Abstract
Background
At cancer diagnosis, it is unclear whether continuity of care (COC) between the patient and GP is safeguarded.
Aim
To identify patient–GP loss of COC around the time of, and in the year after, a cancer diagnosis, together with its determinants.
Design and setting
A post-hoc analysis of data from a prospective cohort of GPs in France, taken from a survey by the Observatoire de la Médecine Générale.
Method
A prospective GP cohort (n = 96) filed data on patients who were diagnosed with incident cancer between 1 January 2000 and 31 December 2010. COC was assessed by ascertaining the frequency of consultations and the maximal interval between them. (In France, patients see their referring/named GP in most cases.) A loss of COC was measured during the trimester before and the year after the cancer diagnosis, and the results compared with those from a 1-year baseline period before cancer had been diagnosed. A loss of COC was defined as a longer interval (that is, the maximum number of days) between consultations in the measurement periods than at baseline. Determinants of the loss in COC were assessed with univariate and multivariate logistic regression models.
Results
In total, 2853 patients were included; the mean age was 66.1 years. Of these, 1440 (50.5%) were women, 389 (13.6%) had metastatic cancer, and 769 (27.0%) had a comorbidity. The mean number of consultations increased up to, and including, the first trimester after diagnosis. Overall, 26.9% (95% confidence interval [CI] = 25.3 to 28.6) of patients had a loss of COC in the trimester before the diagnosis, and 22.3% (95% CI = 20.7 to 23.9) in the year after. Increasing comorbidity score was independently associated with a reduction in the loss of COC during the year after diagnosis (adjusted odds ratio [OR] comorbidity versus no comorbidity 0.61, 95% CI = 0.48 to 0.79); the same was true for metastatic status (adjusted OR metastasis versus no metastasis 0.49, 95% CI = 0.35 to 0.70).
Conclusion
As COC is a core value for GPs and for most patients, special care should be taken to prevent a loss of COC around the time of a cancer diagnosis, and in the year after.
Keywords: cancer, cohort studies, continuity of care, diagnosis, general practice, neoplasm
INTRODUCTION
Continuity of care (COC) is defined as an ongoing therapeutic relationship between a single practitioner and a patient, beyond specific episodes of illness or disease.1 Salisbury et al referred to this as: ‘care from as few professionals as possible at repeated visits’.2 In general practice, COC also integrates interpersonal (or relational) continuity, which implies reciprocal trust and responsibility for preventive and coordinated care.3,4
COC is commonly valued by patients and GPs. Literature on COC in the general practice setting shows consistent associations with better economic, clinical, and patient-reported outcomes. Systematic reviews showed that sustained COC improved the quality of care, particularly for patients with chronic conditions,5,6 while in studies using large US and French databases, longitudinal COC in general practice was associated with a reduced death rate.7,8 From the GP’s point of view, personal COC is closely linked with their role, purpose, and satisfaction at work.9,10
A recent systematic review concluded that GPs and patients supported GPs having a greater role in cancer follow-up.11 Canadian patients with lung cancer wanted their GP to be more involved in all aspects of care and at all cancer phases,12,13 and, in interviews, GPs have shown that they consider themselves to be providers of moral support and crisis management during the cancer treatment phase.14 However, as specialists and hospital cancer teams take the lead in diagnostic procedures and treatments, patients may consider these health professionals to be their regular physicians.15 Many qualitative studies found that GPs and patients perceive that they lose touch with each other when cancer is diagnosed.12,14,16–18
Whether there is an actual loss of patient–GP COC around the time of cancer diagnosis has not been investigated using quantitative patient data. A recent survey suggested the opposite: in a population- based nationwide registry study of 127 210 Danish adults at cancer diagnosis, patients had a higher GP consultation rate than the population who did not have cancer;19 however, this study quantified total GP and specialist care consumption but did not investigate COC.
This article reports on an analysis of a large cohort of patients followed in general practice settings in France. The authors aimed to ascertain whether there was any loss of COC at cancer diagnosis and in the year afterwards, and to identify patient-and cancer-related determinants of that loss.
How this fits in
In qualitative studies, GPs and patients perceive that they lose touch with each other when cancer is diagnosed. This loss of continuity of care (COC) was investigated using quantitative data and it was found that there was a loss of patient–GP COC for approximately a quarter of the patients studied. Such a loss may negatively affect the relationship between the patient and GP, preventive care, or management of other chronic diseases.
METHOD
Study design
This was a post-hoc analysis of a prospective cohort study based on data from the Observatoire de la Médecine Générale (Observatory of General Practice, http://omg.sfmg.org). From 1993 until 2012, 129 French GPs prospectively included every patient consultation in routine practice and provided standardised data on patient characteristics and diagnoses. The GPs are from 11 out of 22 different regions. The participants in this network were largely representative of the French GP population, although a comparison with data from the Ministry of Health showed that doctors working in rural areas were under-represented.20
Using this cohort, the Continuité des Soins en Médecine Générale au Diagnostic de Cancer (Continuity of Care in Cancer Patients in General Practice, COOC-GP) study focused on adult patients diagnosed with an incident cancer between 1 January 2000 and 31 December 2010. The date of the first consultation in which the diagnosis was recorded was used as a proxy for the date of the cancer diagnosis. The period assessed for each patient was from 18 months before diagnosis until 12 months after diagnosis. The baseline period was considered to be the 1-year period from 18 months until 6 months before the cancer diagnosis; this was done to avoid the known increase in general practice care consumption related to the diagnosis.19 Patients were excluded if their usual consultations with a GP were too infrequent — this was defined as there being no consultation during the 1-year baseline period or during the year before diagnosis.
