Abstract
Accumulation of advanced glycation end products (AGEs) on nucleotides, lipids and peptides/proteins are an inevitable component of the aging process in all eukaryotic organisms, including humans. To date, a substantial body of evidence shows that AGEs and their functionally compromised adducts are linked to and perhaps responsible for changes seen during aging and for the development of many age-related morbidities. However, much remains to be learned about the biology of AGE formation, causal nature of these associations and whether new interventions might be developed that will prevent or reduce the negative impact of AGEs-related damage. To facilitate achieving these latter ends, we show how invertebrate models, notably Drosophila melanogaster and Caenorhabditis elegans, can be used to explore AGE-related pathways in depth and to identify and assess drugs that will mitigate against the detrimental effects of AGE-adduct development.
Graphical Abstract
Chaudhuri et al. discuss mechanistic evidence for the role of glycolytic byproducts that lead to accumulation of Advanced Glycation End products (AGEs) in the onset of age-related diseases. They outline how model organisms can unveil these mechanisms that will help develop better therapeutics to overcome diabetic pathologies and neurodegenerative diseases.
Introduction
A heterogeneous group of molecules collectively called advanced glycation end products (AGEs), are produced in the classical Maillard reaction, discovered at the beginning of the 20th century (Maillard, 1912). More than three decades ago, Monnier and Cerami proposed a Maillard theory of aging postulating that slow and continuous accumulation of AGEs was a causal factor in aging (Bjorksten, 1968; Monnier, 1989; Monnier et al., 1988; Sell and Monnier, 1989). Furthermore, they proposed that the protracted buildup of these compounds may alter the structure and function of proteins, thus affecting several of the hallmarks of aging(Gugliucci and Menini, 2017; López-Otín et al., 2013). This process may also contribute to the pathology of metabolic diseases, such as diabetes and atherosclerosis, as well as oxidative stress and inflammation associated with neurodegenerative diseases of aging. Support for this hypothesis includes an age-dependent increase in browning (Maillard reaction), fluorescence, cross-linking, and insolubility, and accrual of AGEs in collagens and lens crystallins (Monnier et al., 1984; Monnier VM, Stevens VJ, 1981). Despite this accumulating evidence, debate continues over whether AGEs are causal or just a consequence of aging and age-related diseases (Gugliucci, 2017).
The link between age-related diseases and AGEs has been difficult to unravel for several reasons: (1) the variety of sources for AGEs, (2) the gradual build-up of AGEs, which can take decades to be detected in humans, (3) lack of accessible and sensitive methods to quantify specific AGEs, (4) a growing number of targets of AGEs, and (5) a lack of models that recapitulate the pathologies resulting from the accumulation of AGEs. These factors have complicated efforts to model causation by connecting an AGE to one specific target and a specific disease relevant to aging.
In this review, we first focus on the chemistry of AGEs, how they accumulate through in situ synthesis or via ingestion of food, the naturally occurring mechanisms that reduce their formation, and their metabolism at the whole-body level. We then explore the causative mechanistic links that impact aging and metabolic diseases via accumulation of AGEs. We suggest considerations for developing model systems to study the impact of AGEs. Finally, we illustrate how modeling of AGEs using model organisms, notably worms and flies, can be used to identify ‘anti-AGE’ drugs and examine their relevance to age-related diseases, including diabetic complications and neurodegeneration.
The Maillard reaction: initiators, propagators, and chemistry
AGEs are produced by glycation in cells or in long-lived extracellular proteins. Protein glycation is a complex series of sequential reactions collectively called the Maillard reaction (Fig. 1), present in all tissues and fluids where significant concentration of glucose, fructose, or more reactive dicarbonyls react with proteins (Stevens et al. 1977, Sell et al. 1991, Monnier et al. 1992). When glucose mediates the reaction, initially the Amadori adduct fructosyl-lysine is formed. In hemoglobin, this adduct is called HbA1c, which revolutionized the diagnosis and follow-up of diabetic patients (Rahbar et al., 1969). However, HbA1c only assesses glucose derived products. There is an urgent need to develop markers of AGEs based on downstream products of glucose metabolism like α-dicarbonyl compounds (α-DCs) that are significantly more reactive than glucose and examine their link with various age-related pathologies.
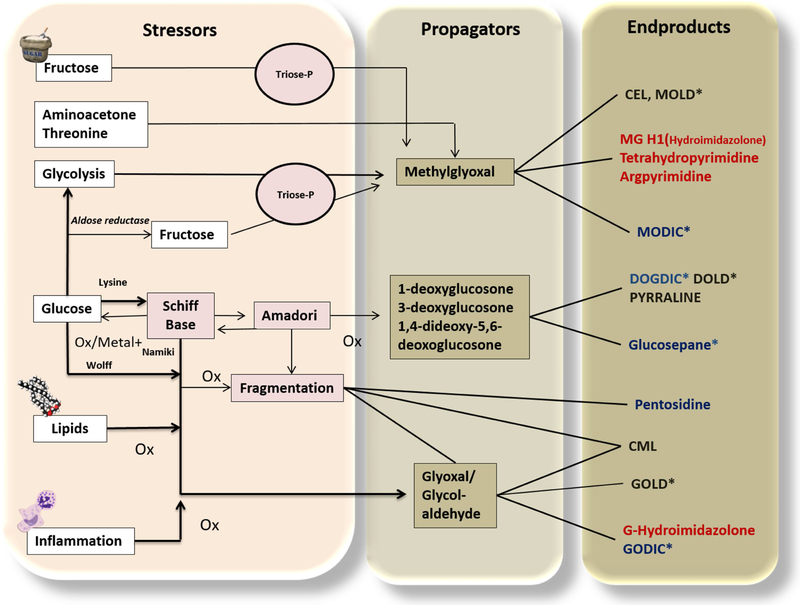
Figure 1. The Maillard reaction or glycation.
Main pathways and main known advanced glycation end products (AGEs) relevant to human pathology. In the left column, we represent the main initiators or stressors, propagators in the center (very potent alpha dicarbonyls, of which methylglyoxal is the most important and reactive) and end products in the right column. Among the endproducts: black represents lysine modifications; red indicates arginine adducts and blue denotes lysine-arginine modifications. CEL: carboxy ethyl lysine; CML: carboxy methyl lysine; MG-H1: methylglyoxal hydroimidazolone; MODIC: methylglyoxal dimer imidazolone crosslink; MOLD: Methylglyoxal lysine dimer; GODIC:glyoxal-derived imidazolium cross-link; DOGDIC, 3-deoxyglucosone-derived imidazolium cross-link; GOLD: glyoxal lysine dimer; DOLD: deoxyglucosone lysine dimer. * indicates crosslinked products. Modified from: (Monnier et al., 2005).
Chronic hyperglycemia results in several metabolic and biochemical perturbations, including elevation of a series of highly reactive α-DCs, such as methylglyoxal (MGO), glyoxal (GO), and 3-deoxyglucosone (3DG) (Henning et al., 2014; Singh et al., 2014). The α-DCs are unavoidable byproducts of anaerobic glycolysis and to a smaller degree lipid peroxidation (Lange et al., 2012; Rabbani and Thornalley, 2014; Thornalley et al., 2003), which react indiscriminately with proteins, lipids, and DNA to yield AGEs (Peppa and Vlassara, 2005; Thornalley et al., 2003). The production of MGO, a key precursor of the AGEs, occurs spontaneously from the triose phosphate isomers glyceraldehyde-3 phosphate and dihydroxyacetone phosphate during glycolysis (Kiefer et al., 2014; Sousa Silva et al., 2013) and is primarily due to β-elimination of a phosphate group from the enediolate phosphate intermediate. The study of the role of α-DCs like MGO in AGEs has lagged due to their unstable nature which makes them hard to detect in standard metabolomics studies and because they are non-enzymatic byproducts of metabolic reactions.
Important sources of AGEs include hydroimidazolones derived from arginine residues modified by GO, MGO, and 3-DG, which we will discuss extensively in this review. Other crucial AGEs compounds are Nε-carboxymethyl-lysine (CML), Nε-carboxyethyl-lysine (CEL), pentosidine, pyrraline and glucosepane (Nemet et al., 2011; Sveen et al., 2015). As discussed in detail below, the catabolism of cellular AGEs yields a new pool of second generation, highly reactive AGE intermediates (peptides and free adducts) named glycotoxins by some authors (Koschinsky et al., 1997; Uribarri et al., 2003a, 2003b), which pass through the bloodstream to compound cellular damage. Some of these serum AGE-small adducts react with new proteins, (e.g., LDL, collagen) perpetuating and propagating oxidative modifications and/or producing new AGE crosslinks in vitro and in vivo (Gugliucci and Bendayan, 1996; Makita et al., 1994;Vlassara et al., 1992).
AGE formation renders irreversible damage to the biological macromolecules, altering their structural and functional integrity (Singh et al., 2014). Kinetics of AGE formation through a reaction between MGO and most susceptible amino acids such as arginine, lysine, and cysteine have been studied in vitro by incubating bovine serum albumin (BSA) and MGO under physiological conditions (Lo et al., 1994). These studies indicate the irreversible formation of MGO adducts mainly on arginine and lysine. As shown in Figure 1,the adducts primarily result from a reaction between MGO and Nα-acetylarginine on the peptide backbone leading to an irreversible imidazolone derivative or hydroimidazolone (MG-H1) through autoxidation of an intermediate 1,5-dihydroimidazolone (Lo et al., 1994). Also, modification of the lysine residues triggered by MGO results in Nε-carboxyethyl-lysine (CEL) (Rabbani and Thornalley, 2015a).
In addition to amino acids, specific nucleotides are also susceptible to modification by MGO. DNA-AGEs such as N2(1-carboxyethyl)-2’-deoxyguanosine (CEdG) has been described as a potential biomarker of chronic hyperglycemia in mice. CEdG levels are significantly elevated in urine collected from hyperglycemic Leprdb/db mice compared to normoglycemic control mice (Jaramillo et al., 2017). Of note, many of the same compounds are produced in baked and roasted foods (Delgado-Andrade and Fogliano, 2018). Administration of dietary AGEs in mice suggests that AGEs consumed through dry heat cooked food (Cai et al., 2012; Goldberg et al., 2004) could potentially enhance the risk for age-related diseases (Goldberg et al., 2004), as depicted further below in Figure 4, via activation of the receptor of AGE (RAGE). The biochemistry of AGE formation and its downstream effects are complex and remain to be fully understood. Nevertheless, it is crucial to understand the dicarbonyl detoxification mechanisms that are potential therapeutic targets for AGE-mediated diseases as well as the pathways for AG Es disposal after they are generated.
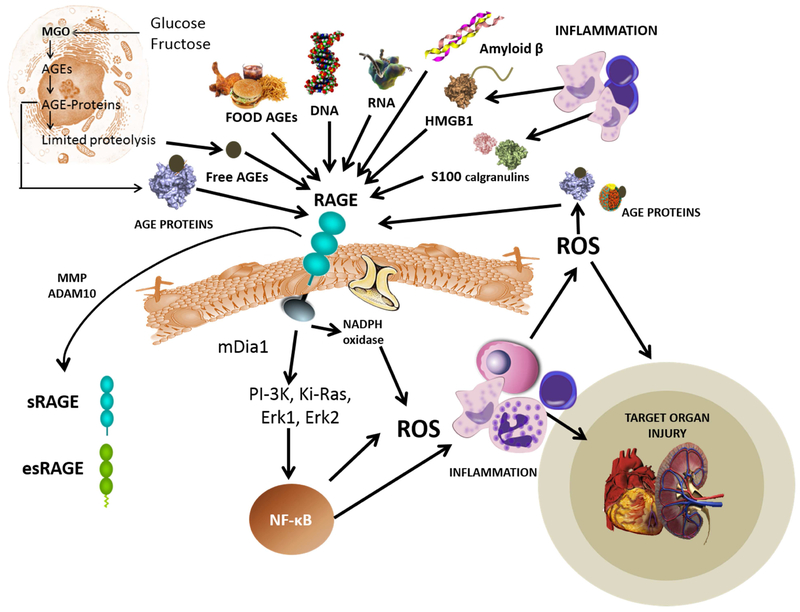
Figure 4. The receptor for advanced glycation endp ro ducts (RAGE) is akey pathway for inflammatory complication sinaging and chronic disease.
