Abstract
Background
Patients who rarely consult a GP in the 19–36 months before a cancer diagnosis have more advanced cancer at diagnosis and a worse prognosis. To ensure more timely diagnosis of cancer, the GP should suspect cancer as early as possible.
Aim
To investigate the GP’s suspicion of cancer according to the patient with cancer’s usual consultation pattern in general practice.
Design and setting
A cross-sectional study based on survey data from general practice of 3985 Danish patients diagnosed with cancer from May 2010 to August 2010, and linked to national register data.
Method
Using logistic regression analysis with restricted cubic splines, the odds ratio (OR) of the GP to suspect cancer as a function of the patient’s number of face-to-face consultations with the GP in the 19–36 months before a cancer diagnosis was estimated.
Results
GPs’ cancer suspicion decreased with higher usual consultation frequency in general practice. A significant decreasing trend in ORs for cancer suspicion was seen across usual consultation categories overall (P<0.001) and for each sex (males: P<0.05; females: P<0.05). GPs’ cancer suspicion was lower in patients aged <55 years in both rare and frequent attenders compared with average attenders.
Conclusion
GPs suspect cancer more often in rare attenders ≥55 years. GPs’ cancer suspicion was lower in younger patients (<55 years), in both rare and frequent attenders. GPs should be aware of possible missed opportunities for cancer diagnosis in young attenders and use safety netting to reduce the risk of missing a cancer diagnosis.
Keywords: accessibility of health services, Denmark, early diagnosis, general practice, healthcare delivery, neoplasms
INTRODUCTION
Early detection of cancer is vital to improve the prognosis of patients with cancer.1 Therefore, many countries, including Denmark and the UK, have implemented strategies for the diagnosis and treatment of cancer.1–3 These strategies seem to provide more timely diagnosis and to improve patients’ prognosis.4–6
Despite recent prognostic improvements, research has shown that patients who rarely consult a GP in the 19–36 months before a cancer diagnosis are diagnosed with more advanced cancer and have a worse prognosis than patients who regularly consult the GP.7 One explanation could be that patients who rarely consult their GP are likely to seek medical advice at a later stage of disease (with more pronounced symptoms) than patients who usually consult their GP on a more regular basis.8 As tumours generally grow exponentially,9 patients are likely to present with more distinct symptoms as the underlying disease evolves, that is, as the tumour reaches a more advanced stage. More distinct symptoms will usually evoke the GP’s suspicion of cancer and lead to a more timely diagnosis.10 To achieve more timely diagnosis, it is thus important that the GP suspects cancer as early as possible. Some authors have argued that GPs are more inclined to suspect serious illness (including cancer) and to act more promptly when seeing a patient who rarely consults in general practice.11,12 Others have suggested that the GP’s limited knowledge of the rare attender’s medical history may obscure the suspicion of cancer.12,13 However, no conclusive evidence exists of the association between a patient’s usual consultation frequency and the GP’s suspicion of cancer.
This study aimed to investigate whether the GP’s suspicion of cancer before diagnosis varies according to the patient’s usual consultation pattern in general practice in a population of patients with cancer.
METHOD
A cross-sectional study was conducted using data from a sub-cohort of the Danish Cancer in Primary Care cohort,14 which were linked to previously reported data on usual consultation frequency in general practice7 and national register data. Information was linked at the individual level using the Danish personal registration number, which is assigned to all citizens in Denmark.15
Setting
The study took place in Denmark, where the publicly funded healthcare system is designed to ensure free access to diagnostic procedures and treatment for all citizens. Almost all citizens (>98%) are registered with a GP, who acts as gatekeeper to the rest of the healthcare system (except for emergencies and privately practising otorhinolaryngologists and ophthalmologists, who can be accessed directly).16 Since 2009, GPs in Denmark have been able to urgently refer patients suspected to have cancer to a standardised cancer patient pathway.2
How this fits in
Rare attenders in general practice who are diagnosed with cancer have more advanced cancers and worse prognosis than more regular attenders. No previous studies have explored whether GPs suspect cancer more often in rare attenders. The results of this study show that GPs are more likely to suspect cancer in rare attenders aged ≥55 years, but not in attenders <55 years in both rare and frequent attenders compared with average attenders. Safety netting is an important diagnostic strategy to reduce the risk of missing a possible cancer diagnosis in younger (<55 years) patients.