Data
The collected data included patient and GP demographic characteristics, a description of the first consultation with the diagnosis of cancer mentioned in the GP medical record, and dates of other consultations. The tumour site was classified by group:
breast;
genital, female;
genital, male;
digestive system (the reference group);
skin;
respiratory system;
lymphoma/leukaemia; and
other locations.
Patients were described as ‘living in rural areas’ or not (because of the difference in access to secondary care); the threshold for rural areas was defined as <2000 inhabitants.
A baseline comorbid condition score was generated using the comorbidities of the Charlson comorbidity21 index documented before the cancer diagnosis, and combined using modified weights (0 = no comorbidity, 29 = maximum score).22 Bannay’s modified Charlson index scores 12 comorbidities with the following weights:
congestive heart failure = 2;
peripheral vascular disease = 1;
cerebrovascular disease = 1;
dementia = 2;
chronic pulmonary disease = 1;
mild liver disease = 2;
moderate or severe renal disease = 1;
hemiplegia = 2;
any tumour (including leukaemia and lymphoma) = 2;
moderate or severe liver disease = 3;
metastatic solid tumour = 11; and
HIV/AIDS = 1.22
The maximum score of 29 is only theoretical.
COC was estimated using the maximal interval, in days, between two consultations to reflect the patient’s usual consultation regularity. It was coded as a categorical variable:
frequent: 0–91 days (0–3 months);
regular: 92–182 days (>3–6 months); and
occasional: ≥183 days (>6 months).
These categories were chosen taking into account French regulatory drug packaging (28–30 days or 84–90 days), and there being a maximum 6-month prescription duration for patients with chronic conditions and a mean of seven medical consultations per patient per year in France.23
The main outcome was loss of COC, defined as a longer interval between consultations with the same GP in the measurement periods than in the baseline period. Patients who died were excluded. In the sensitivity analysis, this outcome was tested with a 30-day and a 20% margin above the longer interval; all patients lost to follow-up were considered a loss of COC. These margins were chosen by the authors to account for there being a 1-month maximum duration for prescriptions given at hospital discharge or for a pharmacy exceptionally dispensing without a new prescription.
The ongoing relationship between the patient and GP was also determined by the number of consultations per patient per trimester, and the proportion of patients seen at least once per trimester. COC was measured during the trimester before cancer diagnosis to observe the diagnostic procedures period, and the year after to observe the cancer management and treatment period. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations were adhered to.
Statistical analysis
Age was coded into four groups:
18–49 years;
50–64 years;
65–74 years; and
≥75 years.
Each patient’s comorbidity score was coded as a three-class variable:
no metastasis and no comorbidity: score 2;
no metastasis with comorbidities: score 3–12;
metastatic cancer: score ≥13.
The paired Student’s t-test and Wilcoxon matched-pairs signed-rank test were used to compare the maximal interval between consultations and the distributions of the frequencies of consultations between the measurement period and baseline period respectively. Differences between groups with frequent, regular, or occasional consultations were tested using a χ2 test or analysis of variance as appropriate, and the Cuzick test, a non-parametric test for trend across ordered groups.24
Factors associated with loss of COC were analysed separately during the two study periods with univariate and multivariate logistic regression models. Variables with a P-value of <0.2 at univariate analysis were introduced in the multivariate analysis after correlations among variables were tested with Cramér’s V and Pearson’s R2 statistics; confounding was also tested. The baseline frequency of consultations was used as a continuous variable (that is, number of days between two consultations per patient) and expressed in months. GPs’ variability was tested in hierarchical mixed models. The discrimination abilities of the multivariate model were checked by area under the ROC curve (AUC) analysis. For all analyses, Stata version 14.2 was used and statistical significance was set at P<0.05.
RESULTS
Population characteristics
The GP population comprised 96 GPs, practising in 11 out of 22 regions in France; 84 (87.5%) were males and 65 (67.7%) had a joint practice.
