Abstract
The idea of developing therapeutic vaccines against cancer has been explored since the early discovery of tumor-specific antigens by Georg Klein in 1967. However, challenges including weak immunogenicity, systematic toxicity, and off-target effects of cancer vaccines remain as barriers to their broad clinical translation. The emerging field of biomaterials has led to advancements in many different biomedical applications, and it may also help cancer vaccines overcome the various aforementioned challenges. Here, we discuss the rational design and clinical status of several classes of cancer vaccines (i.e. DNA, mRNA, peptide/protein, cell-based), along with novel biomaterial-based delivery platforms that improve their safety and efficacy. Further, strategies for designing new platforms for personalized cancer vaccines are also considered.
Keywords: Cancer vaccine, Immunotherapy, Biomaterials, Targeted delivery, Personalized therapy, Translational research
Graphical Abstract
2. Introduction
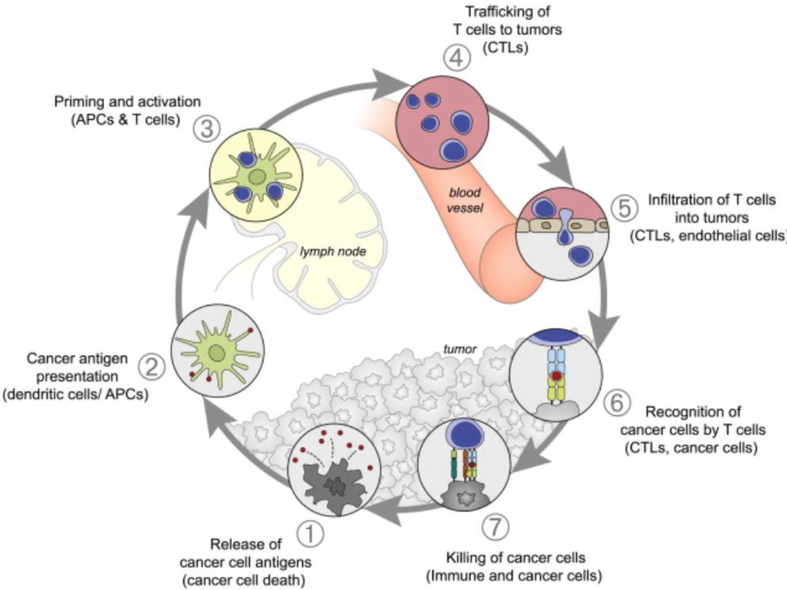
Vaccines have made a tremendous contribution to global health, having led to the elimination of small pox and near eradication of polio and diphtheria[2, 3]. While these traditional whole-pathogen based vaccines against infectious diseases have proven successful, most cancer vaccines have shown disappointing clinical outcomes[4]. This is likely due to a number of factors, including various biological barriers[5, 6], inherently low tumor antigen immunogenicity[7, 8], and the immunosuppressive tumor microenvironment[7, 9]. For a cancer vaccine to be effective, a number of key requirements must be satisfied in order to induce the desired immune response illustrated in Fig 1. First, antigens need to be delivered to antigen presenting cells (APCs), which most notably include dendritic cells (DCs) but also macrophages, neutrophils, and lymphatic endothelial cells to a lesser extent [10, 11]. Subsequently, APCs must process and cross-present tumor antigens to become mature and activate T cells (naïve CD4+ T cells and CD8+ T cells) that reside in lymph nodes (LNs)[12]. Lastly, activated T helper cells (Th cells) and cytotoxic T lymphocytes (CTLs) need to infiltrate the tumor site, shifting the immunosuppressive tumor microenvironment towards a pro-inflammatory environment [13, 14]. This alteration in the microenvironment aids CTLs in killing tumor cells and is accompanied by other mechanisms for tumor cell killing (e.g. natural killer cell killing, antibody-dependent cell-mediated cytotoxicity)[13, 14]. While this approach to treating a range of cancers holds considerable promise, only one cancer vaccine formulation to date has been approved by the US Food and Drug Administration (FDA) over several decades of investigation[15]. A major reason for previous cancer vaccine failures is inefficient delivery in vivo where administered vaccines cannot successfully reach their desired targets[16–19]. Therefore, immunologists, engineers, and clinicians in recent years have focused significant efforts towards developing new delivery materials for the next generation of cancer vaccines[18].
Fig. 1.
This schematic of the cancer-immunity cycle illustrates the immune response to a tumor. Ideally, successful biomaterials-based vaccine delivery technologies would enhance cancer antigen presentation. Adapted from[1]. Reprint with permission from Cell Press.
Over the last decade, there has been exponential growth at the interface of biomaterial science, drug delivery, and cancer vaccines[20–34]. Various delivery approaches, such as nanoparticles[35], microparticles[36], self-assembled materials[37, 38], and biomaterial scaffolds[39] have been widely utilized in combination with various forms of cancer vaccines (e.g. DNA, mRNA, peptide/protein, cell based), and their preclinical outcomes are promising. Researchers have demonstrated that biomaterial-based cancer vaccines have many key advantages over conventional vaccines[21, 39]. Most notably, biomaterial based cancer vaccines can be delivered to the body in a controlled manner where finely tuning vaccine physical properties (e.g. size, shape, charge, or porosity) and targeting moieties can achieve selective delivery to specific tissues with desirable drug release kinetics[40–48]. In this review article, we introduce various classes of vaccines and their clinical status (Table 1), highlight the advances made at the interface of biomaterials and cancer vaccines, summarize key design criteria for biomaterials-based delivery platforms, and provide our insights into the future directions of cancer vaccine development.
Table 1.
Different types of cancer vaccines in clinical development. *Denotes examples mentioned in the text
| Type of Vaccine | Cancer | Design | Biomaterial Delivered |
Delivery Strategy | Trial Number (Phase) |
|---|---|---|---|---|---|
| DNA Vaccine | Melanoma | Plasmid DNA encoding gp100 | Naked plasmids or gold particles | Intramuscular injection or epidermal application of powder (with device) | NCT00398073 (Phase 1) |
| *Plasmid encoding tyrosinase 207–216, 1–17 | Naked Plasmids | Intranodal by pump at varying concentrations | NCT00023647 (Phase 1) | ||
| Metastatic Breast | Plasmid DNA encoding mammaglobin-A | Nakedplasmids | Intramuscular injection with jet delivery device | NCT00807781 (Phase 1) | |
| Breast/Ovarian | Plasmid-based DNA encoding HER-2/neu protein + GM-CSF | Naked DNA | Not specified | NCT00436254 (Phase 1) | |
| Ovarian | DNA encoding HPV E7 antigen | Naked DNA | Intradermal gene gun, intramuscular, intralesional injection | NCT00988559 (Phase 1) | |
| Prostate | *DNA encoding PAP+GM-CSF | Naked DNA | Intradermal injection | NCT00849121 (Phase 2) | |
| Merkel Cell | Plasmid DNA encoding intratumoral IL-2 gene | Naked Plasmid | Intratumoral injection with electroporation | NCT01440816 (Phase 2) | |
| Cervical | *Plasmid DNA encoding Sig and HSP70 | Naked Plasmid | Intramuscular injection | NCT00121173 (Phase 1/2) | |
| Lympohoma-B-Cell | *Plasmid DNA encoding CD20 | Naked Plasmid | Intramuscular injection | NCT00561756 (Phase 1) | |
| mRNA Vaccine | Melanoma | *Melanoma associated antigen mRNA | Naked mRNA | Intranodal injection | NCT01684241 (Phase 1) |
| 4 different mRNA drugs to induce T-Cell response | mRNA in liposomes(Lipo-MERIT) | Intraveneous injection | NCT02410733 (Phase 1) | ||
| mRNA for melanoma associated tumor antigen + GM-CSF | naked mRNA | Subcutaneous injection | NCT00204516 (Phase 1/2) | ||
| Melanoma associated tumor antigen mRNA + GM-CSF | Protamine-stabilized mRNA | Intradermal injection | NCT00204607 (Phase 1/2) | ||
| Breast | *Breast cancer associated tumor antigen mRNA | mRNA in liposomes | Not specified | NCT02316457 (Phase 1) | |
| Prostate | *RNActive (self-adjuvanting mRNA) | Naked and protamine-stabilized mRNA | Intradermal injection | NCT00831467 (Phase 1/2) | |
| Non-Small Cell Lung | * 6 RNAactive (self-adjuvanting mRNA) components | Naked mRNA complex | Intradermal injection and radiation | NCT01915524 (Phase 1) | |
| Peptide/Protein Vaccine | Ovarian/Tubal/Peritoneal | *12 different tumor-rejection peptides known to be presented on ovarian cells | Naked peptide | Intradermal/subcutaneous injection | NCT00437502 (Phase 1) |
| Any Malignant Tumor | NY-ESO-1 protein + CpG + montanide | Naked protein | Intradermal injection | NCT00299728 (Phase 1) | |
| Esophageal, Stomach, Breast, etc | CHP-HER2/CHP-NY-ESO-1 protein + adjuvant OK-432 | Naked proteins | Subcutaneous injection | NCT00291473 (Phase 1) | |
| Multiple Myeloma | MAGE-A3/NY-ESO-1 peptide | Naked peptides | Subcutaneous injection | NCT00090493 (Phase 2/3) | |
| Melanoma | Melanoma associated tumor antigen peptide + GM-CSF | Naked peptides | Subcutaneous injection | NCT01989572 (Phase 3) | |
| gp100 Peptide + anti-CTLA4 | Naked peptides | Intradermal injection intravenous infusion | NCT00094653 (Phase 3) | ||
| *6 Melanoma “helper” peptides + GM-CSF | Naked peptides | Not specified | NCT00089219 (Phase 1/2) | ||
| Colon Adenoma | *MUC1 TAA peptide + adjuvant Poly ICLC | Naked peptide | Subcutaneous injection | NCT00773097 (Phase 2) | |
| Dendritic cell vaccine | Multiple Myeloma | Plasmacytoma cells and DCs from patient injected with GM-CSF | Mixed cells | Intradermal injection | NCT00459069 (Phase 1) |
| Metastatic Breast | Tumor blood vessel antigen pulsed DCs injected after chemotherapy | Modified cells | Intravenous infusion | NCT02479230 (Phase 1) | |
| Prostate | DCs pulsed with tumor lysates expressing cancer/testis antigen | Modified cells | Not specified | NCT01883518 (Phase 1/2) | |
| Renal Cell | *DCs electroporated with RNA | Modified cells | Intradermal injection | NCT01582672 (Phase 3) | |
| Lung | DCs pulsed with lung cancer cells | Modified cells | Intradermal injection | NCT00103116 (Phase 2) | |
| Lymphoma | DCs pulsed with lymphoma cell lysate + IL-2 | Modified cells | Not Specified | NCT00006434 (Phase 3) | |
| Tumor cell Vaccine | Pulmonary Metastases of Melanoma | *Hapten dinitrofluorobenzene modified cancer cells | DNP-modified cells | Intradermal injection | NCT00298298 (Phase 1/2) |
| Ovarian | *Ovarian tumor cells modified with bi-shRNA | Modified cells | Intradermal injection | NCT01867086 (Phase 2) | |
| Kidney | B7–1 gene-modified cancer cells + IL-2 | Modified Cells | Subcutaneous injection | NCT00031564 (Phase 2) | |
| Melanoma | Iradiated melonama cells +Bacillus Calmette Guérin+ GM-CSF +IFN-a2b | Modified and dead cells | Subcutaneous injection | NCT01729663 (Phase 2/3) | |
| Genetically modified melonoma cells expressing HLA A2/4–1BB ligand | Modified cells | Not specified | NCT01861938 (Phase 2/3) | ||
| Colon | Radiated but live colorectal cancer cells | Modified cells | Intradermal injection | NCT02448173 (Phase 3) |
3. Different types of vaccines and their clinical status
DNA vaccines
DNA vaccines were first developed in the early 1990s[49], when researchers found that plasmid DNA can induce potent antibody responses against an encoded antigen[49–51]. The design simplicity and promising pre-clinical studies quickly sparked an interest in developing DNA vaccines for a variety of infectious diseases[52, 53]. Consequently, utilizing DNA vaccines to combat cancer has become an attractive strategy for cancer immunotherapy[54]. When DNA contains unmethylated, repeating “cytosine-guanine” regions, they cause adjuvant effects that stimulate the innate immune system[55]. As such, plasmid DNA can be designed to act as both antigen and adjuvant[56]. However, due to its low molecular weight and negatively charged backbone, naked DNA typically yields low cellular uptake, off-target effects, and systemic dissemination[57–60]. Therefore, various efforts have focused on developing methods of effectively introducing plasmid DNA into antigen presenting cells (APCs). One commonly used strategy to enhance DNA uptake is electroporation (EP), which temporarily permeabilizes cell membranes with an electric pulse[61, 62]. EP has been shown to increase antigen delivery by 100–1,000 fold compared to naked DNA vaccines alone[63]. Moreover, EP has adjuvant-like properties because it induces moderate tissue injury and generates pro-inflammatory cytokines, which recruit APCs at the injection site[64]. Another promising DNA delivery strategy is gene gunning where plasmid DNA is coated with heavy metals (e.g. gold particles) and bombarded into APCs at the injection site, which decreased the required plasmid DNA dose by 100–1,000 fold[65, 66]. Although a variety of strategies have been developed to improve DNA vaccine delivery, these vaccines still possess low immunogenicity profiles in human trials for reasons not yet fully understood[58, 67]. As such, only few DNA vaccines have advanced beyond phase I or phase II clinical trials[68].
