, 1 mM MgCl2, 4 mM Mg-ATP, 2 mM CaCl2, and 10 mM HEPES, adjusted to pH 7.2 with CsOH. After the isolated cardiomyocytes were adequately buffered with 10 mM BAPTA in the internal solution, the isolated cardiomyocytes were superfused with 140 mM TEA-Cl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, adjusted to pH 7.4 with CsOH. For experiments in tsA-201 cells, TEA-Cl was reduced to 130 mM, and 10 mM BaCl2 was used instead of CaCl2. Pipette series resistances were usually less than 1 MΩ after 60% compensation. Leak currents and capacitance transients were subtracted by a P/4 protocol. Voltages were corrected for the liquid junction potential of –10 mV. To measure Ca2+ peak currents, the cell membrane potential was held at −50 mV and stepped to +10 mV for 350 ms every 10 seconds. To evaluate the I-V relationship for Ca2+ currents, the same protocol was repeated with steps between –50 mV to +50 mV in 10-mV increments. All experiments were performed at room temperature, 22°C ± 1°C. The parameters of voltage-dependent activation were obtained using a modified Boltzmann distribution: I(V)= Gmax * (V-Erev)/[1 + exp(Vmid-V)/Vc)], where I(V) is peak current, Gmax is maximal conductance, Erev is reversal potential, Vmid is the midpoint, and Vc is the slope factor.
Whole-cell recordings of virally infected cultured guinea pig ventricular myocytes were conducted at room temperature as previously described (27, 67). Patch pipettes typically had 1–2 MΩ series resistance when filled with internal solution containing 150 mM cesium-methanesulfonate, 10 mM EGTA, 5 mM CsCl, 1 mM MgCl2, 10 mM HEPES, and 4 mM MgATP (pH 7.3). Cells were perfused with normal Tyrode external solution during formation of gigaohm seal. After successful break-in to the whole-cell configuration, the perfusing medium was switched to an external recording solution containing 155 mM N-methyl-D-glucamine-aspartate, 10 mM 4-aminopyridine, 1 mM MgCl2, 5 mM BaCl2, and 10 mM HEPES (pH 7.4). Currents were sampled at 50 KHz and filtered at 5 KHz, and leak and capacitive currents were subtracted using a P/8 protocol.
Fractional shortening of isolated cardiomyocytes.
Freshly isolated myocytes were superfused with a Tyrode’s solution containing 1.0 mM CaCl2 and 300 nM nisoldipine. Myocytes were field stimulated at 1 Hz. Percent contraction of sarcomere length was measured using the SarcLen module (Ionoptix) and calculated as the difference of shortest sarcomere length during a contraction subtracted from the relaxed sarcomere length, divided by the relaxed sarcomere length, all averaged over at least 8 contractions.
Ex vivo cardiac contractility.
The cannulated hearts were retrogradely perfused on a Langendorff system with a modified Krebs solution (118.5 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11 mM glucose, 1.8 mM Ca2+). Left ventricular (LV) pressure was measured using a balloon catheter connected to an APT-300 pressure transducer, which was connected to a PowerLab digitizer (ADInstruments). Hearts were paced at 400 beats per minute using electrodes connected to a pacing stimulator system. After initial assessment of cardiac contractility, 300 nM nisoldipine was perfused to silence endogenous Ca2+ currents. The effects of nisoldipine on contractility were assessed after at least 3 minutes and on stabilization of LV pressures. Thereafter, 200 nM isoproterenol was perfused with 300 nM nisoldipine for at least 3 minutes. Peak LV pressure during the 3-minute period was used for the assessment of β-adrenergic agonist stimulation.
Statistics.
Results are mean ± SEM. For multiple group comparisons, 1-way ANOVA followed by multiple comparison testing was performed. For comparisons between 2 groups, an unpaired Student’s t test was used. Statistical analyses were performed using Prism 6 (Graphpad Software). Differences were considered statistically significant at P values less than 0.05.
Data availability.
The data and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study approval.
The Institutional Animal Care and Use Committee at Columbia University approved all animal experiments.
Author contributions
SOM, HMC, and GSP conceived the study. LY, A Katchman, JK, A Kushnir, SIZ, SV, SOM, HMC, and GSP determined the study methodology. LY, A Katchman, JK, A Kushnir, SIZ, BC, ZS, PS, GL, AP, DR, SOM, and HMC carried out the study investigation. SOM, HMC, and GSP wrote the original draft of the manuscript. LY, A Katchman, JK, A Kushnir, SIZ, BC, SV, GL, AP, DR, GSP, HMC, and SOM reviewed and edited the manuscript. SOM, HMC, and GSP acquired funding for the study. SOM, HMC, and GSP contributed resources to the study.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 HL113136, R01 HL121253, HL126735, and HL 140934. JK was supported by NIH grant T32 HL007343. ZS, AP, and DR were supported by NIH grant T32HL120826.