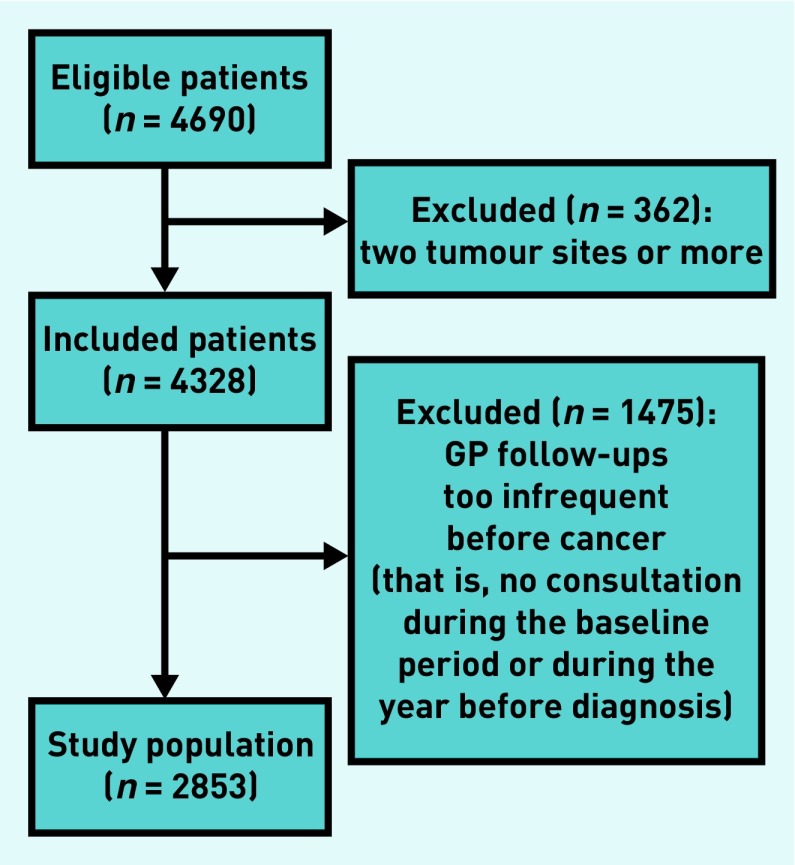
From the 4690 eligible patients, 2853 were included (Figure 1). Patients had a mean age of 66.1 years (standard deviation 13.9 years), 1440 (50.5%) were females, 389 (13.6%) had metastatic disease (Table 1), and 769 (27.0%) had at least one comorbidity (data not shown). During the baseline period, the regularity of consultations was available for 2749 patients: 697 (25.3%) patients had frequent (0–3 months) consultations, 1134 (41.3%) had regular (>3–6 months) consultations, and 918 (33.4%) had occasional (>6 months) consultations (Table 1). Patients with frequent consultations at baseline were older, had more comorbidities, and, proportionally, had home visits more often than patients with regular or occasional consultations; there was no difference according to sex (Table 1).
Figure 1.
Flowchart of patients in the study.
Table 1.
Patient characteristics and consultation frequency at baseline
| Study population, n= 2853 | Maximum interval between consecutive GP consultations, n= 2749 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0–3 months ‘frequent’, n= 697 | >3–6 months ‘regular’, n= 1134 | >6 months ‘occasional’, n= 918 | P-value | P-value for trenda | ||
| Age in years | ||||||
| Mean | 66.09 | 69.04 | 68.01 | 62.2 | <0.001b | <0.001 |
| SD | 13.92 | 14.06 | 12.75 | 13.98 | ||
| Range | 18–110 | 18–102 | 20–98 | 19–110 | ||
|
| ||||||
| Comorbidity score | ||||||
| Mean | 3.96 | 4.33 | 3.81 | 3.78 | <0.001b | <0.001 |
| SD | 3.90 | 4.10 | 3.71 | 3.89 | ||
| Range | 2–19 | 2–19 | 2–18 | 2–16 | ||
|
| ||||||
| Age group, n (%) | ||||||
| 18–49 years | 352 (12.34) | 67 (9.61) | 98 (8.64) | 159 (17.32) | ||
| 50–64 years | 880 (30.84) | 167 (23.96) | 335 (29.54) | 340 (37.04) | <0.001c | <0.001 |
| 65–74 years | 747 (26.18) | 182 (26.11) | 307 (27.07) | 241 (26.25) | ||
| ≥75 years | 874 (30.63) | 281 (40.32) | 394 (34.74) | 178 (19.39) | ||
|
| ||||||
| Comorbidity score (category), n (%) | ||||||
| 2 | 1813 (63.55) | 379 (54.38) | 702 (61.90) | 658 (71.68) | <0.001c | <0.001 |
| 3–12 | 651 (22.82) | 212 (30.42) | 296 (26.10) | 135 (14.71) | ||
| ≥13 (metastatic cancer) | 389 (13.63) | 106 (15.21) | 136 (11.99) | 125 (13.62) | ||
|
| ||||||
| Sex, n (%) | 0.64c | 0.75 | ||||
| Male | 1413 (49.53) | 348 (49.93) | 550 (48.5) | 464 (50.54) | ||
| Female | 1440 (50.47) | 349 (50.07) | 584 (51.50) | 454 (49.46) | ||
|
| ||||||
| Home visit inclusion, n (%) | 430 (15.07) | 140 (20.09) | 176 (15.52) | 98 (10.06) | <0.001c | <0.001 |
|
| ||||||
| Tumour site (n= 2430), n (%) | n = 593d | n = 971d | n = 775d | |||
| Breast | 548 (22.55) | 128 (21.59) | 227 (23.38) | 171 (22.06) | ||
| Genital, female | 128 (5.27) | 34 (5.73) | 48 (4.94) | 40 (5.16) | ||
| Genital, male | 463 (19.05) | 110 (18.55) | 172 (17.71) | 159 (20.52) | ||
| Digestive system | 444 (18.27) | 108 (18.21) | 184 (18.84) | 137 (17.68) | 0.51c | 0.39 |
| Skin | 254 (10.45) | 65 (10.96) | 117 (12.05) | 67 (8.65) | ||
| Respiratory system | 221 (9.09) | 62 (10.46) | 78 (8.03) | 68 (8.77) | ||
| Lymphoma/leukaemia | 102 (4.20) | 22 (3.71) | 43 (4.43) | 34 (4.39) | ||
| Other locations | 270 (11.11) | 64 (10.79) | 102 (10.50) | 99 (12.77) | ||
|
| ||||||
| Living in rural areas (n= 2317) | n = 563 | n = 970 | n = 784 | |||
| 857 (35.80) | 203 (36.06) | 343 (35.36) | 290 (36.99) | 0.78c | 0.68 | |
|
| ||||||
| Standard of livinge (n =2337), n (%) | n = 568 | n = 980 | n = 789 | |||
| 1st quartile | 757 (32.39) | 223 (39.26) | 292 (29.80) | 242 (30.67) | ||
| 2nd quartile | 450 (19.26) | 104 (18.31) | 201 (20.51) | 145 (18.38) | 0.001c | 0.01 |
| 3rd quartile | 558 (23.88) | 102 (17.96) | 251 (25.61) | 205 (25.98) | ||
| 4th quartile | 572 (24.48) | 139 (24.47) | 236 (24.08) | 197 (24.97) | ||
Cuzick non-parametric test for trend across ordered groups.
