Abstract
Objective
Abdominal aortic aneurysm is caused by the accumulation of inflammatory cells in the aortic wall. Our recent studies demonstrated that inhibition of Notch signaling attenuates abdominal aortic aneurysm formation by shifting the macrophage balance towards anti-inflammatory (M2) phenotype. Using mouse model of M2-predominant macrophages (IL12p40−/−; interleukin 12 p40) mice, we investigated their effects in the development of abdominal aortic aneurysm.
Approach and Results
Male (8–10 week-old) wild-type and IL12p40−/− mice (n=15) on C57BL/6 background were infused with Ang II (angiotensin II, 1000 ng/kg per minute) by implanting osmotic pumps subcutaneously for 28 days. In the IL12p40−/− mice, Ang II significantly increased the maximal intraluminal diameter (9/15) as determined by transabdominal ultrasound imaging. In addition, IL12p40-deletion significantly increased aortic stiffness in response to Ang II as measured by pulse wave velocity and atomic force microscopy. Histologically, IL12p40−/− mice exhibited increased maximal external diameter of aorta and aortic lesions associated with collagen deposition and increased elastin fragmentation compared with wild-type mice infused with Ang II. Mechanistically, IL12p40 deficiency by siRNA augmented the Tgfβ2-mediated Mmp2 expression in wild-type bone marrow-derived macrophages without affecting the expression of Mmp9. No such effects of IL12p40 deficiency on MMP2/MMP9 was observed in human aortic smooth muscle cells or fibroblasts. Depletion of macrophages in IL12p40−/− mice by clodronate liposomes significantly decreased the maximal external diameter of aorta and aortic stiffness in response to Ang II as determined by imaging and atomic force microscopy.
Conclusions
IL12p40 depletion promotes the development of abdominal aortic aneurysm, in part, by facilitating recruitment of M2-like macrophages and potentiating aortic stiffness and fibrosis mediated by Tgfβ2.
Keywords: aneurysm, fibrosis, macrophage, mice, transforming growth factor
Abdominal aortic aneurysm (AAA) is a common aortic disorder and 10th leading cause of death among men over 65 years of age.1,2 AAAs tend to expand asymptomatically until aortic rupture or dissection occurs, and once these rupture, the overall mortality rate is >80%.3 The treatment of AAA is confined to surgery and endovascular repair because there are no effective pharmacological therapies to date.2,4 Thus, there is a need to define the molecular and cellular mechanism of the disease to develop effective pharmacological therapies.
The major factors contributing to AAA pathogenesis include infiltration of activated inflammatory cells and degradation of extracellular matrix (ECM) along with loss of vascular smooth muscle cells (SMCs).5–7 It is now well established that innate and adaptive immune cells, including macrophages, neutrophils, B cells, T cells, natural killer cells, and dendritic cells contribute significantly to aortic aneurysm development.8–10 Recent data from human AAA patients and mouse models have reported the presence of macrophages in the aneurysmal aorta wall, accumulating predominantly in the adventitia.11–14
In response to different stimuli, macrophages differentiate towards a spectrum of polarization with distinct functions. The extreme ends of this polarized spectrum are called as proinflammatory (M1) or anti-inflammatory (M2) macrophages and serve distinct functions in the progression of AAA development. Although heterogeneity of these macrophages have been reported in various vascular diseases, recent studies including ours have identified predominance of proinflammatory macrophages within the inflamed aortic tissues.15–17 By contrast, few studies have also reported the presence of anti-inflammatory macrophages in the aneurysmal tissues, which are implicated in ECM remodeling and tissue repair.18,19 Existence of these diverse macrophage phenotypes with pathogenic and reparative roles has provided a complexity in understanding the pathogenesis of AAA. Although inflammatory process seems to contribute to AAA, approaches to limit progression of AAA by use of anti-inflammatory drugs for treating the AAA patients have not been successful.20–22 These observations warrant in-depth studies about the role of inflammatory factors in the development of AAA by examining specific roles of Ml or M2 macrophages.
Among the various key players known to regulate macrophage phenotype is IL12 (interleukin 12), a proinflammatory heterodimeric cytokine composed of p35 and p40 subunits.23,24 Principally expressed by monocytes/macrophages and dendritic cells, it has been shown that macrophages from IL12p40 deficient mice are biased toward M2 activation profile.25,26 It is also reported that IL12p40 deficiency enhances bleomycin-induced fibrosis; however, it protects against silica-induced fibrosis.27 Similarly, deficiency of IL12p35 enhances cardiac fibrosis by promoting M2 macrophages and TGFβ (transforming growth factor-β) production.26 Additionally, IL12p35 deficiency presents robust silica-induced pulmonary inflammation and fibrosis.28 Interestingly, studies on the independent role of M2 macrophages leading to fibrosis and vascular diseases have emerged.29,30 In the recent studies, low dose of Ang II (angiotensin II) infusion differentiated Ly6Chi monocytes into M2 macrophages in the aortic wall.30 Chemokine (C-C motif) receptor 2 antagonist prevented the accumulation of these macrophages and was associated with reduction in fibrosis, elastin loss, and blood pressure in these studies.30 Our lab has previously published that inhibition of Notch signaling protects against the development of AAA associated with decreased M1 polarization and increased M2 polarization of macrophages.15,31 However, direct roles of M2-predominant macrophages in the development of AAA are not yet determined.
In the present study, we sought to determine the impact of M2-like macrophages on AAA development. We hypothesized that deletion of IL12p40 and subsequent enhanced M2 macrophages aortic infiltration would accelerate AAA formation in the setting of Ang II infusion through development of perivascular fibrosis and elastin fragmentation. Our results showed that deficiency of IL12p40 leads to enhanced AAA formation associated with increased Tgfβ2 mediated proteolytic activity.
Materials and Methods
The authors declare that all supporting data are available within the article and in the online-only Data Supplement. The authors also declare that we will make analytic methods and study materials available to other researchers on request from the corresponding author (C.P. Hans).
Animals, Design, and Aneurysmal Model
Eight-week old, male wild-type (WT; C57BL/6J; 000664) and IL12p40−/− mice (B6.129S1; 002693) were purchased from The Jackson Laboratory. In addition to 10 generations crossing with C57BL/6 mice by The Jackson Laboratory, IL12p40−/− mice were further crossed to C57BL/6 mice for 7 to 8 generations in our laboratory to generate on homogenous C57BL/6J background. The WT and IL12p40−/− mice were first bred to generate IL12p40+/− mice, and the male and female IL12p40+/− mice were further interbred to generate WT and IL12p40−/− littermates, which were used for the studies. Genotyping was performed according to The Jackson Laboratory’s protocol.
Only male mice were studied because of low incidence of Ang II-induced AAA in female mice as detailed in an Arteriosclerosis, Thrombosis, and Vascular Biology Council statement.32 Mice were kept on a 12 hours/12 hours light/dark cycle with standard chow. Aneurysmal studies were performed on these mice by infusing Ang II for 28 days using published protocols.13,15 Animals were randomly allocated to Ang II infusion or control. For the end point data analysis, samples and data were blinded wherever possible to reduce bias. Briefly, mice were anesthetized in a closed chamber with 1% to 2% isoflurane in oxygen for 2 to 5 minutes until immobile. Each mouse was then removed and taped on a heated (37±2°C) procedure board with 1.0±1.5% isoflurane administered via nosecone during minor surgery. Mini osmotic pumps (Model 2004; Alzet, Cupertino, CA) containing Ang II (1000 ng/min per kg; Sigma) were implanted subcutaneously in the neck region of anesthetized mice. The mice were observed daily for normal behavior or sudden death. The mice that died in response to Ang II were immediately necropsied to determine the cause of death. At the end of the study, mice were terminated with an overdose of anesthetics ketamine (100 mg/kg) and xylazine (20 mg/kg). The aortas were dissected, fixed in 10% formalin, and processed for macroscopic and histological studies. All the animal-related experiments were approved by the Animal Care and Use Committee at the University of Missouri (Columbia, MO). All the animal experiments conform the National Institutes of Health guidelines (Guide for the care and use of laboratory animals).