Despite the obstacles to their efficacy, the stability, scalability, and inexpensive manufacturing of DNA vaccines have led to their further development and investigation[68]. Because DNA vaccines have been extensively explored, their safety is largely accepted, which has allowed a number of clinical trials to combine phase I and phase II stages to focus on evaluating efficacy over toxicity[69]. Though the first DNA vaccine for cancer (ONCEPT®) was approved in 2010 by the United States Department of Agriculture for canine melanoma based off of data from nonrandomized clinical trials, the same success has not been found using the vaccines to target human cancers[68, 70]. Phase I and II clinical trials have been used to observe the vaccines for numerous cancer types including melanoma[71], prostate[68], lymphoma[72], and cervical[73, 74], but most cases have shown little clinical efficiency[39, 69, 74]. Given that the most common side effects of the vaccines include fever, pain, and redness or swelling of the injection sites rather than more severe consequences like systemic toxicity, it is clear that the main issue in clinical trials continues to be therapeutic efficacy rather than toxicity[68, 69]. The aforementioned methods of EP and gene gunning have been implemented in clinical trials in an attempt to increase therapeutic effects, and both have shown promise. EP has been used in nearly half of the current DNA vaccine clinical trials and has shown an ability to increase the immunological response induced by DNA vaccines for prostate cancer and melanoma[75]. Additionally, promising pre-clinical data has led to phase I and II clinical trials for gene gunning in head and neck squamous cell carcinoma and cervical cancer[73]. Thus, the continued improvement of EP and gene gunning strategies or the investigation of alternative delivery mechanisms such as biomaterial-based vehicles[75–77] and DNA sequence optimization[75, 78] is necessary to improve vaccine immunogenicity for a broader range of cancers.
mRNA vaccines
mRNA vaccines are another promising alternative to conventional vaccine approaches. One of the first reports on mRNA cancer vaccines was from the late 1990s, shortly after the discovery of DNA cancer vaccines[79]. One major advantage of mRNA over DNA vaccines is that mRNA does not need to cross the nuclear barrier to induce protein expression[80]. Therefore, mRNA can be transfected more efficiently than plasmid DNA, especially for slowly dividing cells[81]. Currently two types of mRNA are commonly utilized in vaccines: non-replicating and self-amplifying[82]. While self-amplifying mRNA is commonly used in prophylactic vaccines for infectious diseases[83–87], most mRNA cancer vaccines use non-replicating mRNA [88–92]. One of the most explored topics in non-replicating mRNA vaccines is sequence modification, as the innate immune system can sense unmodified mRNA and induce a robust type 1 interferon response, which reduces mRNA transfection efficacy[89]. Thus, several modifications—such as including 5’ caps, optimized 5’ and 3’ untranslated regions (UTRs), poly(A) tail additions, and the incorporation of pseudouridine sequences—have been utilized to increase mRNA stability and reduce immune sensing by toll-like receptors (TLRs), rig-like receptors (RIG-1), and protein kinase RNA-activated receptors (PKR)[93–96]. Other research also demonstrated that removing double-stranded RNA (dsRNA) from mRNA vaccines is essential for improving their therapeutic effect, as dsRNA is a potent pathogen-associated molecular pattern that significantly suppresses mRNA translation[89, 97–99]. While immune sensing is detrimental to mRNA transfection, it also provides a danger signal to the host which plays an important role in improving vaccine efficacy[100]. Therefore, an important step in the development of mRNA vaccines is finding the appropriate level of immune sensing that will maximize its danger signaling while minimizing its impact on mRNA transfection.[82]. Another critical step in the improvement of mRNA vaccines is addressing delivery challenges similar to those faced with DNA vaccines. Beyond conventional EP and gene gunning approaches, a variety of biomaterial-based delivery systems such as liposomes and polymeric nanoparticles have been extensively studied, and the preclinical outcomes are quite promising[101–103].
More recently, lipid nanoparticles (LNP) have emerged as a promising delivery platform for mRNA vaccines, built off of recent success in delivering siRNAs in vivo and promising phase III clinical trials of siRNA-LNP patisiran by Alnylam Pharmaceuticals[104–108]. Though LNP based mRNA vaccines are in early stages of development, they have shown great promise for a range of disease including multiple types of cancer[80, 90, 92, 109], as well as Zika, Ebola, and influenza[110–113]. The success of LNP delivery platforms in cancer vaccines, such as those for breast cancer[82], is likely due to their ability to increase mRNA cargo retention time in vivo[109] and enhance mRNA cytosolic delivery[114]. Drawbacks to LNPs include their accumulation in off-target organs such as the liver, and some instances of allergic reactions in human patients[82, 109]. In clinical trials using naked mRNA in the absence of a delivery vehicle, such as the intranodally injected mRNA vaccine for advanced melanoma[82], repetitive injections have yielded promising results but present larger issues with convenience, cost, and off-target effects[82]. As with DNA, mRNA vaccine efficacy is highly variable between animal models and human clinical trials, as the method of mRNA uptake into the cytoplasm depends heavily on cell type[82]. Thus, though LNPs have promising preclinical data and have shown some translatability to clinical settings, additional methods for improving efficacy in human trials has been investigated[82]. One major development for mRNA vaccines has been RNActive (first developed by CureVac)—a self-adjuvanted mRNA vaccine that includes both free mRNA and mRNA strands complexed with cationic protamine[115, 116]. In phase I trials for stage IV non-small cell lung cancer and phase I/II trials for prostate cancer, RNActive has shown its ability to induce immune response and encourage longer survival time for patients[115, 116]. With multiple modification methods to improve mRNA preparation, delivery, and overall efficacy, future work must explore how these techniques can come together to fully optimize mRNA cancer vaccines.
Peptide and protein vaccines
Peptide and protein based cancer vaccines employ either fragments of proteins or whole proteins that are specifically expressed on tumor cells as antigen sources[117]. Peptide vaccines are usually chemically synthesized due to their short length, which is both time and cost effective[118]. In contrast, protein vaccines are often obtained by using more complex recombinant protein expression approaches[119]. The distinct advantage of both peptide and protein vaccines is their high level of safety, which has been shown in many pre-clinical and clinical studies[118–120]. However, one major drawback of peptide and protein vaccines is that they usually only target one or few epitopes of tumor associated antigen (TAA)[121]. Because it is generally believed that multivalent antigen-specific CTL responses are necessary for cancer vaccine efficacy, a mixture of multiple antigens (peptides or proteins) is required to achieve desirable effects[121–123]. Additionally, though peptides and proteins do not have negatively charged backbones like DNA and mRNA, delivery vehicles are still necessary to improve vaccine stability and targeting and reduce off-target effects[124–127].
In clinical trials for peptide-based cancer vaccines, a number of the aforementioned limitations remain. Most clinical trials in progress rely primarily on TAA-derived short peptides, with only a few investigating liposome-based delivery or longer peptide formulations[128]. Many of these vaccines fail when they reach phase III trials due to a lack of optimization of peptide formulation, vaccination schedule, peptide combination, or adjuvant selection[39]. However, some early clinical trials have produced promising results. A mucin 1 TAA peptide prophylactic vaccine for colon cancer was highly immunogenic in half the trial’s 39 individuals and was able to elicit a long-term anti-tumor memory, which is important for cancer prevention[129]. Similarly, two phase I/II trials illustrated that administering peptide vaccines for melanoma and ovarian cancer—which used a combination of 6 and 12 peptides, respectively—led to an increase in overall patient survival[130, 131]. Though these promising early-stage results encourage the further investigation of peptide vaccines, most of the vaccines that induce an immune response do not provide enough of a clinical benefit to be used alone[128]. Thus, further optimization of vaccines – along with the development of combination therapies - is needed.
DC vaccines
The major target cell type for the previously described vaccines are DCs, which are essential for initiating anti-tumor immunity[132]. Thus, “vaccinating” DCs ex vivo is likely a more effective approach than administrating vaccines in vivo, where only a small portion of vaccines reach DCs. Those ex vivo treated DCs are called DC vaccines, and to prepare them, a patient’s own DCs are isolated, co-cultured with antigens (e.g. DNA, mRNA, peptides, or proteins) and adjuvants (e.g. TLR agonists or cytokines), matured, and then loaded with TAAs[133, 134]. The treated DCs are then delivered back to the patient, where they migrate to the LN and prime naïve CD8 T cells to initiate anti-tumor immunity[134, 135]. The most distinct advantage of DC vaccines is that the DCs are treated in vitro, so there is less concern over off-target effects than with other vaccines that require vaccine components to be administered directly into patients[135]. However, major challenges of DC vaccine development include the complexity and substantial cost of cell biomanufacturing processes and the batch-to-batch variability between vaccines for individual patients[136]. Although the first DC based cancer vaccine (Sipulencel T) was approved by FDA for the treatment of metastatic prostate cancer in 2010[15], their commercialization is limited to only a few developed countries, in part due to the high cost of treatment and the strict manufacturing requirements for the vaccine production facilities[137].
Because DC vaccine production methods and the resulting composition vary greatly, it is difficult to compare clinical trials or generalize their outcomes. While success has been found with Sipulencel T and promising preliminary data emerges from phase I/II clinical trials[138], there have been a number of notable failures. Argos Therapeutics has had to pause their phase III clinical trial of a DC vaccine for renal cell carcinoma in response to the poor interim evaluation of the patients, which conflicts with promising results from earlier trials[139]. Similarly, phase III results from a clinical trial for a DC vaccine against melanoma showed that the therapy had no significant impact on patient survival or markers of recovery[138, 140]. The failures of these studies however, could be due to the complex process of obtaining, maturing, and treating DCs. Because DCs can be loaded with antigens (e.g. DNA, mRNA, peptide, protein, tumor lysate), or fused with live cancerous cells to generate hybrid cells, there has yet to be a unified, perfected procedure for handling them[138]. Thus, a big focus in DC vaccine development is for the optimization of ex vivo DC protocols[39].
Tumor cell vaccines
Another approach for designing cancer vaccines is utilizing TAAs from isolated tumor cells that have been either resected from patients (autologous tumor cells) or lab-grown (allogeneic tumor cells) as antigen sources[141–143]. Because live tumor cells can produce immune-suppressive cytokines and potentially form new tumors in the body, they must be inactivated before vaccination[144]. The freeze-thaw method is one of the most commonly used strategies for killing tumor cells and obtaining TAAs [142]. The repeated freezing and thawing of tumor cells induces necrotic cell death and releases cellular compartments that contain TAAs[142, 145, 146]. Tumor cell debris and TAAs are then separated by centrifugation, and TAAs are collected from the supernatant[147–149]. Another commonly used method to trigger tumor cell death is irradiation, which induces apoptosis [142]. Instead of obtaining soluble tumor lysate antigens like the freeze-thaw method, irradiation is milder and allows for whole tumor cells to be obtained[142]. Both methods are commonly used to obtain TAAs, and many strategies have streamlined the loading of collected TAAs onto biomaterial delivery platforms such as nanoparticles or scaffolds for applications in cancer vaccines[147–149]. One major advantage of utilizing tumor cells as antigen sources is that, since there is an array of mutated tumor antigens presented on tumor cells, they can generate synergistic immune responses against multiple tumor antigens, reducing the risk of tumor escape[144]. Additionally, if the tumor cells are autologous, anti-tumor immunity can be more individualized, which is considered more immunogenic than using universal tumor antigens [150]. Despite these advantages, drawbacks to using tumor cells also exist. For autologous tumor cells, similar to DC vaccines, the commercialization process can be challenging due to the high cost and strict requirements of production[150]. By contrast, allogeneic tumor cell vaccines—though they can be produced at a lower cost and faster pace[150]—may not contain patient-specific antigens, making them less effective[143, 151].