As shown in the top part of the figure, RAGE is a pattern recognition receptor that participates in primary immunity and has a variety of ligands. Important for our topic are the AGE, AGE-proteins, AGE-peptides and adducts from primary AGE catabolism and dietary AGEs. RAGE activation leads to a signaling cascade that produces NF-kB coordinated inflammatory responses that can lead to target tissue injury. Soluble forms of the receptor (esRAGE and sRAGE) are released to the circulation. Some authors suggest they serve to modulate the response acting as decoy ligands. They are useful as biomarkers of the whole-body AGE-RAGE axis.
The α-DC detoxification network
In the first decade of glycation research, the emphasis was on extracellular glycation of collagens, crystallins and other long-lived proteins. These proteins are correlated with aging and have shown to be important in the pathogenesis of diabetic complications. Since then, more attention has been devoted to intracellular glycation, especially by methylglyoxal, which is between 2 and 3 orders of magnitude more reactive than glucose. The importance of methylglyoxal is underscored by the existence of several conserved enzymes involved in its detoxification.
Glyoxalases such as Glyoxalase I (GLO1) and DJ1/PARK7 are an evolutionarily conserved group of enzymes involved in the detoxification of reactive α-DCs including methylglyoxal and glyoxal that eventually get converted into lactic or glycolic acid as depicted in Figure 2. Studies using knockdown of these enzymes in mice, cell culture, and worms show that these genes play a critical role in avoiding pathologies resulting from the accumulation of α-DC-mediated modification of amino acids or nucleotides (Giacco et al., 2014; Lee et al., 2012; Richarme et al., 2017). The tissue-specificity and sub-cellular localization of the different glyoxalase enzymes are not well-understood. The glyoxalase system can be either glutathione GSH-dependent (e.g., Glo1) or GSH-independent (e.g., Dj-1) (Lee et al., 2012; Thornalley, 1990). In recent years, a number of human cohort studies have provided evidence that polymorphisms associated with genes that detoxify α-DCs are linked to certain age-related diseases. Polymorphisms associated with the glyoxalase Glo1 gene have been associated with the development of nephropathy and retinopathy in Type 2 diabetics (Gale et al., 2004; Kalousová et al., 2008; Wu et al., 2011). Genotyping of whole blood lysates obtained from Type 1 and Type 2 diabetics revealed a marked decrease in the activity of glyoxalase 1 that was predicted to be associated to three SNPs (Peculis et al., 2013). In addition to glyoxalase I, variations within the Dj-1 glyoxalase have also been associated with age-related diseases such as Parkinson’s Disease (PD). A study on 294 PD patients in Southern Italy demonstrated the association of sporadic PD with 5 SNPs within the 3535 bp region extending from the promoter to intron 2 of the Dj-1 gene (De Marco et al., 2010).
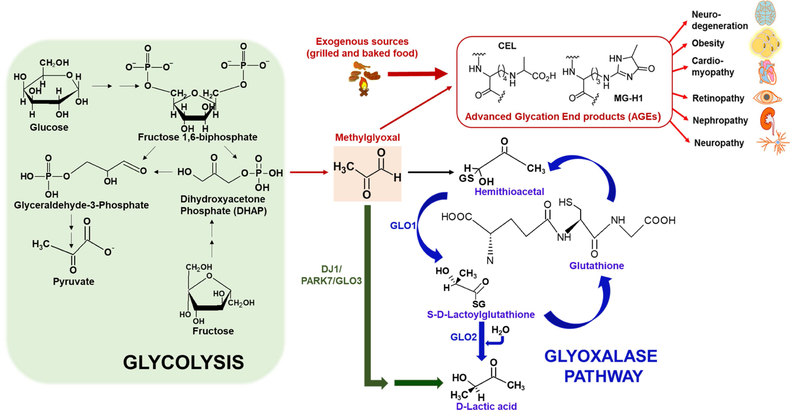
Figure 2. Form ation of AGEs and the glyoxalase system.
The figure illustrates the formation and detoxification of MGO, a glycolytic byproduct that is formed either from glucose or fructose. Endogenously derived glycolytic byproducts (e.g., Methylglyoxal or MGO) or their AGE derivatives (e.g., Nε-carboxyethyl-lysine or CEL and Methylglyoxal-derived hydroimidazolone or MG-H1) lead to a variety of diseases affecting different organs (such as brain, heart, eyes, kidney, lungs) that complicate with age. In addition to endogenous sources such as glucose or fructose derived dihydroxyacetone phosphate (DHAP) (formed during glycolysis), exogenous dietary sources such as dry heat-cooked food predominantly produces AGEs (large red arrow). Detoxification of MGO occurs via glutathione (GSH)-dependent glyoxalase pathway mediated by two mitochondrial enzymes glyoxalase 1 (GLO1) and glyoxalase 2 (GLO2) that eventually converts MGO to lactic acid. Alternatively, MGO can also be converted to lactic acid in a single step mediated by a GSH-independent cytosolic enzyme glyoxalase 3 (GLO3), a mammalianorthologue of the protein DJ1/PARK7.
Recent studies in Escherichia coli, show that the protein DJ1 possesses deglycase activity that restores proteins and amino acids from glycation events induced by methylglyoxal or glyoxal (Richarme et al., 2015). The deglycase activity of DJ1 was not supported in a follow-up study due to a potential artifact arising from TRIS buffer (Pfaff et al., 2017). More recent studies using deglycase-depleted cells reinforce DJ1 as a bona fide deglycase (Richarme and Dairou, 2017). The late stages of the Maillard reaction which form AGEs are considered to be irreversible. However, if the early steps of AGE formation are reversible, then it will provide another step for potential intervention.
Other detoxifying enzymes fo r dicarbonyls and early glycation adducts
Another class of enzymes that play a critical role in the detoxification of the reactive α-DCs are the evolutionarily conserved NADPH-dependent aldo-keto reductases (AKRs) (Vander Jagt et al., 1992). AGE-mediated atherosclerotic lesion formation was enhanced in the absence of AKRs (Baba et al., 2009). Another mechanism of protection against glycation mediated damage has been shown via AGE sequestration by lysozymes resulting in improved renal excretion in mice (Zheng et al., 2001). As stated earlier, due to its stability in the closed ring conformation, glucose is not as strong a glycating agent as MGO. However, its effects are important in erythrocytes and other cells that mainly use GLUT I (non-insulin-dependent) transporter. As shown in Figure 1, the early Schiff base formed between glucose and lysine in proteins stabilizes into a fructosamine. Until the last decade, the fate of fructosamines in mammalian cells was only considered to be their spontaneous conversion into AGEs, as shown in Figure 1. A mammalian fructosamine-3-kinase (FN-3-K), which phosphorylates fructoselysine (FL) residues on glycated proteins to FL-3-phosphate has been isolated and cloned (Delpierre et al., 2004). This unveiled an unsuspected intracellular metabolism of glucose adducts. FN-3-K phosphorylates both low-molecular-mass and protein-bound fructosamines with high affinity for the third carbon of their deoxyfructose moiety, producing fructosamine 3-phosphates. The latter are unstable and spontaneously decompose into inorganic phosphate and 3-deoxyglucosone, regenerating the unglycated amine (Gugliucci, 2005). The presence of proteins related to fructosamine 3-kinase in many prokaryotic and eukaryotic genomes implies that this ‘deglycation’ process is not restricted to erythrocytes nor to just mammals (Collard et al., 2004; Dunmore et al., 2018; Van Schaftingen et al., 2007; Szwergold et al., 2011).
A schematic describing some of the known steps in the formation and detoxification of AGEs is shown in Figure 2. Potentially, several regulators of glyoxalases and alpha keto-reductases in addition to other enzymes that can detoxify AGEs exist and remain to be discovered. Therefore, in addition to our existing body of knowledge on the detoxification pathways, there is a need for studies using model systems that are amenable to large genetic screens, such as worms, flies, and yeast, to determine additional genes and their regulators in the α-dicarbonyl detoxification network. These studies are likely to be pivotal in providing new drug targets that limit the accumulation of AGEs that build up with age.
Systemic metabolism and handling o f AGEs, the key role o f the kidney
AGEs are produced endogenously but are also consumed through the diet especially through dry heat cooked food. Human serum contains partially hydrolyzed AGE peptides and free AGE adducts (Bucala et al., 1994; Gugliucci and Bendayan, 1996). These are increased in diabetes and more so in end-stage renal failure even in the absence of diabetes. In earlier studies, we presented the renal fate of AGEs (Gugliucci and Bendayan 1995, 1996) and have shown that AGE peptides are filtered and then reabsorbed in the proximal tubules followed by excretion of free AGE adducts, as depicted in Figure 3. To determine this fate, AGE-BSA and AGE-peptides were injected in rats, and AGE-products in renal tissue of rats were monitored by colloidal gold post-embedding immunoelectron microscopy (Gugliucci and Bendayan 1995, 1996). We showed that the endo-lysosomal apparatus of the proximal convoluted tubule plays a role in the disposal of AGE-peptides. AGE-peptide clearance in humans and rats is lower than the creatinine clearance which suggests that not all of circulating AGE-peptides are secreted in the urine; reabsorption also occurs. No mammalian enzyme is known to mediate the catabolism of AGE moieties after the lysosomal hydrolysis of peptide bonds. We suggest the existence of a secretory process of resulting AGE-amino acids (free adducts) into urine which could explain the presence of AGE-adducts such as pentosidine in the urine of diabetic patients. These pathways, which depend on glomerular filtration and tubular function, are severely compromised in chronic and end-stage renal failure (Thornalley, 2005a, 2006). Due to this fact, AGE adducts are one of the classical ‘middle toxins’ associated with renal failure (Vlassara, 1994). With the arrival of sensitive LC-MS/MS techniques, a more comprehensive representation of the role of the kidney in AGE control emerged, including the fate of small peptides and free adducts (Agalou et al., 2005; Rabbani and Thornalley, 2009; Rabbani et al., 2007; Thornalley, 2005b; Thornalley and Rabbani, 2009). Key AGEs have been described and shown in Figure 1.
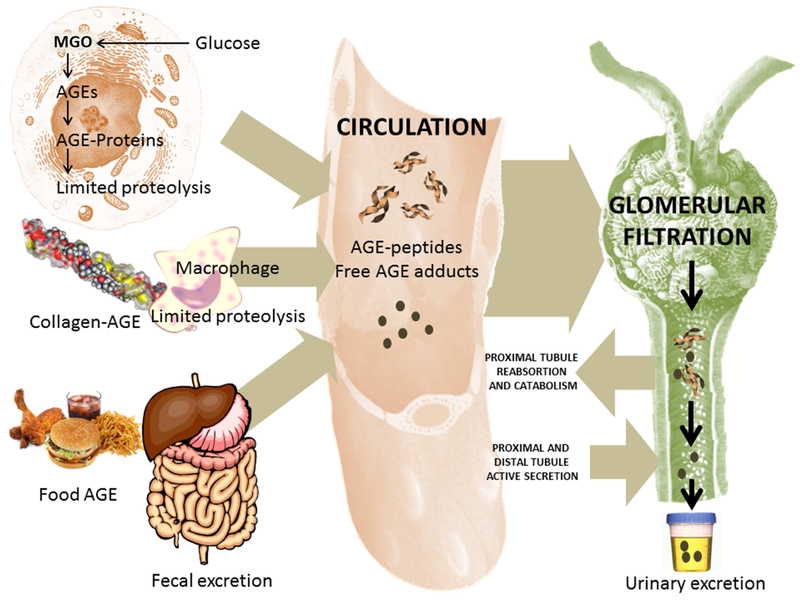
Figure 3. AGE m etabolism.
The diagram represents schematically the production of endogenous AGEs (intra and extracellular) and the intake from certain foods (left), their presence in the circulation (center) and their elimination/catabolism mainly by the kidneys (right). AGEs can be produced intracellularly by multiple pathways as shown in Figure 1. Of special relevance are MGO-generated AGEs. Other AGEs are generated on collagens and other proteins in the ECM as we AGE and more rapidly during diabetes. Partial proteolysis by macrophages, or in cells by ubiquitin-mediated proteasome pathways produce partially digested AGE-peptides as well as free adducts. Some AGEs are thought to also enter the bloodstream from foods. These adducts are very reactive and can damage proteins as they transit in the bloodstream. Renal function is critical for their elimination, as end-stage renal failure patients have very high concentrations of these molecules, which can be lowered by dialysis. These AGEs are filtered and reabsorbed in part in the proximal tube to be detoxified to some extent and then secreted distally for urinary excretion.