Sample Size Calculation and Outcome Variables
Our primary outcome variable in the current study was an increase in the external aortic diameter by ≥50% to define the presence or absence of AAA. Based on our previous work and reports from other labs using this definition, we powered the study with our main outcome variable of maximal external aortic diameter using (α=0.05). We estimated our sample size for this variable to n=10 per group to determine if the data is statistically significant assuming a 5% significance level using t test. For the pathological outcome measures, we included cellular infiltration, elastin fragmentation, abundance of collagen, and increased Mmp2 (matrix metalloproteinase) expression. For the functional outcome, aortic stiffness as determined by 2 independent methods (pulse wave velocity [PWV] and atomic force microscopy [AFM]) was used.33,34
Transabdominal Ultrasound Imaging and Quantification of Aortic Aneurysms
For ultrasonic imaging, mice were restrained for <15 s to put into the anesthesia chamber, followed by anesthetization with oxygen and vaporized isoflurane (≈2%). Loss of spinal reflexes was confirmed via toe pinching, and the loss of corneal reflex was assessed by gentle touch of the eye with a soft tissue paper technique. The animals were placed on a heated (41°C) imaging stage in supine position while under anesthesia. The body temperature, heartbeat, and respiration rates were continuously monitored during the imaging procedure. For abdominal aorta measurements, the abdominal hairs were removed by applying hair removal cream followed by cleaning with wet gauze. Warmed ultrasound gel was applied to the abdominal surface and ultrasound probe (550D MHz) applied to the gelled surface to collect B-mode, M-Mode, ECG-based Kilohertz Visualization mode images, as well as Power Doppler measurements, by the imaging system (Vevo 2100, VisualSonics). Short and long axis scans of aortas were performed on the abdominal aorta from the level of the left renal arterial branch through to the suprarenal region. Cine loops of 100 frames were acquired throughout the renal region on the abdominal aorta and used to determine the maximal diameters of the abdominal aorta in the suprarenal region. To define consistency, all the ultrasound data were collected in a blinded fashion by an experienced faculty member in the core facility at Dalton Cardiovascular Research Center.
The Ang II-induced AAA were defined as having at least 50% increase in the maximal intraluminal and external diameters of the abdominal aorta compared with the control mice.15,35 The maximal intraluminal diameters of the suprarenal abdominal aorta were quantified in vivo by ultrasound imaging. For quantification of the maximal external diameters, suprarenal abdominal aortic diameters were measured using ZEN lite software (Zen 2.3 blue edition; Zeiss, NY) by an independent researcher ex vivo under a microscope. The average suprarenal aortic width was 0.87 mm in control mice, and consequently, we defined AAA as >1.31 mm. For aortic rupture, mice were closely monitored for acute rupture incidences for first 10 days of Ang II infusion. The mice which died post-Ang II infusion immediately underwent autopsy to determine the cause of death. The aortic rupture was defined by the presence of blood clot in the chest cavity and hemorrhage of abdominal aorta between the celiac artery and the left renal artery.36 These aortas were isolated and examined histologically for the presence of disrupted elastic laminae at the site of rupture, with extravasation of blood.
Aortic Stiffness Measurement
In vivo aortic stiffness was measured locally in the abdominal aorta by PWV technique by analyzing ECG-based Kilohertz Visualization data collected at day 14 and 28 of Ang II infusion using VevoVasc software as described previously.34 Briefly, PWV was determined by simultaneous tracking of R-wave of the ECG and the pulse wave along the 2 locations of suprarenal abdominal aorta. VevoVasc software was used to calculate PWV as a ratio of the distance (d) between 2 locations along the aorta and time delay (Δt) of the pulse wave between both locations and is expressed in m/s. The ex vivo aortic stiffness was determined in the abdominal aortic sections by AFM.33 To evaluate the stiffness, a 2 mm segment of the abdominal aorta was obtained from mice. The aorta was opened longitudinally, and the adventitial surface of each explant was fastened to a glass coverslip using cell-tak (BD Biosciences). The aortic stiffness within intact aortic explants was measured using a nano-indentation protocol with AFM according to previously described procedures.33 AFM measurements were conducted at room temperature. The measurements for both PWV and AFM were conducted following the 2-man principle.
Blood Pressure Measurement
Blood pressure was measured noninvasively on conscious mice using a CODA volume pressure recording tailcuff system (Kent Scientific Corporation, Torrington, CT) as described previously.15 The CODA system analyzes 6 measurements: systolic, diastolic pressure, mean pressure, rate, blood flow, and blood volume. Briefly, mice were acclimated for 2 days to restraint tubes and trial measurements. On the third day, after 5 acclimation cycles, 25 individual blood pressure measurements (technical replicates) were taken; all false readings (as determined by the diagnostic software) were excluded, and any animal failing to register at least 20 (80%) true readings were excluded from analysis. Data was trimmed to exclude the lowest and highest 5% of measured values, and the mean was used to represent each animal. The measurement of blood pressure was performed blindly with respect to experimental groups.
Histology and Immunohistochemistry
After fixation, the abdominal aortas from experimental mice were rinsed with PBS and processed for paraffin embedding. Serial sections (5 μm) were prepared by cutting abdominal aorta into 2 equal halves and sectioned throughout the tissue. The sections of the abdominal aorta at regular intervals (200 μm) were subjected to hematoxylin and eosin, elastin, and Masson trichrome stain for histoarchitectural evaluation of aneurysm as described previously.37 The serial tissue sections obtained from these mice were further subjected to immunohistochemistry. For immunohistochemistry, the abdominal aortas were stained with antibodies for Moma2 (ab33451), Cd206 (MCA2235T, BioRad), Tgfβ2 (ab36495), Mmp2 (ab37150), Mmp9 (ab38898), phospho-p38 MAPK (mitogen-activated protein kinase1:800; 9211, cell signaling), and phospho-smad3 (mothers against decapentaplegic homolog 3; 1:200; ab52903) as described.37 The specificity of all the antibodies were confirmed using appropriate IgG controls (I-2000−1, I-4000−1, I-1000−5; Vector laboratories) in place of primary antibodies at same concentrations. The intensity of the immunostaining was evaluated by obtaining 6 images from random areas of interest at ×40 or ×100 from each tissue (n=6) and quantified using Fiji version of Image J following the software directions.38 The images were blinded to reduce bias during quantification.
Cell Isolation, siRNA Transfection, RNA Extraction, and Quantitative Real-Time PCR
Bone marrow−derived macrophages (BMDMs) were isolated from 6- to 8-week old WT or IL12p40T−/− mice by previously established protocol.15,31 Briefly, femur and tibia bones were flushed with culture media under aseptic conditions, and cells were collected and treated with ACK lysis buffer (Gibco) to lyse red blood cells. The remaining bone marrow−derived cells were cultured in ultra-low 6 well tissue culture plates. After 6 hours, cells were treated with macrophage colony stimulating factor (10 ng/mL, R&D systems) in complete medium (RPMI 1640+10% human serum+1% penicillinstreptomycin) with a media change every other day. At day 7, cells were serum starved in 1% FCS-RPMI for 2 hours followed by stimulation with lipopolysaccharide (100 ng/mL, Sigma Aldrich) and IFN-γ (interferon-γ; 20 ng/mL, Biolegend) or IL4/IL13 (10 ng/mL each) for 24 hours to polarize into M1 or M2 states, respectively. For naive macrophages, cells were treated with vehicle only. For gene expression analysis, media was removed, and the cell pellets were mixed with RLT lysis buffer (Qiagen). Total RNA was extracted using the RNeasy kit (Qiagen) following the manufacturers’ instructions. The concentration of extracted RNA was measured using a Nanodrop ND1000 to verify that the 260:280 and 260:230 ratios were ≈2. For transfection studies of BMDMs, IL12p40 siRNA (sc-39641), Tgfβ2 siRNA (sc-39803), and control siRNA (sc-37007) were used with Lipofectamine RNA iMAX reagent (Invitrogen) and the OptiMEM media (31985–070, Invitrogen) using the manufacturer’s protocol. In some wells, IL12p40−/− BMDMs were treated with SB431542, Tgfβ receptor kinase inhibitor (15 nmol/L, Tocris Biosciences), or human recombinant Tgfβ2 protein (10 ng/mL, Fisher) for 48 hours. Quantitative real-time polymerase chain reaction (PCR) was performed on CFX connect real-time PCR detection system (Biorad) in triplicate. CT for Rpl13a was used to normalize gene expression. The primer sequences for genes are available on request.