Study population
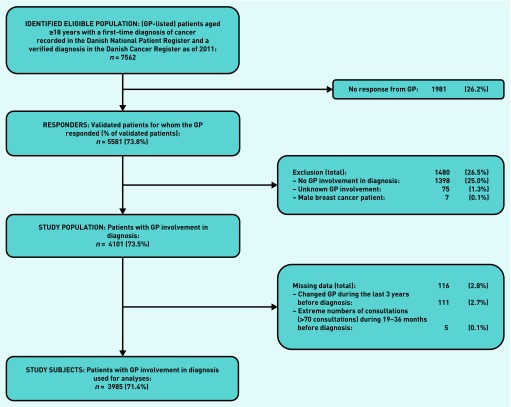
Identification of patients, data collection, and drop-out analysis have been described in detail elsewhere.14 All patients aged ≥18 years with an incident diagnosis of cancer, except for non-melanoma skin cancer, from 1 May 2010 to 31 August 2010 were identified in the Danish National Patient Register.17 The study population was restricted to the 73.5% of patients who had attended general practice as part of the cancer diagnosis according to the GP (Figure 1).10,14
Figure 1.
Flow of patients from identification to inclusion in analyses.
Data collection
A questionnaire on the diagnostic pathway of each patient with cancer was sent to the patient’s GP, who was asked to fill in the questionnaire using the medical records. GPs responded in 5581 cases (73.8%). The response rate was higher for female patients, patients diagnosed with breast cancer, and patients with a high educational level.10,14 Usual GP consultation frequency could not be established for a small proportion of patients (n = 111) because they had been listed with more than one GP in the last 3 years before diagnosis (Figure 1).
Outcome
The outcome was defined as the GP’s reporting of cancer suspicion at the first consultation that led to the patient’s cancer diagnosis. Data were based on information from the questionnaire about the GP’s interpretation of the symptoms presented by the patient.10
Exposure
The exposure in this study was the patient’s usual (that is, customary) consultation frequency in general practice; this was defined as the number of daytime face-to-face consultations with a GP, including home visits, in the 19–36 months before the date of the cancer diagnosis.7 This period was sufficiently close to the date of diagnosis to reflect the patient’s usual consultation frequency according to their age, and to limit the risk of the consultation frequency to be affected by the cancer itself.
Other variables
Demographic and socioeconomic information was collected from Statistics Denmark. Information on age and sex was derived from the Danish personal registration number.15 Household income was categorised into three groups using the Organisation for Economic Co-operation and Development (OECD) modified scale: ‘low’, ‘average’, and ‘high’. The highest attained level of education was categorised into three groups according to the International Standard Classification of Education (ISCED):18 ‘basic’ (ISCED level I–II), ‘short’ (ISCED level III–IV), and ‘long’ (ISCED level V–VI). Comorbidity was defined using the Charlson comorbidity index (CCI)19 and was calculated on the basis of diagnoses registered in the Danish National Patient Register17 during the 10 years preceding study entry. Patient CCI scores were grouped into three levels: ‘none’ (CCI score = 0), ‘moderate’ (CCI score = 1–2), and ‘severe’ (CCI score ≥3).
Analyses
The odds ratio (OR) of the GP to suspect cancer as a function of the patient’s number of face-to-face consultations with the GP during the 19–36 months before the cancer diagnosis was estimated using logistic regression. First, analyses were performed in which the patient’s usual consultation frequency was divided into three categories: ‘rare’ (0–1 consultations), ‘average’ (2–9 consultations), and ‘frequent’ (≥10 consultations). Second, to avoid assuming a linear or piecewise constant association between the patient’s usual consultation frequency and the GP’s cancer suspicion, consultation frequency was treated as a continuous variable by using restricted cubic splines with three knots and the median number of consultations as the reference point. Analyses were adjusted for sex, age, diagnosis, comorbidity, household income, and educational level. Age was centred at the mean. A two-sided P-value ≤0.05 was defined as significant. Analyses were conducted in Stata (version 14).
RESULTS
A total of 3985 eligible incident cancer patients were included, of which 1873 (47%) were females (Table 1). Females had a median of six consultations (interquartile range (IQR) = 3–10) and males had a median of five consultations (IQR = 2–9) with their GP during the 19–36 months before their cancer diagnosis (Table 1).
Table 1.