Analysis of variance test.
Pearson χ2 test.
Available data for this variable.
Standard of living: Institut National de la Statistique et des Etudes Economiques [National Institute for Statistics and Economic Studies] 2013 median standard of living for the GP’s city of practice, used as a proxy of the patient’s living conditions. SD = standard deviation.
Loss of COC
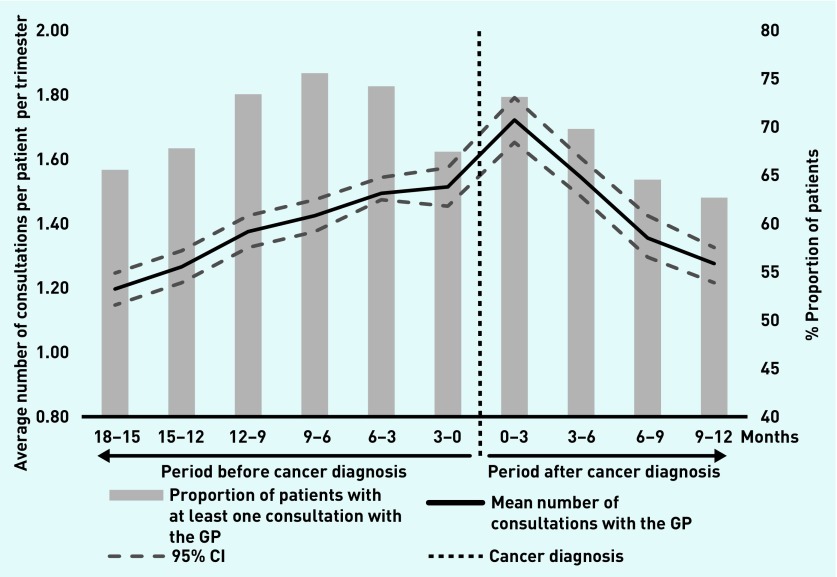
The mean number of consultations ranged from 1.2 to 1.7 per patient per trimester and the proportion of patients seen at least once per trimester ranged from 63% to 76% (Figure 2). A peak frequency of consultations occurred during the first trimester after the cancer diagnosis (Figure 2).
Figure 2.
Number of consultations and proportion of patients with at least one consultation with the GP per trimester.
The mean maximal interval between consultations was statistically significantly shorter during the trimester before cancer diagnosis when compared with the baseline period (122.9 days [95% CI = 119.7 to 126.1] versus 203.1 days [95% CI = 194.2 to 212.0], n = 2794, P<0.001) and during the year after (90.8 days [95% CI = 89.0 to 92.7] versus 195.7 days [95% CI = 186.8 to 204.7], n = 2527, P<0.001) (data not shown).
Overall, 768 (26.9%, n = 2853) patients experienced a loss of COC during the trimester before the diagnosis (95% CI = 25.3 to 28.6) and 583 (22.3%, n = 2616 patients with a follow-up) during the year after (95% CI = 20.7 to 23.9). In the sensitivity analysis, with a 30-day or 20% margin, 15.4% (95% CI = 14.0 to 16.7) and 18.1% (95% CI = 16.7 to 19.5) of patients, respectively, had a loss of COC during the trimester before the diagnosis and 9.5% (95% CI = 8.4 to 10.7) and 13.9% (95% CI = 12.6 to 15.3) during the year after (data not shown). Taking into account the loss of COC for patients lost to follow-up, this proportion equates to 28.5% (95% CI = 26.8 to 30.2) in the year after diagnosis. Among those patients who experienced a loss of COC in the year after diagnosis, 329 (56.4%) had also lost COC during the trimester before the diagnosis (data not shown).
There was no statistical significance in GPs’ variability in the hierarchical models tested (P = 0.18 in the year after diagnosis, P = 0.23 in the trimester before) so non-hierarchical logistic models were tested.
During the trimester before the cancer diagnosis, in univariate analysis, both tumour site and a higher frequency of consultations at baseline were associated with a loss of COC (Table 2). GP characteristics were not statistically significant. In the univariate analysis of data relating to the year after the cancer diagnosis, increased age, sex, tumour site, higher comorbidity score, and frequency of consultations at baseline were associated with a loss of COC (P<0.2, Table 3). In the multivariate analysis, a higher frequency of consultations at baseline was the only significant determinant and, after adjustment for frequency of consultations at baseline, the probability of loss of COC decreased with increasing comorbidity score and with metastatic status (Table 4). The discriminative ability of these models was fair and good25 (AUC 0.73 during the trimester before diagnosis and AUC 0.83 in the year after, [data not shown]).