Western Blot Analysis
The supernatants from BMDMs grown in 60 mm culture dishes were collected, and the media were concentrated 50-fold using Amicon ultra-4 centrifugal filter units (Millipore). In some dishes, the cells were treated with Ang II (200 nm; Sigma) for 24 hours or IL4/IL13 (20 ng/mL each, R&D systems) for 48 hours. The concentrated proteins were mixed with equal volume of 2X Laemmli sample buffer (Biorad) containing β-mercaptoethanol and boiled for 8 minutes at 95°C. After centrifugation, 20 μL of the samples were then loaded onto SDS-PAGE (10% wt/vol) and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes. After blocking, the membranes were probed with antibodies against Tgfβ1 (ab92486;1:2000) and Tgfβ2 (ab36495; 1:2000). Membranes were then incubated with appropriate HRP-conjugated secondary antibodies. Proteins on the membranes were visualized by chemiluminescence detection kit (Super signal west femto maximum sensitivity substrate; Thermo Scientific). The proteins in the media were normalized to total protein contents of the cell extracts.
Aortic Ring Assay and Gelatin Zymography
Aorta from WT and IL12p40−/− mice were isolated, and aortic rings were obtained for transfection or treatment as described previously.37 The aortic rings were treated with nonspecific siRNA or Tgfβ2 siRNA using Lipofectamine as described above. The aortic rings were also treated with SB431542 or human recombinant Tgfβ2 for 48 hours as described above. For gelatin zymography, the aortic tissue lysates and culture supernatants from aortic rings and IL12p40−/− BMDMs with various treatments were used. The supernatants were concentrated to 50-fold using Amicon ultra-10 centrifugal filter units (Millipore). Aortic tissue lysates were prepared in RIPA buffer containing protease and phosphatase inhibitor. Samples were briefly sonicated and centrifuged at 4°C at 10 000 rpm for 20 minutes. After analysis of protein content (BCA Protein assay kit, Pierce) in supernatants, gelatinase activity was detected by loading equal amount of protein (20 μg) onto 7.5% SDS-PAGE gels containing 1 mg/mL gelatin. Bands of lysis in Coomassie Blue-stained gels were derived for bands corresponding to the pro and activated forms of MMP2 and MMP9.
Macrophages Depletion, Flow Cytometry, and Ang II Infusion
The macrophages in IL12p40−/− mice were depleted using liposomes containing clodronate (dichloromethylene diphosphonate; clodronateLiposomes.org). Animals received 150 μL intraperitoneal injections of control or clodronate liposome 2× at 3-day interval. One day after second injection, aneurysmal studies were conducted in these mice for 14 days by methods described above. Some mice were sacrificed after 2 clodronate injections to verify the absence of macrophages in spleens by flow cytometry (Figure IX in the online-only Data Supplement). Flow cytometry cell surface staining with standard procedures was done using BV605 anti-F4/80 antibody as described previously.39 The cells were analyzed by BD LSRFortessa X-20 instrument.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 7.0 (GraphPad Software, Inc, CA) and SAS Proc GLM (SAS 9.4). All the data were assessed for normality and equal variance using GraphPad Prism and SAS Proc GLM. Continuous data were examined by Shapiro-Wilk test for normality. Unpaired 2-tailed Student t test was used to determine statistical difference between 2 groups for normally distributed continuous variables. For comparison of multiple groups, ANOVA followed by Tukey multiple comparison analysis or 2-way ANOVA followed by Bonferroni post hoc tests were used. For data without normal distribution, nonparametric Mann-Whitney U test or Kruskal-Wallis test were applied. In data that revealed unequal variance, Kolmogorov-Smirnov test was applied. For incidence of AAA, Fisher exact test was performed. In the case of blood pressure, repeated measure 2-way ANOVA was used to determine between and within group differences, with time as the repeating factor. All data are presented as mean±SEM. P<0.05 was considered statistically significant for all tests.
Results
IL12p40 Deficiency Induces BMDM Polarization Toward M2 Macrophage Phenotype
We initially characterized the BMDMs from C57BL6 (WT) and IL12p40−/− mice for a panel of M1 and M2 genes by quantitative PCR. In accordance with the published reports,25 BMDMs from IL12p40−/− mice showed significantly decreased expression of proinflammatory (M1) macrophage markers including iNos, Tnfα, and Notch1 (Figure IA in the online-only Data Supplement). Concomitantly, the BMDMs from IL12p40−/− mice showed modest increase in the expression of anti-inflammatory (M2) macrophage markers, including Il10 and Tgfβ1 (Figure IB in the online-only Data Supplement). In addition, the Western blot measurements showed that IL12p40−/− macrophages spontaneously secrete higher amount of Tgfp2 in the supernatant compared with that of WT (Figure IC in the online-only Data Supplement). This increase in Tgfβ2 was consistent with Ang II or IL4/IL13 stimulation of macrophages. However, the Tgfp1 expression by IL12p40−/− macrophages remain unchanged, although it was increased at basal level (Figure IC in the online-only Data Supplement). In addition, the BMDMs were confirmed to be >90% double positive for CD11b and F4/80 (Figure II in the online-only Data Supplement). These results indicate that the BMDMs from the IL12p40−/− mice represent a macrophage profiling with predominance of anti-inflammatory genes (M2) and decreased expression of proinflammatory genes (M1).
IL12p40 Deficiency Exacerbates Ang II-Induced AAAs
To examine the direct role of M2-like macrophages in AAA pathogenesis, WT and IL12p40−/− mice (n=15) were infused with Ang II subcutaneously for 28 days. Transabdominal ultrasound imaging showed increased maximal intraluminal diameter in the suprarenal region of the abdominal aorta of IL12p40−/− mice at day 14 and 28 of Ang II, whereas no such increase in maximal intraluminal diameter in this region was observed in the WT mice infused with Ang II (Figure 1A and1C). Aortic dissection-mediated mortality was observed in 13% of the IL12p40−/− mice (2 out of 15) in response to Ang II, whereas no mortality was observed in WT mice with same treatment (Figure 1D). The macroscopic examination of the aortas at day 28 demonstrated that only 13% of the WT mice (2/15) developed AAA formation in response to Ang II as determined by the maximal external aortic diameter in the suprarenal aortic region (Figure 1B, 1D, and 1E). The extent of AAA development significantly increased to 60% (9/15) in IL12p40−/− mice at day 28 and was significantly higher compared with WT mice in response to Ang II (Figure 1E). No change in the maximal aortic diameter was observed in the saline-treated WT or IL12p40−/− mice (Figure 1D). Ang II infusion increased systolic, diastolic, and mean blood pressure in all mice, and no significant differences were observed between these groups at day 28 (Figure III in the online-only Data Supplement). Similar increase in the total cholesterol, low-density lipids, and high-density lipids contents in the serum of both WT and IL12p40−/− mice was observed in response to Ang II infusion at day 28 (Figure IV in the online-only Data Supplement).
Figure 1.
