Abstract
Background
A growing demand for cancer genetic services has led to suggestions for the involvement of GPs. How, and in which conditions, they can be involved, and whether there are important barriers to implementation should be ascertained.
Aim
To review the tools available, clinician attitudes and experiences, and the effects on patients of genetic cancer risk assessment in general practice.
Design and setting
Systematic review of papers published worldwide between 1996 and 2017.
Method
The MEDLINE (via Ovid), EMBASE, Cochrane Library, CINAHL, and PsycINFO databases and grey literature were searched for entries dating from January 1996 to December 2017. Study quality was assessed with relevant Critical Appraisal Skills Programme tool checklists and a narrative synthesis of findings was conducted.
Results
In total, 40 studies were included in the review. A variety of testing and screening tools were available for genetic cancer risk assessment in general practice, principally for breast, breast–ovarian, and colorectal cancer risk. GPs often reported low knowledge and confidence to engage with genetic cancer risk assessment; however, despite time pressures and concerns about confidentiality and the impact of results on family members, some recognised the potential importance relating to such a development of the GP’s role. Studies found few reported benefits for patients. Concerns about negative impacts on patient anxiety and cancer worries were largely not borne out.
Conclusion
GPs may have a potential role in identifying patients at risk of hereditary cancer that can be facilitated by family-history tools. There is currently insufficient evidence to support the implementation of population-wide screening for genetic cancer risk, especially given the competing demands of general practice.
Keywords: cancer risk assessment, clinician’s attitude, genetic counselling, general practice, systematic review, tool, psychology
INTRODUCTION
According to the World Health Organization, one in six deaths are due to cancer and the number of new cases are expected to rise by 70% over the next two decades.1 In the UK, 5% of patients with bowel cancer have a family history of bowel cancer, 3% of breast cancers are associated with inherited faulty genes, and 10% of melanoma cases are also associated with a family history of the disease.1 In those cases, and other cancers in which genetic risks are involved, earlier detection and treatment could reduce cancer mortality.
There is an increasing demand for cancer genetic services and the potential importance of involving GPs is recognised.2 Patients commonly seek out information regarding their risks and clinicians need to be able to respond to this demand. Direct-to-consumer testing is also increasingly available.3 In addition to this, there are also potential opportunities for systematically or opportunistically screening attendees in general practice, perhaps based on increased familial risk.
The operational definitions of screening and testing are as follows:
screening — aims to identify individuals from asymptomatic groups (those at risk or whole populations) with abnormalities suggestive of a specific cancer or pre-cancer and refer them promptly for diagnosis and treatment;3 and
testing — undertaking tests in response to presentation by patient about symptoms or concerns about risk status.
However, the ways in which GPs might respond to such trends — particularly within the context of everyday practice that is increasingly time pressured and resource constrained — have not been effectively established.4
Family medical history is commonly used in general practice and could be regarded as a genetic screening strategy,5,6 but its use needs to be developed and standardised to optimise health outcomes for those at risk of hereditary cancer. GPs are potentially well placed to recognise individuals at risk, as they have access to longitudinal comprehensive health records and focus on family.7,8 In the UK NHS, a patient is eligible for a genetic test if an inherited faulty gene has already been identified in one of the patient’s relatives or there is a strong family history of cancer. In these scenarios, patients are referred to specialist genetics services (currently 33 across the UK) for consideration of further genetics tests.
Carroll et al suggest that GPs have a potential role as gatekeepers in genetic cancer risk assessment (testing and screening).9 However, GPs may face challenges regarding this expanding role due to a lack of clinical genetics knowledge, perceived lack of confidence in the domain, and time constraints.3,10–15 There may be difficulties in considering genetic cancer risk in routine primary care visits, especially as acute illness is often the priority, and other (for example, cardiovascular) preventive measures have greater prominence than genetic risk of cancer. Testing or screening, leading to preventive measures, is likely to be more successful if cancer genetic risk is assessed in large segments of society and not only in those who are better informed and actively consult their GP.
How this fits in
Cancer incidence is rising across the world and genetic risk is a significant contributor. Cancer risk screening and testing is a potential task to be undertaken by GPs. Several tools are available (although none was found to be superior), but GPs have identified the need for more education to improve their knowledge and confidence regarding cancer genetic risks before wider implementation.
This study aimed to examine and review the tools available, as well as clinicians’ attitudes and the effects on patients of genetic cancer risk assessment, in general practice. From this, the authors aimed also to discuss potential roles that GPs might play in genetic cancer risk assessment, and whether systematic screening may be feasible and effective in general practice.
To meet the specified aims, the following research questions were addressed:
What tests (medical procedure to detect those at high risk) and tools (support or format for those procedures) are available to identify increased genetic risk of cancer in general practice?
What are clinicians’ attitudes towards screening or testing population groups for genetic cancer risk?
What are the levels of patient knowledge, satisfaction, and anxiety in relation to tests and communication by a GP about cancer risk?
What are patients’ risk perceptions following screening or testing for genetic cancer risk in primary care?