Characteristics of the patients with cancer included in the study (N = 3985)
| Females | Males | Total | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| n | % | n | % | n | % | |
| Sex | ||||||
| Female | 1873 | 100 | n/a | n/a | 1873 | 47.0 |
| Male | n/a | n/a | 2112 | 100 | 2112 | 53.0 |
|
| ||||||
| Age group, years | ||||||
| 18–44 | 192 | 10.3 | 107 | 5.1 | 299 | 7.5 |
| 45–54 | 293 | 15.6 | 192 | 9.1 | 485 | 12.2 |
| 55–64 | 404 | 21.6 | 514 | 24.3 | 918 | 23.0 |
| 65–74 | 477 | 25.5 | 726 | 34.4 | 1203 | 30.2 |
| 75–84 | 358 | 19.1 | 484 | 22.9 | 842 | 21.1 |
| ≥85 | 149 | 8.0 | 89 | 4.2 | 238 | 6.0 |
|
| ||||||
| Cancer diagnosis | ||||||
| Colorectal | 293 | 15.6 | 321 | 15.2 | 614 | 15.4 |
| Lung | 212 | 11.3 | 282 | 13.4 | 494 | 12.4 |
| Breast | 563 | 30.1 | n/a | n/a | 563 | 14.1 |
| Prostate | n/a | n/a | 605 | 28.6 | 605 | 15.2 |
| Other | 805 | 43.0 | 904 | 42.8 | 1709 | 42.9 |
|
| ||||||
| Comorbidity | ||||||
| None | 1521 | 81.2 | 1601 | 75.8 | 3122 | 78.3 |
| Moderate | 289 | 15.4 | 436 | 20.6 | 725 | 18.2 |
| Severe | 63 | 3.4 | 75 | 3.6 | 138 | 3.5 |
|
| ||||||
| Household income (disposable) | ||||||
| Low | 639 | 34.1 | 639 | 30.3 | 1278 | 32.1 |
| Average | 667 | 35.6 | 605 | 28.6 | 1272 | 31.9 |
| High | 567 | 30.3 | 868 | 41.1 | 1435 | 36.0 |
|
| ||||||
| Educational level | ||||||
| Basic | 845 | 45.1 | 781 | 37.0 | 1626 | 40.8 |
| Short | 647 | 34.5 | 912 | 43.2 | 1559 | 39.1 |
| Long | 381 | 20.3 | 419 | 19.8 | 800 | 20.1 |
|
| ||||||
| Face-to-face contactsa | ||||||
| Median (IQR) | 6 (3–10) | 5 (2–9) | 5 (2–10) | |||
| ‘Rare’ | 303 | 16.2 | 445 | 21.1 | 748 | 18.8 |
| ‘Average’ | 1036 | 55.3 | 1183 | 56.0 | 2219 | 55.7 |
| ‘Frequent’ | 534 | 28.5 | 484 | 22.9 | 1018 | 25.5 |
|
| ||||||
| Cancer suspicion | ||||||
| Yes | 939 | 50.1 | 887 | 42.0 | 1826 | 45.8 |
| No | 857 | 45.8 | 1164 | 55.1 | 2021 | 50.7 |
| Missing | 77 | 4.1 | 61 | 2.9 | 138 | 3.5 |
19–36 months before the cancer diagnosis. IQR = interquartile range (25th to 75th percentiles). n/a = not applicable.
GPs suspected cancer in 45.8% of the cases; this was seen more often in females than males (50.1% versus 42.0%, P<0.001) (Table 1). The proportion of cancer suspicion was 48.3% among rare attenders (52.1% among females versus 45.6% among males, P<0.001) (Table 2). Patients in whom the GP suspected cancer had a significantly shorter primary care interval (the time from when the patient presented to general practice until referral to secondary care) than patients in whom the GP did not suspect cancer (median = 0 days (IQR = 0–3) versus median = 11 days (IQR = 0–39), P<0.001).
Table 2.