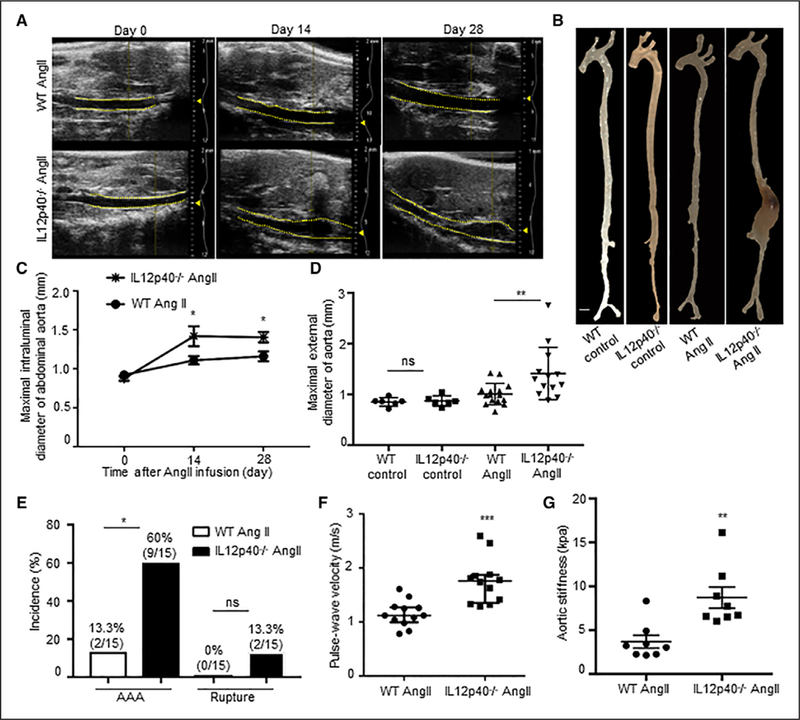
IL12p40 (interleukin 12 p40) deficiency increases Ang II (angiotensin II)-induced abdominal aortic aneurysm (AAA) development and aortic stiffness. A, Representative transabdominal ultrasound images obtained at day 0, 14, and 28 using a VisualSonics Vevo2100 showing increase in the luminal expansion of abdominal aorta in the wild-type (WT) mice (top) and IL12p40−/− mice (bottom) in response to Ang II. Dashed yellow lines outline the lumen. B, Representative aortas from the experimental mice showing maximal aortic width in the suprarenal region of the aorta. Images were taken using Zeiss Stemi 2000-C microscope. Scale bar, 1 mm. C, Quantification of maximal intraluminal diameters of abdominal aorta by ultrasound. D, Quantification of ex vivo maximal external diameters of abdominal aortas by microscopy (n=6; control mice and n=15; Ang II mice). E, Incidence of AAA and aortic aneurysm rupture in WT and IL12p40−/− mice in response to Ang II. F, Pulse wave velocity calculated from measurements of abdominal aortic pulse pressure as determined by ECG-based Kilohertz Visualization in response to Ang II at day 28 (n=12). G, Bar graph showing increased aortic stiffness in IL12p40−/− mice compared with WT-Ang II obtained by atomic force microscopy (n=8 per group). Two-way repeated measures ANOVA followed by Bonferroni post hoc analysis was used for statistics in C. Kruskal-Wallis test was applied for statistics in D. Fisher exact test was used for comparing AAA incidence and mortality because of rupture in E. MannWhitney test was applied for statistics in F and Kolmogorov-Smirnov test was applied in G.*P<0.05, **P<0.01, ***P<0.001. ns indicates nonsignificant.
Next, we investigated whether increase in maximal intraluminal diameter in abdominal aorta of IL12p40−/− mice is correlated with the aortic wall functions. Aortic stiffness as measured by PWV using VevoVasc software was significantly higher in IL12p40−/− compared with WT at day 28 of Ang II infusion (1.76±0.40 versus 1.15±0.23 m/s; Figure 1F). Furthermore, AFM confirmed the increase in the aortic stiffness of the abdominal aorta in IL12p40−/− mice than WT mice in response to Ang II (8.72±3.21 versus 3.70±1.94 kpa; Figure 1G). These results demonstrate that IL12p40 deficiency is associated with aortic dysfunctions and increased AAA incidence.
IL12p40 Deficiency Increases Structural Damages of Aorta in Response to Ang II
The tissue sections from different groups were subjected to histological analysis to characterize the abdominal aortic lesions using hematoxylin and eosin, Verhoeff-Van Gieson (elastin), and Masson trichrome (collagen) staining of aorta. Hematoxylin and eosin, elastin staining, and quantitative data demonstrated that infusion of Ang II in WT mice induced marginal adventitial thickening with minimal infiltration of inflammatory cells and no visible elastin fragmentation (Figure 2C, 2G, and 2M). IL12p40 deficiency increased structural deformity as evidenced by thickening and noticeable cellular infiltration in adventitial layer and increased fragmentation of elastin layer in response to Ang II (Figure 2D, 2H, and 2M). In addition, the degree of collagen deposition was significantly higher in the adventitial region of IL12p40−/− compared with that of WT mice infused with Ang II indicating that IL12p40 deficiency promotes aortic fibrosis (Figure 2L and 2N and Figure VH in the online-only Data Supplement). WT and IL12p40−/− control mice did not show any observable difference in the structure of aorta in the suprarenal region (Figure 2A, 2B, 2E, 2F, 2I, and 2J). These data demonstrate that deficiency of IL12p40 in C57BL/6 mice induces elastin fragmentation and increased collagen deposition in the abdominal aorta exhibiting the characteristics features of AAA in response to Ang II.
Figure 2.
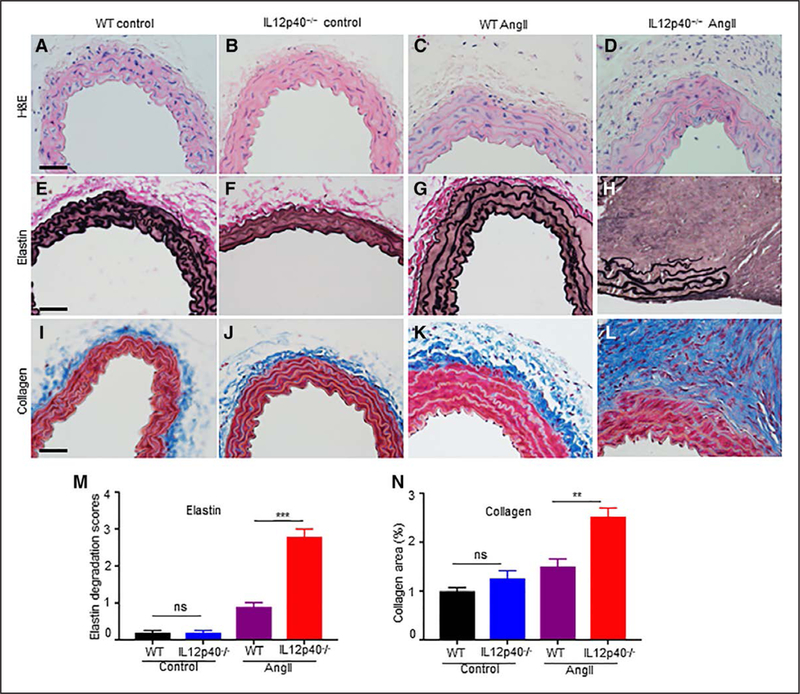
IL12p40 (interleukin 12 p40) deficiency increases structural damage of aorta in response to Ang II (angiotensin II). A–D, Transverse sections of abdominal aorta stained with H&E (hematoxylin and eosin) illustrating the extent of abdominal aortic aneurysm progression at day 28 in response to saline (A–B) or Ang II (C–D). E–H, Representative images of Verhoeff-Van Gieson staining demonstrating the extent of elastin fragmentation in IL12p40−/− mice infused with Ang II at day 28. I–L, Representative collagen staining in the abdominal aorta of experimental groups visualized in blue using trichrome staining. M–N, Quantification of medial elastin degradation (M) and collagen deposition (N) in experimental groups (n=6 for each group). Scale bar=50 μm. **P<0.01, ***P<0.001. ns denotes nonsignificant in Tukey multiple comparisons test. WT indicates wild-type
IL12p40 Deficiency Increases Infiltration of M2 Macrophages in Aortic Lesions
Next, we characterized the immune cells infiltrated into AAA lesions in IL12p40−/− mice using immunohistochemistry. Aortic sections were stained with antibodies against common macrophage marker (Moma2) and M2-macrophage specific markers (Cd206 and Tgfβ2). Increased immunostaining of Moma2 positive macrophages was observed within the remodeled adventitia of IL12p40−/− mice (arrows in Figure 3D), whereas no such Moma2 positive cells were observed in WT mice infused with Ang II (Figure 3C). Furthermore, the infiltrated cells in the aorta of IL12p40−/− mice in response to Ang II were found to be positive for both M2 macrophage markers, Cd206 and Tgfβ2 (Figure 3H and3L). No marked increase in the immunostaining for iNos (an M1 polarization marker) was observed in the adventitia of IL12p40−/− mice or WT mice infused with Ang II (data not shown). Interestingly, Tgfp2 positive M2 macrophages were found to be penetrating towards the medial region of the abdominal aortic sections in IL12p40−/− mice (arrows in Figure 3H and3L). WT and IL112p40−/− control mice did not show any observable difference in cellular immunostaining for Moma2, Cd206, or Tgfp2 in the aortic tissue (Figure 3A, 3B, 3E, 3F, 3I, and 3J). These data demonstrated that macrophages and Cd206-positive as well as Tgfβ2-positive cells (markers of M2 macrophages) seem to be infiltrating in the adventitial and medial regions of the aorta in IL12p40−/− mice during pathogenesis of AAA.