Table 2.
Determinants of loss in continuity of care during the trimester before cancer diagnosis (univariate analysis)
| Loss of COC (n= 768) | No loss of COC (n= 2085) | ORa | 95% CI | P-valueb | |
|---|---|---|---|---|---|
| Patient age in years | 0.57 | ||||
| Mean | 66.33 | 66.00 | 1.00 | 0.99 to 1.00 | |
| SD | 14.37 | 13.75 | |||
|
| |||||
| GP age in years | 0.32 | ||||
| Mean | 51.20 | 51.5 | 0.99 | 0.98 to 1.00 | |
| SD | 7.82 | 27.59 | |||
|
| |||||
| Patient age group, n (%) | 0.77 | ||||
| 18–49 years | 97 (12.63) | 255 (12.23) | Ref | – | |
| 50–64 years | 226 (29.43) | 654 (31.37) | 0.91 | 0.69 to 1.20 | |
| 65–74 years | 202 (26.30) | 545 (26.14) | 0.97 | 0.73 to 1.29 | |
| ≥75 years | 243 (31.64) | 631 (30.26) | 1.01 | 0.77 to 1.34 | |
|
| |||||
| Patient sex, n (%) | 0.23 | ||||
| Male | 366 (47.66) | 1047 (50.22) | Ref | – | |
| Female | 402 (52.34) | 1038 (49.78) | 1.11 | 0.94 to 1.31 | |
|
| |||||
| Comorbidity score (category), n (%) | 0.33 | ||||
| 2 | 500 (65.10) | 1313 (62.97) | Ref | – | |
| 3–12 | 175 (22.79) | 476 (22.83) | 0.97 | 0.79 to 1.18 | |
| ≥13 (metastatic cancer) | 93 (12.11) | 296 (14.20) | 0.83 | 0.64 to 1.06 | |
|
| |||||
| Tumour site (n= 2430), n (%) | n = 650 | n = 1780 | 0.16 | ||
| Breast | 158 (24.31) | 390 (21.91) | 1.15 | 0.86 to 1.52 | |
| Genital, female | 43 (6.62) | 85 (4.78) | 1.43 | 0.94 to 2.18 | |
| Genital, male | 117 (18.00) | 346 (19.44) | 0.96 | 0.71 to 1.29 | |
| Digestive system | 116 (17.85) | 328 (18.43) | Ref | – | |
| Skin | 76 (11.69) | 178 (10.00) | 1.21 | 0.86 to 1.70 | |
| Respiratory system | 57 (8.77) | 164 (9.21) | 0.98 | 0.68 to 1.42 | |
| Lymphoma/leukaemia | 20 (3.07) | 82 (4.60) | 0.69 | 0.40 to 1.17 | |
| Other locations | 63 (9.69) | 207 (11.63) | 0.69 | 0.40 to 1.17 | |
|
| |||||
| Frequency of consultations at baseline, n (%) | <0.001 | ||||
| 0–3 months: frequent | 350 (45.67) | 347 (17.52) | Ref | – | |
| >3–6 months: regular | 317 (41.28) | 817 (41.24) | 0.39 | 0.32 to 0.47 | |
| >6 months: occasional | 101 (13.15) | 817 (41.24) | 1.22 | 0.87 to 1.17 | |
|
| |||||
| GP sex, n (%) | 0.38 | ||||
| Male | 700 (91.15) | 1878 (90.07) | Ref | – | |
| Female | 68 (8.85) | 207 (9.93) | 0.88 | 0.66 to 1.17 | |
|
| |||||
| GP joint practice, n (%) | 0.83 | ||||
| Yes | 556 (72.40) | 1518 (72.81) | 0.98 | 0.81 to 1.18 | |
| No | 212 (27.60) | 567 (27.19) | Ref | – | |
|
| |||||
| Living in rural areas (n= 2394), n (%) | 650 | 1744 | 0.20 | ||
| No | 404 (62.15) | 1133 (64.97) | Ref | – | |
| Yes | 246 (37.85) | 611 (35.03) | 1.13 | 0.94 to 1.36 | |
Univariate logistic regression.
Wald test. COC = continuity of care. OR = odds ratio. SD = standard deviation.
Table 3.