As with DC vaccines, tumor cell vaccines vary widely in preparation and ex vivo treatment, making clinical trials very challenging to directly compare or generalize[126]. However, highly individualized vaccines have had a number of notable successes[7]. The GVAX vaccine—an allogeneic prostate tumor cell line that has been modified to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF)—has had successful phase I and II trials that were able to increase the mean survival of patients with prostate cancer by 26 months[152]. The results have led to GVAX being investigated in a broader array of cancer types in a number of phase I clinical trials[142]. Similarly, the FANG vaccine—a whole tumor cell vaccine with plasmid DNA as well as RNA incorporated into it—showed promising phase I results when used to treat a number of cancer types including ovarian, breast, colorectal, and small cell lung cancer, and it has progressed into a phase II trial for treating melanoma, ovarian cancer, and colorectal carcinoma[153]. While these vaccines tend to have varying efficacies based on individual patients and cancer types, they may also provide insight into future optimization[142]. For example, a phase II clinical trial on the regression of pulmonary metastases in patients with melanoma reported anti-tumor responses in only 11 of the 89 patients in the study[154]. However, the study was able to correlate small volume lung metastases with an increased likelihood of responding the vaccine[154]. Thus, future studies using such vaccines should focus on specific subpopulations where the vaccine will likely prove effective while potentially exploring other treatment modalities for patients outside of that subpopulation[142].
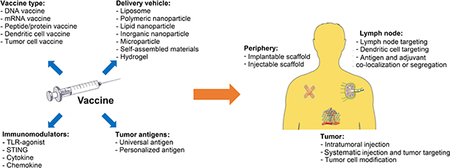
4. Bridging Biomaterials and Cancer vaccines
As discussed in the previous section, a considerable number of cancer vaccine trials have shown negative outcomes, in part due to a lack of effective delivery methods [155]. Peptide cancer vaccines provide a prime example. When unmodified and naked peptides are delivered, the overall clinical response rate is roughly 3%[156]. However, if a patient’s DCs are isolated, treated with peptides ex vivo, and infused back into the patient, an improved clinical response rate is observed[157]. This difference in patient response indicates that naked peptides have difficulty reaching DCs in vivo, which may be one reason for their low efficacy in the absence of a DC delivery platform[157]. Therefore, biomaterial-based delivery systems are required to help overcome the biological barriers of cancer vaccines in vivo, and enhance cancer vaccine efficacy [158]. Because of the diversity in cancer vaccine approaches, multiple classes of biomaterials are needed to overcome the varying obstacles faced by different vaccine types. Thus, biomaterials used in cancer vaccines range from the nanoscale (e.g. liposomes, polymeric nanoparticles) to larger implantable or injectable scaffolds [159, 160].
Nanoparticle-based delivery systems
Nanoparticle-based cancer vaccines refer to a range of delivery systems—including liposomes, polymeric nanoparticles, self-assembled nanoparticles, and lipid nanoparticles [18]. Incorporating nanoparticles into a vaccine can lead to enhanced delivery to certain organs or tissues such as the lymph nodes, spleen, or solid tumors and can elevate on-target effects [18]. Liposomes are perhaps one of the first studied nanoparticles for cancer vaccines[161], with some formulations featured in ongoing clinical trials (Table 1). Because of their FDA approval, liposomes are an attractive option for fast clinical translation, and they are consistently shown to improve delivery compared to free drug[162]. However, these early generation liposomes have several disadvantages including low loading capacity, relatively low stability, and toxicity[163–165]. Another type of widely studied and FDA-approved nanoparticle-based drug carrier is PLGA nanoparticles—a type of polymeric nanoparticle—but they suffer from similar issues with low cargo encapsulation[166, 167]. However, one distinct advantage of using PLGA nanoparticles is that they can be accurately and consistently generated using well-established protocols that create a wide range of particle sizes[168]. One notable difference between liposomes and PLGA nanoparticles that affects their use as delivery platforms is the hydrophilicity and/or hydrophobicity of the therapeutic cargo[169]. Liposomes contain both a hydrophilic core and a hydrophobic bilayer that make them suitable for carrying hydrophobic and hydrophilic compounds in the same nanoparticle[169]. PLGA nanoparticles, however, have a relatively high overall hydrophilic content, which results in low encapsulation rates for hydrophobic compounds[170].
To overcome the obstacles still faced by commonly used delivery systems, significant work has been done to chemically modify liposome or PLGA formulations in order to improve stability and cargo encapsulation rates[171, 172]. From this work, rationally-designed, new classes of nanoparticles have been developed[18]. For example, self-assembled nanoparticles often have high loading capacities and have shown to successfully deliver peptide or nucleic acid-based vaccines[173–176]. Similarly, lipid nanoparticles, with their history of successful siRNA delivery, have been used extensively for mRNA vaccine delivery[82, 177]. However, both self-assembled and lipid nanoparticles are limited to specific antigen types as their nanoparticle formulations rely on the charge complexation[82, 173, 176]. Therefore, the self-assembled nanoparticles may be more suitable for antigens with easily modified sequences (e.g. peptide) or defined charges (e.g. DNA or mRNA), while liposomes or polymeric particles may be more suitable for complexed and undefined antigen types (e.g. protein, tumor lysate or tumor cell)[161, 178, 179].
Biomaterial scaffold-based delivery systems
Scaffold-based cancer vaccines refer to locally delivered vaccines, such as polymeric scaffolds and hydrogel scaffolds[18]. Due to their large size, these cancer vaccines usually remain at the peripheral injection site after vaccination[18]. The scaffolds often encapsulate a variety of molecules, such as antigens and immunomodulators, that can efficiently program the peripheral tissue and facilitate immune cell infiltration (the vaccine mechanisms are more extensively discussed in Section 6)[180, 181]. Commonly used cancer vaccine scaffolds include PLGA, alginate-based hydrogels, and mesoporous silica micro-rods (MSRs), all three materials are degradable and highly biocompatible[182]. PLGA is FDA approved but is not injectable and must be implanted due to its stiffness[149]. Alginate-based hydrogels can be processed under cryogenic conditions to form cryogels, which have strong shape-memory properties that allow them to be injected instead of implanted into patients[182]. However, cryogels require large gauge needles that result in wounds at the injection site[180]. Both PLGA and cryogel scaffolds have shown great success in encapsulating tumor cell derived antigens, but PLGA is more commonly used for encapsulating tumor lysate antigen while cryogels more commonly carry irradiated whole tumor cells[149, 181, 182]. MSRs with high aspect ratios are perhaps the most injectable form of scaffold because they assemble to form three-dimensional structures in situ after injection[181]. However, MSRs have only shown success in encapsulating relatively small cargo (e.g. nucleic acids, peptides, or proteins), so they may not be suitable for encapsulating whole tumor cells as antigen sources[183]. Because scaffold-based cancer vaccines need to encapsulate a significant number of molecules while allowing for immune cell infiltration, they are often designed to have porous structures[180, 181]. These pores can be adjusted to accommodate different types of cargo. For example, cryogels with larger pores are well-suited for antigens of a larger size, such as whole tumor cells, as they allow for immune cell infiltration while still efficiently encapsulating large cargo[184, 185]. Additionally, chemical modifications allow scaffold-based cancer vaccines to load almost all vaccine types, including nucleic acids, peptides, tumor lysates, and whole tumor cells[18, 186].
One distinct difference between nanoparticle-based and scaffold-based cancer vaccine delivery is the longevity of cancer vaccine and immune cell interaction they provide[182]. Nanoparticle-based cancer vaccines, because of their small size, can be internalized easily by APCs in the tissue or LN shortly after interstitial immunization[6]. By contrast, scaffold-based cancer vaccines, because of their large size, interact with immune cells via encapsulated therapeutic cargos that can be released over a prolonged period of time[158]. Therefore, nanoparticle-based cancer vaccines often require repeated vaccination to achieve effective anti-tumor immunity while scaffold-based cancer vaccines can achieve desirable anti-tumor responses with a single dose or few doses[123, 187]. Nevertheless, similar to other prophylactic single-dose vaccines (e.g. microparticle-based vaccines), questions may be raised against scaffold-based vaccines regarding whether encapsulated antigens or adjuvants remain stable in scaffolds after administration in vivo [188]. However, for antigens that are difficult to collect or processed frequently (i.e autologous tumor cell antigens), the scaffold-based cancer vaccines may still be more desirable as their vaccination schedule generally requires less frequent dosing than nanoparticle-based cancer vaccines[123].
5. Biomaterial Vaccines for Lymph Node (LN) delivery
LN targeting
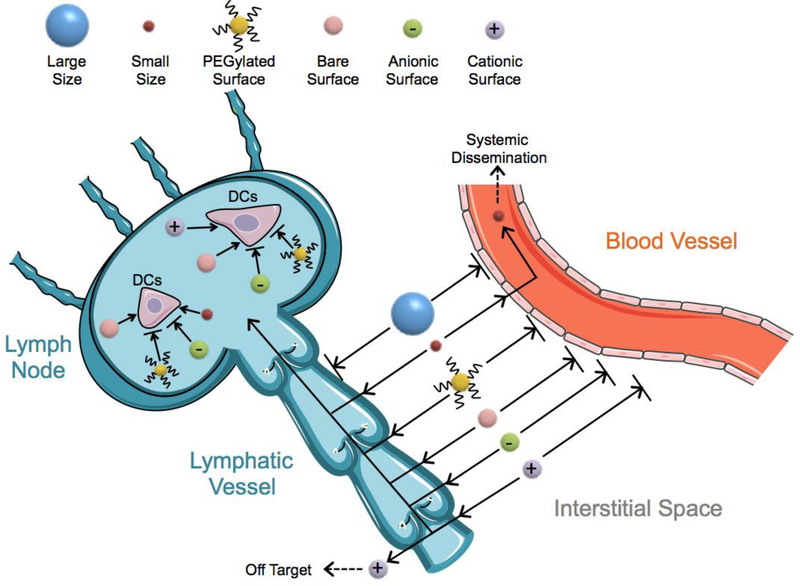
LNs and their surrounding areas contain a large, diverse population of cells types (e.g. APCs, T cells, and lymphatic endothelial cells) that orchestrate immune responses[189, 190]. Therefore, targeting LNs is a promising strategy for controlling the magnitude of vaccine efficacy in both prophylactic and therapeutic settings[27, 191]. Although intranodal injections have shown great promise in effectively delivering vaccines to LNs[192], this technique typically requires an invasive surgical procedure[193, 194], making it less appealing. Instead, interstitial injections (e.g. subcutaneous, intradermal, intramuscular) are one of the most commonly utilized vaccination strategies[195–197]. Though successful in preventing some diseases (e.g. hepatitis B, smallpox, and measles-mumps-rubella[198–200]), their broader applications have been severely limited due to pre-existing biological barriers that prevent interstitially administered vaccines from reaching LNs. Over many years of investigation, researchers have demonstrated that physical and chemical parameters of vaccines, such as size, charge, surface properties, and material chemistry, can dramatically shift a vaccine’s bio-distribution (Fig. 2).
Fig. 2.
The role of biophysical properties (such as size, charge, and PEGylation) of biomaterials on the fate of interstitially administrated vaccines targeting LNs. Large vaccines (over 200 nm) exhibit reduced uptake in the lymphatic vessels. However, small vaccines (less than 2 nm) can easily enter blood vessels and result in systemic dissemination. High density PEGylation or anionic surfaces can enhance vaccine accumulation in LNs, but can also hinder their uptake by DCs. Cationic vaccines can exhibit stronger uptake by DCs, but suffer from off-target effects and toxicity.