Figure 3.
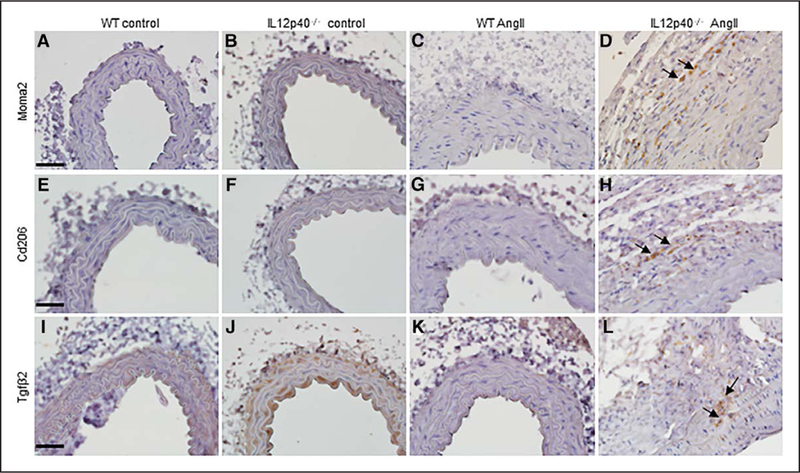
Recruitment of M2 macrophages in the aorta of IL12p40−/− (interleukin 12 p40) mice after 28 d of Ang II (angiotensin II) Infusion. A–D, Immunohistochemistry showing expression of Moma2, a macrophage marker, in the aortic tissue at day 28 of saline or Ang II treated mice. E–H, Immunohistochemistry showing expression of Cd206, an M2-macrophage marker in the aortic tissue at day 28 of saline or Ang II mice. I–L, Immunostaining of Tgfβ2 (transforming growth factor β) in the abdominal aorta of experimental mice. Some of the immunopositive cells are pointed with arrows (scale bar=50 μm). WT indicates wild-type.
IL12p40 Deficiency Induces Upregulation of Tgfβ2 and Mmp2 Expression
TGFβ is one of the upstream signaling proteins known to alter the structure and composition of ECM and known to play an important role in vascular remodeling and fibrosis.40 We examined if Tgfβ signaling could be associated with pathological process of extensive matrix degradation in IL12p40−/− mice in response to Ang II. To this end, we inhibited IL12p40 in WT BMDMs with IL12p40 siRNA and checked for expression of Tgfβ and downstream Mmps. As expected, increased expression of Tgfβ2, not the Tgfβ1 mRNA was observed in the WT BMDMs with IL12p40 deficiency (Figure 4A). IL12p40 deficiency in WT BMDMs also increased the expression of Mmp2 mRNA without affecting Mmp9 expression (Figure 4A). Deficiency of IL12p40 in fibroblasts and SMCs using IL12p40-specific siRNA did not change the gene expression of Tgfβ2 and Mmp2 (Figure VII in the online-only Data Supplement and data not shown). To confirm the increase in Mmp2 is mediated through increased Tgfβ2, we treated IL12p40−/− BMDMs with either Tgfβ pathway inhibitor (SB431542) or human recombinant TGFβ2 and checked the expression of Mmps and Timps (tissue inhibitor of metalloproteinase). Interestingly, we observed increased expression of Mmp2 with recombinant TGFβ2 treatment (Figure 4B). We confirmed the increased expression of TgfβRl with recombinant TGFβ2 treatment (Figure VIII in the online-only Data Supplement). To further validate these observations, we immunostained the abdominal aortic sections from WT and IL12p40−/− mice with antibodies specific to Mmp2 and Mmp9. As expected, significant increase in the immunostaining of Mmp2 was observed in the adventitial region of IL12p40−/− mice than WT mice in response to Ang II (Figure 4C–4F and 4K). However, there was no change in the expression of Mmp9 in the aortic sections of WT and IL12p40−/− mice infused with Ang II (Figure 4G–4J and 4L). Taken together, it is conceivable that increase in Tgfβ2 may play a role in increasing the expression of MMP2.
Figure 4.
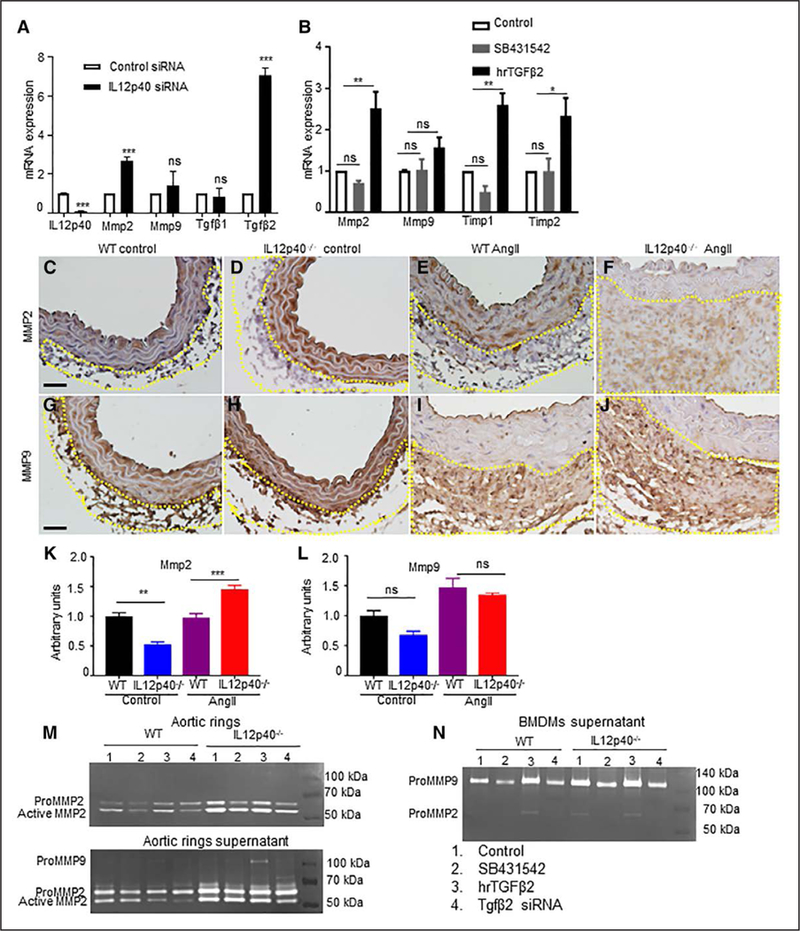
IL12p40 (interleukin 12 p40) deficiency increases Tgfβ2 (transforming growth factor β) dependent Mmp (matrix metalloproteinase) activity. A, mRNA expression of IL12p40, Mmp2, Mmp9, Tgfβ1, and Tgfβ2 in wild-type (WT) bone marrow-derived macrophage (BMDMs) transfected with IL12p40-specific siRNA or negative control siRNA as measured by quantitative real-time polymerase chain reaction. B, mRNA expression of Mmps and Timps in IL12p40−/− BMDMs treated with Tgfβ receptor kinase inhibitor (SB431542) or human recombinant TGFβ2. Representative immunohistochemistry images of MMP2 (C–F) and MMP9 (G–J) in abdominal aorta of experimental mice. Quantification of MMP2 (K) and MMP9 (L) immunostaining in the adventitial region of abdominal aorta from experimental mice. M and N, Representative gelatin zymography for pro-MMP2 (matrix metalloproteinase 2), active MMP2 and pro-MMP9 in aortic rings (M, top), supernatants from aortic rings (M, bottom), and supernatants from BMDMs of indicated groups (N). Scale bar=50 μm. *P<0.05, **P<0.01, ***P<0.001, ns denotes nonsignificant in paired 2-tailed Student t test (A and B) or in Tukey multiple comparisons test (K–L). Ang II indicates angiotensin II.