What are the outcomes of referrals to secondary care following genetic cancer risk identification in general practice?
METHOD
Literature search
The MEDLINE (via Ovid), EMBASE, Cochrane Library, CINAHL, and PsycINFO databases were electronically searched for entries dating from January 1996 to December 2017. The grey literature was also searched via OpenGrey and the Health Management Information Consortium database (also to December 2017). The search strategy (available from the authors on request) was adapted to each database, with layers of terms around general practice, cancer, genetics, testing and tools, attitudes, outcomes, and effectiveness. Hand searching of key journals (for example, Family Practice, Genetics in Medicine, and British Journal of General Practice) and reference lists of relevant papers was also conducted. The search outputs were downloaded and merged into Zotero software (http://www.zotero.org), and duplicates were removed.
Study selection
Inclusion criteria were set to include studies involving adults (aged ≥18 years) of either sex, who were considered to be at high risk of hereditary cancer. As advocated by Scheuner et al,16 high-risk family-history characteristics include the presence of multiple affected first-degree relatives (FDRs) or an FDR with age of onset of ≤50 years. Studies were then included according to intervention or outcome variables, as follows:
intervention — strategies used for cancer genetic risk testing or screening within general practice. As suggested by Olesen et al,17 general practice (known as family practice in some countries) was defined as care involving: ‘the general practitioner [who] is a specialist trained to work in the front line of a healthcare system and to take the initial steps to provide care for any health problem(s) that patients may have’; or
outcome variables — clinician attitudes to tests for cancer genetic risk assessment, patient outcomes following such tests, or the outcomes of referrals to secondary care after the intervention in primary care.
A range of study designs — observational, cross-sectional, cluster randomised controlled trials (RCTs), implementation studies, or qualitative studies involving focus groups or semi-structured interviews — was included to address the different review questions.
Exclusion criteria were as follows:
study not based primarily in general practice or separate data relating to GPs not presented;
non-cancerous conditions only;
cancers without a known familial component;
description of test activity (that is, number of tests undertaken) only;
population-based screening application only (not involving primary care); and
non-English-language studies.
The authors wanted to investigate scenarios involving either the identification of patients at high risk of hereditary cancer in opportunistic health visits with their GP, or potential broad systematic or opportunistic screening of patient populations in general practice to identify those at a high genetic cancer risk.
The selection criteria were initially applied independently to all titles and/or abstracts by two authors. Once narrowed down to references that were potentially relevant, full-copy papers were assessed by a third reviewer to determine inclusion and exclusion. Disagreements were resolved through discussion.
Data extraction
Two reviewers extracted all data into an Excel spreadsheet, recording the study title, aims, design, setting, participants, inclusion and exclusion criteria, nature of intervention (where applicable), methods, outcome measures, analysis, key findings, and limitations.
Assessment of methodological quality
The quality of all eligible studies was assessed using the relevant Critical Appraisal Skills Programme (CASP) tool checklists18 for qualitative studies and trials. As there is no CASP tool for cross-sectional studies, common points included in the checklists for observational cohort and case–control study designs were selected and combined.
Data synthesis
Due to the heterogeneous nature of the studies included, a narrative synthesis was undertaken to collate the evidence relating to each of the research questions, as proposed by Pope et al.19 Specific subgroups of studies were assessed and are presented regarding testing and screening for genetic cancer risk.
RESULTS
Description of studies
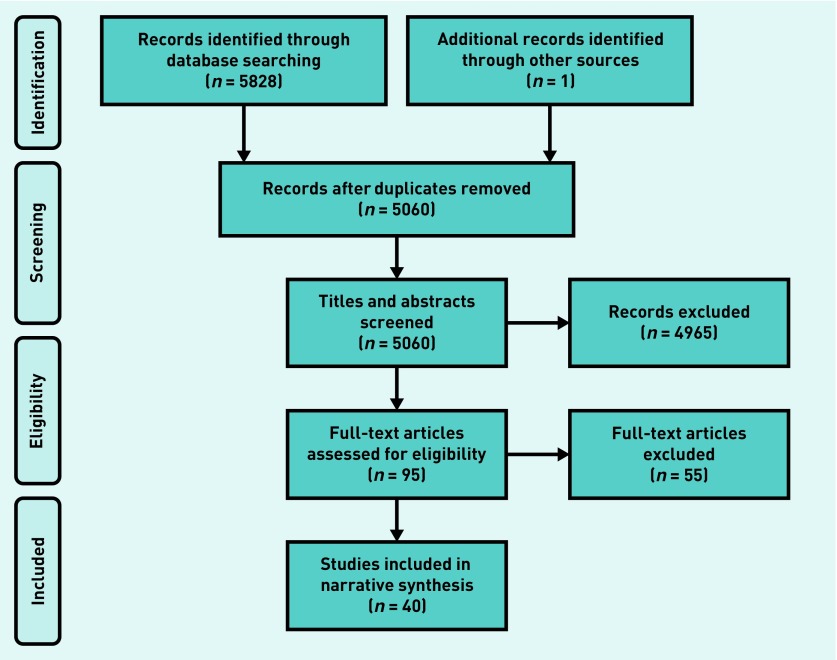
The study selection process is summarised in Figure 1. In total, 40 articles were included in the review. Fourteen of these were cross-sectional studies,2,20–32 two were retrospective studies,15,33 and six were qualitative4,9,34–37 (of which four involved semi-structured interviews4,34,35,37). There were 13 intervention studies,38–50 comprising three validation studies,38,47,50 one before–after study,49 three hybrid implementation studies,42,45,48 one comparison against standard care,40 two comparative studies,41,44 and three observational studies.39,43,46 Three studies were cluster RCTs51–53 and two were descriptive feasibility studies.54,55
Figure 1.