Determinants of loss in continuity of care during the year after cancer diagnosis (univariate analysis)
| Loss of COC (n= 583) | No loss of COC (n= 2033) | ORa | 95% CI | P-valueb | |
|---|---|---|---|---|---|
| Patient age in years | |||||
| Mean | 67.6 | 65.9 | 1.01 | 1.00 to 1.02 | 0.01 |
| SD | 13.2 | 13.9 | |||
|
| |||||
| GP age in years | |||||
| Mean | 51.5 | 51.5 | 1.00 | 0.99 to 1.01 | 0.98 |
| SD | 7.9 | 7.4 | |||
|
| |||||
| Patient age group, n (%) | 0.09 | ||||
| 18–49 years | 56 (9.61) | 256 (12.59) | Ref | – | |
| 50–64 years | 169 (28.99) | 631 (31.04) | 1.22 | 0.88 to 1.71 | |
| 65–74 years | 160 (27.44) | 530 (26.07) | 1.38 | 0.98 to 1.94 | |
| ≥75 years | 198 (33.96) | 616 (30.30) | 1.47 | 1.06 to 2.04 | |
|
| |||||
| Patient sex, n (%) | 0.10 | ||||
| Male | 273 (46.83) | 1030 (50.66) | Ref | – | |
| Female | 310 (53.17) | 1003 (49.34) | 1.17 | 0.97 to 1.40 | |
|
| |||||
| Comorbidity score (category), n (%) | 0.02 | ||||
| 2 | 379 (65.01) | 1262 (62.08) | Ref | – | |
| 3–12 | 145 (24.87) | 475 (23.36) | 1.02 | 0.82 to 1.26 | |
| ≥13 (metastatic cancer)c | 59 (10.12) | 296 (14.56) | 0.66 | 0.49 to 0.90 | |
|
| |||||
| Tumour site (n= 2254), n (%) | n = 483 | n = 1771 | 0.03 | ||
| Breast | 120 (24.84) | 385 (21.74) | 1.16 | 0.85 to 1.59 | |
| Genital, female | 33 (6.83) | 83 (4.69) | 1.48 | 0.93 to 2.37 | |
| Genital, male | 92 (19.05) | 347 (19.59) | 0.99 | 0.71 to 1.38 | |
| Digestive system | 86 (17.81) | 321 (18.13) | Ref | – | |
| Skin | 60 (12.42) | 183 (10.33) | 1.22 | 0.84 to 1.78 | |
| Respiratory system | 29 (6.00) | 173 (9.77) | 0.63 | 0.40 to 0.99 | |
| Lymphoma/leukaemia | 21 (4.35) | 76 (4.29) | 1.03 | 0.60 to 1.77 | |
| Other locations | 42 (8.70) | 203 (11.46) | 0.77 | 0.51 to 1.16 | |
|
| |||||
| Frequency of consultations at baseline (n= 2539), n (%) | n = 583 | n = 1956 | <0.001 | ||
| 0–3 months: frequent | 347 (59.52) | 312 (15.95) | Ref | – | |
| >3–6 months: regular | 216 (37.05) | 856 (43.76) | 0.23 | 0.18 to 0.28 | |
| >6 months: occasional | 20 (3.43) | 788 (40.29) | 0.02 | 0.01 to 0.04 | |
|
| |||||
| GP sex, n (%) | 0.36 | ||||
| Male | 534 (91.60) | 1837 (90.36) | Ref | – | |
| Female | 49 (8.40) | 196 (9.64) | 0.86 | 0.62 to 1.19 | |
|
| |||||
| GP joint practice, n (%) | 0.38 | ||||
| No | 168 (28.82) | 548 (26.96) | Ref | – | |
| Yes | 415 (71.18) | 1485 (73.04) | 0.91 | 0.74 to 1.12 | |
|
| |||||
| Living in rural areas, n (%) | 0.44 | ||||
| No | 319 (65.77) | 1090 (63.85) | Ref | – | |
| Yes | 166 (34.23) | 617 (36.15) | 0.92 | 0.74 to 1.14 | |
Univariate logistic regression.
Wald test.
Weight of metastasis in comorbidity score was 11 points. COC = continuity of care. OR = odds ratio. SD = standard deviation.
Table 4.
Independent determinants of a loss in continuity of care (multivariate logistic regression)
| Loss of COC Mean (SD) | No loss of COC Mean (SD) | ORa | 95% CI | |
|---|---|---|---|---|
| Trimester before cancer (n= 2539) | ||||
|
| ||||
| Frequency of consultations at baseline (expressed by units of 1 month) | 3.59 (2.21) | 7.87 (8.80) | 0.73 | 0.71 to 0.76 |
|
| ||||
| Year after cancer (n= 2539) | ||||
|
| ||||
| Frequency of consultations at baseline (expressed by units of 1 month) | 2.91 (1.82) | 7.50 (8.25) | 0.51 | 0.48 to 0.55 |
|
| ||||
| Comorbidity score (category), n (%) | ||||
| 2 | 379 (14.93) | 1208 (47.58) | Ref | – |
| 3–12 | 145 (5.71) | 469 (18.47) | 0.61 | 0.48 to 0.79 |
| ≥13 (metastatic cancer) | 59 (2.32) | 279 (10.99) | 0.49 | 0.35 to 0.70 |
Multivariate logistic regression. COC = continuity of care. OR = odds ratio. SD = standard deviation.
DISCUSSION
Summary
The study presented here showed an overall increase in the number and frequency of GP consultations around the time of cancer diagnosis and in the year afterwards. It demonstrated that approximately three-quarters of patients did not experience a loss of COC with their GP — however, 27% did during the trimester before the diagnosis and 22% did in the year after. Of those who experienced a loss of COC in the year after diagnosis, 56% had also experienced loss of COC during the trimester before their diagnosis. The risk of loss of COC in the year after diagnosis was reduced for patients with comorbidities and with metastatic cancer. The age and sex of patients, tumour site, or GP characteristics were not associated with COC loss.