The influence of AGE build up during aging and its im pact on aging
There is significant evidence for the buildup of AGEs with aging in multiple species. Contributions from the Baynes, Thorpe and Monnier laboratories over the past three decades have been key in this area (Monnier and Taniguchi, 2016). The characterization of several cross-links present in collagen and their association with arterial disease has underscored their importance in diabetic complications as well as in aging (Baynes, 2001; Baynes et al., 1989; Dyer et al., 1991; Thorpe and Baynes, 2003). Based on a population-based study on adults aged 65 and older, high plasma carboxymethyl-lysine (CML) levels were found to be positively correlated with an increased risk of mortality in older adults due to all-cause mortality or cardiovascular disease (CVD) (Semba et al., 2009). Interestingly, the association of CML with increased mortality risk (all-cause or CVD) was found to be independent of diabetes mellitus (Semba et al., 2009). Thus, AGEs may not just be a biomarker but also a potential driver of aging.
As depicted in Figure 1, AGEs are a heterogeneous group of compounds that include more than 20 different products. These products that have been described further potentially mediate a wide variety of pathological effects (Monnier et al., 2014, 2015; Piperi et al., 2012; Sveen et al., 2015). Evidence of their accumulation in crystallins, collagens and basement membranes has accumulated over the years. Several studies have addressed the buildup of AGEs in long-lived proteins (Hammes et al., 1999; Sell et al., 1992; Vashishth, 2009). One human study that quantified autofluorescence of eye lens as an indicator of AGE accumulation with age (Cahn et al., 2014). The buildup of different AGEs (e.g., pentosidine) in the skin and increased crosslinking of collagen due to AGEs has been observed in the skin biopsies of diabetic patients (Monnier et al., 2005; Sell et al., 1992). Pentosidine, a pentose-mediated protein cross-linking is present in multiple types of human tissues including plasma proteins and red blood cells (Sell and Monnier, 1989). Similar work has underscored the importance of AGEs in bone health with age (Yamagishi, 2011). In particular, the extracellular matrix is an important compartment for the buildup of AGEs due to the presence of long-lived proteins like collagen (Singh et al., 2014). Accumulation of AGEs has also been suggested to contribute to the accumulation of lipofuscin with age (Nowotny et al., 2014).
The above evidence although suggestive is merely correlative. Evidence for a direct and causal relationship of AGEs with lifespan comes from studies in C. elegans. Overexpression of the glyoxalase GLOD-4 in worms extends lifespan and also inhibits dicarbonyl-mediated modification of mitochondrial proteins by AGEs (Morcos et al., 2008). Loss of function of GLOD-4, on the other hand, shortens lifespan which is exacerbated under high glucose conditions in C. elegans (Chaudhuri et al., 2016). Interestingly, a recent study has shown that low concentrations of MGO, formed via inactivation of the enzyme glycine-C-acetyltransferase (GCAT) involved in threonine catabolism, can promote lifespan through proteohormesis (Ravichandran et al., 2018). Reduction of age-related accumulation of AGEs by the FDA-approved (for the treatment of tuberculosis (TB)) drug Rifampicin also enhances worm lifespan through activation of DAF-16/FOXO (Golegaonkar et al., 2015). The additional evidence underlying the role of AGEs in determining lifespan comes from studies correlating the levels of AGEs in different species. Previous results from the Monnier group have shown that the accumulation of pentosidine in skin samples varies at a rate inversely related to maximum lifespan. This has been studied across eight mammalian species (Sell et al., 1996). Thus, there is significant evidence on the effect of AGEs in the aging process and for their causal influence on rates of organismal aging. We further discuss the evidence for the role of AGEs in age-associated diseases below.
AGEs and their link with age-related diseases in humans
Prolonged hyperglycemia in diabetes leads to a number of pathologies collectively termed diabetic complications. These include neuropathy, cardiomyopathy, nephropathy and retinopathy (Singh et al., 2014). One of the most compelling explanations for the molecular basis of diabetic complications is the buildup of glucose catabolism-derived reactive by products, the α-DCs (Henning et al., 2014). Notably, accumulation of MGO greater than 600 nM in the plasma distinguishes diabetics with pain and no pain in humans (Bierhaus et al., 2012). Variations in susceptibility to diabetic complications under similar glycemic levels could potentially be due to differences in detoxification of these α-DCs and associated AGEs. Notably, the active involvement of AGEs has also been implicated in age-related neurodegenerative diseases (Castellani et al., 1996). A key question in the field is whether AGEs are passive bystanders or if they actively contribute to cellular damage. Direct role for AGEs has been shown in mice by administering synthetic AGEs (derived from MGO treatment of BSA) that enhanced the accumulation of triglycerides and premature development of insulin resistance due to the reduction in anti-AGE receptor 1 (AGER1) and sirtuin 1 (SIRT1) in various tissues (Cai et al., 2012). Diet-derived AGEs has also been shown to exacerbate conditions of diabetic complications resulting in accumulation of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the serum of streptozotocin-induced diabetic mice (LV et al., 2016). AGEs also lead to significant vascular complications and damage to organs such as the heart and kidney (LV et al., 2016). These studies provide evidence behind the direct involvement of α-DCs such as MGO in mediating a variety of age-related disease complications.
Pathways underlying AGE-mediated effects on aging and age-related diseases
The RAGE pathway and oxidative stress: a key mechanism by which AGEs participate in the pathogenicity o f chronic diseases
AGEs likely contribute to the age-related increase in inflammation or ‘inflammaging’ (Franceschi and Campisi, 2014). They are ligands to several pro- and anti-inflammatory cellular receptors. A key pro-inflammatory receptor is the receptor for advanced glycation end-products (RAGE), a multi-ligand protein discovered and isolated from bovine lung (Coughlan et al., 2007; Miyata et al., 1996; Rodríguez-Ayala et al., 2005; Schmidt and Stern, 2000; Schmidt et al., 1996; Tanji et al., 2000; Yamagishi, 2011). RAGE belongs to the immunoglobulin superfamily of receptors and coordinates intracellular RAGE signaling (Daffu et al., 2013). As depicted in Figure 4, besides AGE adducts, RAGE binds a wide array of ligands including the leukocyte integrin Mac-1, S100 / calgranulins, high mobility group box 1 protein (HMGB1), modified LDL, DNA, RNA and amyloid fibrils. These ligands bear similar structural features: multiple β-sheets. RAGE identifies its ligands through them (Ramasamy et al., 2011;Yan et al., 2010a). Upon ligand binding, RAGE starts a signaling cascade with activation of nuclear factor-kB (NF-kB), oxidative stress and inflammation as shown in Figure 4. RAGE signals via phosphatidylinositol-3 kinase (PI-3K), Ki-Ras and the MAPKs, Erk1, and Erk2 (Ramasamy et al., 2011;Yan et al., 2010a). These pathways orchestrate the translocation of NF-kB from the cytoplasm to the nucleus stimulating inflammation and tissue injury operated by the RAGE-dependent expression of pro-inflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1). Through these pathways, RAGE activity is associated with diabetic microvascular complications including nephropathy, retinopathy, and neuropathy (Ramasamy et al., 2011;Yan et al., 2010a). Besides these cascades leading to inflammation, in endothelial (glomerular) and mesangial cells, RAGE activation increases reactive oxygen species (ROS). As we illustrate in Figure 4, a protagonist role is taken by reduced nicotinamide adenine dinucleotide phosphate, NAD(P)H oxidase, which in analogy to what happens in neutrophils, is a significant player in oxidative stress and dysfunction (D’Agati and Schmidt, 2010). The extracellular domain of RAGE is present in the bloodstream and may play a role in cardiovascular disease and chronic diseases (Figure 3). The C-terminal truncated form of RAGE mRNA lacks the encoding sequences for the transmembrane and intracytoplasmic domains (Forbes et al., 2005; Kalousová et al., 2007; Nakashima et al., 2010). The truncated version (endogenous secretory RAGE: esRAGE) is released and found in the circulation in humans. This esRAGE cancels the effects of AGEs on cells in culture (Bowman and Schmidt, 2013) and overexpression of esRAGE in mice reverses diabetic vascular dysfunction. Hence, esRAGE may play a decoy function: a feedback mechanism has been proposed by which esRAGE prevents RAGE signaling. Another form of soluble RAGE (sRAGE) (Figure 4) stems from proteolytic cleavage of the native RAGE expressed on the cell surface, both may act as decoys protecting from excessive ligand binding to RAGE and its consequent inflammatory cascades (Leonardis et al., 2012; Raposeiras-Roubín et al., 2010; Vazzana et al., 2009; Yan et al., 2010b). Further, the protective effect of esRAGE against dopaminergic neuronal death through inhibition of AGE-albumin build-up suggests the direct link between AGE-RAGE interaction and neurodegeneration (Bayarsaikhan et al., 2016).
Is AMPK a key target for damage by MGO that impairs metabolism?
Another mechanism by which MGO stress may be implicated in metabolic disease and especially in the metabolic syndrome (MetS) is by its putative action against AMPK. AMPK induces a cascade of events within cells in response to the fluxes and availability of metabolites. The role of AMPK in regulating cellular energy status (by sensing low energy using [AMP] as its signal) and activating catabolic pathways while inhibiting anabolic routes, places this enzyme at a central control point in maintaining energy homeostasis. Once activated, AMPK-mediated downstream phosphorylation events switch cells from active ATP consumption to active ATP production. Thus, it increases glucose transport, glycolysis, beta-oxidation, and inhibits lipogenesis and cholesterol biosynthesis. The mammalian AMPK is a trimeric enzyme composed of a catalytic α subunit and non-catalytic β and γ subunits. The N-terminal half of the α subunits contains a typical serine/threonine kinase catalytic domain. Direct AMP-binding studies have shown that AMP is bound to the γ subunits by a pair of so-called Bateman’s domains. Three arginine residues are the binding site for AMP (the binding is electrostatic and not the AMP-Mg++ complex, but free AMP is sensed), making this allosteric site vulnerable to carbonyl attack, especially by MGO.
A very small rise in AMP levels can induce a dramatic increase in the activity of AMPK which suggests that blocking of the AMP allosteric sites, even minimally, can have amplified reduction in AMPK activation. We proposed that an increased flux of MGO could achieve this effect. This increased flux may be produced by fructose surges (excess sugar in the diet, especially liquid), hyperglycemia, as well as an overflow of the glycolytic pathway caused by inactivation of glyceraldehyde-3-P dehydrogenase (GAPDH), in turn induced by its direct oxidation by reactive oxygen species (ROS) or indirectly by their activation of polyadenorybosyl polymerase (PARP) as proposed by Michael Brownlee (Brownlee, 2005). Inactivation of AMPK would favor lipogenesis, insulin resistance, and hyperglycemia, all hallmarks of MetS and diabetes (Gugliucci, 2009, 2016, 2017).