Furthermore, to confirm that Tgfβ2 is the mediator for Mmp2 activity, we performed gelatin zymography studies in aortic rings and macrophages with Tgfβ modulation. As shown in Figure 4M, we found increased activity of Mmp2 in aortic rings as well as supernatants from IL12p40−/− mice compared with WT both at basal level and with stimulation of TGFβ2. The Mmp2 activity was decreased with SB431542 or Tgfβ2 siRNA (Figure 4M). Similarly, macrophages from IL12p40−/− mice showed increased Mmp2 at basal level and with stimulation with TGFβ2 (Figure 4N). This Mmp2 activity was alleviated with SB431542 or Tgfp2 siRNA treatment. Finally, to confirm that Tgfβ pathway is activated in aortic tissues, we immunostained the abdominal aortic sections with phospho-smad3 and phospho-p38 MAPK from all 4 experimental groups (Figure 5). Interestingly, we found significantly increased number of nuclei positive for phosphorylated forms of both smad3 and p38 in the IL12p40−/− tissues in response to Ang II (Figure 5D, 5H, 5I, and 5J). This data suggested that Tgfβ pathway is activated and may play a role in increasing the AAA in IL12p40−/− mice.
Figure 5.
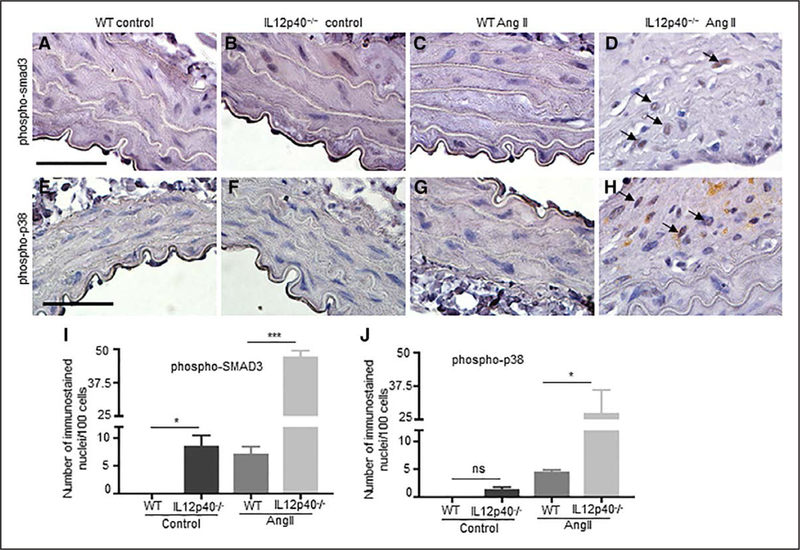
Tgfβ (transforming growth factor β) pathway is activated in IL12p40−/− (interleukin 12 p40) mice in response to Ang II (angiotensin II). Representative ¡mmunohistochemistry images of phospho-smad3 (mothers against decapentaplegic homolog 3; A–D) and phospho-p38 MAPK (mitogen-activated protein kinase) staining (E–H) in abdominal aorta of experimental mice. Quantification of phospho-smad3 (I) and phospho-p38 (J) nuclear immunostaining in the abdominal aorta from experimental mice. Scale bar=50 μm. *P<0.05, ***P<0.001, ns denotes nonsignificant in Tukey multiple comparisons test (I and J). WT indicates wild-type.
Depletion of Macrophages in IL12p40−/− Mice Reduces Fibrosis and Aortic Stiffness
Finally, to confirm the active contributions of M2 macrophages in AAA pathogenesis, we depleted macrophages using clodronate liposomes in IL12p40−/− mice and characterized Ang II-induced aneurysm formation. Intraperitoneal injection of clodronate-containing liposomes resulted in a decrease in F4/80+ macrophages in the mouse spleen evident by flow cytometry for macrophage marker, F4/80 (Figure VII in the online-only Data Supplement). At day 14 of Ang II infusion, a significant decrease in the maximal external diameter of aorta was observed in IL12p40−/− mice treated with clodronate than control group. (Figure 6A and6B). Aortic stiffness was also significantly attenuated in the clodronate-treated IL12p40−/− mice than control IL12p40−/− mice in response to Ang II (Figure 6C). Histological analysis of the aortic tissues revealed decreased adventitial remodeling and reduced cellular infiltration in the macrophages depleted group of IL12p40−/− mice (Figure 6D– 6I). Interestingly, there was less collagen deposition in the adventitial region of clodronate injected IL12p40−/− Ang II mice compared with IL12p40−/− Ang II alone as determined by Trichrome staining (Figure 6H-6I). These results suggest that increased fibrosis and arterial stiffness in the IL12p40−/− mice may primarily be mediated by macrophages.
Figure6.
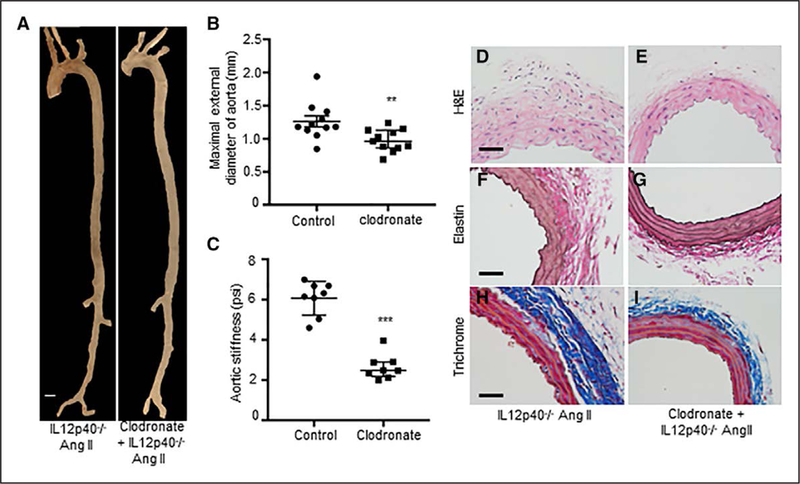
Depletion of macrophages In IL12p40−/− (Interleukin 12 p40) mice attenuates structural damage of aorta. A, Representative aorta from IL12p40−/− mice treated with PBS liposome and clodronate liposome followed by Ang II (angiotensin II) infusion for 14 d. B, Quantification of maximal aortic width from aortas of IL12p40−/− control and clodronate-treated mice at day 14 of Ang II infusion (n=11). C, Abdominal aortic stiffness as measured by atomic force microscopy at day 14 of Ang II infusion in IL12p40−/− control and clodronate-treated mice (n=8). Representative H&E (hematoxylin and eosin) image (D–E), elastin staining (F–G), and Trichrome staining (H–I) from IL12p40−/− experimental mice at day 14 of Ang II infusion. **P<0.01, ***P<0.001 in Mann-Whitney test. Scale bar=50 μm.
Discussion
The goal of the current study was to examine the direct role of M2-like macrophages in the pathogenesis of Ang II-induced AAA using IL12p40−/− mice as a model. We preferred C57BL/6 mice over the well-known Apoe−/− mouse model because Apoe−/− mice have been shown to have a skew towards increased expression of M1-associated genes even at naive state.41 Moreover, the development of AAA in Apoe−/− mice is critically driven by M1-polarized macrophages. However, we aimed to determine the role of M2 macrophages in the fibrotic events independent of inflammation observed in Apoe−/− mice in response to Ang II. We demonstrated that predominance of M2-like macrophages in IL12p40−/− mice increases Tgfβ2 mediated fibrosis resulting in increased Mmp2 expression. These events lead to focal aortic enlargement along with medial destruction thus causing AAA development. IL12, composed of p35 and p40 subunits, is a well-known proin-flammatory cytokine important in controlling macrophage phenotype and T-cell effector functions.24 Previous studies have illustrated that the macrophages from p35 or p40 deficient mice have a bias towards the M2 activation profile.2527 Although, both the p35 and p40 deficient mice are associated with extensive cardiac fibrosis mediated by Tgfβ, this is the first study reporting the role of M2-like macrophages in the pathogenesis of AAA in IL12p40−/− mice.
The M2 macrophages are characterized by their involvement in tissue remodeling, immune regulation, tumor promotion, and phagocytic activity. These cells are generally intended to create anti-inflammatory environment and promote healing. However, when the lesion is persistent, M2 macrophages secrete large amounts of profibrotic factor, such as Tgfβ. The activation of Tgfβ signaling, a multifunctional cytokine, is implicated in various cellular process, such as proliferation, angiogenesis, and wound healing. Tgfβ signaling, a modulator of the structure and composition of the ECM, contributes to pathological tissue fibrosis of the heart, lung, and liver in many vascular diseases.42–44 Here, we demonstrated that in response to Ang II, activation of Tgfβ signaling and fibrotic events in the aorta of IL12p40−/− mice are associated with increased collagen deposition in the adventitial region. Similarly, we observed infiltration of macrophages in the aorta of IL12p40−/− mouse that was positive for Tgfp2 immunostaining. Significant increase in Tgfβ2 expression in the WT BMDMs transfected with IL12p40 siRNA suggested that Tgfβ2 is the major pathway affected by M2 macrophages associated with IL12p40 deficiency. Overall, these results implicated that Tgfβ2 may be one of the major players during pathogenesis of AAA in IL12p40−/− mouse.