Distribution and odds ratios (ORs) for the GP to suspect cancer according to the patient’s usual GP consultation frequencya
| GP-suspected cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Observed | Crude | Adjustedb | |||||||
|
|
|
|
|||||||
| Usual GP consultation frequencya | n | % | OR | (95% CI) | Trendc | OR | (95% CI) | Trendc | |
| Overall | |||||||||
| Rare (n = 748) | 361 | 48.3 | 1.09 | 0.92 to 1.30 | 1.14 | 0.95 to 1.37 | |||
| Average (n = 2219) | 1031 | 46.5 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 1018) | 434 | 42.6 | 0.83 | 0.71 to 0.96 | P= 0.018 | 0.78 | 0.66 to 0.91 | P<0.001 | |
|
| |||||||||
| Females | |||||||||
| Rare (n = 303) | 158 | 52.1 | 1.02 | 0.79 to 1.32 | 1.03 | 0.77 to 1.39 | |||
| Average (n = 1036) | 536 | 51.7 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 534) | 245 | 45.9 | 0.77 | 0.63 to 0.96 | P= 0.059 | 0.72 | 0.57 to 0.91 | P= 0.032 | |
|
| |||||||||
| Males | |||||||||
| Rare (n = 445) | 203 | 45.6 | 1.16 | 0.93 to 1.44 | 1.19 | 0.95 to 1.50 | |||
| Average (n = 1183) | 495 | 41.8 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 484) | 189 | 39.0 | 0.88 | 0.71 to 1.10 | P= 0.044 | 0.83 | 0.66 to 1.04 | P= 0.011 | |
|
| |||||||||
| Age 18–44 years | |||||||||
| Rare (n = 63) | 26 | 41.3 | 0.80 | 0.44 to 1.45 | 0.86 | 0.46 to 1.62 | |||
| Average (n = 192) | 89 | 46.4 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 44) | 15 | 34.1 | 0.61 | 0.31 to 1.19 | P= 0.522 | 0.56 | 0.27 to 1.16 | P= 0.334 | |
|
| |||||||||
| Age 45–54 years | |||||||||
| Rare (n = 121) | 56 | 46.3 | 0.81 | 0.52 to 1.25 | 0.90 | 0.55 to 1.46 | |||
| Average (n = 295) | 149 | 50.5 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 69) | 26 | 37.7 | 0.56 | 0.33 to 0.95 | P= 0.233 | 0.55 | 0.27 to 1.16 | P= 0.144 | |
|
| |||||||||
| Age 55–64 years | |||||||||
| Rare (n = 227) | 102 | 44.9 | 1.12 | 0.82 to 1.53 | 1.20 | 0.87 to 1.65 | |||
| Average (n = 525) | 218 | 41.5 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 166) | 68 | 41.0 | 0.96 | 0.66 to 1.40 | P= 0.474 | 0.82 | 0.55 to 1.23 | P= 0.104 | |
|
| |||||||||
| Age 65–74 years | |||||||||
| Rare (n = 229) | 116 | 50.7 | 1.34 | 0.98 to 1.82 | 1.39 | 1.00 to 1.93 | |||
| Average (n = 640) | 278 | 43.4 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 334) | 144 | 43.1 | 1.00 | 0.76 to 1.31 | P= 0.091 | 0.97 | 0.73 to 1.29 | P= 0.057 | |
|
| |||||||||
| Age 75–84 years | |||||||||
| Rare (n = 87) | 50 | 57.5 | 1.33 | 0.82 to 2.16 | 1.39 | 0.83 to 2.34 | |||
| Average (n = 454) | 231 | 50.9 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 301) | 129 | 42.9 | 0.72 | 0.54 to 0.96 | P<0.05 | 0.69 | 0.50 to 0.94 | P<0.05 | |
|
| |||||||||
| Age ≥85 years | |||||||||
| Rare (n = 21) | 11 | 52.4 | 0.78 | 0.31 to 1.96 | 0.49 | 0.18 to 1.32 | |||
| Average (n = 113) | 66 | 58.4 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 104) | 52 | 50.0 | 0.71 | 0.41 to 1.24 | P= 0.841 | 0.63 | 0.34 to 1.18 | P= 0.618 | |
|
| |||||||||
| No comorbidity | |||||||||
| Rare (n = 665) | 318 | 47.8 | 1.07 | 0.89 to 1.28 | 1.15 | 0.95 to 1.40 | |||
| Average (n = 1803) | 830 | 46.0 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 654) | 283 | 43.3 | 0.89 | 0.74 to 1.07 | P= 0.101 | 0.79 | 0.65 to 0.96 | P<0.01 | |
|
| |||||||||
| Moderate comorbidity | |||||||||
| Rare (n = 72) | 37 | 51.4 | 1.09 | 0.67 to 1.80 | 1.31 | 0.75 to 2.27 | |||
| Average (n = 361) | 173 | 47.9 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 292) | 123 | 42.1 | 0.79 | 0.58 to 1.08 | P= 0.213 | 0.80 | 0.58 to 1.11 | P= 0.093 | |
|
| |||||||||
| High comorbidity | |||||||||
| Rare (n = 11) | 6 | 54.5 | 1.07 | 0.23 to 4.04 | 2.34 | 0.24 to 22.98 | |||
| Average (n = 55) | 28 | 50.9 | 1.00 | ref | 1.00 | ref | |||
| Frequent (n = 72) | 28 | 38.9 | 0.57 | 0.28 to 1.17 | P= 0.342 | 0.49 | 0.21 to 1.17 | P= 0.168 | |
Usual consultation frequency defined according to the patient’s number of face-to-face contacts in the 19–36 months before the cancer diagnosis.