The impact of AGEs on diabetes-associated complications
Peripheral neuropathy
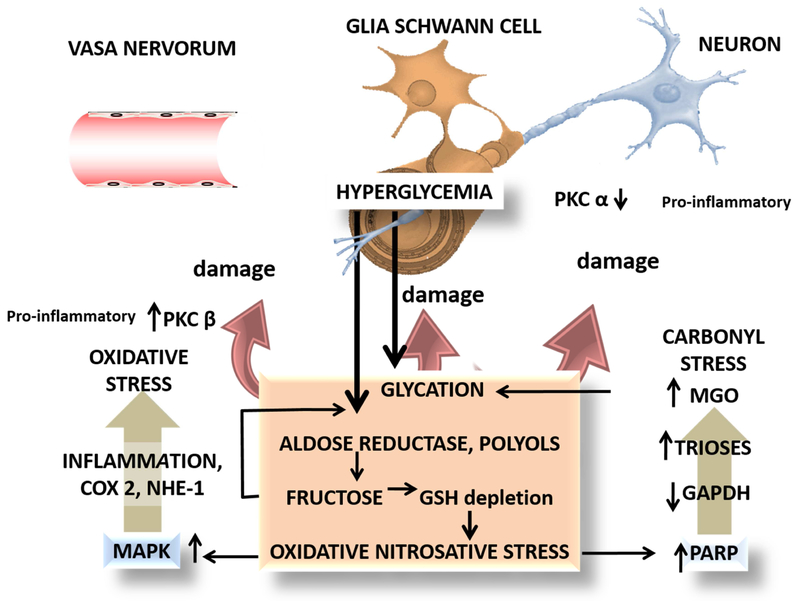
Some of the strongest evidence of the role of AGEs in diabetic complications comes from mice studies in peripheral neuropathy. As an example, we depict a summary of those pathways in Figure 5. To understand the important role of glycation in diabetic neuropathy one should consider that the neural deficit is the consequence of a metabolic shift at three levels: the endothelium of the vasa nervorum, the sensory neuron (dorsal root ganglia) axon and the Schwann cell (glia) (Münch et al., 2012). Figure 5 illustrates some characteristics of this triple hit, which bear common pathways in all cells as well as specific differences in others. Hyperglycemia leads to polyol and fructose accumulation (fructose is 7 times more reactive than glucose), NADPH and GSH depletion and oxidative/nitrosative stress. This damages DNA which induces the repair enzyme poly (ADP-ribose) polymerase (PARP) activity. PARP inactivates GAPDH, blocking the second phase of glycolysis, leading to MGO accumulation and more glycation and damage. Protein kinase C beta (PKC β) is activated in the vasa nervorum as a result of these metabolic shifts, and inflammatory damage to the endothelium ensues, leading to increased permeability and cell death. Conversely, protein kinase C alpha (PKC α) is inactivated in neuronal axons, which induces dysfunction. Glycation of the extracellular matrix molecules impairs nerve regeneration, compounding the problem. Dorsal root neuron RAGE is increased, and so are the NF-kB cascade responses. When protracted these changes are damaging to neurons, which do not reproduce and therefore accumulate the damage. Lastly, MAPK pathways are activated by the same metabolic shifts leading to COX 2 activation with potent pro-inflammatory components (Münch et al., 2012). In support of these general pathways, variation in Glo1 abundance among mice strains has been shown to lead to a differential response of sensory neurons to dicarbonyl stress (Jack et al., 2011). In another study in mice, it was found that post-translational modifications in the voltage-gated sodium channel Na(v)1.8 induced by plasma methylglyoxal, results in hyperalgesia (Bierhaus et al., 2012). This study was crucial in delineating a novel therapeutic target for dicarbonyl-mediated diabetic neuropathy. In addition, and consistent with the latter, the dicarbonyl sensitive neuronal receptor TRPA1 (Transient Receptor Potential cation channel A1), was shown to mediate pain in Trpa1 null mice and the agonist, methylglyoxal, to activate the receptor in mammalian cell culture (Andersson et al., 2013). More recently, hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels in mice have also been identified to be a potential therapeutic target for neuropathy, a condition that develops progressively with age in diabetic patients (Tsantoulas et al., 2017). In conclusion, AG Es participate in the triple hit at the basis of diabetic neuropathy by acting on vasa nervorum, neurons and glia.
Figure 5: Glycation and diabetic neuropathy: an example of pathways and targets where AGEs, MGO, and carbonyl stress play an important role.
Evidence shows that a triple hit may be operating to produce the final damage: vasa vasorum, the Schwann cell and the neuron axon all three are targets for the damage. Hyperglycemia produces AGEs via the mechanisms shown here and in detail in Figure 1. The interplay with oxidative stress (depletion of glutathione) activates MAPK and its inflammatory cascades which via PKC beta produces vascular damage. Oxidative stress on DNA induces the repair enzyme PARP which has the drawback of inactivating GAPDH, therefore blocking glycolysis at the triose level with the upstream consequence of further MGO accumulation, compounding the problem (Brownlee’s hypothesis, see text). Final damage occurs to the neuronal axon, neuronal mitochondria, and the glial Schwann cells.
Diabetic nephropathy
In addition to diabetic neuropathy, the role of α-DC-derived AGEs has also been implicated in diabetic nephropathy (Rabbani and Thornalley, 2018a). Knockdown of the α-DC detoxification gene glyoxalase I (Glo1) in non-diabetic mice recapitulates diabetic nephropathy symptoms without hyperglycemia (Giacco et al., 2014). Some features of AGE-mediated diabetic nephropathy, namely myofibroblast-based fibrosis, has also been modeled in a rat proximal tubule cell line by inducing trans-differentiation of epithelial cells into myofibroblasts using labeled AGE (125I-AGE-BSA). AGE-RAGE signaling elicits this response by stimulating the production of TGF-β and other cytokines which, in turn, trigger transdifferentiation (Oldfield et al., 2001). Endothelial damage and early renal dysfunction also seem to result from AGE accumulation. Thus, the appearance of this phenotype was inhibited in streptozotocin-treated Glo1 overexpressing rats (Brouwers et al., 2014). Similarly, overexpression of Glo1 completely prevented hyperglycemia-induced renal pathology, possibly by lowering induced oxidative stress mediated by α-DC induced glomerular protein modifications (Giacco et al., 2014).
Diabetic macroangiopathy
The detoxification of α-DCs, a precursor of AGE, also underlies the prevention of diabetes-mediated micro and macro vascular damage that may contribute to atherosclerosis and stroke (Brownlee, 2001, 2005). In support of the role of AGEs in vascular derangement, intravenous(IV) administration of AGEs in healthy rats significantly triggered the expression of vascular endothelial growth factor (VEGF) and inhibited pigment epithelium-derived factor (PEDF) levels that lead to diabetic retinopathy due to enhanced vascular permeability (Yamagishi et al., 2006). AGEs also induce diabetic macroangiopathy primarily by interacting with the endothelial cells lining the walls of the blood vessels. The latter activity results in a pro-inflammatory cascade followed by vascular dysfunction (Basta et al., 2004). Along similar lines but involving a different endpoint, it was earlier reported that both in vitro and in vivo administration of aminoguanidine (a nucleophilic hydrazine compound, that prevents AGE crosslinking) inhibits AGE formation and subsequent age-related increase in collagen cross-linking in the wall of the arteries (Brownlee et al., 1986).
Dietary AGEs and obesity
In addition to AGE accumulation via the endogenous route, exogenous sources such as diet also contribute significantly to a number of AGE-related pathologies. Thus, in mice, dietary AGEs supplemented with high fat (high AGE-high fat or HAGE-HF) resulted in hepatosteatosis and steatohepatitis, both characteristics of Non-alcoholic Fatty Liver Disease or NAFLD (Sayej et al., 2016). In addition to gaining weight, treated animals also showed secretion of various inflammatory cytokines from the adipose tissue (Sayej et al., 2016). The latter finding is consistent with the effects of, standard western diet, a major source of AGEs as a causative factor behind chronic inflammation and oxidative stress leading to insulin resistance (IR) (Gugliucci, 2017; Uribarri et al., 2015; Vlassara and Uribarri, 2014).
Modeling the influence of AGEs in neurodegenerative diseases
Parkinson’s Disease (PD)
In addition to diabetic complications, AGEs are also known to contribute to age-related neurodegeneration (reviewed in (Münch et al., 2012)). For example, a diet with high glycemic index, when fed to mice, has been shown to cause a significant increase in the formation of AGEs in the brain, primarily in the substantia nigra (Uchiki et al., 2012). In addition, glycation mediated AGE formation has been reported at the periphery of Lewy bodies in PD patients (Castellani et al., 1996). Lewy bodies are aggregations of intracytoplasmic inclusions in the subcortical neurons in PD patients (Trojanowski et al., 1998). In cases of incidental Lewy body disease, AGEs appear in newly formed Lewy bodies further suggesting that AGEs may play a critical role in triggering Lewy body formation in pre-PD individuals (Münch et al., 2000).
Further evidence of the role of AGEs in neurodegenerative diseases comes from the findings that DJ1, glutathione-independent glyoxalase, involved in the detoxification of AGEs, is associated with familial, early-onset and sporadic forms of PD (Lee et al., 2012). This protective role of DJ-1 against dicarbonyls was modeled using mouse embryonic fibroblast and human SH-SY5Y cell models for dopaminergic neurons (Lee et al., 2012). In addition, studies using cell culture and C. elegans suggest that the glyoxalase function of DJ-1 is critical for neuroprotection against toxic oxaldehydes (Lee et al., 2012). Further, using human and mice primary cells, DJ-1 has also been identified as a stabilizer of Nrf2 under conditions of toxicity related stress (Clements et al., 2006; Im et al., 2012; Xue et al., 2012). Thus, the loss of DJ-1 function may lead to increased levels of methylglyoxal and AGEs, making it an attractive therapeutic target for diseases associated with oxidative stress (Ariga et al., 2013). At the mechanistic level, the relationship between AGEs and PD could also be due to the ability of AGEs to cross-link alpha-synuclein, as has been shown using in vitro studies (Shaikh and Nicholson, 2008). A recent study has shown that glycation of α-synuclein, a protein whose aggregation is the hallmark of PD, enhanced the aggregation and reduced clearance of toxic oligomers. In vitro and in vivo studies using flies and mice showed that enhanced glycation is toxic while glycation inhibitors reduce aggregation and enhance clearance of α-synuclein, and rescue behavioral phenotypes (Vicente Miranda et al., 2017). Furthermore, these studies also explain the significantly increased risk of PD amongst diabetics.
Alzheimer’s Disease (AD)
In addition to PD, AGEs have also been associated with AD. Previous studies suggest that the glycation of such AD-associated proteins such as Aβ and tau play a critical role in the pathogenesis of this disorder. For example, plaques extracted from AD brains show a 3-fold increase in AGE content compared to age-matched healthy individuals (Vitek et al., 1994). Also, AGEs have been suggested to stabilize and promote the formation of aggregated forms of Aβ and tau (Chen et al., 2006; Ledesma et al., 1994; Woltjer et al., 2003). More specifically like PD, glycation is considered to be responsible for the increase in the cross-linking of Aβ and tau proteins and the subsequent formation of stable oligomeric forms. Additional direct evidence favoring an AGE-AD connection comes from, in vitro studies using neuroblastoma cell lines and in vivo experiments involving mice. These experiments show that glycation (AGE formation) also upregulates the expression of the amyloid precursor protein (APP), an enhancement that increases Aβ peptides levels (Ko et al., 2010) that can in turn trigger phosphorylation of tau proteins as characterized in rat septal cholinergic neurons (Zheng et al., 2002). In addition to phosphorylating tau proteins, an important hallmark of the disease, AGEs also co-localize with other markers of neurodegeneration, such as nNOS (a nitrooxidative stress marker) and caspase-3 (an apoptotic cell-death marker) (Lüth et al., 2005). The connection between glycated protein levels in the cerebrospinal fluid (CSF) of AD patients (Ahmed et al., 2005; Li et al., 2013) further reinforces the importance of glycated Aβ as a potential mechanism for disease progression. It has been shown consistently that there is a significant buildup of AGEs in serum and CSF of patients with AD compared to controls (Ahmed et al., 2005). Thus, it seems reasonable to postulate that the age-dependent decrease in glyoxalase levels and a concomitant increase in AGE buildup, contributes, at least in part, to the increased incidence of Alzheimer’s and other neurodegenerative diseases (Kuhla et al., 2007). These studies may also help explain mechanisms underlying the epidemiological evidence that suggest link between type 2 diabetes and AD (Li et al., 2015).
Potential factors underlying AGE-mediated effects on neurodegeneration
A. Inflammation
Inflammation downstream of AGE production has been implicated as the key mechanism contributing to diseases. For instance, AGE-albumin in the extracellular space of human microglial cells triggers the generation of multi-ligand receptors for AGE or RAGE on cell membranes (Bayarsaikhan et al., 2016). The RAGE interaction with the secreted AGE-albumin complexes has been shown to mediate an apoptotic cascade leading to the death of human dopaminergic neurons. Elevated AGE-albumin accumulation from activated microglia has been suggested as potential biomarkers for neurodegenerative diseases (Bayarsaikhan et al., 2016). Activation of macrophages leads to an exacerbated release of AGE-albumin complexes, a major AGE derivative that drives shared pathways resulting in the progression of neurodegeneration (Byun et al., 2017). Upregulation of RAGE-mediated signaling has also been shown in the 6-hydroxydopamine (6-OHDA) induced rat model for neurotoxicity (Serratos et al., 2016).
