Introduction
When treating a psoriasis patient, underlying complications such as latent viral infection and chronic kidney disease could affect the selection of an appropriate therapy. Here, we present a case of psoriasis and progressive psoriatic arthritis who was a hepatitis B virus (HBV) carrier and also a patient of end-stage kidney disease (ESKD) undergoing hemodialysis (HD), successfully and safely treated with ixekizumab, an interleukin (IL)-17A inhibitor.
Case report
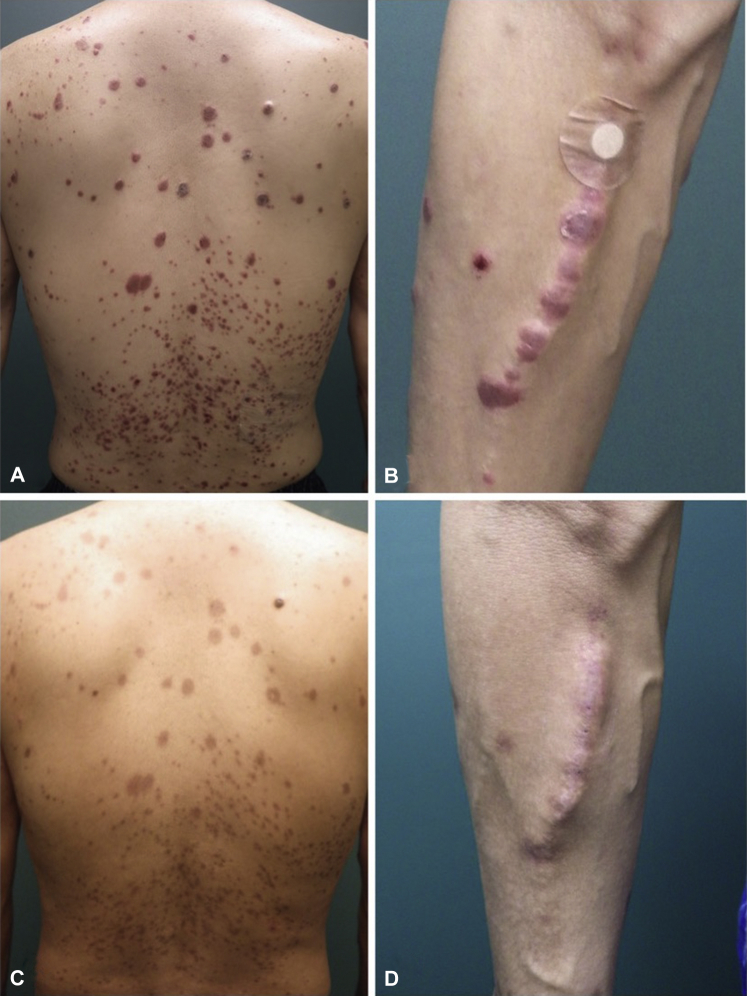
A 48-year-old man presented with a systemic eruption that lasted for 2 months. He had a 4-year history of regular HD for ESKD because of hypertensive nephropathy and had been identified as an HBV carrier. Scaly erythema and plaques were seen on his scalp, face, torso, and extremities (Fig 1, A). Linear scaly plaques were seen at the HD injection site on his right forearm (Fig 1, B). A skin biopsy found typical histopathologic features of psoriasis in which mounds of parakeratosis, markedly elongated rete ridges, and dilated blood vessels at the top of dermal papillae with inflammatory cells were observed. He also had dactylitis of his fingers, which showed bone erosions in joint radiographs. In addition, his right Achilles tendon was swollen and painful, and pelvic magnetic resonance imaging showed asymptomatic sacroiliac arthritis. Blood tests for anticitrullinated peptide antibodies and rheumatoid factor were negative, and elevated serum levels of C-reactive protein (1.87 mg/dL [<0.30]) and matrix metalloproteinase 3 (252.3 ng/mL [36.9-121.0]) indicated active arthritis. Psoriasis and psoriatic arthritis were subsequently diagnosed. Tests were positive for hepatitis B surface antigen and HBV DNA (2.8 Log IU/mL), but there was no impairment of liver function. After consultation with a hepatologist, the patient was placed on ixekizumab for psoriasis and psoriatic arthritis and simultaneously started on entecavir for HBV infection. After 1 month of ixekizumab therapy, cutaneous lesions and joint pain were dramatically reduced (Fig 1, C and D), and circulating HBV DNA were not detected without impaired liver function. Serum levels of C-reactive protein and matrix metalloproteinase 3 decreased in normal ranges. The patient arbitrarily discontinued ixekizumab therapy after 4 months of treatment. After another 4 months, the patient returned to our clinic because of a mild recurrence of cutaneous lesions without arthritis. After confirming that the patient was negative for HBV DNA, ixekizumab and entecavir were restarted. After 18 months of treatment, he shows no skin eruptions or joint symptoms and safely continues therapies without HBV reactivation and disturbance of liver function or adverse events caused by HD.