Adjusted for sex, age group, diagnosis, comorbidity, disposable household income, and educational level. Estimates marked by bold were statistically significant at P≤0.05.
Test for linear trend across categories. ref = reference
A significant decreasing trend in ORs for cancer suspicion was observed across usual consultation categories overall (P-trend <0.001) and for each sex (males: P-trend <0.05; females: P-trend <0.05) (Table 2). The likelihood of the GP to suspect cancer in males was 1.19 (95% confidence intervals (CI) = 0.95 to 1.50) times higher in rare attenders and 0.83 (95% CI = 0.66 to 1.04) times lower in frequent attenders compared with average attenders (Table 2). The corresponding ORs in females were 1.03 (95% CI = 0.77 to 1.39) times higher in rare attenders and 0.72 (95% CI = 0.57 to 0.91) times lower in frequent attenders (Table 2).
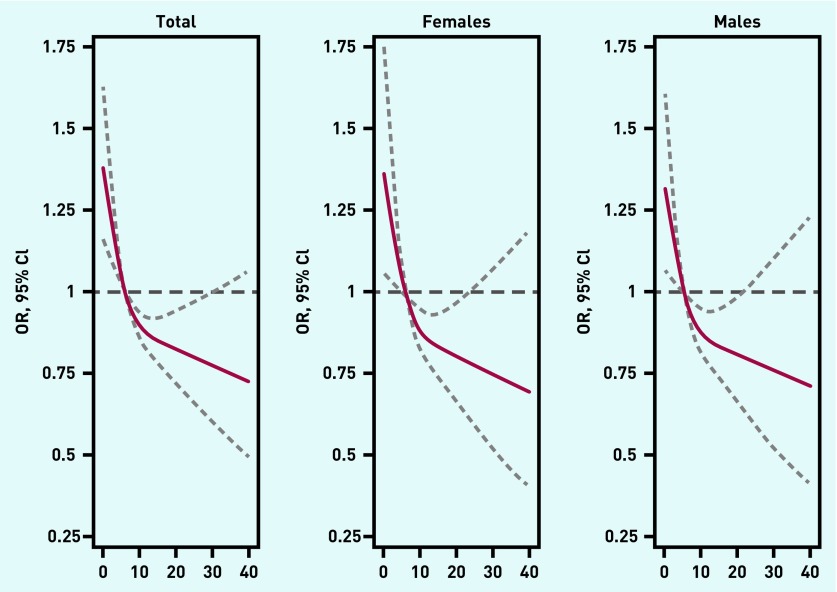
The association between usual consultation frequency and the GP’s cancer suspicion displayed a hyperbolic relationship when consultation frequency was treated as a continuous variable (Figure 2). Statistically significant higher odds for the GP to suspect cancer were seen in patients who consulted less frequently compared with the median number of consultations. The more frequently the patient consulted the GP, the more decreasing odds were observed; meaning that the odds of the GP suspecting cancer reduced with the more times the patient consulted; this was seen overall and for both sexes (Figure 2).
Figure 2.
Association between the GP’s suspicion of cancer (y-axis) and the patient’s usual consultation frequency (defined according to the patient’s number of face-to-face contacts in the 19–36 months before cancer diagnosis) (x-axis) for all cases and by sex as estimated using cubic spline models with three knots (reference = median number of usual consultations). The solid lines in figures depict estimated odds ratio, dashed lines depict 95% confidence limits.
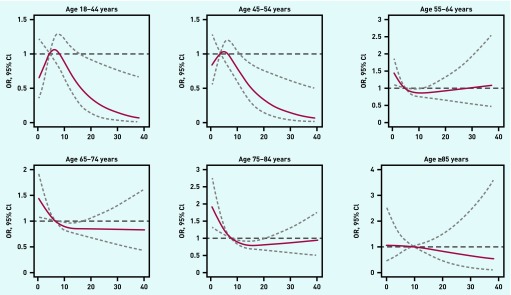
The association between the patient’s usual consultation frequency and the GP’s cancer suspicion showed different patterns across age groups (Figure 3). In patients aged 18–44 and 45–54 years, GPs suspected cancer less often in patients with both below and above the median number of consultations, although this was not statistically significant (Figure 3). The association for the three age groups of 55–84 years was similar to the overall analysis, and no association was seen among patients aged >85 years.