B. Cellular stress and decreased proteostasis
Other than AGE-RAGE interaction enhancing inflammation, one of the key mechanisms that lead to AGE-mediated neurodegeneration is the generation of cellular stress and decline in proteostasis with age. This stems from the damage caused by AGEs to proteins and its subsequent crosslinking and accumulation in aggregates. Accumulation of AGEs potentially through such cross-linking events results in sustained cellular stress eventually leading to neuronal cell death (Guerrero et al., 2012). Such increased cellular stress can also happen due to activation of RAGE-like receptors (Hipkiss et al., 2013; Peppa and Vlassara, 2005).
C. Glycation o f neurotransmitters
Moreover MGO, besides proteins, may selectively modify dopamine. The reaction leads to toxic metabolites like 1-acetyl-6.7-dihydroxy-1,2,3,4,-tetrahydroisoquinoline (ADTIQ) (Deng et al., 2012). This active metabolite is present in human brain tissue, including the substantia nigra. ADTIQ levels are increased in PD patients (Deng et al., 2012), and the structure of ADTIQ resembles that of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) elucidated using hyperglycemic cell and rat models (Song et al., 2014). Interestingly, in a separate study, ADTIQ has been shown as an endogenous neurotoxin in SH-SY5Y neuroblastoma cells, and the levels of both MGO and ADTIQ are increased in the striatum of a streptozotocin-induced diabetic rat model (Xie et al., 2015).
Despite our mechanistic understanding of the AGE-mediated effects in the pathology of age-related diseases, designing viable therapeutic strategies can be a challenge without modeling the disease in experimental organisms and without a better understanding of the pathways targeted by AGES and those involved in AGE formation and detoxification.
Using invertebrate models to study the impact of AGEs
To better understand the role of AGEs in modulating age-related disease, there is an urgent need for suitable models to study the biological mechanisms of AGE formation, detoxification, and link to the disease pathology in a short time-frame. This is a major bottleneck in our current understanding of the biochemistry of AGE formation and its consequences, and hence rapid drug development to combat the associated pathologies. To that end, there has been a considerable effort to model AGE accumulation in a variety of model organisms ranging from invertebrates to vertebrates in order to gain a mechanistic insight (Table 1). These studies also highlight the importance of model organisms in designing and delineating putative therapeutic targets that could have otherwise been extremely challenging to determine. A good model to study long-term AGE-related complications should have the following characteristics: (1) Recapitulate, at least some of the pathologies associated with α-DC and AGE buildup, (2) Recapitulate the build-up of AGEs in an age-dependent manner in a short time frame and (3) Be amenable to high-throughput genetic and drug screens.
Table 1.
Summary of model organisms, phenotypes and genes / proteins / pathways related to α-DC or AGE-mediated pathologies that exacerbate with age
| Model Organism | Phenotypes | Genes / Proteins / Pathways |
Citations |
|---|---|---|---|
| Mice / Rats | Neuropathy / Nephropathy / Neurodegeneration / Cardiomyopathy / Retinopathy / Vasculopathy | TGF-βa / VCAM1b / RAGEc,d / VEGFe / Nav 1.8f / GLO1g / TRPA1h / HCN2i / DJ-1j / NF-kBk | (Oldfield et al., 2001)a; (Schmidt et al., 1995)b; (Bayarsaikhan et al., 2016)c; (Ma et al., 2009)d; (Yamagishi et al., 2006)e; (Bierhaus et al., 2012)f; (Jack et al., 2011)g; (Andersson et al., 2013)h; (Tsantoulas et al., 2017)i; (Lee et al., 2012)j; (Zhou et al., 2016)k |
| Fly | Neurodegeneration / Paralysis / Fat accumulation / Lifespan | Glo1∫a, ∫b / FASN∫a / Tpi∫b / wstd∫b | (Garrido et al., 2015)∫a; (Gnerer et al., 2006)∫b |
| Worm | Neurodegeneration / Hyperesthesia / Lifespan | TRPA-1ⱡa / Nrfⱡa / p38 MAPKⱡa / DJ-1 related (djr-1.1 & djr-1.2)ⱡa, ⱡb, ⱡc, ⱡd, ⱡe / GLOD-4ⱡa, ⱡb, ⱡc, ⱡf, ⱡg / DAF-18ⱡc / JNK-1ⱡc / DAF-16ⱡc, ⱡf | (Chaudhuri et al., 2016)ⱡa; (Chen et al., 2015)ⱡb;(Golegaonkar et al., 2015)ⱡc; (Lee et al., 2012)ⱡd; (Lee et al., 2013, 2012)ⱡe;(Mendler et al., 2014)ⱡf; (Morcos et al., 2008)ⱡg |
| Yeast | Proteotoxicity / Proteostasis | Glo1ⱡ∫a, ⱡ∫b , ⱡ∫c / Tpiⱡ∫a / Hsp26pⱡ∫b / Hsp104 ⱡ∫c / ALKR ⱡ∫c / Sln1 ⱡ∫c / Hog1ⱡ∫c | (Vicente Miranda et al., 2016)ⱡ∫a; (Gomes et al., 2008)ⱡ∫b; (Zemva et al., 2017)ⱡ∫c |
Vertebrate model organisms such as rodents are a natural first choice to model AGE-mediated pathologies, due to the relatively close link to human disease biology and a track record as a model for studying disease pathology in diabetes (King, 2012). However, a rodent’s relatively long life span and associated cost of maintenance pose limitations to conduct rapid genetic or drug screens or to serve as a model that recapitulates the effects of aging while studying AGE-mediated pathologies. Using C. elegans, it has been shown that glod-4, an orthologue of mammalian Glo1 glyoxalase, plays a critical role in regulating worm lifespan (Morcos et al., 2008). This study enabled the understanding of the long-term effects of dicarbonyl-mediated damage of mitochondrial proteins via generation of enhanced mitochondrial ROS (reactive oxygen species) that was rescued by the glyoxalase overexpressing animals (Morcos et al., 2008). Also, C. elegans displayed endogenous accumulation of dicarbonyls and AGEs as the glyoxalase-1 activity declined with age (Morcos et al., 2008). In our recent study, we have utilized the C. elegans mutant for glod-4 as a model to study diabetic complications. The C. elegans glod-4 mutant accumulates toxic α-DCs and exhibits several diabetes-related pathologies akin to the mouse model. These include accumulation of α-DCs, hyperesthesia (or hyper sensitivity to touch), neuronal damage and early mortality, in a glucose-dependent fashion (Chaudhuri et al., 2016). The short-lived glod-4 mutant has also been shown to accumulate a broad variety of AGE-modified proteins (Golegaonkar et al., 2015). Furthermore, the powerful genetic tools available to study C. elegans led to the identification of a conserved and critical role for TRPA-1, in sensing MGO and also in SKN-1/Nrf2 activation to detoxify α-DCs. In addition, we utilized the worm to undertake a phenotypic drug screen for neuropathy by screening for compounds that reduce the increased hypersensitivity to touch in glod-4 mutants. This screen has identified several novel pharmacological leads, including Podocarpic and Lipoic acid as activators of TRPA1 that ameliorate the deleterious consequences of MGO accumulation in C. elegans (Chaudhuri et al., 2016). The relevance of a worm model to study diabetic complications is underscored by the facts that Nrf2 has been previously shown to play an important role in ameliorating diabetic pathologies in mice (Jiang et al., 2010; Li et al., 2012; Zheng et al., 2011),and that lipoic acid is used clinically for diabetic nephropathy (McIlduff and Rutkove, 2011; Ziegler et al., 1999).
Other studies have also utilized C. elegans or D. melanogaster to study MGO and AGE stress.C. elegans has been modeled to study glucose toxicity due to AGE-mediated modification of mitochondrial proteins (Schlotterer et al., 2009). A reduction of AGE-mediated glycation by the Rifampicin has been shown to extend C. elegans lifespan through the activation of DAF-16/FOXO signaling (Golegaonkar et al., 2015). A study in fruit flies demonstrated that the mutant for the glycolytic enzyme, triose phosphate isomerase (TPI) leads to increased neurodegeneration (Gnerer et al., 2006). TPI is a key isomerase that converts dihydroxyacetone phosphate (DHAP) to glyceraldehyde-3-phosphate (GAP), and in its absence, DHAP non enzymatically generates increased concentrations of MGO and thus AGEs (Gnerer et al., 2006). In another study using flies, a mutant for the fatty acid synthase (FASN) gene was used to understand the interplay between dietary sugar, MGO, and lipogenesis (Garrido et al., 2015). Their study demonstrates the requirement for fatty acid synthesis to prevent the potentially detrimental intracellular build-up of MGO-derived AGEs (Garrido et al., 2015). In addition to invertebrate animal models such as worm and flies, another model system frequently utilized to characterize glycation mediated protein damage is S. cerevisiae (yeast). In a recent study, mutants that enhance MGO production were used to understand glycation-mediated protein misfolding associated with huntingtin protein in yeast, flies and human cells (Vicente Miranda et al., 2016). The key finding in the study was that glycation enhanced the aggregation and inhibited clearance of HTT suggesting a direct role of protein glycation in neurodegeneration (Vicente Miranda et al., 2016). In a recent study utilizing flies and mice, it was also shown that accumulation of toxic oligomers due to age-related glycation of α-synuclein resulted in disruption of synaptic transmission in the neurons (Vicente Miranda et al., 2017).
The vertebrate, invertebrate, and cell culture models suggest the direct involvement of AGEs in mediating a broad spectrum of disease pathologies and also the feasibility to gain a mechanistic understanding of these processes. In particular, the use of short-lived animal models has allowed one to monitor the progression of pathology and its link with AGEs. Overall, the evidence linking AGEs with diabetic complications and neurodegenerative diseases is emerging, but the mechanisms by which they influence disease progression and genetic networks involved in modulating AGEs remain poorly understood. Greater utilization of expedient genetic models should help reveal the mechanisms in place to reduce the accumulation of AGEs.
Glyoxalases as a target for therapy
Thus given the evidence implicating AGEs in diabetic complications, detoxification of AGEs represents an orthogonal approach to the treatment of diabetes in addition to lowering glucose. Given the success of aminoguanidine (also known as Pimagedine) in animal models, a human clinical trial was conducted to prevent diabetic kidney disease (Freedman et al., 1999). Unfortunately, the trial was discontinued due to side effects (Thornalley, 2003), which has led to reduced enthusiasm in the field that lowering AGEs would be a successful strategy in treating age-related diseases. However, there is need to devise novel approaches to lower AGEs by harnessing the endogenous defenses in place, which are likely to prevent AGE formation and potentially pose fewer side effects. In a recent promising clinical trial, a combination therapy of trans-Resveratrol (tRes) and Hesperetin (HESP) helped improve glycemic control and vascular inflammation in healthy overweight and obese individuals via induction of Glo1 (Rabbani and Thornalley, 2018b). This suggests that inhibition of MGO accumulation via induction of glyoxalases could be a potential therapeutic strategy for glycation-mediated diseases.
Glyoxalase expression and activity are shown to be regulated by the nuclear factor erythroid 2 related factor 2 (Nrf2) (Xue et al., 2012), a basic leucine zipper (bZIP) protein that also regulates the expression of other antioxidant proteins (Rabbani and Thornalley, 2015b). This is due to the presence of stress-response-related regulatory elements such as AREs (antioxidant-response elements) on the 5’ flanking region of the Glo1 gene (Xue et al., 2012). Urine samples from 10 week old Nrf2−/− mice compared with age-matched controls showed significant accumulation of AGEs such as MGdG and MG-H1 as well as macromolecular damage (Xue et al., 2012). Similar results were described by our group using C. elegans (Chaudhuri et al., 2016). Furthermore, we found that the Nrf2 mediated regulation of both the glutathione-dependent and independent glyoxalases (GLO1 and DJ1) is mediated by the activation of the cation channel TRPA1, an upstream sensor for α-DCs (Chaudhuri et al., 2016). Sensing of dicarbonyls by TRPA1 is relayed to the transcription factor SKN-1/Nrf via MAP kinase-mediated signaling and ultimately enhances the expression of Glo1 and Dj1 to initiate the detoxification program (Chaudhuri et al., 2016). Chronic activation in vitro in the vascular endothelial cells of diabetic rats of the nuclear factor-kappaB (NF-kappaB), downstream of AGE activation of RAGE, has also been shown to regulate glyoxalases (Bierhaus et al., 2001). Thus given the evidence for Nrf2 in detoxifying AGEs across species, it represents an excellent target to mitigate AGEs and associated diseases.