Version 1. 11/13/2018
In-Press Preview
Version 2. 01/07/2019
Electronic publication
Version 3. 02/01/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
License: Copyright 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(2):647–658.https://doi.org/10.1172/JCI123878.
See the related Commentary at The L-type calcium channel current modulation mechanism: the plot thickens and fogs.
Contributor Information
Lin Yang, Email: ly44@columbia.edu.
Alexander Katchman, Email: ank2121@columbia.edu.
Alexander Kushnir, Email: ak2033@columbia.edu.
Sergey I. Zakharov, Email: siz2101@columbia.edu.
Bi-xing Chen, Email: bc4@columbia.edu.
Zunaira Shuja, Email: zs2305@cumc.columbia.edu.
Prakash Subramanyam, Email: prakash.subramanyam@gmail.com.
Guoxia Liu, Email: gl2018@columbia.edu.
Arianne Papa, Email: ap3452@cumc.columbia.edu.
Geoffrey S. Pitt, Email: geoffrey.pitt@med.cornell.edu.
Henry M. Colecraft, Email: hc2405@columbia.edu.
Steven O. Marx, Email: Sm460@cumc.columbia.edu.
References
- 1.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Min D, et al. The alterations of Ca2+/calmodulin/CaMKII/CaV1.2 signaling in experimental models of Alzheimer’s disease and vascular dementia. Neurosci Lett. 2013;538:60–65. doi: 10.1016/j.neulet.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22(3):549–558. doi: 10.1016/S0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 4.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31(6):973–985. doi: 10.1016/S0896-6273(01)00438-X. [DOI] [PubMed] [Google Scholar]
- 5.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J. 2000;79(5):2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Reyes E, et al. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992;267(3):1792–1797. [PubMed] [Google Scholar]
- 7.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel beta subunit. J Biol Chem. 1993;268(17):12359–12366. [PubMed] [Google Scholar]
- 8.Lacerda AE, et al. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991;352(6335):527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- 9.Bichet D, et al. The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25(1):177–190. doi: 10.1016/S0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 10.Chien AJ, et al. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270(50):30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 11.Brice NL, et al. Importance of the different beta subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci. 1997;9(4):749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 12.Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35(6):599–620. doi: 10.1023/B:JOBB.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 13.Buraei Z, Yang J. The ß subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90(4):1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13(3):298–307. doi: 10.1016/S0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 15.Weissgerber P, et al. Reduced cardiac L-type Ca2+ current in Ca(V)beta2-/- embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ Res. 2006;99(7):749–757. doi: 10.1161/01.RES.0000243978.15182.c1. [DOI] [PubMed] [Google Scholar]
- 16.Altier C, et al. The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci. 2011;14(2):173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 17.Fang K, Colecraft HM. Mechanism of auxiliary β-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J Physiol (Lond) 2011;589(Pt 18):4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waithe D, Ferron L, Page KM, Chaggar K, Dolphin AC. Beta-subunits promote the expression of Ca(V)2.2 channels by reducing their proteasomal degradation. J Biol Chem. 2011;286(11):9598–9611. doi: 10.1074/jbc.M110.195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meissner M, et al. Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the cacnb2 gene. J Biol Chem. 2011;286(18):15875–15882. doi: 10.1074/jbc.M111.227819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marbán E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007;101(2):166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 21.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87(12):1095–1102. doi: 10.1161/01.RES.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 22.Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol (Lond) 1977;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katchman A, et al. Proteolytic cleavage and PKA phosphorylation of α1C subunit are not required for adrenergic regulation of CaV1.2 in the heart. Proc Natl Acad Sci USA. 2017;114(34):9194–9199. doi: 10.1073/pnas.1706054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandmayr J, et al. Deletion of the C-terminal phosphorylation sites in the cardiac β-subunit does not affect the basic β-adrenergic response of the heart and the Ca(v)1.2 channel. J Biol Chem. 2012;287(27):22584–22592. doi: 10.1074/jbc.M112.366484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemke T, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283(50):34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98(2):e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ Res. 2008;102(7):e54–e64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 28.Chen YH, et al. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429(6992):675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 29.Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42(3):387–399. doi: 10.1016/S0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 30.Van Petegem F, Clark KA, Chatelain FC, Minor DL. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429(6992):671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL. Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaValpha1 AID-CaVbeta interaction site that is critical for channel modulation. Structure. 2008;16(2):280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He M, Bodi I, Mikala G, Schwartz A. Motif III S5 of L-type calcium channels is involved in the dihydropyridine binding site. A combined radioligand binding and electrophysiological study. J Biol Chem. 1997;272(5):2629–2633. doi: 10.1074/jbc.272.5.2629. [DOI] [PubMed] [Google Scholar]