Flowchart of study selection process.
Populations studied
All studies involved both male and female patients or their GPs, except for one,35 which comprised only female patients and female practitioners. Fourteen studies were carried out in the UK,2,4,20–22,25,34,41,44,49–51,54,55 17 in North America,9,15,23,27–31,35,37,39,42,43,45,48,52,53 two in South America,38,47 and two in Australia.33,36 The remaining four were conducted in the European Union26,32,40,46 (the Netherlands, Belgium, and Spain), and one study reported data from four countries across Europe,24 namely the UK, France, Germany, and the Netherlands.
Methodological quality
Study details are available from the authors on request. The included studies were generally well designed and reported. Recruitment of participants was suitable, and methods and analyses were described clearly. Studies varied in the generalisability of their findings to populations beyond those studied.
Screening
Screening method
A variety of tools that could be used in general practice for screening genetic cancer risk was desc ribed.15,29,30,38,39,42–45,47–50,52–55 Examples of family-history collection tools included Family Healthware,52,53 a self-administered web-based tool, and the seven-item Family History Screen (FHS-7) tool,38 both of which cover family history of breast, ovarian, and colorectal cancer. MeTree — a computerised tool stratifying risk of hereditary cancer syndromes (that is, breast, ovarian, and colorectal cancer) to be completed at routine visits and to support clinical decisions — was used in three studies.42,45,48 Two studies examined an office screening form for familial breast cancer alone,29,30 whereas one, by Walter et al,50 developed a family-history questionnaire assessing breast and colorectal cancer to be completed at a planned data-collection session in the general practice surgery.
In 2013, the US Preventive Services Task Force updated its recommendations and recognised the Ontario Family History Assessment Tool, Manchester Scoring System, Breast Cancer Genetics Referral Screening Tool, Pedigree Assessment Tool, and FHS-7 as suitable for primary care providers to screen women and suggest testing for BRCA1 or BRCA2 genes.56
The Gail risk model provides the basis for a questionnaire implemented by Owens et al, and which identifies patients deemed to be at high risk of developing breast cancer.43 Four studies described simple postal questionnaires,44,49,54,55 with Leggatt et al 49,54 screening for genetic risk assessment of breast and colorectal cancer, House et al 55 identifying those at risk of colorectal cancer alone, and Qureshi et al 44 collecting non-specific cancer family-history information. Biswas et al 15 developed and tested a two-stage approach with three simplified versions of the BRCAPRO model and software — which assesses the probability that an individual carries a germline deleterious mutation of the BRCA1 and BRCA2 genes, based on family history of breast and ovarian cancer — to reduce the genetic counselling burden in general practice. Flória-Santos et al described self-reported cancer family history as a tool to detect breast, prostate, and colon cancer — and potentially also useful to screen other hereditary cancer syndromes.47
Clinicians’ attitudes
Of the five studies examining GP attitudes, three addressed attitudes towards the process of screening patients for inherited cancer risk in general27,28,37 and two reported attitudes towards specific screening tools.43,45 Gramling et al 28 reported that 87% of 300 GPs who were surveyed agreed that screening patients for inherited cancer risk was important to their practice, but only 62% were confident in their own screening effectiveness. Caroll et al 37 showed that primary care providers are prepared to discuss personalised medicine. A further study by Gramling et al,27 conducted with a small sample of US family physicians, showed that the importance physicians placed on screening was positively related to their beliefs that a high-risk genetic test result would motivate behaviour change in patients; the methods for screening in question were not described.27,28
In contrast, Owens et al 43 discussed that some providers were concerned with the accuracy of the Gail model formula in identifying patients at high risk of hereditary cancer. Furthermore, there were concerns over the time needed to counsel patients who were newly determined to be at high risk, and concerns regarding liability for not successfully providing risk counselling.
Wu et al 45 showed that physicians at two primary care clinics initially felt that they were already collecting high-quality family histories and that MeTree would negatively impact their workflow. They believed that patients would redirect discussions away from physician priorities and, instead, focus on MeTree recommendations. However, post MeTree integration, 86% of physicians believed that the tool improved the way they practised medicine, thereby making practice easier, and none reported that it adversely affected their workflow.45
Box 1 summarises clinicians’ attitudes to screening for genetic cancer risk.