Figure 3.
Association between the GP’s suspicion of cancer (y-axis) and the patient’s usual consultation frequency (defined according to the patient’s number of face-to-face contacts in the 19–36 months before the cancer diagnosis) (x-axis) for six age groups as estimated using cubic spline models with three knots (reference = median number of usual consultations). The solid lines in figures depict estimated odds ratio, dashed lines depict 95% confidence limits.
The association between the patient’s usual consultation frequency and the GP’s cancer suspicion stratified by comorbidity, educational level, and income showed similar patterns for all subgroups as those seen in the overall analysis (data not shown).
DISCUSSION
Summary
This study of nearly 4000 patients with cancer shows that GPs were generally more likely to suspect cancer in rare consulters; the more infrequently the patient usually consulted the GP, the higher the GP’s suspicion of cancer. However, at the same time, GPs were less inclined to suspect cancer in patients aged <55 years. Thus, the relationship between the patient’s usual consultation frequency and the GP’s cancer suspicion is modified by the patient’s age. Interestingly, younger patients had a lower likelihood of being suspected of cancer if they were either rare or frequent attenders.
Strengths and limitations
The strengths of this study include a well-defined study population with minimal selection bias, as all cases were identified through the Danish National Patient Register, and the high response rate of 74%.14,20,21 In fact, the cohort population is similar to patients in the Danish Cancer Registry,14 which indicates minimal selection bias.
Another major strength was that the consultation frequency used in this study reflects the true usual GP consultation pattern among patients with cancer in Denmark as their consultation rates are comparable to sex-and age-matched comparison subjects without cancer.7 Using the period 19–36 months before diagnosis seems to be a reasonable way of categorising a cancer patient’s usual consultation frequency, although this interval could be different for other diseases.
The most important limitation of this study is the risk of information bias caused by GP recall bias when filling in the questionnaire. Still, the risk of recall bias was reduced as GPs consulted their continuously updated electronic medical records while filling in the questionnaire. However, the retrospective nature of the questionnaire may mean that some of the GPs could have misinterpreted the symptoms of a particular case and have overestimated the proportion of cases with ‘alarm’ symptoms. However, this seems unlikely because the proportion of patients with ‘alarm’ symptoms in this study is similar to the proportion of patients reported in other studies.22,23
Although 3985 cancer cases were analysed, the lack of statistical significance in some of the sub-analyses may be related to the relatively smaller sample sizes in the sub-groups.
Comparison with existing literature
Direct comparison with other large studies into the association between low usual consultation frequency in general practice and suspicion of cancer by the GP is not possible because of the absence of similar studies. The only study the authors were able to identify was a German study that reported contrasting results, but only 5% of the cancer cases in that study were suspected to have cancer.24 Qualitative studies report that GPs are more likely to suspect serious illness in patients who rarely consult, although the evidence is equivocal.11–13 The current study adds to these findings because it suggests that GPs’ ‘alertness’ towards serious disease is generally higher if the patient rarely consults the GP. However, the premise that GPs are always more alert when seeing rare consulters is not in line with the results of the current study, which found lower cancer suspicion among GPs when the patients were younger than 55 years of age.
A lower level of cancer suspicion among GPs is the main contributor to a prolonged time to cancer diagnosis.10 One reason could be that the GP ascribes a patient’s symptoms to comorbidities.12 This could partly explain the longer time to diagnosis in patients with colorectal cancer who had comorbidities reported in a UK study.25 However, there were no differences in the GPs’ suspicion of cancer between different levels of comorbidity in the current study. Among the 55–84-year-old patients in the current study, a higher likelihood of GP cancer suspicion was also observed in frequent attenders, which could indicate that the GPs use age as an important risk factor for cancer in their clinical triage.