- 33.Hockerman GH, Peterson BZ, Sharp E, Tanada TN, Scheuer T, Catterall WA. Construction of a high-affinity receptor site for dihydropyridine agonists and antagonists by single amino acid substitutions in a non-L-type Ca2+ channel. Proc Natl Acad Sci USA. 1997;94(26):14906–14911. doi: 10.1073/pnas.94.26.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermair GJ, et al. Reciprocal interactions regulate targeting of calcium channel beta subunits and membrane expression of alpha1 subunits in cultured hippocampal neurons. J Biol Chem. 2010;285(8):5776–5791. doi: 10.1074/jbc.M109.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colyer J. Phosphorylation states of phospholamban. Ann N Y Acad Sci. 1998;853:79–91. doi: 10.1111/j.1749-6632.1998.tb08258.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;38(32):10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- 37.Neuberger G, Schneider G, Eisenhaber F. pkaPS: prediction of protein kinase A phosphorylation sites with the simplified kinase-substrate binding model. Biol Direct. 2007;2:1. doi: 10.1186/1745-6150-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iakoucheva LM, et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32(3):1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou FF, Xue Y, Chen GL, Yao X. GPS: a novel group-based phosphorylation predicting and scoring method. Biochem Biophys Res Commun. 2004;325(4):1443–1448. doi: 10.1016/j.bbrc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294(5):1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 41.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31(13):3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci USA. 2010;107(22):10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domes K, et al. Truncation of murine CaV1.2 at Asp-1904 results in heart failure after birth. J Biol Chem. 2011;286(39):33863–33871. doi: 10.1074/jbc.M111.252312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y, et al. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286(14):12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muth JN, et al. Cardiac-specific overexpression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem. 1999;274(31):21503–21506. doi: 10.1074/jbc.274.31.21503. [DOI] [PubMed] [Google Scholar]
- 46.Beetz N, et al. Transgenic simulation of human heart failure-like L-type Ca2+-channels: implications for fibrosis and heart rate in mice. Cardiovasc Res. 2009;84(3):396–406. doi: 10.1093/cvr/cvp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang M, et al. Enhanced basal contractility but reduced excitation-contraction coupling efficiency and beta-adrenergic reserve of hearts with increased Cav1.2 activity. Am J Physiol Heart Circ Physiol. 2010;299(2):H519–H528. doi: 10.1152/ajpheart.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97(10):1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, et al. Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50(3):460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca(V)beta modulatory properties are AID-independent. Nat Struct Mol Biol. 2005;12(4):372–377. doi: 10.1038/nsmb909. [DOI] [PubMed] [Google Scholar]
- 51.Findeisen F, Minor DL. Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J Gen Physiol. 2009;133(3):327–343. doi: 10.1085/jgp.200810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negroni JA, et al. β-adrenergic effects on cardiac myofilaments and contraction in an integrated rabbit ventricular myocyte model. J Mol Cell Cardiol. 2015;81:162–175. doi: 10.1016/j.yjmcc.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aalkjaer C, Nilsson H. Vasomotion: cellular background for the oscillator and for the synchronization of smooth muscle cells. Br J Pharmacol. 2005;144(5):605–616. doi: 10.1038/sj.bjp.0706084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kushnir A, Betzenhauser MJ, Marks AR. Ryanodine receptor studies using genetically engineered mice. FEBS Lett. 2010;584(10):1956–1965. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 57.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94(6):e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 58.Shan J, et al. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120(12):4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muraski JA, et al. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci USA. 2008;105(37):13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eisner DA, Kashimura T, O’Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46(4):474–481. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92(6):609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 62.Hambleton M, et al. Inducible and myocyte-specific inhibition of PKCalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293(6):H3768–H3771. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valencik ML, McDonald JA. Codon optimization markedly improves doxycycline regulated gene expression in the mouse heart. Transgenic Res. 2001;10(3):269–275. doi: 10.1023/A:1016601928465. [DOI] [PubMed] [Google Scholar]
- 64.Colecraft HM, et al. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol (Lond) 2002;541(Pt 2):435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Katchman A, Samad T, Morrow J, Weinberg R, Marx SO. β-adrenergic regulation of the L-type Ca2+ channel does not require phosphorylation of α1C Ser1700. Circ Res. 2013;113(7):871–880. doi: 10.1161/CIRCRESAHA.113.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 67.Xu X, Marx SO, Colecraft HM. Molecular mechanisms, and selective pharmacological rescue, of Rem-inhibited CaV1.2 channels in heart. Circ Res. 2010;107(5):620–630. doi: 10.1161/CIRCRESAHA.110.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.