Background
Light-chain amyloidosis (AL) is the most common form of systemic amyloid disease. In the US, there are an estimated 4000 new cases each year. Although novel immunotherapies are in clinical trials [1], the rapid development of effective treatments and novel imaging agents has suffered due to the lack of experimental animal models with amyloid-infiltrated organs. The NIH recently acknowledged the importance of developing animal models of human disease, stating that ‘‘Such models provide valuable insights into the basic biology of disease, diagnosis, and treatment in humans’’ and that ‘‘further studies are needed to develop new animal models that better recapitulate human disease phenotypes’’ (PA-13– 135). Almost 25 y ago, Solomon et al. [2] and, more recently, Teng et al. [3], have attempted to develop experimental models of AL amyloidosis; however, although amyloid deposits were observed, the models were impractical and intractable. Therefore, we and another group have developed transgenic mice that constitutively express human intact λ6 LC proteins (Wall, unpublished data; [4]). The model generated by Ward et al. expressed a human Vλ6 protein that developed amyloid in gastric vacuoles in a subset of 18 month-old female mice. Amyloid formation in these mice was sporadic, took 18 months to develop, and the amyloid deposits were not accessible to the circulation based on the histological appearance [4]. This latter observation rendered the model of little use to study novel amyloid targeting agents delivered IV. Therefore, at present, there is no experimental mammalian system that effectively models the complexity of AL amyloidogenesis in a reproducible fashion. Our goal is to develop new targeting agents for AL amyloidosis that allow whole body imaging of patients for early diagnosis and disease monitoring. To assist with the validation of these imaging agents, we have recently developed a model of highly vascularized human AL amyloidoma in mice.
Materials and methods
Synthetic amyloid fibrils were generated using recombinant λ6 variable domain isolated from patient Wil (rVλ6Wil protein) by shaking for 72 h in phosphate buffered saline (150 mM NaCl, pH7.2). To generate the intrahepatic or intrasplenic amyloidoma, mice were anesthetized with isofluorane and placed supine atop a warming pad and administered 125 µg of ketoprofen sc. The belly was clipped and cleaned before a 1 cm incision was made in the skin and abdominal wall. The liver or spleen was visualized and 200 µL (∼0.6–6 mg) of fibril suspension injected into the organs using a 20 g needle. The abdominal wall and skin were then closed with one or two interrupted 5–0 monocryl stitches, and a running stitch of 5–0 monocryl, respectively. Skin glue was then applied to assure waterseal and avoid contamination of the wound. After 5 d, the distribution of 125I-labeled amyloidophilic tracer p5 + 14 was assessed by SPECT imaging and microautoradiography.
Results
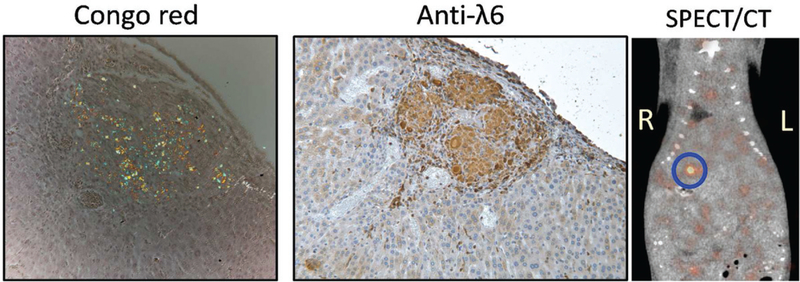
Injections of amyloid fibrils were well tolerated, and the mice recovered well. Intrahepatic injection was invariably more successful than intrasplenic. Amyloid deposited within the liver became highly vascularized (Figure 1); however, some amyloid fibrils extruded out of the injection site and formed adhesions on the abdominal organ surfaces. Both the intrahepatic and adhesion amyloid deposits labeled with 125I-p5 + 14 peptide, when given IV, as evidenced by microautoradiography (not shown). SPECT imaging revealed focal uptake of 125I-p5 + 14 in the liver, consistent with the presence of a focal amyloid lesion (Figure 1, circle).
Figure 1.
Synthetic λ6 fibrils surgically implanted into a mouse liver are detectable using radiolabeled amyloid-reactive peptides. The rVλ6 amyloid mass was visible in the liver by using Congo red and immunohistochemically using anti-λ6 antibodies. SPECT/CT imaging of the mouse following IV injection of 125I-p5 + 14 peptide revealed uptake in a focal lesion in the liver, consistent with the presence of amyloid. Mouse was 5 days post-surgery. Original magnification 160×.
Discussion and conclusions
Intrahepatic injection of human immunoglobulin-related rVλ6Wil fibrils resulted in pathologic lesions that appeared more highly vascularized as compared to standard subcutaneous amyloidomas generated in mice [5]. Increased vascularity of the amyloid deposits would allow greater and more clinically relevant accessibility to targeting agents delivered IV.The amyloidomas bound 125I-p5 + 14 as evidenced by SPECT imaging and microautoradiography and validated the use of this radiotracer for imaging AL amyloid deposits in patient organs. Although this model does not recapitulate aspects of the onset and progression of AL, it might afford a robust model of vascularized AL amyloid in the abdominal organs of a mouse that may be used for validating the efficacy of novel amyloid-targeting agents in vivo.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Gertz MA, Landau H, Comenzo RL, Seldin D, Weiss B, Zonder J, Merlini G, et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol 2016;34:1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon A, Weiss DT, Pepys MB. Induction in mice of human light-chain-associated amyloidosis. Am J Pathol 1992;140: 629–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Teng J, Turbat-Herrera EA, Herrera GA. An animal model of glomerular light-chain-associated amyloidogenesis depicts the crucial role of lysosomes. Kidney Int 2014;86:738–46. [DOI] [PubMed] [Google Scholar]
- 4.Ward JE, SooHoo P, Toraldo G, Jasuja R, Connors LH, O’Hara C, Seldin DC. Metabolic phenotype in an AL amyloidosis transgenic mouse model. Amyloid 2011;18:40–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hrncic R, Wall J, Wolfenbarger DA, Murphy CL, Schell M, Weiss DT, Solomon A. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol 2000;157: 1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]