Conclusions
We have a limited understanding of the causal effects of both dietary and endogenous AGEs on aging and age-related diseases. More importantly, many aspects of AGE accumulation including how they are formed, sensed and detoxified remains poorly understood. Regulatory mechanisms that underlie these pathways are potential targets for human diseases. There is a significant need to develop therapeutics that lower AGEs and can serve as an orthogonal approach to treat various age-related human metabolic diseases, like metabolic syndrome, diabetic complications as well as neurodegenerative diseases. Vertebrate and invertebrate model systems with a genetically impaired glyoxalase system have been instrumental in assessing the extent of α-DC and AGE-mediated disease pathologies and mechanisms. Invertebrate model organisms have the advantage to recapitulate AGE-mediated pathologies over a short time frame which otherwise takes years to build up in humans. The worm, fly and yeast models with their relatively short lifespans and easy-to-use genetic tools, could help answer some of the complex mechanistic questions associated with diseases such as obesity, neurodegeneration and diabetic complications in a much more rapid manner. The combination of AGE-mediated stress with existing human disease models is likely to better mimic the age-related disease pathology in humans. They could also be used as a tool for high-throughput drug screening platform to identify novel pharmaceutical leads for these diseases. A key limitation to using certain invertebrate models could be trying to model AGE-related disease complications associated with organs, such as the bone, the heart, and the vascular system. The initial discoveries of disease mechanisms or mode of action for drug molecules from high-throughput screens made possible in invertebrates could be further validated in vertebrate model systems in pre-clinical trials. Importantly, despite certain drawbacks from a physiological point of view, utilizing the strength of invertebrate model systems in gaining mechanistic insights and performing high-throughput small molecule and genetic screens could potentially fuel major discoveries. The use of invertebrate models allows the examination of these complex diseases through the lens of aging, which is normally harder to recapitulate in traditional pre-clinical models.
Acknowledgments
This work was funded by grants from the American Federation of Aging Research (P.K.), Larry L. Hillblom Foundation (PK), the impact circle award (Buck), and the NIH grants R21 AG053066 & RO1 AG045835 (P.K.) and Touro University California. J.C. was supported by NIH/NIA T32 award (T32 AG000266), N.B was supported by the Hillblom Foundation Postdoctoral Fellowship, AG is supported by NIH RO1 5RO1HL113887. We also thank the members of the Kapahi lab and Gugliucci labs for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Agalou S, Ahmed N, Babaei-Jadidi R, Dawnay A, and Thornalley PJ (2005). Profound mishandling of protein glycation degradation products in uremia and dialysis. J. Am. Soc. Nephrol 16,1471–1485. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Ahmed U, Thornalley PJ, Hager K, Fleischer G, and Münch G (2005). Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem 92, 255–263. [DOI] [PubMed] [Google Scholar]
- Andersson D. a, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, and Bevan S (2013). Methylglyoxal evokes pain by stimulating TRPA1. PLoS One 8, e77986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, and Iguchi-Ariga SMM (2013). Neuroprotective function of dj-1 in Parkinson’s disease. Oxid. Med. Cell. Longev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba SP, Barski OA, Ahmed Y, O’Toole TE, Conklin DJ, Bhatnagar A, and Srivastava S (2009). Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes 58, 2486–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta G, Schmidt AM, and De Caterina R (2004). Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res 63, 582–592. [DOI] [PubMed] [Google Scholar]
- Bayarsaikhan E, Bayarsaikhan D, Lee J, Son M, Oh S, Moon J, Park HJ, Roshini A, Kim SU, Song BJ, et al. (2016). Microglial AGE-albumin is critical for neuronal death in Parkinson???s disease: A possible implication for theranostics. Int. J. Nanomedicine 10, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW (2001). The role of AGEs in aging: Causation or correlation. Exp. Gerontol 36, 1527–1537. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Watkins NG, Fisher CI, Hull CJ, Patrick JS, Ahmed MU, Dunn JA, and Thorpe SR (1989). The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res 304, 43–67. [PubMed] [Google Scholar]
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, et al. (2001). Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 50, 2792–2808. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, et al. (2012). Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med 18, 926–933. [DOI] [PubMed] [Google Scholar]
- Bjorksten J (1968). The crosslinkage theory of aging. J. Am. Geriatr. Soc 16, 408–427. [DOI] [PubMed] [Google Scholar]
- Bowman MAH, and Schmidt AM (2013). The next generation of RAGE modulators: Implications for soluble RAGE therapies in vascular inflammation. J. Mol. Med 91,1329–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers O, Niessen PMG, Miyata T, Østergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, Sieber J, Mundel PH, Brownlee M, Janssen BJA, et al. (2014). Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 57, 224–235. [DOI] [PubMed] [Google Scholar]
- Brownlee M (2001). Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820. [DOI] [PubMed] [Google Scholar]
- Brownlee M (2005). The pathobiology of diabetic complications: A unifying mechanism. In Diabetes, pp. 1615–1625. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, and Cerami A (1986). Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science (80-.). 232, 1629–1632. [DOI] [PubMed] [Google Scholar]
- Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, and Vlassara H (1994). Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc. Natl. Acad. Sci. U. S. A 91,9441–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun K, Yoo YC, Son M, Lee J, Jeong GB, Park YM, Salekdeh GH, and Lee B (2017). Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther [DOI] [PubMed] [Google Scholar]
- Cahn F, Burd J, Ignotz K, and Mishra S (2014). Measurement of lens autofluorescence can distinguish subjects with diabetes from those without. J. Diabetes Sci. Technol 8, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ramdas M, Zhu L, Chen X, Striker GE, and Vlassara H (2012). From the Cover: Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci 109, 15888–15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani R, Smith MA, Richey PL, and Perry G. (1996). Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 737, 195–200. [DOI] [PubMed] [Google Scholar]
- Chaudhuri J, Bose N, Gong J, Hall D, Rifkind A, Bhaumik D, Peiris TH, Chamoli M, Le CH, Liu J, et al. (2016). A Caenorhabditis elegans Model Elucidates a Conserved Role for TRPA1-Nrf Signaling in Reactive ??-Dicarbonyl Detoxification. Curr. Biol 26, 3014–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Maley J, and Yu PH (2006). Potential inplications of endogenous aldehydes in beta-amyloid misfolding, oligomerization and fibrillogenesis. J. Neurochem 99,1413–1424. [DOI] [PubMed] [Google Scholar]
- Chen P, DeWitt MR, Bornhorst J, Soares FA, Mukhopadhyay S, Bowman AB, and Aschner M (2015). Age- and manganese-dependent modulation of dopaminergic phenotypes in a C. elegans DJ-1 genetic model of Parkinson’s disease. Metallomics 7, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, and Ting JP-Y (2006). DJ-1, a cancer-and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci 103, 15091–15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard F, Wiame E, Bergans N, Fortpied J, Vertommen D, Vanstapel F, Delpierre G, and Van Schaftingen E (2004). Fructosamine 3-kinase-related protein and deglycation in human erythrocytes. Biochem. J 382, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan MT, Cooper ME, and Forbes JM (2007). Renal microvascular injury in diabetes: RAGE and redox signaling. Antioxid. Redox Signal 9, 331–342. [DOI] [PubMed] [Google Scholar]
- D’Agati V, and Schmidt AM (2010). RAGE and the pathogenesis of chronic kidney disease. Nat. Rev. Nephrol 6, 352–360. [DOI] [PubMed] [Google Scholar]
- Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, and Schmidt AM (2013). Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci 14,19891–19910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Andrade C, and Fogliano V (2018). Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol 9, [DOI] [PubMed] [Google Scholar]
- Delpierre G, Vertommen D, Communi D, Rider MH, and Van Schaftingen E (2004). Identification of fructosamine residues deglycated by fructosamine-3-kinase in human hemoglobin. J. Biol. Chem 279, 27613–27620. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang Y, Li Y, Xiao S, Song D, Qing H, Li Q, and Rajput AH (2012). Occurrence and distribution of salsolinol-like compound, 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (ADTIQ) in Parkinsonian brains. J. Neural Transm 119, 435–441. [DOI] [PubMed] [Google Scholar]
- Dunmore SJ, Al-Derawi AS, Nayak AU, Narshi A, Nevill AM, Hellwig A, Majebi A, Kirkham P, Brown JE, and Singh BM (2018). Evidence that differences in fructosamine-3-kinase activity may be associated with the glycation gap in human diabetes. Diabetes 67, 131–136. [DOI] [PubMed] [Google Scholar]
- Dyer DG, Blackledge JA, Katz BM, Hull CJ, Adkisson HD, Thorpe SR, Lyons TJ, and Baynes JW (1991). The Maillard reaction in vivo. Z. Ernahrungswiss 30, 29–45. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Thorpe SR, Thallas-Bonke V, Pete J, Thomas MC, Deemer ER, Bassal S, El-Osta A, Long DM, Panagiotopoulos S, et al. (2005). Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J. Am. Soc. Nephrol 16, 2363–2372. [DOI] [PubMed] [Google Scholar]
- Franceschi C, and Campisi J (2014). Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 69, S4–S9. [DOI] [PubMed] [Google Scholar]
- Freedman BI, Wuerth JP, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, and Spinowitz BS (1999). Design and baseline characteristics for the aminoguanidine clinical trial in overt type 2 diabetic nephropathy (ACTION II). Control. Clin. Trials 20, 493–510. [DOI] [PubMed] [Google Scholar]
- Gale CP, Futers TS, and Summers LKM (2004). Common polymorphisms in the glyoxalase-1 gene and their association with pro-thrombotic factors. Diab. Vasc. Dis. Res 1, 34–39. [DOI] [PubMed] [Google Scholar]
- Garrido D, Rubin T, Poidevin M, Maroni B, Le Rouzic A, Parvy J-P, and Montagne J (2015). Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity. PLoS Genet.11,e1004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Du X, D’Agati VD, Milne R, Sui G, Geoffrion M, and Brownlee M (2014). Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnerer JP, Kreber R. a, and Ganetzky B (2006). wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc. Natl. Acad. Sci 103, 14987–14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, and Vlassara H (2004). Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc 104, 1287–1291. [DOI] [PubMed] [Google Scholar]
- Golegaonkar S, Tabrez SS, Pandit A, Sethurathinam S, Jagadeeshaprasad MG, Bansode S, Sampathkumar S-G, Kulkarni MJ, and Mukhopadhyay A (2015). Rifampicin reduces advanced glycation end products and activates DAF-16 to increase lifespan in Caenorhabditis elegans. Aging Cell 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RA, Vicente Miranda H, Sousa Silva M, Graça G, Coelho AV, Do Nascimento Ferreira AE, Co rdeiro C, and Freire AP (2008). Protein glycation and methylglyoxal metabolism in yeast: Finding peptide needles in protein haystacks. FEMS Yeast Res. 8, 174–181. [DOI] [PubMed] [Google Scholar]
- Guerrero E, Vasudevaraju P, Hegde ML, Britton GB, and Rao KS (2012). Recent Advances in α-Synuclein Functions, Advanced Glycation, and Toxicity: Implications for Parkinson’s Disease. Mol. Neurobiol 1–12. [DOI] [PubMed] [Google Scholar]
- Gugliucci A (2005). Alternative antiglycation mechanisms: Are spermine and fructosamine-3-kinase part of a carbonyl damage control pathway? Med. Hypotheses 64, 770–777. [DOI] [PubMed] [Google Scholar]
- Gugliucci A (2009). “Blinding” of AMP-dependent kinase by methylglyoxal: A mechanism that allows perpetuation of hepatic insulin resistance? Med. Hypotheses 73, 921–924. [DOI] [PubMed] [Google Scholar]
- Gugliucci A (2016). Fructose surges damage hepatic adenosyl-monophosphate-dependent kinase and lead to increased lipogenesis and hepatic insulin resistance. Med. Hypotheses 93, 87–92. [DOI] [PubMed] [Google Scholar]