Box 1. Clinicians’ attitudes to screening for genetic cancer risk.
| Domain | Attitude |
|---|---|
| Role of GP |
|
| Knowledge and ability |
|
| Impact on patient | – |
| Restriction in practice |
|
Patient outcomes
Six studies assessed patient outcomes following the various methods of screening for genetic cancer risk.29,30,39,45,49,53 There was some evidence that screening can lead to higher accuracy for risk perceptions with risk feedback following an office screening form; there were greater odds of a patient correctly rating their breast cancer risk as ‘high’ in those who had an FDR with breast cancer.30 Wang et al 53 found that, in comparison with patients in the control group, those who underestimated their risk and who were screened using the Family Healthware tool increased their perceptions of colon cancer risk, but not that relating to breast or ovarian cancer. Baer et al 39 found that, compared with the control group, a higher percentage of patients who had been screened via Your Health Snapshot reported their perceived risk of colon cancer to be above average — and possibly incorrectly. Wu et al 45 found that 85% of 1184 patients believed MeTree generally raised their awareness of both their personal and family health risk, changing the way they think about health.
Gramling et al 29 also showed that risk feedback following screening was associated with lower perceived severity of breast cancer, but not with the perceived likelihood of developing breast cancer in the future.29 They also found that patients who had recently undertaken family medical history screening were less likely to be worried about developing breast cancer than those who had had no screening. This association was present even in those at high risk, although it was stronger for women with a lower-risk family history. In contrast, Leggatt et al found that completing a screening questionnaire and receiving an assessment of high genetic risk had no significant impact on general anxiety and cancer worries.49
Outcomes of referrals
Only one study — that by Rubinstein et al 52 — assessed the effectiveness of referrals following screening for genetic cancer risk. They found that, in those at high risk of hereditary cancer, consultation rates with genetic specialists did not differ between the group that completed Family Healthware and the control group. Furthermore, both groups equally increased their adherence to risk-based colon cancer screening and mammography schedules.
Testing
Available tests and tools
Eight tools were described that could be used in general practice for assessing a genetic risk of cancer. These tools all incorporated family history into their assessment, and some included further decision support and recommendations.
Studies also used the Gail model,43 MeTree,42,45,48 and FHS-738 for testing. The Genetic Risk Assessment in an Intranet and Decision Support (GRAIDS) software provides risk estimates of breast, ovarian, and colorectal cancer; this was used in two studies.20,51 Risk Assessment in Genetics (RAGs), which addresses familial breast and ovarian cancer, was used in two studies,34,41 whereas Your Health Snapshot,39 which calculates inherited susceptibility to colon, lung, breast, and prostate cancer, was used in one study. The set of GP guidelines by de Bock et al 40 assesses breast cancer risk and Qureshi et al ’s Family History Questionnaire44 identifies the presence of relatives with cancer in general.
In relation to genomic tests, four studies reported testing for inherited susceptibility to breast cancer,23,24,26,31 one study included ovarian cancer,31 and one study related to predictive testing more broadly.9 The remaining studies referred to the use of a standard family history for identifying individuals at risk of hereditary breast cancer21,22,25,29,43 and non-specific cancer.2,4,23
Clinician attitudes
A range of GPs’ views regarding genetic cancer risk assessment and testing was evident. Overall, GPs considered undertaking genetic risk assessment to be a potentially important role for them,2,9,24–26,37 but the extent to which they believed they should be involved with genetics varied. Genetic counselling of patients with regard to their risk and making management decisions was thought to be ‘not always appropriate’ for GPs, whereas providing emotional support following testing was acknowledged to be part of their job.2,21,23,25,26
GPs admitted that they found assessing genetic risk difficult34,37 and, consequently, felt uncomfortable when doing so because of their lack of knowledge.2,4,22 For instance, Hapgood et al 22 showed that 89.5% of GPs included in their study incorrectly categorised a low-risk breast cancer family history as either moderate (52.9%) or high (36.6%) risk. GPs also lacked confidence in their ability to interpret genetic test results and explain them to patients.2,4,21,22,34 Furthermore, inadequate skills in taking an appropriate family history were highlighted, with GPs often failing to get sufficient information from patients to appropriately assess their risk of developing hereditary cancer.4,23,24 Statistically significant proportions of GPs were unfamiliar with their local cancer genetics guidelines and knew little of the services that were available to them.25,34
From the studies included, it appeared that clinicians were commonly also not confident in discussing the benefits, risks, and limitations of genetic testing with patients.2,21,37 They were concerned by the unnecessary anxiety caused by the process of genetic testing itself, as well as patients receiving a result indicating increased risk.4,9,20,25,32 The belief that results of decreased risk would create a false sense of security was also expressed by some GPs. Another further theme that arose was about ethical implications and fears of legal repercussions after genetic tests;9,44 this particularly derived from concerns about confidentiality and how best to inform other family members of their risk when a positive result from testing was received.9
Overall, GPs expressed concern regarding the validity of genomic testing and its clinical utility. Time constraints were a further reason they gave for not being able to sufficiently counsel patients regarding the benefits and risks of genomic testing, or being able to interpret test results sufficiently.2,20,34,43,45 Some GPs believed that they needed education before exploring an expanded role,37 but studies conflicted regarding their intentions in seeking further education.4,21,31 Walter et al 25 reported that only one-third of practitioners had attended education about risk management for breast cancer in the previous 3 years.