Implications for practice
Clinically and epidemiologically, GPs’ lower suspicion of cancer among the youngest rare attenders may be explained by the low incidence of cancer in people aged <40 years,26 which makes a cancer diagnosis highly unlikely in this age group. This is also exemplified by a more than two-fold higher likelihood for young patients to have multiple contacts before diagnosis.27 However, as the incidence of cancer rises rapidly in patients aged >40 years,26 the low incidence cannot explain the unexpected finding of a lower likelihood for the GP to suspect cancer in both rare and frequent attenders than in average attenders aged <55 years. In fact, 16% of all tumours in Denmark are diagnosed in patients <55 years.26 This indicates that some diagnoses could be missed initially as some of the contacts from younger patients might be attributed to other medical conditions than cancer. Consequently, although relatively uncommon in patients aged <55 years, cancer remains a potential diagnosis whenever a patient consults the GP. This stresses the importance of safety netting in general practice to ensure reliable follow-up of patients with persisting symptoms.28
It has been suggested that the organisation of general practice with its gatekeeping function and fixed patient lists frames the clinical encounter in general practice and potentially restricts care seeking.29 It has been argued that the current structure of the clinical encounter in general practice, which is characterised by strict time limitations and focus on only one medical complaint at a time, does not encourage all patient groups to seek general practice for health problems.30,31 Some patients may thus be discouraged from seeking care on a regular basis, which questions the accessibility of general practice. European patients generally assess the accessibility of general practice/GPs as low.32,33 Moreover, recent evidence indicates that the dissatisfaction with accessibility in general practice is lower than it used to be, at least among patients with cancer.34 The results of this study thus underpin the need for general practice to focus on accessibility.
It is well established that recognition and classification of symptoms by GPs is influenced by both the dominating medical discourses and the different social contexts in which the negotiation of the sick role takes place.35,36 Hence, it could be argued that patients who do not frequently contact general practice are less familiar with general practice as a cultural and organisational site of interaction. These patients may thus engage in the clinical encounter in a way that does not bring the cancer symptoms into the spotlight.
The results from this study indicate that GPs do react with more cancer suspicion to rare attenders. However, in younger patients, the likelihood of suspicion was low in both rare and frequent attenders. GPs should thus engage with rare attenders to help inexperienced patients present the complexity of their medical problems and worries in the GP setting. Additionally, GPs should be aware of the risk of possible missed opportunities for cancer diagnosis in young frequent attenders to ensure that a reliable safety net is set up for this group of patients.
Funding
This study was funded by the Danish Cancer Society (file no. R130-A8212-15-S38) and the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation, and administered by the Danish Regions. The funders did not have any influence on the study.
Ethical approval
The study was approved by the Danish Data Protection Agency (reference no. 2009-41-3471). According to Danish law, the study did not require approval from the Committee on Health Research Ethics of the Central Denmark Region as no biomedical intervention was performed. The data that support the findings of this study are stored and maintained electronically at Statistics Denmark and are not publicly available.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231–1272. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 2.Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians: a national Danish project. Health Policy. 2012;105(1):65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Emery J, Vedsted P. New NICE guidance on diagnosing cancer in general practice. Br J Gen Pract. 2015 doi: 10.3399/bjgp15X686401. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal RD, Din NU, Hamilton W, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110(3):584–592. doi: 10.1038/bjc.2013.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen H, Tørring ML, Olesen F, et al. Diagnostic intervals before and after implementation of cancer patient pathways — a GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer. 2015;15:308. doi: 10.1186/s12885-015-1317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen H, Vedsted P, Møller H. Prognosis of cancer in persons with infrequent consultations in general practice: a population-based cohort study. Int J Cancer. 2017;141(12):2400–2409. doi: 10.1002/ijc.30916. [DOI] [PubMed] [Google Scholar]
- 8.Sheringham J. Not visiting the GP and the risk of cancer: what are the possible implications for research, policy and practice? Int J Cancer. 2017;141(12):2378–2378. doi: 10.1002/ijc.31033. [DOI] [PubMed] [Google Scholar]
- 9.Ringborg U, Henriksson R, Friberg S, et al. Kræftsygdomme — onkologi. 1st edn. Copenhagen: FADL; 2004. [Cancer diseases — oncology] [Google Scholar]
- 10.Jensen H, Tørring ML, Olesen F, et al. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14:636. doi: 10.1186/1471-2407-14-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen ML, Holtedahl KA, Rudebeck CE. How does the thought of cancer arise in a general practice consultation? Interviews with GPs. Scand J Prim Health Care. 2012;30(3):135–140. doi: 10.3109/02813432.2012.688701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridd M, Shaw A, Salisbury C. ‘Two sides of the coin’: the value of personal continuity to GPs: a qualitative interview study. Fam Pract. 2006;23(4):461–468. doi: 10.1093/fampra/cml010. [DOI] [PubMed] [Google Scholar]
- 13.Hjortdahl P. The influence of general practitioners’ knowledge about their patients on the clinical decision-making process. Scand J Prim Health Care. 1992;10(4):290–294. doi: 10.3109/02813439209014076. [DOI] [PubMed] [Google Scholar]
- 14.Jensen H, Tørring ML, Larsen MB, Vedsted P. Existing data sources for clinical epidemiology: Danish Cancer in Primary Care cohort. Clin Epidemiol. 2014;6:237–246. doi: 10.2147/CLEP.S62855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen KM, Andersen JS, Søndergaard J. General practice and primary health care in Denmark. J Am Board Fam Med. 2012;25(Suppl 1):S34–S38. doi: 10.3122/jabfm.2012.02.110216. [DOI] [PubMed] [Google Scholar]
- 17.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 18.United Nations Educational, Scientific and Cultural Organization International Standard Classification of Education. ISCED. 2011. 2012. http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf (accessed 7 Jan 2019).