- Gugliucci A (2017). Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. An Int. Rev. J 8, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliucci A, and Bendayan M (1996). Renal fate of circulating advanced glycated end products (AGE): Evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia 39,149–160. [DOI] [PubMed] [Google Scholar]
- Gugliucci A, and Menini T (2017). Advanced Glycation endproducts and aging In Mechanisms Linking Aging, Diseases and Biological Age Estimation, Zapico SC, ed. (CRC Press; ), pp. 68–79. [Google Scholar]
- Hammes HP, Alt A, Niwa T, Clausen JT, Bretzel RG, Brownlee M, and Schleicher ED (1999). Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia 42, 728–736. [DOI] [PubMed] [Google Scholar]
- Henning C, Liehr K, Girndt M, Ulrich C, and Glomb M.a (2014). Extending the Spectrum of α-Dicarbonyl Compounds in vivo. J. Biol. Chem 289, 28676–28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss AR, Cartwright SP, Bromley C, Gross SR, and Bill RM (2013). Carnosine: can understanding its actions on energy metabolism and protein homeostasis inform its therapeutic potential? Chem. Cent. J 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JY, Lee KW, Woo JM, Junn E, and Mouradian MM (2012). Dj-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet 21,3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack M., Ryals J., and Wright DE (2011). Characterization of Glyoxalase I in Streptozocin-Induced Diabetic Mouse Models of Painful and Insensate Neuropathy. Diabetologia 54, 2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Jagt DL, Robinson B, Taylor KK, and Hunsaker LA (1992). Reduction of trioses by NADPH-dependent Aldo-Keto reductases: Aldose reductase, methylglyoxal, and diabetic complications. J. Biol. Chem 267, 4364–4369. [PubMed] [Google Scholar]
- Jaramillo R, Shuck SC, Chan YS, Liu X, Bates SE, Lim PP, Tamae D, Lacoste S, O’Connor TR, and Termini J (2017). DNA Advanced Glycation End Products (DNA-AGEs) Are Elevated in Urine and Tissue in an Animal Model of Type 2 Diabetes. Chem. Res. Toxicol 30, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, and Zhang DD (2010). The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59, 850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousová M, Jáchymová M, Mestek O, Hodková M, Kazderová M, Tesar V, and Zima T (2007). Receptor for advanced glycation end products--soluble form and gene polymorphisms in chronic haemodialysis patients. Nephrol. Dial. Transplant 22, 2020–2026. [DOI] [PubMed] [Google Scholar]
- Kalousová M, Germanová A, Jáchymová M, Mestek O, Tesař V, and Zima T (2008). A419C (E111A) polymorphism of the glyoxalase I gene and vascular complications in chronic hemodialysis patients. In Annals of the New York Academy of Sciences, pp. 268–271. [DOI] [PubMed] [Google Scholar]
- Kiefer AS, Fleming T, Eckert GJ, Poindexter BB, Nawroth PP, and Yoder MC (2014). Methylglyoxal concentrations differ in standard and washed neonatal packed red blood cells. Pediatr. Res 75, 409–414. [DOI] [PubMed] [Google Scholar]
- King AJF (2012). The use of animal models in diabetes research. Br. J. Pharmacol 166, 877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S-Y, Lin Y-P, Lin Y-S, and Chang S-S (2010). Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic. Biol. Med 49, 474–480. [DOI] [PubMed] [Google Scholar]
- Koschinsky T, He C-J, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, and Vlassara H (1997). Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci 94, 6474–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhla B, Boeck K, Schmidt A, Ogunlade V, Arendt T, Münch G, and Lüth HJ (2007). Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer’s disease brains. Neurobiol. Aging 28, 29–41. [DOI] [PubMed] [Google Scholar]
- Lange JN, Wood KD, Knight J, Assimos DG, and Holmes RP (2012). Glyoxal formation and its role in endogenous oxalate synthesis. Adv. Urol 2012, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, Bonay P, Colaço C, and Avila J (1994). Analysis of microtubule-associated protein tau glycation in paired helical filaments. J. Biol. Chem 269, 21614–21619. [PubMed] [Google Scholar]
- Lee J-Y, Kim C, Kim J, and Park C (2013). DJR-1.2 of Caenorhabditis elegans is induced by DAF-16 in the dauer state. Gene 524, 373–376. [DOI] [PubMed] [Google Scholar]
- Lee JY, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, and Park C (2012). Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet 21,3215–3225. [DOI] [PubMed] [Google Scholar]
- Leonardis D, Basta G, Mallamaci F, Cutrupi S, Pizzini P, Tripepi R, Tripepi G, De Caterina R, and Zoccali C (2012). Circulating soluble receptor for advanced glycation end product (sRAGE) and left ventricular hypertrophy in patients with chronic kidney disease (CKD). Nutr. Metab. Cardiovasc. Dis 22, 748–755. [DOI] [PubMed] [Google Scholar]
- Li B, Liu S, Miao L, and Cai L (2012). Prevention of diabetic complications by activation of Nrf2: Diabetic cardiomyopathy and nephropathy. Exp. Diabetes Res 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-H, Du L-L, Cheng X-S, Jiang X, Zhang Y, Lv B-L, Liu R, Wang J-Z, and Zhou X-W (2013). Glycation exacerbates the neuronal toxicity of ß-amyloid. Cell Death Dis 4, e673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Song D, and Leng SX (2015). Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo TWC, Westwood ME, Mclellan AC, Selwood T, and Thornalleys PJ (1994). Binding and Modification of Proteins by Methylglyoxal under Physiological Conditions. J. Bol. Chem 269, 32299–32305. [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G (2013). The hallmarks of aging. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüth HJ, Ogunlade V, Kuhla B, Kientsch-Engel R, Stahl P, Webster J, Arendt T, and Münch G (2005). Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb. Cortex 15, 211–220. [DOI] [PubMed] [Google Scholar]
- LV X, LV GH, DAI GY, SUN HM, and XU HQ (2016). Food-advanced glycation end products aggravate the diabetic vascular complications via modulating the AGEs/RAGE pathway. Chin. J. Nat. Med 14, 844–855. [DOI] [PubMed] [Google Scholar]
- Ma H, Li SY, Xu P, Babcock SA, Dolence EK, Brownlee M, Li J, and Ren J (2009). Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J. Cell. Mol. Med 13,1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maillard LC (1912). Action des Acides Aminés sur les Sucres; Formation des Mélanoïdines par Voie Méthodique.
- Makita Z, Bucala R, Rayfield EJ, Fuh H, Manogue KR, Cerami A, Viassara H, Friedman EA, Kaufman AM, Korbet SM, et al. (1994). Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet 343, 1519–1522. [DOI] [PubMed] [Google Scholar]
- De Marco EV, Annesi G, Tarantino P, Nicoletti G, Civitelli D, Messina D, Annesi F, Arabia G, Salsone M, Condino F, et al. (2010). DJ-1 is a Parkinson’s disease susceptibility gene in southern Italy. Clin. Genet 77,183–188. [DOI] [PubMed] [Google Scholar]
- McIlduff CE, and Rutkove SB (2011). Critical appraisal of the use of alpha lipoic acid (thioctic acid) in the treatment of symptomatic diabetic polyneuropathy. Ther. Clin. Risk Manag 7, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler M, Schlotterer A, Ibrahim Y, Kukudov G, Fleming T, Bierhaus A, Riedinger C, Schwenger V, Herzig S, Hecker M, et al. (2014). daf-16/FOXO and glod-4/glyoxalase-1 are required for the life-prolonging effect of human insulin under high glucose conditions in Caenorhabditis elegans. Diabetologia 58, 393–401. [DOI] [PubMed] [Google Scholar]
- Miyata T, Hori O, Zhang J, Yan SD, Ferran L, Iida Y, and Schmidt AM (1996). The receptor for advanced glycation end products (RAGE) is a central mediator of the interaction of AGE-beta2microglobulin with human mononuclear phagocytes via an oxidant-sensitive pathway. Implications for the pathogenesis of dialysis-related amyloidosi. J Clin Invest 98, 1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM (1989). Toward a Maillard reaction theory of aging. Prog. Clin. Biol. Res 304, 1–22. [PubMed] [Google Scholar]
- Monnier VM, and Taniguchi N (2016). Advanced glycation in diabetes, aging and age-related diseases: editorial and dedication. Glycoconj. J 33, 483–486. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Kohn RR, and Cerami A (1984). Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci 81,583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Sell DR, Abdul-Karim FW, and Emancipator SN (1988). Collagen browning and cross-linking are increased in chronic experimental hyperglycemia. Relevance to diabetes and aging. Diabetes 37, 867–872. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Sell DR, and Genuth S (2005). Glycation products as markers and predictors of the progression of diabetic complications. In Annals of the New York Academy of Sciences, pp. 567–581. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Sun W, Sell DR, Fan X, Nemet I, and Genuth S (2014). Glucosepane: A poorly understood advanced glycation end product of growing importance for diabetes and its complications. Clin. Chem. Lab. Med 52, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Sun W, Gao X, Sell DR, Cleary PA, Lachin JM, and Genuth S (2015). Skin collagen advanced glycation endproducts (AGEs) and the long-term progression of sub-clinical cardiovascular disease in type 1 diabetes. Cardiovasc. Diabetol 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Stevens VJ, C.A. (1981). Maillard reactions involving proteins and carbohydrates in vivo: relevance to diabetes mellitus and aging. Prog Food Nutr Sci. 5, 315–327. [PubMed] [Google Scholar]
- Morcos M, Du X, Pfisterer F, Hutter H, Sayed A. a R., Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, et al. (2008). Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 7, 260–269. [DOI] [PubMed] [Google Scholar]
- Münch G, Westcott B, Menini T, and Gugliucci A (2012). Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids 42,1221–1236. [DOI] [PubMed] [Google Scholar]
- Münch G, Lüth HJ, Wong a, Arendt T, Hirsch E, Ravid R, and Riederer P (2000).Crosslinking of alpha-synuclein by advanced glycation endproducts--an early pathophysiological. step in Lewy body formation? J. Chem. Neuroanat 20, 253–257 [DOI] [PubMed] [Google Scholar]
- Nakashima A, Carrero JJ, Qureshi AR, Miyamoto T, Anderstam B, Barany P, Heimbürger O, Stenvinkel P, and Lindholm B (2010). Effect of circulating soluble receptor for advanced glycation end products (sRAGE) and the proinflammatory RAGE ligand (EN RAGE, S100A12) on mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol 5, 2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet I, Strauch CM, and Monnier VM (2011). Favored and disfavored pathways of protein crosslinking by glucose: Glucose lysine dimer (GLUCOLD) and crossline versus glucosepane. Amino Acids 40,167–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny K, Jung T, Grune T, and Höhn A (2014). Reprint of “Accumulation of modified proteins and aggregate formation in aging.” Exp. Gerontol 59, 3–12. [DOI] [PubMed] [Google Scholar]
- Oldfield MD, Bach L. a, Forbes JM, Nikolic-Paterson D, McRobert a, Thallas V, Atkins RC, Osicka T, Jerums G, and Cooper ME (2001). Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J. Clin. Invest 108, 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peculis R, Konrade I, Skapare E, Fridmanis D, Nikitina-Zake L, Lejnieks A, Pirags V, Dambrova M, and Klovins J (2013). Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene 515, 140–143. [DOI] [PubMed] [Google Scholar]
- Peppa M, and Vlassara H (2005). Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens). 4, 28–37. [DOI] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, and Buck LB (2007). An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556. [DOI] [PubMed] [Google Scholar]
- Pfaff DH, Fleming T, Nawroth P, and Teleman AA (2017). Evidence Against a Role for the Parkinsonism-associated Protein DJ-1 in Methylglyoxal Detoxification. J. Biol. Chem 292, 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, and Papavassiliou AG (2012). Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab 97, 2231–2242. [DOI] [PubMed] [Google Scholar]
- Rabbani N, and Thornalley PJ (2009). Quantitation of markers of protein damage by glycation, oxidation, and nitration in peritoneal dialysis. In Peritoneal Dialysis International, p. [PubMed] [Google Scholar]
- Rabbani N, and Thornalley PJ (2014). Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc 9, 1969–1979. [DOI] [PubMed] [Google Scholar]
- Rabbani N, and Thornalley PJ (2015a). Hidden complexities in the measurement of fructosyllysine and advanced glycation end products for risk prediction of vascular complications of diabetes. Diabetes 64, 9–11. [DOI] [PubMed] [Google Scholar]
- Rabbani N, and Thornalley PJ (2015b). Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun 458, 221–226. [DOI] [PubMed] [Google Scholar]
- Rabbani N, and Thornalley PJ (2018a). Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. [DOI] [PubMed] [Google Scholar]
- Rabbani N, and Thornalley PJ (2018b). Glyoxalase 1 Modulation in Obesity and Diabetes. Antioxid. Redox Signal. [DOI] [PubMed] [Google Scholar]