Of these factors, the size of a vaccine and its delivery vehicle is one of the most studied characteristics[44, 201, 202]. Vaccine size is a key factor that affects biodistribution upon interstitial injection, due to the differences between the blood and lymphatic vessels that both reside in the interstitial space [203]. While vascular endothelial cells form tight junctions (less than 10 nm in size) around blood capillaries, lymphatic endothelial cells form discontinuous junctions (hundreds of nanometers in size) surrounding the lymphatic capillaries [16]. Additionally, blood flow rates through vascular capillaries are 100–500 times greater than lymphatic capillaries [16]. As a result, when a vaccine is less than 2 nm in size, it can typically cross tight junctions between vascular endothelial cells and preferentially enter blood vessels[204]. Upon entering a blood vessel, the vaccine faces many obstacles to delivery, including serum-induced instability and the mononuclear phagocyte system that rapidly clears vaccines [205]. In contrast, vaccines over 200 nm in size are excluded form directly entering lymphatic vessels via passive diffusion[206] and must rely on tissue-resident APCs for transport to LNs. Therefore, the ideal vaccine size ranges from 2–200 nm, which reduces blood vessel entry and systemic dissemination while enabling entry into lymphatic vessels.
In addition to size, charge is an important factor that affects vaccine trafficking to LNs and their transport via APCs within the LNs. Because of the negatively charged phospholipid bilayer structure of cell membranes, anionic vaccines create a repulsion force with cells that decreases cell-vaccine interaction. Though the surface repulsion decreases cell contact to help vaccines travel smoothly within the lymphatic vessel [201, 207], it also inhibits uptake by APCs after they reach LNs. In contrast, cationic vaccines exhibit stronger cell-vaccine interactions, but may become trapped within the interstitium or lymphatic endothelium before reaching LNs[16, 201, 207]. Despite their limitations, cationic vaccines are heavily investigated and have shown promising experimental results [208–211]. Dampening of surface charge via incorporation of polyethylene glycol (PEG) in vaccine formulations also impacts cell-vaccine interactions, which ultimately influence LN drainage[212]. Additionally, PEG is hydrophilic, therefore PEGylated vaccines have decreased interactions with hydrophobic cell membranes [213, 214] and excel at accumulating in LNs [215, 216]. To increase vaccine uptake in cells of interest (i.e. DCs), further surface modifications, such as incorporation of cell penetrating peptides or DC ligand targeting sequences, are worth consideration[217, 218].
DC targeting
DCs have been extensively studied as a crucial immune cell type that can cross present antigens [219–222]. Therefore, targeting DCs is a promising strategy for priming naïve CD8 T cells and initiating anti-tumor immunity[223, 224]. However, there are several challenges in delivering vaccines to DCs. First, other phagocytic cells (i.e. macrophages and neutrophils) compete with DCs to phagocytose exogenous antigens, which reduces the amount of antigens taken by DCs[92, 225]. Additionally, mature DCs have reduced phagocytic properties that lower their capacity to internalize and process antigens[226]. Further, many vaccines utilize PEGylation or anionic surface to improve biodistribution to LNs[27, 212, 227], but their internalization by DCs can then be hindered due to the strong hydrophilicity difference or electrostatic repulsion between cell membranes and the PEGylated or anionic vaccine (Fig. 2)[201, 228]. Therefore, once vaccines reach the LNs, additional strategies are required to enhance uptake into DCs residing in LNs. DCs can be classified into several subtypes, such as CD8α+ DCs, plasmacytoid DCs, and Langherans cells, based on their different marker expression (e.g. CD11c, MHC-I, MHC-II, DEC-205, DC-SIGN, and CD40, comprehensively reviewed in [229]). Thus, actively targeting specific DC ligands has become an attractive approach to reduce off-target effects in vaccines[230]. Among those receptors expressed on DCs, DEC-205, DC-SIGN, and CD40 have been the most successful as targeting moieties utilizing antibodies[231–234]. Antibody-functionalized vaccines allow for not only improved targeting specificity, but also the capacity to enhance antigen cross-presentation[218, 235, 236]. Though promising, antibody production can be time and cost intensive[237]. Therefore, recent work has focused on utilizing short peptide fragments, such as the WH peptide[238] and NW peptide[239], to target DC surface receptors and improve vaccine efficacy[240].This strategy has been extraordinarily successful for peptide-based vaccines, as a DC-targeting peptide can be tethered to the peptide epitope during the vaccine’s synthesis[239, 241]. The fast and inexpensive peptide production process makes peptide-based DC targeting strategies a promising strategy for developing cancer vaccines.
Antigen and adjuvant co-localization or segregation
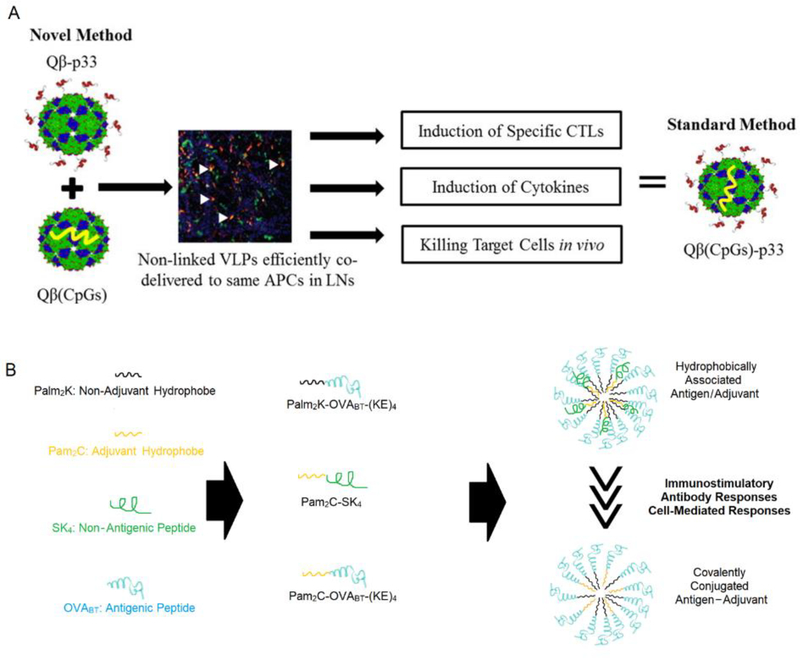
Recent advancements in molecular adjuvants, including TLR agonists, have accelerated the development of cancer vaccines, as traditional adjuvants (e.g. alum and Freund’s adjuvant) fail to induce potent CTL and Th1 immune responses[26, 242–244]. One of the most important discoveries from the last decade is that the co-delivery of antigen and molecular adjuvants encapsulated within a biomaterial carrier tends to induce stronger immune responses than the delivery of soluble antigens and adjuvants in the absence of a carrier[245]. This concept prompted the development of many different types of biomaterial-based vaccines, including polymeric nanoparticles[246–248], inorganic nanoparticles[176, 208, 249], and biomimetic nanoparticles[250–253], where antigens and adjuvants are encapsulated within a single nanoparticle platform. Though these vaccines have been successful, recent results demonstrated that encapsulating antigens and adjuvants in separate particles induced similar or even stronger immune responses than platforms containing both antigen and adjuvant within a single nanoparticle platform[254–256] (Fig. 3A).
Fig. 3.
Co-localizing antigen and adjuvant to the same APCs is important and can be achieved without the need for physical (A) or chemical linkage (B). (A). Antigen and adjuvant are delivered separately by different nanoparticles but are still co-localized within the same APCs in LNs, thereby inducing similar immune responses as when antigens and adjuvants are engineered into the same nanoparticle. (B). When antigen and adjuvant are covalently tethered, the resulting vaccine formulation induced lower immune responses than when antigens and adjuvants are hydrophobically associated.
In the above mentioned studies, it is important to note that co-encapsulation of antigens and adjuvants into the same nanoparticles or separated into different nanoparticles does not impact the biodistribution of the cargo[254]. Both delivery strategies include nanoparticles that can be trafficked to LNs and subsequently taken up by APCs in a similar manner[254]. However, once the antigen and adjuvant reach to the same APCs, they may still need to separate because antigens and adjuvants may function in different cell compartments[257–262]. Therefore, segregating antigens and adjuvants into separate nanoparticles allows them to be easily divided and transported to the desirable cell compartments after they both reach to APCs. The importance of antigen and adjuvant segregation is further supported by recent work, where a vaccine with chemically-tethered antigens and adjuvants induce weaker immune responses than the vaccine with hydrophobically associated antigens and adjuvants[260] (Fig. 3B). Hence, while antigens and adjuvants need to be taken up by the same APC within the same LN, the antigen and adjuvant may not necessarily have to be encapsulated in the same particle or chemically linked together to be effective.
Chemical modifications of antigens and adjuvants are frequently utilized to induce their co-localization[208, 263–266]. However, several factors need to be taken into consideration when modifying antigens and adjuvants for cancer vaccines. For instance, peptide terminus modification can affect the capacity of peptides to be cross-presented on major histocompatibility complex 1 (MHC-I)[267]. Therefore, it is important to consider the effect of cross-presentation when modifying cancerous epitopes (e.g. neoantigens), as potent antigen-specific CTL responses are a key factor of anti-tumor immunity. Additionally, terminus modification methods for adjuvants can greatly affect their activity—as shown by CpG, a TLR-9 agonist. As one of the most commonly used adjuvants for cancer vaccine development, it has been modified using various strategies, and experimental results demonstrated that the 5’ end of CpG is critically important for interacting with its receptor, TLR-9[268]. Thus, modifications on the 3’ end of CpG maintain its bioactivity, while 5’ modifications diminish adjuvanticity[268–271] with limited exceptions[225, 272]. Another commonly utilized adjuvant, Pam2C, a TLR-2 agonist, has also demonstrated changes in bioactivity resulting from chemical modifications. The structure of Pam2C includes a –COOH group that makes it easy to conjugate with peptides, but the adjacent amino acid residues appear to play an important role in modulating Pam2C adjuvanticity[273–276]. Therefore, directly conjugating antigens to Pam2C can decrease Pam2C adjuvanticity (Figure 3B)[260, 275], so strategies such as using an extra linker between Pam2C and the peptide epitope must be explored to prevent diminished adjuvanticity.
6. Biomaterial Scaffolds for Localized Vaccine Delivery
From physical adjuvant “scaffold” to biomaterial scaffold
Although DCs are abundant in secondary LNs, significant numbers of DCs also reside in the skin and circulate in the blood[277, 278]. While these DCs are accessible targets for therapeutic delivery, it remains challenging to selectively deliver antigens while avoiding off target cells and tissues. To overcome this, delivery technologies that recruit DCs to specific peripheral tissue can concentrate these cells at a given site to deliver antigen cargo while avoiding systemic toxicity[279, 280]. Subsequently, these DCs can be activated in situ with additional reagents and then migrate to LNs to initiate immune responses[281].