Strengths and limitations
To the authors’ knowledge, this is the first study to explore the impact of a cancer diagnosis on COC in general practice routine care. Several studies have explored COC from the patient’s point of view12,15,16 and, in qualitative studies, the GP’s point of view,14,16,26,27 but no known study has quantified the loss of COC using a national GP database. The large cohort of patients included in this study enabled the provision of accurate estimations. However, care should be taken with generalisability outside of the French or comparable-care context.
Among the multiple dimensions of COC, this study used a new approach to assessing personal COC based on the frequency of patient–GP consultations. This study may also assess longitudinal COC, defined as a pattern of consultations ‘that occurs in the same place, with the same medical record, and with the same professionals’.4 These aspects of COC highlight the potential clinical impact on the patient’s follow-up and the care coordination.
Excluded patients were probably seen occasionally by the GP instead of a colleague or were patients who do not usually come to see their physician; as such, extrapolation of the results should be limited to patients who had been seen at least once or twice over the 18 months before diagnosis.
Comparison with existing literature
In a Danish study, patients who had been diagnosed with cancer were seen by a GP more often than those who had not.19 The results presented here illustrate a similar pattern in the number of GP consultations. In another study, this time conducted in England, of 65 337 patients with cancer, a third declared having had contact with a GP in the previous 2 weeks.28 These results concur with the notion that GPs are involved at the time of cancer diagnosis to meet patients’ needs, including: conducting consultations or home visits; providing psychological support; managing pain, anxiety, comorbidities, and side effects of treatment; and help with family issues or economic problems.13,14,16 According to most patients, the GP is the doctor they trust to be responsible for them as a whole person over time. It has been demonstrated that patients wish to see ‘their’ GP when dealing with chronic or emotional problems so they do not have to repeat their whole story at each consultation;29 this situation can also apply when patients have cancer.
Despite the overall increased number of consultations, approximately a quarter of patients with cancer did not see their GP during a longer period than they ever had during 12 months of usual follow-up. These data reflect what GPs subjectively perceive — namely: ‘when patients have cancer, they stop seeing me’,16 ‘from the moment the diagnosis is made, I’m completely out of the game’.17 Although GP perceptions are borne out by these results in some instances, there is also a discrepancy with the fact that three-quarters of patients did not lose touch with the GP. Some studies assessing COC at a cancer diagnosis made assumptions about the causes of this loss of COC. A study conducted in England found that only half of patients with cancer were told to contact their GP post-discharge,28 whereas a literature review found that patients with cancer felt more confident with hospital-based follow-up, as it meant they had possible access to expertise and tests if needed.30 In a Canadian survey of patients with lung cancer, some patients did not identify the role their GP could play with regard to their cancer, especially if they had no other health problems.13
In the study presented here, comorbidities and metastatic status were found to be independently associated with reduced probability of loss of COC in the year after a diagnosis of cancer diagnosis. Patients who have existing comorbidities when they are diagnosed with cancer may be used to trusting their GP for all of their health care.29
Implications for research and practice
The loss of COC that affected some patients around the time of their cancer diagnosis and in the year after may negatively affect the patient–GP relationship, preventive care, or management of other chronic diseases. COC is a core value for GPs and for most patients, notably those with chronic conditions. Further qualitative research could be conducted to better understand what GPs need to get more involved in cancer care. After the treatment phase, most cancers do, or will, require long-term follow-up by GPs, along with disease management — just as with other chronic diseases. Preserving patient–GP COC would be an asset for all patients with cancer, at the diagnostic and treatment phases; as such, it may be useful to ensure that such patients have named GPs, and to educate and support cancer specialists in referring patients to their GP periodically.
Acknowledgments
The authors would like to thank Emmanuelle Boutin and Richard Layese for their statistical support, and Laura Smales for editorial support.
Funding
The survey by the Observatoire de la Médecine Générale (Observatory of General Practice) was funded by the Société Française de Médecine Générale (French Society for General Practice) and Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés (National Health Insurance Fund for Employees).