The role of Tgfβ in AAA pathogenesis has been shown to be variable depending on the stage of the disease or the environment milieu of the vascular injury.45 The systemic inhibition of Tgfβ during Ang II infusion augmented expansion and rupture of abdominal aorta.46 Similarly, protective role of Tgfβ was reported in the C57BL/6 mice in response to Ang II.47 In addition, our previous studies have shown that Notch1 haploinsufficiency stabilizes AAA by promoting macrophage differentiation towards M2 phenotype mediated by Tgfβ2.31 However, genetic deletion of Tgfβ receptor signaling in SMCs did not accelerate AAA in elastase model of mice.48 Moreover, increased Tgfβ signaling is pathogenic in experimental Marfan syndrome of aortic aneurysm and is associated with abnormal aortic wall remodeling, dilatation, and aneurysm expansion.49 In the present study, we observed infiltration of M2-like macrophages is associated with increased Tgfβ2 and fibrosis leading to augmented AAA. Potential explanation for these variable results could be the cellular source of Tgfβ because many cells, including immune cells, vascular SMCs, (myo)fibroblasts, and platelets, contribute to Tgfβ production. Thus, the pathological effect of Tgfβ could be intrinsic to particular cell type. Similarly, a baseline level of Tgfβ signaling may be necessary to preserve aortic structure, but the excessive production may be pathogenic in vascular pathologies. In our study, excessive production of M2-macrophage specific Tgfβ2 could be a key process leading to fibrosis and enhancing the pathogenesis of AAA in IL12p40−/− mice. Both the classic and alternative pathways of Tgfβ signaling are known to play a role in regulating ECM remodeling.40 We observed increased immunostaining of both phospho-smad3 and phospho-p38 in IL12p40−/− tissues in response to Ang II. Because alternative Tgfβ signaling pathway is known to induce Mmp2 and Mmp9 expression,40,50 our study indicates that Tgfβ signaling leads to proteolytic destruction of elastin fragments by the production of Mmps. This is evident by increased activity of Mmp2 in macrophages and aortic rings from IL12p40−/− mice. Although Mmp9 activity was higher compared with Mmp2 in macrophages, Mmp2 was present only in IL12p40−/− BMDMs, which was eliminated by inhibition of Tgfp2. Similarly, the aortic rings experiment clearly showed the increased activity of Mmp2 in IL12p40−/− aortas. The increased Mmp2 activity in the aortic rings might also be contributed by vascular SMC-rich medial layer. It will be interesting to determine if macrophages directly influence vascular SMCs to secrete Mmps or it is mediated through secreted factors, such as Tgfp2. Evidently, further studies will highlight such interactions between macrophages and SMCs. Taken together, increased expression of Mmp2 in the aortic sections of IL12p40−/− mice and BMDMs with IL12p40 deficiency suggest that Mmp2 is the major contributor for elastin degradation. Thus, increased Tgfβ signaling associated Mmp2 may be one of the most important mechanisms leading to elastin degradation during AAA development in IL12p40−/− mice.
A critical balance between proinflammatory (M1) and anti-inflammatory (M2) macrophages may be a decisive factor in the initiation and progression of AAA over time.17 In the well-established Ang II-induced Apoe−/− mice model, proinflammatory Ml macrophages are the major vascular insult and are known to play a critical role in the initiation of AAA and is followed by fibrotic events modulated by M2 macrophages at the later stage of the disease.14 In contrast, in IL12p40−/− mice, fibrosis seems to be an early event and play a causal role during the AAA development. These observations are supported by the evidence that depletion of macrophage in IL12p40−/− mice led to decreased collagen deposition and reduced aortic stiffness. Aortic stiffness has been shown to be an early event in the development of AAA51; however, direct relationship between fibrosis and aortic stiffness has not been established. It is likely that the early fibrotic event mediated by Tgfp2 and increased Mmp2 expression may be leading to elastin degradation and structural abnormality in the IL12p40−/− mice. This might be true as we did not see a major structural abnormality and elastin degradation in response to macrophage depletion in IL12p40−/− mice at day 14. These data support the role of M2 macrophages in the fibrosis of aortic tissue and remodeling followed by the structural abnormality leading to AAA.
In conclusion, deficiency of IL12p40 is associated with exacerbated AAA in C57BL/6 mice as shown by excessive accumulation of ECM leading to pathological matrix remodeling, elastin degradation, and fragile vascular wall. The development of AAA in IL12p40−/− mice in response to Ang II may be a useful experimental model to address the role of M2 macrophages in AAA progression. It will be interesting to examine the precise and direct role of M2 macrophages and Tgfβ2 activity in chemical (elastase and CaCl2) models of AAA. Similarly, it will be interesting to examine the downstream mechanisms and targets of M2 macrophage-mediated Tgfβ activity in promoting the AAA. Overall, these novel roles of M2 macrophages demonstrated in our study open up new avenues to understand AAA pathogenesis. Although there is limited information available on the direct roles of IL12 in human AAA, our study indicate that a careful examination of the aortic aneurysm is required with regard to the inflammatory and fibrotic component to treat AAA in the clinical settings.
Supplementary Material
Highlights.
IL12p40−/− (interleukin 12 p40) mice depict M2-predominant macrophage phenotype.
Deficiency of IL12p40 augments Ang II (angiotensin II)—induced abdominal aortic aneurysm in C57BL/6 mice.
The development of abdominal aortic aneurysm in IL12p40−/− mice is associated with enhanced fibrotic events and medial layer degeneration via upregulation of Tgfβ2 (transforming growth factor β) and Mmp2 (matrix metalloproteinase 2).
Our findings suggest that M1/M2 macrophage balance rather than predominance of 1 phenotype is necessary for tissue homeostasis.
Acknowledgments
We thank Zhe Sun at imaging core facility at Dalton Cardiovascular Research Center for capturing the ultrasound images and Cell and Immunology Core facility, University of Missouri for flow cytometry data.
Sources of Funding
This work was supported by Grants R01HL124155 (C.P. Hans), 1R01HL118376 (L. Pulakat), 1R01HL138988 (L. Pulakat), Veterans Affairs Merit System (BX003391; A. Whaley-Connell), and funding from the Research Institute at the University of Missouri to C.P. Hans.
Nonstandard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- AFM
atomic force microscopy
- Ang II
angiotensin II
- BMDM
bone marrow-derived macrophage
- ECM
extracellular matrix
- IFN-γ
interferon-γ
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- Mmp
matrix metalloproteinase
- PCR
polymerase chain reaction
- PWV
pulse wave velocity
- Smad
mothers against decapentaplegic homolog 3
- SMC
smooth muscle cell
- Tgfβ
transforming growth factor β
- Timp
tissue inhibitor of metalloproteinase
- WT
wild type
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.118.311969.
Disclosures
None.
Contributor Information
Neekun Sharma, Department of Cardiovascular Medicine, University of Missouri, Columbia.
Rishabh Dev, Department of Cardiovascular Medicine, University of Missouri, Columbia.
Anthony M. Belenchia, Department of Cardiovascular Medicine, University of Missouri, Columbia
Annayya R. Aroor, Department of Medical Pharmacology and Physiology, University of Missouri, Columbia
Adam Whaley-Connell, Harry S. Truman Memorial Veterans’ Hospital, University of Missouri, Columbia.
Lakshmi Pulakat, Department of Cardiovascular Medicine, University of Missouri, Columbia; Dalton Cardiovascular Research Center, University of Missouri, Columbia.