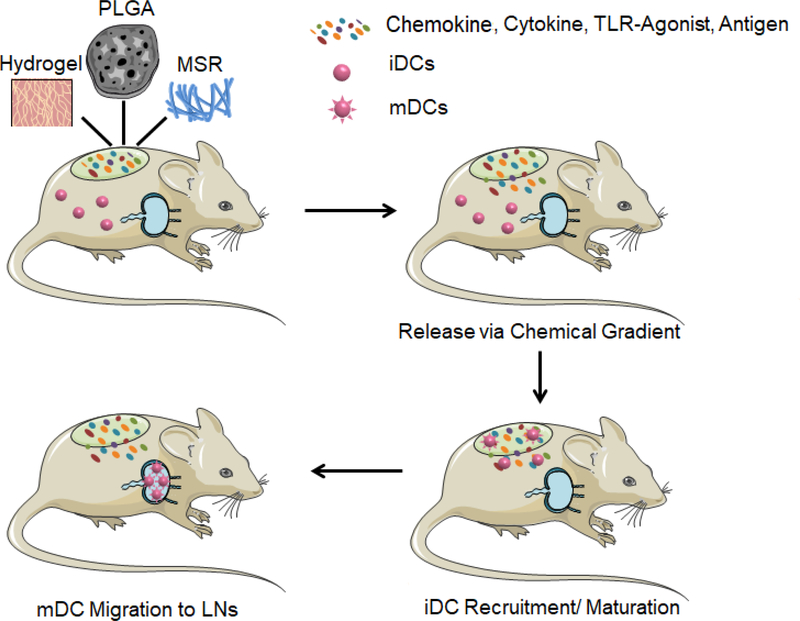
The strategy of recruiting DCs to peripheral tissue was early employed in the 1920s with alum adjuvant[282]. It was originally believed that alum would function as a depot that sustainably releases antigen to LNs[283]. However, recent research has found that alum acts like a “scaffold”, as it stimulates chemokine and cytokine induction at the injection site, which subsequently recruits and activates DCs in situ[284–286]. Other types of physical adjuvants including Freund’s adjuvant, Montanide, MF59, and ASO4 have also been used in this strategy[287, 288]. However, the application of those physical adjuvants in cancer vaccines is quite limited because they typically initiate strong Th2 but weak Th1 and CTL responses[289]. Nevertheless, potent Th1 and CTL responses are essential for cancer vaccines because Th1 cells produce large amounts of pro-inflammatory cytokines (most notably IFN-γ) that alter the immune-suppressive microenvironment, while CTLs are responsible for direct killing of tumor cells[290–293]. Therefore, new vaccine scaffolds capable of triggering potent Th1 and CTL responses are an emerging need in cancer vaccine development[294, 295]. The design of these cancer vaccine scaffolds must address several engineering criteria: first, the scaffold should contain chemical signals, such as cytokines or chemokines, that enable DC recruitment[296]. Additionally, a 3D macroporous structure is required that enables DC infiltration[297]. After infiltration, DCs need to be able to uptake antigen within the scaffold and undergo maturation. Therefore, TAAs are incorporated within the scaffold, and function as antigen sources[180, 181]. Additionally, TLRs agonists are usually included to help induce potent Th1 and CTL responses[123, 298]. Collectively, the design requirements for this ideal system are too complex to be addressed by traditional physical adjuvants. As an alternative approach, biomaterials have recently been used to develop cancer vaccine scaffolds to address these needs, and their design is being continuously improved to enhance vaccine delivery (Fig. 4.).
Fig. 4.
Schematic of biomaterial-based scaffold vaccines. Various classes of biomaterial scaffolds, such as hydrogels, poly(lactic-co-glycolic acid) (PLGA), and mesoporous silica microrods (MSR), encapsulating antigens and immunomodulators (e.g. chemokines, cytokines, and TLR-agonists) can be implanted or injected to peripheral tissue as scaffold vaccines. Immature DCs (iDCs) are then recruited to the scaffold, become mature DCs (mDCs), and migrate to lymph nodes (LNs) to initiate anti-tumor immunity.
Implantable scaffold
One of the first studies employing biomaterial scaffold-based vaccines was conducted in 2002, using EVA-based biomaterials[299]. In this study, macrophage inflammatory protein 3 b (MIP-3b) and TAA were entrapped in separate ethylene vinyl acetate (EVA) tubes and co-implanted subcutaneously in mice[299]. MIP-3b was used to recruit Langerhans cells (LCs) that subsequently load TAA in situ[299]. Three different tumor models, E.G7-OVA tumor, fibrosarcoma, and Lewis lung carcoma, were evaluated[299]. Mice that received multiple doses of EVA vaccine showed significantly inhibited tumor growth in both prophylactic and therapeutic settings[299]. The success of this proof-of-concept study encouraged researchers to develop other rationally designed cancer vaccine scaffolds, which led to the utilization of poly(lactic-co-glycolic acid) (PLGA)—an FDA approved, biodegradable polymer[167]. In this model, PLGA scaffolds were formed using a gas-foaming process[300], where GM-CSF, TLR agonists (CpG or poly(I:C)), and tumor lysate antigen were incorporated into the structure[149, 301, 302]. GM-CSF was released from the PLGA scaffold over a 30-day period, which created the cytokine gradient to recruit DCs[149]. The recruited DCs then encountered TAA and danger signals, matured, and subsequently migrated to LNs[18, 294]. This PLGA scaffold-based cancer vaccine induced synergistic anti-tumor immunity by elevating CTL responses and attenuating TGF-β, IL-10, and FoxP3 regulatory T cells[149]. The scaffold has shown great efficacy in mouse xenograft model of melanoma: a single dose implantation protects over 70% of mice from melanoma cell challenging, while two doses led to complete melanoma regression in nearly 40% of the mice[149].
Injectable hydrogel scaffold
Although PLGA scaffolds have shown great promise as a cancer vaccine, one drawback of this approach is that it requires surgical implantation, the procedure is painful, and it can leave large scars on patients[303]. Therefore, recent studies have focused on developing injectable cancer vaccine scaffolds[180, 181, 298]. Cryogels are one of the first injectable scaffolds developed for cancer vaccine applications[180, 304]. To form cryogels, methacrylated-alginate is first polymerized at −20 °C, allowing ice crystals to form within the cryogel structure[180, 304]. Subsequently, the cryogels are exposed to room temperature, allowing ice crystals thaw and leave behind macropores[180, 304]. An important feature of cryogels are their shape-memory properties, which allow the gels to recover their intended configuration after a conventional 16-gauge needle injection[304, 305]. To test their bioactivity as a cancer vaccine, cryogels were loaded with GM-CSF, CpG, and irradiated tumor cells, then injected subcutaneously in a mouse model of melanoma. Results indicated that the cryogel cancer vaccine scaffold induced a higher survival rate in mice than the previously investigated PLGA cancer vaccine scaffold, when the same immunization schedule was applied[180]. However, the first generation cryogel was not mechanically robust enough to fit in a needle smaller than 16-gauge without damaging the cryogel[184]. Therefore, this cryogel required a more invasive 16 gauge needle, which created large wounds at the injection sites[184]. To improve injectability, a second generation cryogel was developed by incorporating additional ionic crosslinks to improve its elasticity, and was injected through an 18-gauge needle without any damage to its structure[184].
Another attractive strategy for designing injectable cancer vaccine scaffolds is inspired by stimuli-responsive hydrogels, which have been widely used in biomedical research[306–309]. One thermo-responsive polymer, monomethoxypoly (ethylene glycol) – co–poly(lactic-co-glycolic acid) copolymer (mPEG-PLGA) has shown great promise as an injectable scaffold as it is injectable at 4°C, but turns to gel within 5 mins at body temperature[298, 310]. When the scaffold is loaded with GM-CSF, it is released over a 15 day period and recruits DCs to the scaffold site[298]. Interestingly, the most potent anti-tumor immunity was generated when a lentivirus-encoding antigen and adjuvant (CpG or MPLA) were administered 7 days post injection of a hydrogel loaded with GM-CSF, which extended survival in a mouse model of melanoma[298]. This indicates that there may be a time period between DC recruitment signaling and DC activation signaling from the scaffold that can affect therapeutic efficacy. More recently, researchers demonstrated that these hydrogels can be used to encapsulate nanoparticles loaded with antigen, in addition to soluble antigen[311]. This dual delivery system enhanced antigen uptake by recruited DCs and induced potent CTL responses, indicating that it is a platform worth further investigation for vaccine delivery [311].
Injectable mesoporous silica micro-rod (MSR) scaffold
MSRs have recently emerged as another system for cancer vaccine scaffolds[312–314]. Mesoporous silica has been widely used in many biomaterials because of its high biocompatibility[315–317]. For cancer vaccine scaffolds, hexagonal MSRs with certain aspect ratios (88 μm × 4.5 μm) were synthesized. MSRs are injectable after reconstitution in cold PBS, but because of their high aspect ratio, they non-specifically self-assemble after injection, and generate pores that are larger than cells to allow cell infiltration[181, 312]. A single dose of MSRs loaded with TAA, GM-CSF, and CpG, induced potent anti-tumor immunity, which protected 90% mice from EG7.OVA lymphoma cell challenging[181]. Interestingly, a single dose MSR vaccine also induced durable antibody responses[181], indicating that MSRs may also be used for other types of vaccines, such as Zika, Ebola, and Plasmodium falciparum, where the circulation of high-tier antibodies are crucial for the disease prevention[318–320]. The second generation of MSR scaffold was further modified by mixing Polyethylenimine (PEI) and MSR to form PEI-MSR scaffolds[123]. PEI-MSR scaffolds alone have been shown to stimulate multiple damage-associated molecular pattern (DAMP) receptors and exert potent adjuvanticity, which assists in DC activation and cross-presentation[123, 321, 322]. When loaded with GM-CSF, CpG, and TAAs, a single dose vaccination of PEI-MSR scaffolds eradicated established, large TC-1 tumors in 80% of mice[123]. Moreover, when treating more aggressive type tumors, such as B16-F10 or CT26 lung metastases, PEI-MSR scaffolds were shown to eradicate established lung metastasis when synergized with anti-CTLA4 therapy[123].
7. Biomaterials for tumor targeting and tumor modification
Immunomodulators turning tumor site into antigen depot
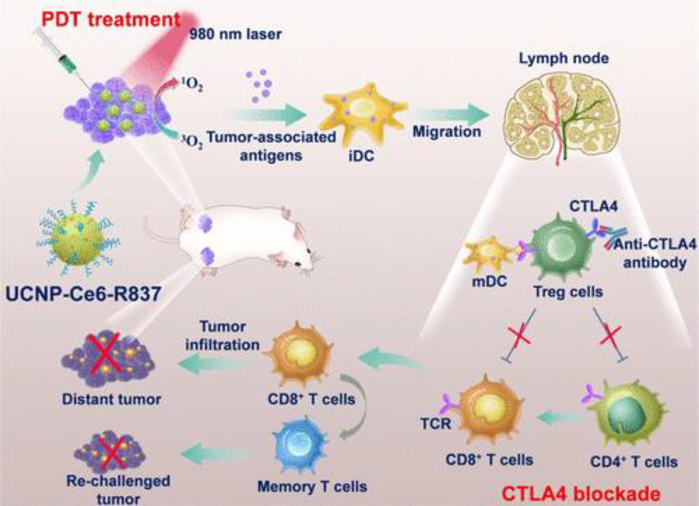
In the 19th century, a surgeon named William Coley discovered that repeated intratumoral injections of bacterial lysate reduced the progression of carcinomas[323]. However, it was not until almost a century later that researchers identified CpG, a special immunomodulator, is the key component of the lysate that induced tumor regression[324, 325]. Since this discovery, delivering immunomodulators directly to tumors has become an attractive strategy for cancer immunotherapy[326–331]. Although immunomudulators do not display antigen, they can turn tumor sites into antigen depots by inducing tumor cell death and releasing tumor antigens in situ[332–335]. Subsequently, tumor antigens are taken up by DCs that either reside in the tumor stromal area or are recruited to this area. After DCs mature, they migrate to LNs and generate systematic anti-tumor immunity in a vaccine-like manner[332, 335] (Fig. 5).
Fig. 5.
Immunomodulators turn tumor sites into antigen depots and induce anti-tumor immunity in a vaccine-like manner. Photodynamic therapy (PDT) destructs tumor cells and effectively generates TAAs. TAAs are then captured by DCs and transported to LNs, which promote strong anti-tumor immunity with the help of an anti-CTLA-4. Adapted from [335]. Reprinted with permission from ACS Publications.