Box 2 summarises the main findings regarding clinicians’ attitudes towards testing.
Box 2. Clinicians’ attitudes to testing for genetic cancer risk.
| Domain | Attitude |
|---|---|
| Role of GP |
|
| Knowledge and ability |
|
| Impact on patient |
|
| Restriction in practice |
|
Patient outcomes
Data about patient knowledge, satisfaction, and anxiety in relation to tests and risk communication were limited. For the GRAIDS software, there were no statistically significant differences in knowledge scores, but patients referred from intervention practices had statistically significantly lower cancer worry scores.51 There was also no difference in mean risk perception, though there was a statistically non-significant trend towards higher accuracy in risk perception, with fewer intervention patients overestimating their risk at the point of referral.51
Outcomes of referrals
There were few data evaluating the effectiveness of referrals to secondary care following the identification of high genetic risk of cancer in general practice. One study43 reported that, of the patients referred to the breast centre after a high-risk consultation, only half actually attended for their visits. A retrospective audit in Australia found that GPs referred the majority of patients to the genetics service and were also the most likely to refer inappropriately (compared with gynaecologists, for example).33
DISCUSSION
Summary
There are several tools available to GPs that can enable them to identify genetic risk of cancer; most of these involve a family-history component as an effective way of determining a patient’s risk. Regarding the questions to be addressed by this review, there was most evidence about clinicians’ attitudes to cancer genetics, whereby GPs consider undertaking the assessment of genetic risk to be a potentially important job for them. However, lack of confidence and knowledge may be reasons for their reluctance to undertake a role expanded beyond that of gatekeeper.
GPs were worried about the impact of genetic risk assessment on patient anxiety,32 particularly if discussions with whole families would then be required. Furthermore, their ability to adequately explain risk and its implications in short, routine appointments was raised as a concern. The results regarding patient outcomes show that there may be a link between genetic risk assessment in primary care and lower cancer worry in patients, but there were not enough data to accurately describe the relationship in the general practice setting.
Strengths and limitations
A comprehensive search strategy was developed for high recall (sensitivity), with a range of databases, grey literature, and hand searches of reference lists conducted. Studies of various designs were included in order to gather evidence that addressed all of the researchers’ questions. There was considerable heterogeneity in the results, making statistical analysis unfeasible and a narrative synthesis was conducted. The inclusion criteria were applied strictly, with a particular effort made only to include studies that specified results from general practice.
The main weakness of this literature review is the limited number of studies that were identified. The heterogeneity of outcomes reported adds further to difficulties in drawing conclusions. The authors recognise that the nature of primary care is likely to vary across the many countries in which the included studies were conducted. This is particularly the case in North America (concerning ‘family medicine’, which is the equivalent of general practice in Europe), from which almost half of the studies derived.
Knowledge about genetic cancer risk and referrals have dramatically changed over the 20-year period13 covered by those studies that were reviewed. It is also recognised that the studied population is a sub-population in primary care. Nevertheless, this review still highlights where evidence is lacking.
Comparison with existing literature
Many studies have shown that GPs lack confidence in their skills involving cancer genetics.2,3,57 The results presented here regarding clinicians’ attitudes towards cancer genetics are similar to those of Mathers et al,12 who reported GPs’ resistance to clinical genetics in general. They too showed that GPs believed genetic conditions required complex knowledge that should be covered by specialist services, as they were worried about the accuracy of their own knowledge. A review by Emery and Hayflick7 in 2001 identified family history as important and indicated that GPs needed to gain generic knowledge and skills to ascertain genetic cancer risk. The review presented here confirms the current evidence that clinicians’ confidence in their knowledge is usually suboptimal.
McClain et al 58 investigated six family-history screening protocols for breast and/or ovarian cancer by applying them to family histories taken from four cohorts of women in a variety of settings. They showed that each of these protocols used alone gave too many screen-positive results, but when all six protocols agreed there was a more acceptable screen-positive rate. Similarly, if some of the genetic cancer risk screening tools identified in this review were compared directly, a singular composite screening tool including key items could potentially be identified; that is, different tools can be used to extract key items.
Implications for research and practice
Advances in genetic medicine are expected to lead to a shift towards general practice being more involved with the provision of genetic services. GPs are potentially important in identifying patients at increased risk of hereditary cancers to ensure suitable subsequent management. The value of taking a comprehensive family history, or using the many other tools identified in this review that could potentially be used in practice, should not be overlooked by clinicians. None of the tools identified can be recommended for use over another at this stage, but a GP being able to use one of these tools also implies that they are capable of discussing advantages and disadvantages of such screening and testing, along with any results, with patients; as such, improving clinicians’ awareness of the tools’ existence could support future implementation.