Fig 1.
Photographs of the psoriasis patient's back (A) and right forearm (B). The well-circumscribed scaly erythema on the forearm corresponded to the hemodialysis injection sites. The Psoriasis Area Severity Index score was 21.6. One month after the initiation of ixekizumab therapy, the eruptions had regressed (C, D).
Discussion
There are a few reports describing the treatment of psoriasis patients who are HBV carriers with IL-17 inhibitors; anti–IL-17A antibody monotherapy1 or anti–IL-17A antibody and nucleoside analogs combination therapy.2 Consultation with a hepatologist is strongly recommended when treating psoriasis patients with biologic therapy who have HBV.3 Prophylaxis with nucleoside analogs should be considered for preventing HBV reactivation in HBV carrier patients when treating with immunosuppressive therapy.4 Because IL-17 is a pro-inflammatory cytokine, which commonly mediates allergic responses, psoriasis patients receiving IL-17 inhibitors might have lower immunocompetence, although whether systemic IL-17 inhibition has a negative impact on HBV-associated liver disease remains controversial.5
To the best of our knowledge, this is the first report of a psoriasis patient on HD successfully treated with an anti–IL-17A antibody. There are pharmacokinetic concerns related to treatment of psoriasis patients on HD with therapeutic antibodies: should the concentration of the antibody be increased to compensate for the delayed renal clearance, or decreased because the HD itself clears the drug? First, antibody-based drugs, similar to endogenous antibodies, are generally degraded through intracellular catabolism, in which the biological half-life of antibodies is about 14 to 21 days, rather than cleared through the kidney or liver. Second, biological agents are high molecular weight proteins and are therefore not thought to be cleared by HD. Kusakari et al6 reviewed the previous literature in which 5 psoriasis patients on HD showed improvement after treatment with biologics, and no severe adverse events were reported. Larquey et al7 also reported 5 psoriasis cases receiving HD and treated with biologics, and only 1 patient treated with ustekinumab showed a decreased plasma concentration of therapeutic antibody.7 Because some systemic therapies for psoriasis such as cyclosporine, methotrexate, and retinoids could affect renal function or be contraindicated in ESKD patients on HD,8 biologics like anti–IL-17A antibodies might be preferable for psoriasis patients with severe renal disorders.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Yuta Koike has received honoraria for serving as a speaker for Elililly and Novartis. The rest of the authors have no conflicts to disclose.
References
- 1.Bevans S.L., Mayo T.T., Elewski B.E. Safety of secukinumab in hepatitis B virus. J Eur Acad Dermatol Venereol. 2018;32:e120–e121. doi: 10.1111/jdv.14608. [DOI] [PubMed] [Google Scholar]
- 2.Yanagihara S., Sugita K., Yoshida Y., Tsuruta D., Yamamoto O. Psoriasis vulgaris in a hepatitis B virus carrier successfully treated with secukinumab and entecavir combination therapy. Eur J Dermatol. 2017;27:185–186. doi: 10.1684/ejd.2016.2939. [DOI] [PubMed] [Google Scholar]
- 3.Smith C.H., Jabbar-Lopez Z.K., Yiu Z.Z. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628–636. doi: 10.1111/bjd.15665. [DOI] [PubMed] [Google Scholar]
- 4.Oketani M., Ido A., Uto H., Tsubouchi H. Prevention of hepatitis B virus reactivation in patients receiving immunosuppressive therapy or chemotherapy. Hepatol Res. 2012;42:627–636. doi: 10.1111/j.1872-034X.2012.00998.x. [DOI] [PubMed] [Google Scholar]
- 5.Arababadi M.K., Bidaki M.Z., Kennedy D. IL-17A in hepatitis B infection: friend or foe? Arch Virol. 2014;159:1883–1888. doi: 10.1007/s00705-014-2002-x. [DOI] [PubMed] [Google Scholar]
- 6.Kusakari Y., Yamasaki K., Takahashi T. Successful adalimumab treatment of a psoriasis vulgaris patient with hemodialysis for renal failure: a case report and a review of the previous reports on biologic treatments for psoriasis patients with hemodialysis for renal failure. J Dermatol. 2015;42:727–730. doi: 10.1111/1346-8138.12901. [DOI] [PubMed] [Google Scholar]
- 7.Larquey M., Girard C., Sbidian E., Richard M.A., Aubin F., Schmutz J.L. Efficacy of biologics in psoriasis patients under hemodialysis. Eur J Dermatol. 2017;27:531–533. doi: 10.1684/ejd.2017.3064. [DOI] [PubMed] [Google Scholar]
- 8.Nast A., Amelunxen L., Augustin M. S3 Guideline for the treatment of psoriasis vulgaris, update - Short version part 1 - Systemic treatment. J Dtsch Dermatol Ges. 2018;16:645–669. doi: 10.1111/ddg.13516. [DOI] [PubMed] [Google Scholar]