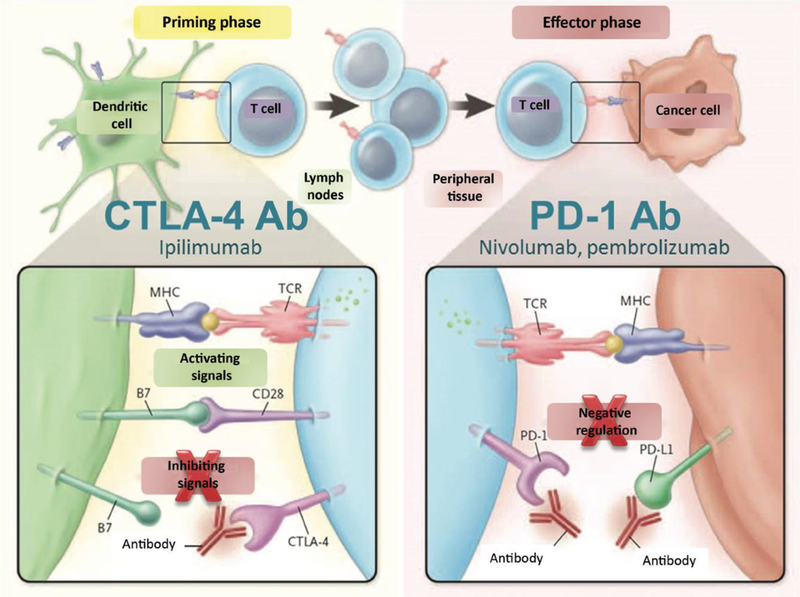
In many studies, checkpoint blockade therapies are combined in order to improve the therapeutic outcomes of cancer vaccines by reducing the immunosuppressive tumor microenvironment[336]. For instance, CTLA-4 antibodies are used to block CTLA-4 receptors (highly expressed on exhausted T cells) that reside in tumors, which strengthens the co-stimulatory signals (CD28 and B7 engagement) for T cell activation and subsequent enhancement of T cell effector function[336]. In addition, blocking PD-1 (highly expressed on exhausted T cells) and its ligand PD-L1 (highly expressed on cancer cells) serves the same purpose—to improve T cell activation (Fig 6) [337].
Fig. 6.
This schematic shows the use of CTLA-4 and PD-1 antibodies to improve signaling in both the priming and effector phase of the immune response. Adapted from[338], Reprinted with permission from Wiley Online Library.
Intratumoral injection
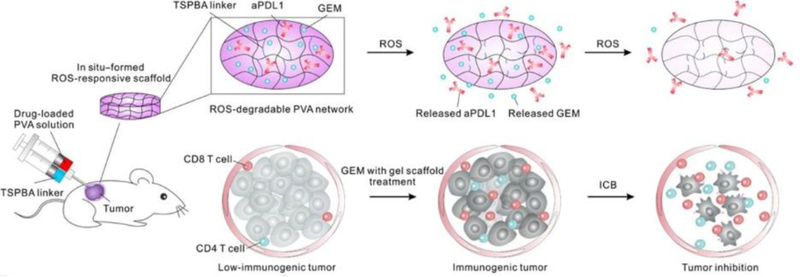
Intratumoral injection is one of the earliest and most direct methods for delivering immunomodulators to tumor sites[339]. Many types of immunomodulators, such as TLR agonists, stimulator of interferon gene (STING), chemotherapeutics, cytokines, and antibodies have been used in intratumoral injections[326, 340, 341]. However, because of their small size, these therapeutics can rapidly leak out of the tumor and enter the circulatory system within minutes, causing systemic toxicity[342–344]. Thus, various types of biomaterials have been developed to increase the retention time of immunomodulators at tumor sites[345]. Several particle-based delivery systems, such as liposomes[346, 347], polymeric nanoparticles[348–350], and inorganic nanoparticles[351, 352] have been shown to enhance retention of immunomodulators in the tumor microenvironment and reduce systemic toxicity. Hydrogel based delivery systems are also an attractive platform to increase drug retention at tumor sites[45, 353, 354], and their distinct degradation profiles allow for therapeutics to be slowly released with finely tuned kinetics[158, 355–357]. As prior reports have shown that chemotherapy enhances immunotherapy efficacy [358–360], a recent study designed a hydrogel system to release chemotherapeutics faster than immunomodulators[45]. In the design, gemcitabine (GEM), a chemo drug, had a smaller molecular weight as the checkpoint blockade (anti-PD-L1), allowing it to release faster from the hydrogel[45] (Fig. 7). The results indicated that a single dose injection significantly prolonged survival in mouse xenograft models of melanoma and breast cancer[45]. Therefore, intratumorally-injected hydrogels formulated to release immunomodulators in a controlled manner to generate anti-tumor immunity have become a promising direction in cancer vaccine development.
Fig. 7.
Schematic of an intratumorally injected hydrogel vaccine. GEM and anti-PD-L1 are encapsulated within reactive oxygen species-responsive hydrogel that degrades post-injection. The smaller molecular weight GEM is released faster than larger molecular weight anti-PD-L1, which is a desirable kinetic difference to ultimately induce potent anti-tumor immunity. Adapted from [45]. Reprinted with permission from AAAS.
Systemic injection and tumor targeting
Though intratumoral injections show promising efficacy, one challenge to their broad implementation is that they are not a viable option for less accessible and disseminated metastatic cancers[345]. To overcome this obstacle, new strategies have been developed that utilize systemically administered vaccines that are able to reach the tumor site[345]. Targeting solid tumors via systemic injection requires long drug circulation times in the blood, increasing the chances that the drug will reach the tumor[361].
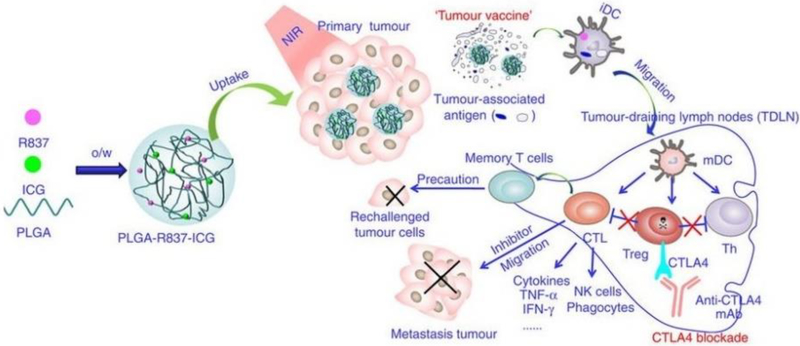
Several factors affect the circulation time of drugs in the blood. Size is one of the most frequently studied topics, as drugs that are too small (less than 8 nm) are vulnerable to renal clearance while particles that are too large (over 200 nm) tend to accumulate in the spleen and liver where they are processed by MPS cells[362, 363]. PEGylation is another important parameter in increasing circulation time, as PEGylated surfaces help decrease non-specific interactions with the large population of phagocytic cells in the blood that work to opsonize foreign substances[364–367]. However, highly PEGylated nanoparticles may cause an accelerated blood clearance (ABC) phenomenon in later dosing[368]. The ABC phenomenon occurs when repeated exposures to PEG (on particles or in therapeutic modifications) leads to the increased production of anti-PEG antibodies, which mark PEGylated substances for endocytosis or phagocytosis[368]. PEGylated nanoparticles are then cleared to the liver more rapidly, and the decreased blood circulation time limits vaccine efficacy[369, 370]. Therefore, newly developed non-fouling materials, such as zwitterionic peptides or polymers, are worth consideration for incorporation in future nanoparticle platforms to reduce the effects of the ABC phenomenon [369, 371–373]. Although drug shape also plays an important role in drug circulation time[374–377], its effect in tumor accumulation is still under investigation. Because different tumor types possess different vascular wall pore shapes[376], specific drug shapes may accumulate differently depending on the type of tumor[362, 378]. Overall, nanoparticles approximately 100 nm in diameter with a densely PEGylated surface tend to accumulate more at tumor sites, in a process known as passive targeting[362, 379]. One recent example was a study using a highly PEGylated, 100 nm PLGA particle carrying TLR-7 agonist, which accumulated in the tumor following systemic injection[334]. When combined with photodynamic therapy (PDT) with Indocyanine green (ICB), it inhibited tumor growth and induced immunological memory in mouse models of breast and colorectal cancer[334] (Fig. 8). Although passive targeting has shown great promise in helping drugs accumulate in tumor sites, further strategies—such as actively targeting cancer cells or cancer endothelium—are worth consideration to further increase the tumor targeting efficacy of vaccines[191, 380–384].
Fig. 8.
Schematic of passive tumor targeting. 100nm PLGA nanoparticles with a densely PEGylated surface were injected intravenously and passively accumulated in tumors. When combined with PDT therapy, tumor cells were disrupted and released TAAs. The TAAs were subsequently captured by DCs and transported to LNs, which promoted strong anti-tumor immunity with the help of anti-CTLA-4. Adapted from [334]. Reprinted with permission from Nature Publishing Group.
Leveraging tumor cell membranes for nanoparticle-mediated vaccine delivery
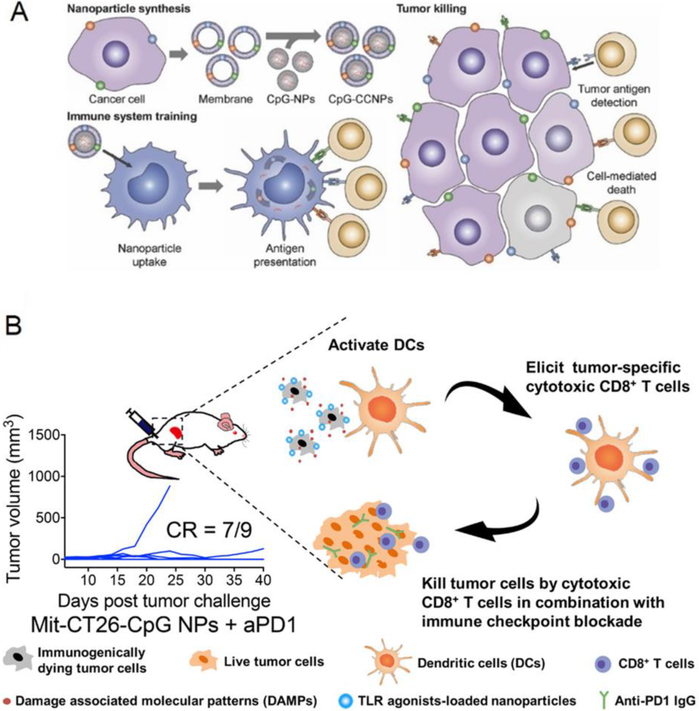
Another important strategy for utilizing tumor sites as antigen sources, as reviewed in tumor cell vaccine section, is processing resected tumor cells. Common methods for obtaining TAAs from tumor cells include freeze-thawing and irradiation[142, 145, 146]. Many strategies have streamlined the subsequent step of loading the collected TAAs onto biomaterial delivery platforms such as nanoparticles or scaffolds for applications in cancer vaccines[147–149]. In a manner similar to obtaining TAAs from tumor cells, tumor cell membranes have recently been isolated through hypotonic lysing and mechanical disruption for coating drug-loaded nanoparticles for in vivo delivery[385]. In one example, TAA-abundant tumor cell membranes were coated onto the surface of adjuvant-loaded nanoparticles to create a tumor membrane-coated nanoparticle vaccine[253, 385] (Fig. 9A). When utilized in a prophylactic setting, three doses of the vaccine protected 80% of mice from a melanoma cell challenge[253]. When used in a therapeutic setting, four doses of the vaccine combined with checkpoint blockades induced long-term survival in 50% of melanoma tumor-bearing mice[253].
Fig. 9.
Schematic of modifying tumor cells to formulate cancer vaccines. (A) Cancer membranes were isolated and coated onto the surface of CpG-loaded nanoparticles. (B). Mitoxantrone, a chemo drug, was used to induce immunogenic cell death. The surfaces of dead tumor cells were coated with CpG-loaded nanoparticles. Part A is adapted from [187], part B is adapted from [252]. Reprinted with permission from Wiley Online Library and ACS Publications.
In addition to coating nanoparticle surfaces with tumor cell membranes, another recent study investigated the reverse strategy, where tumor cell surfaces were coated with nanoparticles[252]. In this study, isolated tumor cells were first treated with the chemotherapeutic mitoxantrone to trigger immunogenic cell death[252]. Subsequently, the dying tumor cells were purified and decorated with adjuvant-loaded nanoparticles to form a nanoparticle-coated tumor cell vaccine [252] (Fig. 9B). In this study, a single dose of the vaccine protected all of the mice from melanoma cell challenging[252]. Additionally, a single dose of the vaccine combined with multiple doses of a checkpoint blockade induced complete tumor regression in almost 80% of mice with established colon carcinoma[252]. The major advantage of the tumor membrane-coated nanoparticle vaccine and the nanoparticle-coated tumor cell vaccine is that they closely mimic many natural properties of cancer cells[251, 385]. However, several challenges, such as large scale production and batch-to-batch variability, do currently exist and can hinder their future commercialization [126].