Not being able to discuss results is a challenge that might also come into play when individuals take results from direct-to-consumer tests (for example, 23andMe) to a GP. There is little evidence that GPs have the combined knowledge, confidence, skills, experience, or capacity to have such discussions in usual practice.
Further studies are needed to evaluate patient outcomes — particularly the psychological impact — of genetic cancer risk screening, especially if it this is to be offered routinely to patients in general practice. Moreover, it is important to consider the acceptability of such screening to patients in primary care. Research with hard-to-reach groups, who may be less likely to take up screening when offered, is also needed.
GPs have a potential role in identifying patients at risk of hereditary cancer; however, family history-taking practices are often inadequate to assess risk. Consequently, several tools have been developed to help facilitate and improve genetic risk assessment in general practice. However, at this point in time it is difficult to support the adoption of routinely available testing or population-wide screening practices in primary care. Before the implementation of such genetic risk assessment tools is recommended in practice, further well-conducted studies are needed to provide evidence of their benefits, particularly on patient outcomes. GP knowledge and confidence regarding cancer genetics are barriers that must also be addressed if these practitioners are to consider an expanded role.
Funding
None.
Ethical approval
Not required.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.World Health Organization . Cancer. Geneva: WHO; 2018. http://www.who.int/cancer/en/ (accessed 22 Nov 2018) [Google Scholar]
- 2.Fry A, Campbell H, Gudmundsdottir H, et al. GPs’ views on their role in cancer genetics services and current practice. Fam Pract. 1999;16(5):468–474. doi: 10.1093/fampra/16.5.468. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32(3):315–324. doi: 10.1007/s11606-016-3943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Habsi H, Lim JN, Chu CE, Hewison J. Factors influencing the referrals in primary care of asymptomatic patients with a family history of cancer. Genet Med. 2008;10(10):751–757. doi: 10.1097/GIM.0b013e318185212a. [DOI] [PubMed] [Google Scholar]
- 5.Guttmacher AE, Collins FS, Carmona RH. The family history: more important than ever. N Engl J Med. 2004;351(22):2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 6.Rich EC, Burke W, Heaton CJ, et al. Reconsidering the family history in primary care. J Gen Intern Med. 2004;19(3):273–280. doi: 10.1111/j.1525-1497.2004.30401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery J, Hayflick S. The challenge of integrating genetic medicine into primary care. BMJ. 2001;322(7293):1027–1030. doi: 10.1136/bmj.322.7293.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson BJ, Islam R, Francis JJ, et al. Supporting genetics in primary care: investigating how theory can inform professional education. Eur J Hum Genet. 2016;24(11):1541–1546. doi: 10.1038/ejhg.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll JC, Brown JB, Blaine S, et al. Genetic susceptibility to cancer. Family physicians’ experience. Can Fam Physician. 2003;49:45–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Watson E, Austoker J, Lucassen A. A study of GP referrals to a family cancer clinic for breast/ovarian cancer. Fam Pract. 2001;18(2):131–134. doi: 10.1093/fampra/18.2.131. [DOI] [PubMed] [Google Scholar]
- 11.Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract. 1999;16(4):426–445. doi: 10.1093/fampra/16.4.426. [DOI] [PubMed] [Google Scholar]
- 12.Mathers J, Greenfield S, Metcalfe A, et al. Family history in primary care: understanding GPs’ resistance to clinical genetics — qualitative study. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X501868. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jbilou J, Halilem N, Blouin-Bougie J, et al. Medical genetic counseling for breast cancer in primary care: a synthesis of major determinants of physicians’ practices in primary care settings. Public Health Genomics. 2014;17(4):190–208. doi: 10.1159/000362358. [DOI] [PubMed] [Google Scholar]
- 14.Sussner KM, Jandorf L, Valdimarsdottir HB. Educational needs about cancer family history and genetic counseling for cancer risk among frontline healthcare clinicians in New York City. Genet Med. 2011;13(9):785–793. doi: 10.1097/GIM.0b013e31821afc8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas S, Atienza P, Chipman J, et al. A two-stage approach to genetic risk assessment in primary care. Breast Cancer Res Treat. 2016;155(2):375–383. doi: 10.1007/s10549-016-3686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheuner MT, Wang SJ, Raffel LJ, et al. Family history: a comprehensive genetic risk assessment method for the chronic conditions of adulthood. Am J Med Genet. 1997;71(3):315–324. doi: 10.1002/(sici)1096-8628(19970822)71:3<315::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Olesen F, Dickinson J, Hjortdahl P. General practice — time for a new definition. BMJ. 2000;320(7231):354–357. doi: 10.1136/bmj.320.7231.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Critical Appraisal Skills Programme. CASP Tools & Checklists. http://www.casp-uk.net/casp-tools-checklists (accessed 22 Nov 2018)