- Rabbani N, Sebekova K, Sebekova K, Heidland A, and Thornalley PJ (2007). Accumulation of free adduct glycation, oxidation, and nitration products follows acute loss of renal function. Kidney Int. 72,1113–1121. [DOI] [PubMed] [Google Scholar]
- Rahbar S, Blumenfeld O, and Ranney HM (1969). Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem. Biophys. Res. Commun 36, 838–843. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, and Schmidt AM. (2011). Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci 1243, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposeiras-Roubin S, Rodino-Janeiro BK, Grigorian-Shamagian L, Moure-Gonzalez M, Seoane-Blanco A, Varela-Român A, Alvarez E, and González-Juanatey JR (2010). Soluble receptor of advanced glycation end products levels are related to ischaemica etiology and extent of coronary disease in chronic heart failure patients, independent of advanced glycation end products levels. Eur. J. Heart Fail 12,1092–1100. [DOI] [PubMed] [Google Scholar]
- Ravichandran M, Priebe S, Grigolon G, Rozanov L, Groth M, Laube B, Guthke R, Platzer M, Zarse K, and Ristow M (2018). Impairing L-Threon ine Catabolism Promotes Healthspan through Methylglyo xal-Mediated Proteohormesis. Cell Metab. 27, 914–925.e5. [DOI] [PubMed] [Google Scholar]
- Richarme G, and Dairou J (2017). Parkinsonism-associated protein DJ-1 is a bona fide deglycase. Biochem. Biophys. Res. Commun 483, 387–391. [DOI] [PubMed] [Google Scholar]
- Richarme G, Mihoub M, Dairou J, Chi Bui L, Leger T, and Lamouri A (2015). Parkinsonism-associated protein DJ-1/park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem 290, 1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart J-C, Jurkunas UV, Nadal M, Bouloc P, et al. (2017). Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science (80-.). 357, 208–211. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ayala E, Anderstam B, Suliman ME, Seeberger A, Heimbürger O, Lindholm B, and Stenvinkel P (2005). Enhanced RAGE-mediated NFKB stimulation in inflamed hemodialysis patients. Atherosclerosis 180, 333–340. [DOI] [PubMed] [Google Scholar]
- Sayej WN, Knight PR, Guo WA, Mullan B, Ohtake PJ, Davidson BA, Khan A, Baker RD, and Baker SS (2016). Advanced Glycation End Products Induce Obesity and Hepatosteatosis in CD-1 Wild-Type Mice. Biomed Res. Int 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E, Delpierre G, Collard F, Fortpied J, Gemayel R, Wiame E, and Veigada-Cunha M (2007). Fructosamine 3-kinase and other enzymes involved in protein deglycation. Adv. Enzyme Regul 47, 261–269. [DOI] [PubMed] [Google Scholar]
- Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, et al. (2009). C. elegans as Model for the Study of High Glucose- Mediated Life Span Reduction. Diabetes 58, 2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, and Stern D (2000). Atherosclerosis and diabetes: the RAGE connection. Curr. Atheroscler. Rep 2, 430–436. [DOI] [PubMed] [Google Scholar]
- Schmidt a M., Hori O, Cao R, Yan SD, Brett J, Wautier JL, Ogawa S, Kuwabara K, Matsumoto M, and Stern D (1996). RAGE: a novel cellular receptor for advanced glycation end products. Diabetes 45, S77–80. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, and Stern D.(1995). Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Invest 96,1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell DR, and Monnier VM (1989). Structure Elucidation of a Senescence Cross-link from Human Extracellular Matrix. 264, 21597–21602. [PubMed] [Google Scholar]
- Sell DR, Lapolla A, Odetti P, Fogarty J, and Monnier VM (1992). Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes 41,1286–1292. [DOI] [PubMed] [Google Scholar]
- Sell DR, Lane M. a, Johnson W. a, Masoro EJ, Mock OB, Reiser KM, Fogarty JF, Cutler RG, Ingram DK, Roth GS, et al. (1996). Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc. Natl. Acad. Sci. U. S. A 93, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Bandinelli S, Sun K, Guralnik JM, and Ferrucci L (2009). Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J. Am. Geriatr. Soc 57,1874–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serratos IN, Castellanos P, Pastor N, Millan-Pacheco C, Colin-Gonzalez AL, Rembao D, Pérez-Montfort R, Cabrera N, Sánchez-García A, Gómez I, et al. (2016). Early expression of the receptor for advanced glycation end products in a toxic model produced by 6-hydroxydopamine in the rat striatum. Chem. Biol. Interact 249, 10–18. [DOI] [PubMed] [Google Scholar]
- Shaikh S, and Nicholson LFB (2008). Advanced glycation end products induce in vitro cross-lin king of α-synuclein and accelerate the process of intracellular inclusion body formation.J. Neurosci. Res 86, 2071–2082. [DOI] [PubMed] [Google Scholar]
- Singh VP, Bali A, Singh N, and Jaggi AS (2014). Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol 18,1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DW, Xin N, Xie BJ, Li YJ, Meng LY, Li HM, Schlappi M, and Deng YL (2014). Formation of a salsolinol-like compound, the neurotoxin, 1-acetyl-6,7-dihydroxy −1,2,3,4 tetrahydroisoquinoline, in a cellular model of hyperglycemia and a rat model of diabetes. Int. J.Mol. Med 33, 736–742. [DOI] [PubMed] [Google Scholar]
- Sousa Silva M, Gomes RA, Ferreira AEN, Ponces Freire A, and Cordeiro C (2013). The glyoxalase pathway: the first hundred years… and beyond. Biochem. J 453, 1–15. [DOI] [PubMed] [Google Scholar]
- Sveen KA, Dahl-Jørgensen K, Stensaeth KH, Angel K, Seljeflot I, Sell DR, Monnier VM, and Hanssen KF (2015). Glucosepane and oxidative markers in skin collagen correlate with intima media thickness and arterial stiffness in long-term type 1 diabetes. J. Diabetes Complications 29, 407–412. [DOI] [PubMed] [Google Scholar]
- Szwergold BS, Bunker RD, and Loomes KM (2011). The physiological substrates of fructosamine-3-kinase-related-protein (FN3KRP) are intermediates of nonenzymatic reactions between biological amines and ketose sugars (fructation products). Med. H ypothe ses 77, 739–744. [DOI] [PubMed] [Google Scholar]
- Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi a, Pischetsrieder M, Stern D, Schmidt a M., and D’Agati VD (2000). Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J. Am. Soc. Nephrol 11,1656–1666. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ (1990). The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J 269,1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ (2003). Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys 419, 31–40. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ (2005a). Glycation free adduct accumulation in renal disease: The new AGE. Pediatr. Nephrol 20,1515–1522. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ (2005b). Measurement of protein glycation, glycated peptides, and glycation free adducts. Perit. Dial. Int 25, 522–533. [PubMed] [Google Scholar]
- Thornalley PJ (2006). Advanced glycation end products in renal failure. J. Ren. Nutr 16,178–184. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, and Rabbani N (2009). Highlights and hotspots of protein glycation in end-stage renal disease. Semin. Dial 22, 400–404. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, and Dawnay A (2003). Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J 375, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe SR, and Baynes JW (2003). Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 25, 275–281. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Goedert M, Iwatsubo T, and Lee VMY (1998). Fatal attractions: Abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differ. 5, 832–837. [DOI] [PubMed] [Google Scholar]
- Tsantoulas C, Laínez S, Wong S, Mehta I, Vilar B, and Mcnaughton PA (2017). Hyperpolarization-activated cyclic nucleotide - gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci. Transl. Med 9, eaam6072, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, Handa JT, Brownlee M, Nagaraj R, and Taylor A (2012). Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic indx, aging, and age-related disease (in nondiabetics). Aging Cell 11,1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, Vassalotti JA, and Vlassara H (2003a). Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am. J. Kidney Dis 42, 532–538. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, He C, and Vlassara H (2003b). Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J. Am. Soc. Nephrol 14, 728–731. [DOI] [PubMed] [Google Scholar]
- Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, et al. (2015). Dietary advanced glycation end products and their role in health and disease. Adv. Nutr 6, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishth D (2009). Advanced glycation end-products and bone fractures. IBMS Bonekey 6, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazzana N, Santilli F, Cuccurullo C, and Davi G (2009). Soluble forms of RAGE in internal medicine. Intern. Emerg. Med 4, 389–401. [DOI] [PubMed] [Google Scholar]
- Vicente Miranda H, Gomes MA, Branco-Santos J, Breda C, Lazaro DF, Lopes LV, Herrera F, Giorgini F, and Outeiro TF (2016). Glycation potentiates neurodegeneration in models of Huntington’s disease. Sci Rep 6, 36798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente Miranda H, Szego ÉM, Oliveira LMA, Breda C, Darendelioglu E, De Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, et al. (2017). Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain 140, 1399–1419. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, and Cerami A (1994). Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A 91,4766–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H.(1994). Serum advanced glycosylation end products: A new class of uremic toxins? Blood Purif. 12, 54–59. [DOI] [PubMed] [Google Scholar]
- Vlassara H, and Uribarri J (2014). Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diab. Rep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, and Bucala R (1992). Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc. Natl. Acad. Sci 89,12043–12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjer RL, Maezawa I, Ou JJ, Montine KS, and Montine TJ (2003). Advanced glycation endproduct precursor alters intracellular amyloid-beta/A beta PP carboxy-terminal fragment aggregation and cytotoxicity. J. Alzheimers. Dis 5, 467–476. [DOI] [PubMed] [Google Scholar]
- Wu JC, Li XH, Peng YD, Wang JB, Tang JF, and Wang YF (2011). Association of two glyoxalase I gene polymorphisms with nephropathy and retinopathy in Type 2 diabetes. J. Endocrinol. Invest 34. [DOI] [PubMed] [Google Scholar]
- Xie B, Lin F, Ullah K, Peng L, Ding W, Dai R, Qing H, and Deng Y (2015). A newly discovered neurotoxin ADTIQ associated with hyperglycemia and Parkinson’s disease. Biochem. Biophys. Res. Commun 459, 361–366. [DOI] [PubMed] [Google Scholar]
- Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, et al. (2012). Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J 443, 213–222. [DOI] [PubMed] [Google Scholar]
- Yamagishi S (2011). Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr. Drug Targets 12, 2096–2102. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Nakamura K, Matsui T, Inagaki Y, Takenaka K, Jinnouchi Y, Yoshida Y, Matsuura T, Narama I, Motomiya Y, et al. (2006). Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J. Biol. Chem 281, 20213–20220. [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, and Schmidt AM (2010a). The RAGE axis a fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res 106, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, and Schmidt AM (2010b). Soluble RAGE: Therapy and biomarker in unraveling the RaGe axis in chronic disease and aging. Biochem. Pharmacol 79,1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemva J, Andreas Fink C, Henry Fleming T, Schmidt L, Loft A, Herzig S, André Knieß R, Mayer M, Bukau B, Paul Nawroth P, et al. (2017). Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox Biol 13, 674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Cai W, Mitsuhashi T, and Vlassara H (2001). Lysozyme enhances renal excretion of advanced glycation endproducts in vivo and suppresses adverse age-mediated cellular effects in vitro: a potential AGE sequestration therapy for diabetic nephropathy? Mol. Med 7, 737–747. [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, and Zhang DD (2011). Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60, 3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W-H, Bastianetto S, Mennicken F, Ma W, and Kar S (2002). Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 115, 201–211. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Tang Y, Jin X, Chen C, Lu Y, Liu L, and Shen C (2016). Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFKB Pathway Suppression. J. Diabetes Res 2016, 4847812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Reljanovic M, Mehnert H, and Gries FA (1999). Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp. Clin. Endocrinol. Diabetes 107, 421–430. [DOI] [PubMed] [Google Scholar]