8. Outlook – Towards personalized cancer vaccines
Identification of TAAs has long been a central driving force behind the development of tumor-specific cancer vaccines[7, 386, 387]. Most TAAs currently in clinical use are self-tumor antigens, as they are derived from healthy cells with a normally expressed protein that is overexpressed on cancer cells[7]. This strategy has led to the successful discovery of many TAAs, such as MAGE1 (a melanoma associated antigen), NY-ESO-1 (a cancer-testis antigen), and HER-2 (a breast cancer associated antigen)[388–390]. Though the identification process has proven promising, early clinical investigations have had limited success, likely due to several important factors[7, 8]. First, every tumor has a unique pattern of somatic mutation that generates many different copies of TAAs, but identified self-tumor antigens are usually only a small fraction of the TAAs that share common features between individual patients[8, 391, 392]. Therefore, administration of only self-tumor antigens can result in tumor escape[393]. Second, as the self-tumor antigens are also expressed in healthy tissue, they are subjective to a certain degree of central tolerance and are often recognized by T cells with low affinity, resulting in low-immunogenicity[7]. Moreover, the antitumor-immunity developed against those self-tumor antigens can also attack antigens expressed on normal cells, which may cause off-target autoimmune effects[8]. Collectively, new strategies are needed to discover patient-specific TAAs that are expressed exclusively on cancer cells.
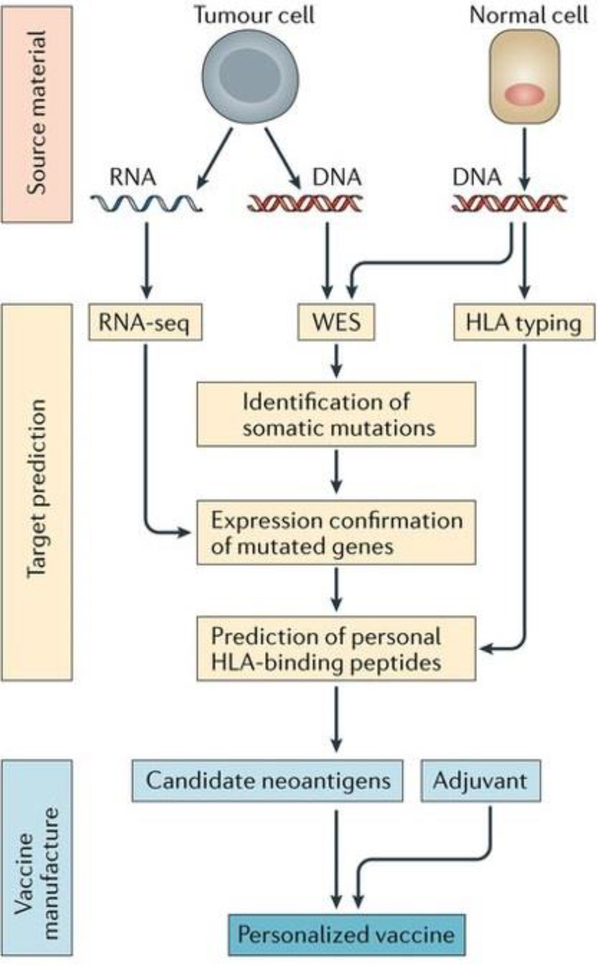
Next generation sequencing has revolutionized our understanding of cancer mutations[394, 395]. More importantly, recent advancements allowing for a reduction in the time and cost provide unique opportunities for researchers to identify tumor antigens on an individual patient basis[7, 8, 396–398]. Thus, experimental and computational pipelines have been generated to identify personalized tumor antigens in real-time (Fig. 10)[7]. In one approach to formulate personalized cancer vaccines, the DNA and RNA from both normal cells and cancer cells have been extracted[7]. Subsequently, whole exosome sequencing (WES) and RNA sequencing (RNA-seq) are conducted to identify mutated genes and their corresponding mutated antigens[388]. Thereafter, human leukocyte antigen (HLA) typing is carried out to determine which mutated genes have a strong binding affinity for the individual patient to then predict and personalize the cancer vaccine epitope, known as neoantigens[7, 399]. Lastly, those selected neoantigens are synthesized and combined with other immunomodulators (e.g. adjuvants) and delivery vehicles to create the final vaccine formulation[388]. A recent study demonstrated that utilizing nanodiscs, a novel biomaterial based vaccine delivery vehicle, to deliver predicted neoantigens has significant potential as a cancer therapeutic [124]. When the nanodisc vaccine is combined with checkpoint blockade therapy in murine models, it has been shown to eradicate established colon carcinoma or melanoma in 90% of mice[124].
Fig. 10.
Schematic of neoantigen selection and personalized cancer vaccine formulation. The mutated antigens are selected by whole-exome sequencing, RNA sequencing, and HLA typing. The selected epitopes are synthesized and formulated with other immunomodulators, yielding personalized cancer vaccines. Adapted from [124]. Reprinted with permission from Nature Publishing Group.
Another strategy for developing personalized cancer vaccines is utilizing patient-derived tumors[117]. Compared with the previously described neoantigen vaccine, utilizing a patient’s own tumor cells eliminates the need for tumor antigen selection and synthesis, thus reducing vaccine production time[151]. A recent mice study embedded autologous dead breast cancer cells, thienotriazolodiazepine (a bromodomain-containing protein 4 inhibitor), and ICG (a photothermal therapy agent) in hydrogels as a personalized cancer vaccine[151]. The vaccine induced complete remission in all mice with breast tumors, indicating that personalized vaccines developed from a patient’s own tumor, coupled with novel biomaterial delivery system, may be a promising direction for personalized cancer vaccines[151]. This strategy is especially applicable for patients with solid tumors that require surgical resection as the excised tumors can be modified and used in biomaterial based vaccines that are capable of preventing tumor recurrence and metastasis post-surgery[151]. This new approach may be an attractive alternative to traditional chemotherapy and radiotherapy—the current standard-of-care therapy post-surgery—as both traditional methods dramatically decrease a patient’s quality of life[400]. Though promising, the production processes for personalized vaccines derived from either neoantigens or a patient’s own tumor are often time consuming, costly, and complex[7, 151, 401]. Therefore, future efforts should focus on reducing production time and costs during the vaccine manufacturing process so that personalized cancer vaccines can become widely commercialized.
9. Conclusion
Though many advances have been made at the interface of cancer vaccines, biomaterials, and bioinformatics, the final key step is to effectively translate these novel techniques from academic laboratories into the clinic, where several challenges exist. First, the differences in immune systems between laboratory animals and humans need to be taken into consideration[386]. Although humanized mouse models are utilized to better simulate a human immune system, they are unable support the development of human innate immune cells[402]. Additionally, animal models for melanoma are mostly commonly used due to their ease of tumor manipulation and assessment[402]. However, melanoma may differ significantly from other types of solid tumor or hematological cancers, which may impact the translatability of the model to other types of cancer in the clinic[403]. Lastly, the capacity of large scale production, and batch-to-batch quality control are also important factors that need to be addressed before biomaterial-based vaccines can be widely commercialized[404]. One strategy to improve the potential for clinical translation is to develop delivery technologies comprised of FDA-approved materials, as a means to reduce the length of the approval process[405–407]. A prime example is the PLGA-based scaffold vaccine (WDVAX), developed by Mooney and colleagues, which has recently been licensed by Novartis for commercial use[408]. Additionally, using existing and future clinical trial data to compare vaccine efficacy across patient subpopulations may allow for vaccines to be optimized more quickly for specific groups of patients, based on factors determined such as biomarker expression[409].
This review article has covered various aspects of biomaterial-based cancer vaccines that are capable of training immune systems to selectively attack tumor cells. Different engineering approaches, including improving lymph node delivery, enhancing immune cell recruitment, tumor targeting, and tumor cell modification, have been extensively discussed. Moreover, the identification of optimized tumor antigen sequences has been indicated as a crucial step in improving cancer vaccine efficacy. Thus, extensive collaborations between immunologists, computational scientists, and bioengineers, entrepreneurs are necessary to design safer, more effective, and more translatable next generation cancer vaccines.
Highlights.
Cancer vaccines aim to reprogram a patient’s own immune cells to target and kill cancer cells.
Barriers to the broad clinical translation of cancer vaccines include weak immunogenicity, systemic toxicity, and off-target effects.
Advances in biomaterial technologies can overcome the challenges faced in cancer vaccine delivery.
Nanoparticle- and implantable scaffold-based technologies improve cancer vaccine safety and efficacy.