- 19.Pope C, Mays N, Popay J. How can we synthesize qualitative and quantitative evidence for healthcare policy-makers and managers? Healthc Manage Forum. 2006;19(1):27–31. doi: 10.1016/S0840-4704(10)60079-8. [DOI] [PubMed] [Google Scholar]
- 20.Braithwaite D, Sutton S, Smithson WH, Emery J. Internet-based risk assessment and decision support for the management of familial cancer in primary care: a survey of GPs’ attitudes and intentions. Fam Pract. 2002;19(6):587–590. doi: 10.1093/fampra/19.6.587. [DOI] [PubMed] [Google Scholar]
- 21.Campbell H, Holloway S, Cetnarskyj R, et al. Referrals of women with a family history of breast cancer from primary care to cancer genetics services in South East Scotland. Br J Cancer. 2003;89(9):1650–1656. doi: 10.1038/sj.bjc.6601348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hapgood R, Qureshi N, Allen J. Breast cancer genetics in primary care which GPs most accurately categorise patients at low risk? Eur J Gen Pract. 2002;8(4):146–150. [Google Scholar]
- 23.Kelly KM, Love MM, Pearce KA, et al. Cancer risk assessment by rural and Appalachian family medicine physicians. J Rural Health. 2009;25(4):372–377. doi: 10.1111/j.1748-0361.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nippert I, Julian-Reynier C, Harris H, et al. Cancer risk communication, predictive testing and management in France, Germany, the Netherlands and the UK: general practitioners’ and breast surgeons’ current practice and preferred practice responsibilities. J Community Genet. 2014;5(1):69–79. doi: 10.1007/s12687-013-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter FM, Kinmonth AL, Hyland F, et al. Experiences and expectations of the new genetics in relation to familial risk of breast cancer: a comparison of the views of GPs and practice nurses. Fam Pract. 2001;18(5):491–494. doi: 10.1093/fampra/18.5.491. [DOI] [PubMed] [Google Scholar]
- 26.Welkenhuysen M, Evers-Kiebooms G. General practitioners and predictive genetic testing for late-onset diseases in Flanders: what are their opinions and do they want to be involved? Community Genet. 2002;5(2):128–137. doi: 10.1159/000065170. [DOI] [PubMed] [Google Scholar]
- 27.Gramling R, Nash J, Siren K, Culpepper L. Predictive genetics in primary care: expectations for the motivational impact of genetic testing affects the importance family physicians place on screening for familial cancer risk. Genet Med. 2003;5(3):172–175. doi: 10.1097/01.GIM.0000068986.03217.BB. [DOI] [PubMed] [Google Scholar]
- 28.Gramling R, Nash J, Siren K, et al. Family physician self-efficacy with screening for inherited cancer risk. Ann Fam Med. 2004;2(2):130–132. doi: 10.1370/afm.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gramling R, Anthony D, Lowery J, et al. Association between screening family medical history in general medical care and lower burden of cancer worry among women with a close family history of breast cancer. Genet Med. 2005;7(9):640–645. doi: 10.1097/01.gim.0000187123.76699.e9. [DOI] [PubMed] [Google Scholar]
- 30.Gramling R, Anthony D, Simmons E, Bowen D. Self-rated breast cancer risk among women reporting a first-degree family history of breast cancer on office screening questionnaires in routine medical care: the role of physician-delivered risk feedback. Genet Med. 2006;8(10):658–664. doi: 10.1097/01.gim.0000237769.59166.ad. [DOI] [PubMed] [Google Scholar]
- 31.O’Malley MS, Klabunde CN, McKinley ED, Newman B. Should we test women for inherited susceptibility to breast cancer? What do NC primary care physicians think. N C Med J. 1997;58(3):176–180. [PubMed] [Google Scholar]
- 32.Bouhnik A-D, N’Diaye K, Evans DG, et al. Validation of a scale for assessing attitudes towards outcomes of genetic cancer testing among primary care providers and breast specialists. PloS One. 2017;12(6):e0178447. doi: 10.1371/journal.pone.0178447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aitken L, Warwick L, Davis A. Breast and ovarian cancer referrals to the ACT Genetic Service: are we meeting guidelines? Intern Med J. 2017;47(3):311–317. doi: 10.1111/imj.13357. [DOI] [PubMed] [Google Scholar]
- 34.Emery J, Walton R, Coulson A, et al. Computer support for recording and interpreting family histories of breast and ovarian cancer in primary care (RAGs): qualitative evaluation with simulated patients. BMJ. 1999;319(7201):32–36. doi: 10.1136/bmj.319.7201.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60–69. doi: 10.1038/gim.2013.75. [DOI] [PubMed] [Google Scholar]
- 36.Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311–324. doi: 10.1007/s10689-013-9695-y. [DOI] [PubMed] [Google Scholar]
- 37.Carroll JC, Makuwaza T, Manca DP, et al. Primary care providers’ experiences with and perceptions of personalized genomic medicine. Can Fam Physician. 2016;62(10):e626–e635. [PMC free article] [PubMed] [Google Scholar]