Chetan P. Hans, Department of Cardiovascular Medicine, University of Missouri, Columbia Department of Medical Pharmacology and Physiology, University of Missouri, Columbia; Dalton Cardiovascular Research Center, University of Missouri, Columbia.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)664598 [DOI] [PubMed] [Google Scholar]
- 3.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. CurrProbl Surg. 2002;39:110–230. [DOI] [PubMed] [Google Scholar]
- 4.Moxon JV, Parr A, Emeto TI, Walker P, Norman PE, Golledge J. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol. 2010;35:512–548. doi: 10.1016/j.cpcardiol.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel JB, Martin-Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U, Cockerill G, Swedenborg J; FAD EU Consortium. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuivaniemi H, Platsoucas CD, Tilson MD III. Aortic aneurysms: an immune disease with a strong genetic component. Circulation. 2008;117:242–252. doi: 10.1161/CIRCULATI0NAHA.107.690982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correction to: heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e493. [DOI] [PubMed] [Google Scholar]
- 8.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 9.Forester ND, Cruickshank SM, Scott DJ, Carding SR. Functional characterization of T cells in abdominal aortic aneurysms. Immunology. 2005;115:262–270. doi: 10.1111/j.1365-2567.2005.02157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale MA, Ruhlman MK, Baxter BT. Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol. 2015;35:1746–1755. doi: 10.1161/ATVBAHA.115.305269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raffort J, Lareyre F, Clément M, Hassen-Khodja R, Chinetti G, Mallat Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017;14:457–471. doi: 10.1038/nrcardio.2017.52 [DOI] [PubMed] [Google Scholar]
- 12.Turner GH, Olzinski AR, Bernard RE, Aravindhan K, Boyle RJ, Newman MJ, Gardner SD, Willette RN, Gough PJ, Jucker BM. Assessment of macrophage infiltration in a murine model of abdominal aortic aneurysm. J Magn Reson Imaging. 2009;30:455–460. doi: 10.1002/jmri.21843 [DOI] [PubMed] [Google Scholar]
- 13.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin II in apoE(−/−) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hans CP, Koenig SN, Huang N, Cheng J, Beceiro S, Guggilam A, Kuivaniemi H, Partida-Sanchez S, Garg V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arterioscler Thromb Vasc Biol. 2012;32:3012–3023. doi: 10.1161/ATVBAHA.112.254219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Z, Bagley J, Sukhova G, Baur WE, Park HJ, Beasley D, Libby P, Zhang Y, Galper JB. Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J Mol Cell Cardiol. 2015;87:160–170. doi: 10.1016/j.yjmcc.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, Casale GP, Baxter BT. Elastin-derived peptides promote abdominal aortic aneurysm formation by modulating M1/M2 macrophage polarization. J Immunol 2016;196:4536–4543. doi: 10.4049/jimmunol.1502454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boytard L, Spear R, Chinetti-Gbaguidi G, Acosta-Martin AE, Vanhoutte J, Lamblin N, Staels B, Amouyel P, Haulon S, Pinet F. Role of proinflammatory CD68(+) mannose receptor(−) macrophages in peroxiredoxin-1 expression and in abdominal aortic aneurysms in humans. Arterioscler Thromb Vasc Biol. 2013;33:431–438. doi: 10.1161/ATVBAHA.112.300663 [DOI] [PubMed] [Google Scholar]
- 19.Dutertre CA, Clement M, Morvan M, Schäkel K, Castier Y, Alsac JM, Michel JB, Nicoletti A. Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta. PLoS One. 2014;9:e89983. doi: 10.1371/journal.pone.0089983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATI0NAHA.107.735274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH; Pharmaceutical Aneurysm Stabilisation Trial Study Group. Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med. 2013;159:815–823. doi: 10.7326/0003-4819-159-12-201312170-00007 [DOI] [PubMed] [Google Scholar]
- 22.Lindeman JH, Rabelink TJ, van Bockel JH. Immunosuppression and the abdominal aortic aneurysm: Doctor Jekyll or Mister Hyde? Circulation. 2011;124:e463–e465. doi: 10.1161/CIRCULATI0NAHA.110.008573 [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 24.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 2002;71:271–278. [PubMed] [Google Scholar]
- 26.Li Y, Zhang C, Wu Y, Han Y, Cui W, Jia L, Cai L, Cheng J, Li H, Du J. Interleukin-12p35 deletion promotes CD4 T-cell-dependent macrophage differentiation and enhances angiotensin II-Induced cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1662–1674. doi: 10.1161/ATVBAHA.112.249706 [DOI] [PubMed] [Google Scholar]
- 27.Huaux F, Arras M, Tomasi D, Barbarin V, Delos M, Coutelier JP, Vink A, Phan SH, Renauld JC, Lison D. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol. 2002;169:2653–2661. [DOI] [PubMed] [Google Scholar]
- 28.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJ, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–269. [DOI] [PubMed] [Google Scholar]
- 29.Carlson S, Helterline D, Asbe L, Dupras S, Minami E, Farris S, Stempien-Otero A. Cardiac macrophages adopt profibrotic/M2 phenotype in infarcted hearts: role of urokinase plasminogen activator. J Mol Cell Cardiol. 2017;108:42–49. doi: 10.1016/j.yjmcc.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 30.Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am J Physiol Heart Circ Physiol. 2015;309:H906–H917. doi: 10.1152/ajpheart.00821.2014 [DOI] [PubMed] [Google Scholar]
- 31.Cheng J, Koenig SN, Kuivaniemi HS, Garg V, Hans CP. Pharmacological inhibitor of notch signaling stabilizes the progression of small abdominal aortic aneurysm in a mouse model. J Am Heart Assoc. 2014;3:e001064. doi: 10.1161/JAHA.114.001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from ATVB council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. doi: 10.1161/ATVBAHA.117.309524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aroor AR, Jia G, Habibi J, Sun Z, Ramirez-Perez FI, Brady B, Chen D, Martinez-Lemus LA, Manrique C, Nistala R, Whaley-Connell AT, VG Demarco, GA Meininger, JR Sowers. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in western diet fed mice. Metabolism. 2017;74:32–40. doi: 10.1016/j.metabol.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1281–H1287. doi: 10.1152/ajpheart.00232.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutshumba J, Liu S, Zhong Y, Hou T, Daugherty A, Lu H, Guo Z, Gong MC. Deletion of BMAL1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38:1063–1075. doi: 10.1161/ATVBAHA.117.310153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64 [DOI] [PubMed] [Google Scholar]
- 37.Sachdeva J, Mahajan A, Cheng J, Baeten JT, Lilly B, Kuivaniemi H, Hans CP. Smooth muscle cell-specific Notch1 haploinsufficiency restricts the progression of abdominal aortic aneurysm by modulating CTGF expression. PLoS One. 2017;12:e0178538. doi: 10.1371/journal.pone.0178538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma N, Trinidad CV, Trembath AP, Markiewicz MA. NKG2D signaling between human NK cells enhances TACE-mediated TNF-a release. J Immunol. 2017;199:2865–2872. doi: 10.4049/jimmunol.1700647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones JA, Spinale FG, Ikonomidis JS. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J Vasc Res. 2009;46:119–137. doi: 10.1159/000151766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baitsch D, Bock HH, Engel T, Telgmann R, Müller-Tidow C, Varga G, Bot M, Herz J, Robenek H, von Eckardstein A, Nofer JR. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783. doi: 10.1172/JCI94753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, Elias JA, Hogaboam CM, Herzog EL. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43:154–162. doi: 10.1016/j.biocel.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 44.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 45.Daugherty A, Chen Z, Sawada H, Rateri DL, Sheppard MB. Transforming growth factor-ß in thoracic aortic aneurysms: good, bad, or irrelevant? J Am Heart Assoc. 2017;6:e005221. doi: 10.1161/JAHA.116.005221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. TGF-β neutralization enhances angII-induced aortic rupture and aneurysm in both thoracic and abdominal regions. PLoS One. 2016;11:e0153811. doi: 10.1371/journal.pone.0153811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadière C, Rénia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao F, Chambon P, Offermanns S, Tellides G, Kong W, Zhang X, Li W. Disruption of TGF-β signaling in smooth muscle cell prevents elastase-induced abdominal aortic aneurysm. Biochem Biophys Res Commun. 2014;454:137–143. doi: 10.1016/j.bbrc.2014.10.053 [DOI] [PubMed] [Google Scholar]
- 49.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116 [DOI] [PubMed] [Google Scholar]
- 50.Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGF-β 1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. doi: 10.1186/1471-2407-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raaz U, Zöllner AM, Schellinger IN, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation. 2015;131:1783–1795. doi: 10.1161/CIRCULATIONAHA.114.012377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