Ethical approval
This study was approved by the Société Française de Médecine Générale (French Society for General Practice) and received administrative authorisation from the Commission Nationale de l’Informatique et des Libertés (National Commission on Informatics and Liberty, approval no: 311668).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Haggerty JL, Reid RJ, Freeman GK, et al. Continuity of care: a multidisciplinary review. BMJ. 2003;327(7425):1219–1221. doi: 10.1136/bmj.327.7425.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salisbury C, Sampson F, Ridd M, Montgomery AA. How should continuity of care in primary health care be assessed? Br J Gen Pract. 2009 doi: 10.3399/bjgp09X420257. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie B, Saultz JW, Freeman GK, Haggerty JL. Continuity of care matters. BMJ. 2008;337:a867. doi: 10.1136/bmj.a867. [DOI] [PubMed] [Google Scholar]
- 4.Saultz JW. Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1(3):134–143. doi: 10.1370/afm.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? J Fam Pract. 2004;53(12):974–980. [PubMed] [Google Scholar]
- 6.Sans-Corrales M, Pujol-Ribera E, Gené-Badia J, et al. Family medicine attributes related to satisfaction, health and costs. Fam Pract. 2006;23(3):308–316. doi: 10.1093/fampra/cmi112. [DOI] [PubMed] [Google Scholar]
- 7.Leleu H, Minvielle E. Relationship between longitudinal continuity of primary care and likelihood of death: analysis of national insurance data. PloS One. 2013;8(8):e71669. doi: 10.1371/journal.pone.0071669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolinsky FD, Bentler SE, Liu L, et al. Continuity of care with a primary care physician and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(4):421–428. doi: 10.1093/gerona/glp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokes T, Tarrant C, Mainous AG, et al. Continuity of care: is the personal doctor still important? A survey of general practitioners and family physicians in England and Wales, the United States, and the Netherlands. Ann Fam Med. 2005;3(4):353–359. doi: 10.1370/afm.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridd M, Shaw A, Salisbury C. ‘Two sides of the coin’: the value of personal continuity to GPs — a qualitative interview study. Fam Pract. 2006;23(4):461–468. doi: 10.1093/fampra/cml010. [DOI] [PubMed] [Google Scholar]
- 11.Meiklejohn JA, Mimery A, Martin JH, et al. The role of the GP in follow-up cancer care: a systematic literature review. J Cancer Surviv. 2016;10(6):990–1011. doi: 10.1007/s11764-016-0545-4. [DOI] [PubMed] [Google Scholar]
- 12.Aubin M, Vézina L, Verreault R, et al. Family physician involvement in cancer care follow-up: the experience of a cohort of patients with lung cancer. Ann Fam Med. 2010;8(6):526–532. doi: 10.1370/afm.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubin M, Vézina L, Verreault R, et al. Patient, primary care physician and specialist expectations of primary care physician involvement in cancer care. J Gen Intern Med. 2012;27(1):8–15. doi: 10.1007/s11606-011-1777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farquhar MC, Barclay SI, Earl H, et al. Barriers to effective communication across the primary/secondary interface: examples from the ovarian cancer patient journey (a qualitative study) Eur J Cancer Care (Engl) 2005;14(4):359–366. doi: 10.1111/j.1365-2354.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 15.McWhinney IR, Hoddinott SN, Bass MJ, et al. Role of the family physician in the care of cancer patients. Can Fam Physician. 1990;36:2183–2186. [PMC free article] [PubMed] [Google Scholar]
- 16.Anvik T, Holtedahl KA, Mikalsen H. ‘When patients have cancer, they stop seeing me’: the role of the general practitioner in early follow-up of patients with cancer — a qualitative study. BMC Fam Pract. 2006;7:19. doi: 10.1186/1471-2296-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisler JJ, Brown JB, Stewart M. Family physicians’ roles in cancer care: survey of patients on a provincial cancer registry. Can Fam Physician. 2004;50:889–896. [PMC free article] [PubMed] [Google Scholar]
- 18.Chicoulaa B, Balardy L, Stillmunkes A, et al. French general practitioners’ sense of isolation in the management of elderly cancer patients. Fam Pract. 2016;33(5):551–556. doi: 10.1093/fampra/cmw034. [DOI] [PubMed] [Google Scholar]
- 19.Christensen KG, Fenger-Gron M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis: a population-based nationwide registry study of 127 000 incident adult cancer patients. BMC Health Serv Res. 2012;12:224. doi: 10.1186/1472-6963-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosman S, Le Vaillant M, Pelletier-Fleury N. Gaining insight into benzodiazepine prescribing in general practice in France: a data-based study. BMC Fam Pract. 2011;12:28. doi: 10.1186/1471-2296-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Bannay A, Chaignot C, Blotière PO, et al. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care. 2016;54(2):188–194. doi: 10.1097/MLR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 23.Organisation for Economic Co-operation and Development Doctors’ consultations. 2018. https://data.oecd.org/healthcare/doctors-consultations.htm (accessed on 7 Jan 2019).
- 24.Cuzick J. A method for analysing case-control studies with ordinal exposure variables. Biometrics. 1985;41(3):609–621. [PubMed] [Google Scholar]
- 25.Božikov J, Zaletel-Kragelj L. Test validity measures and receiver operating characteristic (ROC) analysis. In: Zaletel-Kragelj L, Božikov J, editors. Methods and tools in public health. Lage: Hans Jacobs Verlag; 2010. pp. 749–770. [Google Scholar]
- 26.Guassora AD, Jarlbaek L, Thorsen T. Preparing general practitioners to receive cancer patients following treatment in secondary care: a qualitative study. BMC Health Serv Res. 2015;15(1):202. doi: 10.1186/s12913-015-0856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell GK, Burridge LH, Colquist SP, Love A. General practitioners’ perceptions of their role in cancer care and factors which influence this role. Health Soc Care Community. 2012;20(6):607–616. doi: 10.1111/j.1365-2524.2012.01075.x. [DOI] [PubMed] [Google Scholar]
- 28.Allgar VL, Neal RD. General practitioners’ management of cancer in England: secondary analysis of data from the National Survey of NHS Patients — Cancer. Eur J Cancer Care (Engl) 2005;14(5):409–416. doi: 10.1111/j.1365-2354.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie B, Wyke S. Personal continuity and access in UK general practice: a qualitative study of general practitioners’ and patients’ perceptions of when and how they matter. BMC Fam Pract. 2006;7:11. doi: 10.1186/1471-2296-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis RA, Neal RD, Williams NH, et al. Follow-up of cancer in primary care versus secondary care: systematic review. Br J Gen Pract. 2009 doi: 10.3399/bjgp09X453567. . [DOI] [PMC free article] [PubMed] [Google Scholar]