- 19.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 20.Larsen MB, Jensen H, Hansen RP, et al. Identification of patients with incident cancers using administrative registry data. Dan Med J. 2014;61(2):A4777. [PubMed] [Google Scholar]
- 21.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 22.Ingebrigtsen SG, Scheel BI, Hart B, et al. Frequency of ‘warning signs of cancer’ in Norwegian general practice, with prospective recording of subsequent cancer. Fam Pract. 2013;30(2):153–160. doi: 10.1093/fampra/cms065. [DOI] [PubMed] [Google Scholar]
- 23.Scheel BI, Ingebrigtsen SG, Thorsen T, Holtedahl K. Cancer suspicion in general practice: the role of symptoms and patient characteristics, and their association with subsequent cancer. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X671614. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostev K, Meister U, Kalder M, Jacob L. Suspected cancer diagnoses made by general practitioners in a population with subsequently confirmed cancer diagnoses in Germany: a retrospective study of 31,628 patients. Oncotarget. 2017;8(48):84540–84545. doi: 10.18632/oncotarget.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mounce LTA, Price S, Valderas JM, Hamilton W. Comorbid conditions delay diagnosis of colorectal cancer: a cohort study using electronic primary care records. Br J Cancer. 2017;116(12):1536–1543. doi: 10.1038/bjc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engholm G, Ferlay J, Christensen N, et al. NORDCAN: a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 27.Mendonca SC, Abel GA, Lyratzopoulos G. Pre-referral GP consultations in patients subsequently diagnosed with rarer cancers: a study of patient-reported data. Br J Gen Pract. 2016 doi: 10.3399/bjgp16X683977. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson BD, Goyder CR, Bankhead CR, et al. Responsibility for follow-up during the diagnostic process in primary care: a secondary analysis of International Cancer Benchmarking Partnership data. Br J Gen Pract. 2018 doi: 10.3399/bjgp18X695813. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen RS, Vedsted P, Olesen F, et al. Does the organizational structure of health care systems influence care-seeking decisions? A qualitative analysis of Danish cancer patients’ reflections on care-seeking. Scand J Prim Health Care. 2011;29(3):144–149. doi: 10.3109/02813432.2011.585799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen RS, Tørring ML, Vedsted P. Global health care-seeking discourses facing local clinical realities: exploring the case of cancer. Med Anthropol Q. 2015;29(2):237–255. doi: 10.1111/maq.12148. [DOI] [PubMed] [Google Scholar]
- 31.Merrild CH, Risør MB, Vedsted P, Andersen RS. Class, social suffering, and health consumerism. Med Anthropol. 2016;35(6):517–528. doi: 10.1080/01459740.2015.1102248. [DOI] [PubMed] [Google Scholar]
- 32.Heje HN, Vedsted P, Sokolowski I, Olesen F. Patient characteristics associated with differences in patients’ evaluation of their general practitioner. BMC Health Serv Res. 2008;8:178. doi: 10.1186/1472-6963-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grol R, Wensing M, Mainz J, et al. Patients in Europe evaluate general practice care: an international comparison. Br J Gen Pract. 2000;50(460):882–887. [PMC free article] [PubMed] [Google Scholar]
- 34.Dahl TL, Vedsted P, Jensen H. The effect of standardised cancer pathways on Danish cancer patients’ dissatisfaction with waiting time. Dan Med J. 2017;64(1):A5322. [PubMed] [Google Scholar]
- 35.Shilling C. Culture, the ‘sick role’ and the consumption of health. Br J Sociol. 2002;53(4):621–638. doi: 10.1080/0007131022000021515. [DOI] [PubMed] [Google Scholar]
- 36.Mik-Meyer N, Obling AR. The negotiation of the sick role: general practitioners’ classification of patients with medically unexplained symptoms. Sociol Health Illn. 2012;34(7):1025–1038. doi: 10.1111/j.1467-9566.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]