- 38.Ashton-Prolla P, Giacomazzi J, Schmidt AV, et al. Development and validation of a simple questionnaire for the identification of hereditary breast cancer in primary care. BMC Cancer. 2009;9:283. doi: 10.1186/1471-2407-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baer HJ, Schneider LI, Colditz GA, et al. Use of a web-based risk appraisal tool for assessing family history and lifestyle factors in primary care. J Gen Intern Med. 2013;28(6):817–824. doi: 10.1007/s11606-013-2338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bock GH, Vliet Vlieland TP, Hageman GC, et al. The assessment of genetic risk of breast cancer: a set of GP guidelines. Fam Pract. 1999;16(1):71–77. doi: 10.1093/fampra/16.1.71. [DOI] [PubMed] [Google Scholar]
- 41.Emery J, Walton R, Murphy M, et al. Computer support for interpreting family histories of breast and ovarian cancer in primary care: comparative study with simulated cases. BMJ. 2000;321(7252):28–32. doi: 10.1136/bmj.321.7252.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlando LA, Wu RR, Beadles C, et al. Implementing family health history risk stratification in primary care: impact of guideline criteria on populations and resource demand. Am J Med Genet C Semin Med Genet. 2014;166C(1):24–33. doi: 10.1002/ajmg.c.31388. [DOI] [PubMed] [Google Scholar]
- 43.Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85–88. doi: 10.1200/JOP.2010.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qureshi N, Bethea J, Modell B, et al. Collecting genetic information in primary care: evaluating a new family history tool. Fam Pract. 2005;22(6):663–669. doi: 10.1093/fampra/cmi073. [DOI] [PubMed] [Google Scholar]
- 45.Wu RR, Orlando LA, Himmel TL, et al. Patient and primary care provider experience using a family health history collection, risk stratification, and clinical decision support tool: a type 2 hybrid controlled implementation-effectiveness trial. BMC Fam Pract. 2013;14:111. doi: 10.1186/1471-2296-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuevas-Cuerda D, Salas-Trejo D. Evaluation after five years of the cancer genetic counselling programme of Valencian Community (Eastern Spain) Fam Cancer. 2014;13(2):301–309. doi: 10.1007/s10689-013-9693-0. [DOI] [PubMed] [Google Scholar]
- 47.Flória-Santos M, Lopes-Júnior LC, Alvarenga L de M, et al. Self-reported cancer family history is a useful tool for identification of individuals at risk of hereditary cancer predisposition syndrome at primary care centers in middle-income settings: a longitudinal study. Genet Mol Biol. 2016;39(2):178–183. doi: 10.1590/1678-4685-GMB-2014-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orlando LA, Wu RR, Myers RA, et al. Clinical utility of a web-enabled risk-assessment and clinical decision support program. Genet Med. 2016;18(10):1020–1028. doi: 10.1038/gim.2015.210. [DOI] [PubMed] [Google Scholar]
- 49.Leggatt V, Mackay J, Marteau TM, Yates JR. The psychological impact of a cancer family history questionnaire completed in general practice. J Med Genet. 2000;37(6):470–472. doi: 10.1136/jmg.37.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walter FM, Prevost AT, Birt L, et al. Development and evaluation of a brief self-completed family history screening tool for common chronic disease prevention in primary care. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X668186. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emery J, Morris H, Goodchild R, et al. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br J Cancer. 2007;97(4):486–493. doi: 10.1038/sj.bjc.6603897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinstein WS, Acheson LS, O’Neill SM, et al. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial. Genet Med. 2011;13(11):956–965. doi: 10.1097/GIM.0b013e3182241d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Sen A, Ruffin MT, et al. Family history assessment: impact on disease risk perceptions. Am J Prev Med. 2012;43(4):392–398. doi: 10.1016/j.amepre.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leggatt V, Mackay J, Yates JR. Evaluation of questionnaire on cancer family history in identifying patients at increased genetic risk in general practice. BMJ. 1999;319(7212):757–758. doi: 10.1136/bmj.319.7212.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.House W, Sharp D, Sheridan E. Identifying and screening patients at high risk of colorectal cancer in general practice. J Med Screen. 1999;6(4):205–208. doi: 10.1136/jms.6.4.205. [DOI] [PubMed] [Google Scholar]
- 56.US Preventive Services Task Force Final recommendation statement: BRCA-related cancer — risk assessment, genetic counseling, and genetic testing. 2013. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing (accessed 22 Nov 2018)
- 57.Watson EK, Shickle D, Qureshi N, et al. The ‘new genetics’ and primary care: GPs’ views on their role and their educational needs. Fam Pract. 1999;16(4):420–425. doi: 10.1093/fampra/16.4.420. [DOI] [PubMed] [Google Scholar]
- 58.McClain MR, Palomaki GE, Hampel H, et al. Screen positive rates among six family history screening protocols for breast/ovarian cancer in four cohorts of women. Fam Cancer. 2008;7(4):341–345. doi: 10.1007/s10689-008-9188-6. [DOI] [PubMed] [Google Scholar]