Looking forward, these biomaterials-based delivery technologies can be exploited to design platforms for personalized cancer vaccines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity, 39 (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [2].van Riet E, Ainai A, Suzuki T, Kersten G, Hasegawa H, Combatting infectious diseases; nanotechnology as a platform for rational vaccine design, Advanced drug delivery reviews, 74 (2014) 28–34. [DOI] [PubMed] [Google Scholar]
- [3].Mortellaro A, Ricciardi‐Castagnoli P, From vaccine practice to vaccine science: the contribution of human immunology to the prevention of infectious disease, Immunology and cell biology, 89 (2011) 332–339. [DOI] [PubMed] [Google Scholar]
- [4].Bodey B, Bodey JB, Siegel SE, Kaiser HE, Failure of cancer vaccines: the significant limitations of this approach to immunotherapy, Anticancer research, 20 (2000) 2665–2676. [PubMed] [Google Scholar]
- [5].Mitragotri S, Devices for overcoming biological barriers: the use of physical forces to disrupt the barriers, Advanced drug delivery reviews, 65 (2013) 100–103. [DOI] [PubMed] [Google Scholar]
- [6].Reddy ST, Van Der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA, Exploiting lymphatic transport and complement activation in nanoparticle vaccines, Nature biotechnology, 25 (2007) 1159. [DOI] [PubMed] [Google Scholar]
- [7].Hu Z, Ott PA, Wu CJ, Towards personalized, tumour-specific, therapeutic vaccines for cancer, Nature Reviews Immunology, 18 (2018) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang X, Sharma PK, Goedegebuure SP, Gillanders WE, Personalized cancer vaccines: Targeting the cancer mutanome, Vaccine, 35 (2017) 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gajewski TF, Schreiber H, Fu Y-X, Innate and adaptive immune cells in the tumor microenvironment, Nature immunology, 14 (2013) 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Radford KJ, Tullett KM, Lahoud MH, Dendritic cells and cancer immunotherapy, Current opinion in immunology, 27 (2014) 26–32. [DOI] [PubMed] [Google Scholar]
- [11].Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA, VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics, Cell reports, 1 (2012) 191–199. [DOI] [PubMed] [Google Scholar]
- [12].Mempel TR, Henrickson SE, Von Andrian UH, T-cell priming by dendriticcells in lymph nodes occurs in three distinct phases, Nature, 427 (2004) 154. [DOI] [PubMed] [Google Scholar]
- [13].Nobuoka D, Yoshikawa T, Takahashi M, Iwama T, Horie K, Shimomura M, Suzuki S, Sakemura N, Nakatsugawa M, Sadamori H, Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy, Cancer Immunology, Immunotherapy, 62 (2013) 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang C, Sun W, Wright G, Wang AZ, Gu Z, Inflammation‐Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti‐PD1 Antibody, Advanced Materials, 28 (2016) 8912–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheever MA, Higano CS, PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine, Clinical Cancer Research, (2011). [DOI] [PubMed] [Google Scholar]
- [16].Jiang H, Wang Q, Sun X, Lymph node targeting strategies to improve vaccination efficacy, Journal of Controlled Release, 267 (2017) 47–56. [DOI] [PubMed] [Google Scholar]
- [17].Andorko JI, Hess KL, Jewell CM, Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance, The AAPS journal, 17 (2015) 323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Koshy ST, Mooney DJ, Biomaterials for enhancing anti-cancer immunity, Current opinion in biotechnology, 40 (2016) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheung AS, Mooney DJ, Engineered materials for cancer immunotherapy, Nano today, 10 (2015) 511–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmed KK, Geary SM, Salem AK, Applying biodegradable particles to enhance cancer vaccine efficacy, Immunologic research, 59 (2014) 220–228. [DOI] [PubMed] [Google Scholar]
- [21].Mehta NK, Moynihan KD, Irvine DJ, Engineering new approaches to cancer vaccines, Cancer immunology research, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsoras AN, Champion JA, Cross-linked peptide nanoclusters for delivery of oncofetal antigen as a cancer vaccine, Bioconjugate chemistry, 29 (2018) 776–785. [DOI] [PubMed] [Google Scholar]
- [23].Purwada A, Tian YF, Huang W, Rohrbach KM, Deol S, August A, Singh A, Self‐Assembly Protein Nanogels for Safer Cancer Immunotherapy, Advanced healthcare materials, 5 (2016) 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wafa EI, Geary SM, Goodman JT, Narasimhan B, Salem AK, The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response, Acta biomaterialia, 50 (2017) 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jeanbart L, Ballester M, De Titta A, Corthésy P, Romero P, Hubbell JA, Swartz MA, Enhancing efficacy of anti-cancer vaccines by targeted delivery to tumor-draining lymph nodes, Cancer immunology research, (2014) canimm. 0019.2013. [DOI] [PubMed] [Google Scholar]
- [26].Black M, Trent A, Kostenko Y, Lee JS, Olive C, Tirrell M, Self‐Assembled Peptide Amphiphile Micelles Containing a Cytotoxic T‐Cell Epitope Promote a Protective Immune Response In Vivo, Advanced Materials, 24 (2012) 3845–3849. [DOI] [PubMed] [Google Scholar]
- [27].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines, Nature, 507 (2014) 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kuai R, Sun X, Yuan W, Ochyl LJ, Xu Y, Najafabadi AH, Scheetz L, Yu M-Z, Balwani I, Schwendeman A, Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy, Journal of Controlled Release, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Acharya AP, Sinha M, Ratay ML, Ding X, Balmert SC, Workman CJ, Wang Y, Vignali DA, Little SR, Localized Multi‐Component Delivery Platform Generates Local and Systemic Anti‐Tumor Immunity, Advanced Functional Materials, 27 (2017) 1604366. [Google Scholar]
- [30].Acharya AP, Little SR, Stapled endosome disrupting alginate particles for cytosolic delivery of cations, Journal of drug targeting, 23 (2015) 690–697. [DOI] [PubMed] [Google Scholar]
- [31].Balmert SC, Little SR, Biomimetic delivery with micro‐and nanoparticles, Advanced materials, 24 (2012) 3757–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karlsson J, Vaughan HJ, Green JJ, Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments, Annual review of chemical and biomolecular engineering, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ, Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy, Nanomedicine: Nanotechnology, Biology and Medicine, 14 (2018) 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xie YQ, Wei L, Tang L, Immunoengineering with biomaterials for enhanced cancer immunotherapy, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, (2018) e1506. [DOI] [PubMed] [Google Scholar]
- [35].Park Y-M, Lee SJ, Kim YS, Lee MH, Cha GS, Jung ID, Kang TH, Han HD, Nanoparticle-based vaccine delivery for cancer immunotherapy, Immune network, 13 (2013) 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Joshi VB, Geary SM, Gross BP, Wongrakpanich A, Norian LA, Salem AK, Tumor lysate-loaded biodegradable microparticles as cancer vaccines, Expert review of vaccines, 13 (2014) 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hainline KM, Fries CN, Collier JH, Progress Toward the Clinical Translation of Bioinspired Peptide and Protein Assemblies, Advanced healthcare materials, 7 (2018) 1700930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chesson CB, Huante M, Nusbaum RJ, Walker AG, Clover TM, Chinnaswamy J, Endsley JJ, Rudra JS, Nanoscale Peptide Self-assemblies Boost BCG-primed Cellular Immunity Against Mycobacterium tuberculosis, Scientific reports, 8 (2018) 12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wong KK, Li WA, Mooney DJ, Dranoff G, Advances in therapeutic cancer vaccines, in: Advances in immunology, Elsevier, 2016, pp. 191–249. [DOI] [PubMed] [Google Scholar]
- [40].Meyer RA, Sunshine JC, Green JJ, Biomimetic particles as therapeutics, Trends in biotechnology, 33 (2015) 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Singh A, Peppas NA, Hydrogels and scaffolds for immunomodulation, Advanced Materials, 26 (2014) 6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Joshi VB, Geary SM, Salem AK, Biodegradable particles as vaccine delivery systems: size matters, The AAPS journal, 15 (2013) 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sunshine JC, Perica K, Schneck JP, Green JJ, Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells, Biomaterials, 35 (2014) 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kang S, Ahn S, Lee J, Kim JY, Choi M, Gujrati V, Kim H, Kim J, Shin E-C, Jon S, Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses, Journal of Controlled Release, 256 (2017) 56–67. [DOI] [PubMed] [Google Scholar]
- [45].Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, Ye Y, Bomba H, Hu X, Liu Z, In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy, Science translational medicine, 10 (2018) eaan3682. [DOI] [PubMed] [Google Scholar]
- [46].He Y, Li J, Turvey ME, Funkenbusch MT, Hong C, Uppu DS, He H, Irvine DJ, Hammond PT, Synthetic Lift-off Polymer beneath Layer-by-Layer Films for Surface-Mediated Drug Delivery, ACS Macro Letters, 6 (2017) 1320–1324. [DOI] [PubMed] [Google Scholar]
- [47].Rhodes KR, Green JJ, Nanoscale artificial antigen presenting cells for cancer immunotherapy, Molecular immunology, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang R, Ulery BD, Synthetic vaccine characterization and design, Journal of Bionanoscience, 12 (2018) 1–11. [Google Scholar]
- [49].Kutzler MA, Weiner DB, DNA vaccines: ready for prime time?, Nature Reviews Genetics, 9 (2008) 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tang D.-c., DeVit M, Johnston SA, Genetic immunization is a simple method for eliciting an immune response, Nature, 356 (1992) 152. [DOI] [PubMed] [Google Scholar]
- [51].Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki V, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, Heterologous protection against influenza by injection of DNA encoding a viral protein, Science, 259 (1993) 1745–1749. [DOI] [PubMed] [Google Scholar]
- [52].Leitner WW, Hwang LN, Zhou A, Silverman RH, Williams BR, Dubensky TW, Ying H, Restifo NP, Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways, Nature medicine, 9 (2003) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu M, DNA vaccines: a review, Journal of internal medicine, 253 (2003) 402–410. [DOI] [PubMed] [Google Scholar]
- [54].Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH, Therapeutic cancer vaccines, The Journal of clinical investigation, 125 (2015) 3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Klinman DM, Yamshchikov G, Ishigatsubo Y, Contribution of CpG motifs to the immunogenicity of DNA vaccines, The Journal of Immunology, 158 (1997) 3635–3639. [PubMed] [Google Scholar]
- [56].Kojima Y, Xin K-Q, Ooki T, Hamajima K, Oikawa T, Shinoda K, Ozaki T, Hoshino Y, Jounai N, Nakazawa M, Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine, Vaccine, 20 (2002) 2857–2865. [DOI] [PubMed] [Google Scholar]
- [57].Lee S-H, Danishmalik SN, Sin J-I, DNA vaccines, electroporation and their applications in cancer treatment, Human vaccines & immunotherapeutics, 11 (2015) 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yang B, Jeang J, Yang A, Wu T, Hung C-F, DNA vaccine for cancer immunotherapy, Human vaccines & immunotherapeutics, 10 (2014) 3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kuang H, Ku SH, Kokkoli E, The design of peptide-amphiphiles as functional ligands for liposomal anticancer drug and gene delivery, Advanced drug delivery reviews, 110 (2017) 80–101. [DOI] [PubMed] [Google Scholar]
- [60].Li NK, Kuang H, Fuss WH, Zauscher S, Kokkoli E, Yingling YG, Salt Responsive Morphologies of ssDNA‐Based Triblock Polyelectrolytes in Semi ‐Dilute Regime: Effect of Volume Fractions and Polyelectrolyte Length, Macromolecular rapid communications, 38 (2017) 1700422. [DOI] [PubMed] [Google Scholar]
- [61].Sun Y, Peng S, Yang A, Farmer E, Wu T, Hung C-F, Coinjection of IL2 DNA enhances E7-specific antitumor immunity elicited by intravaginal therapeutic HPV DNA vaccination with electroporation, Gene therapy, 24 (2017) 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chew WL, Tabebordbar M, Cheng JK, Mali P, Wu EY, Ng AH, Zhu K, Wagers AJ, Church GM, A multifunctional AAV–CRISPR–Cas9 and its host response, Nature methods, 13 (2016) 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sardesai NY, Weiner DB, Electroporation delivery of DNA vaccines: prospects for success, Current opinion in immunology, 23 (2011) 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, Signori E, Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration, Expert opinion on biological therapy, 8 (2008) 1645–1657. [DOI] [PubMed] [Google Scholar]
- [65].Nguyen-Hoai T, Pezzutto A, Westermann J, Gene gun Her2/neu DNA vaccination: evaluation of vaccine efficacy in a syngeneic Her2/neu mouse tumor model, Gene Therapy of Solid Cancers: Methods and Protocols, (2015) 17–37. [DOI] [PubMed] [Google Scholar]
- [66].Raska M, Turanek J, DNA Vaccines for the Induction of Immune Responses in Mucosal Tissues, in: Mucosal Immunology (Fourth Edition), Elsevier, 2015, pp. 1307–1335. [Google Scholar]
- [67].Coban C, Kobiyama K, Aoshi T, Takeshita F, Horii T, Akira S, J Ishii K, Novel strategies to improve DNA vaccine immunogenicity, Current gene therapy, 11 (2011) 479–484. [DOI] [PubMed] [Google Scholar]
- [68].Zahm CD, Colluru VT, McNeel DG, DNA vaccines for prostate cancer, Pharmacology & therapeutics, 174 (2017) 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fioretti D, Iurescia S, Fazio VM, Rinaldi M, DNA vaccines: developing new strategies against cancer, BioMed Research International, 2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, Hess PR, Jankowski MK, Jones PD, Leibman NF, Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor, American journal of veterinary research, 72 (2011) 1631–1638. [DOI] [PubMed] [Google Scholar]
- [71].Tagawa ST, Lee P, Snively J, Boswell W, Ounpraseuth S, Lee S, Hickingbottom B, Smith J, Johnson D, Weber JS, Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma, Cancer, 98 (2003) 144–154. [DOI] [PubMed] [Google Scholar]
- [72].Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, Rajapaksa R, Caspar CB, van Beckhoven A, Levy R, Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma, Cancer Research, 62 (2002) 5845–5852. [PubMed] [Google Scholar]
- [73].Trimble C, Lin C-T, Hung C-F, Pai S, Juang J, He L, Gillison M, Pardoll D, Wu L, Wu T-C, Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe, Vaccine, 21 (2003) 4036–4042. [DOI] [PubMed] [Google Scholar]
- [74].Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, Pardoll D, Wu T, A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3, Clinical Cancer Research, 15 (2009) 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tiptiri-Kourpeti A, Spyridopoulou K, Pappa A, Chlichlia K, DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy, Pharmacology & therapeutics, 165 (2016) 32–49. [DOI] [PubMed] [Google Scholar]
- [76].Kasturi SP, Qin H, Thomson KS, El-Bereir S, Cha S.-c., Neelapu S, Kwak LW, Roy K, Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles, Journal of Controlled Release, 113 (2006) 261–270. [DOI] [PubMed] [Google Scholar]
- [77].Farris E, Brown DM, Ramer-Tait AE, Pannier AK, Micro-and nanoparticulates for DNA vaccine delivery, Experimental Biology and Medicine, 241 (2016) 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Stachyra A, Redkiewicz P, Kosson P, Protasiuk A, Góra-Sochacka A, Kudla G, Sirko A, Codon optimization of antigen coding sequences improves the immune potential of DNA vaccines against avian influenza virus H5N1 in mice and chickens, Virology journal, 13 (2016) 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhou W-Z, Hoon D, Huang S, Fujii S, Hashimoto K, Morishita R, Kaneda Y, RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization, Human gene therapy, 10 (1999) 2719–2724. [DOI] [PubMed] [Google Scholar]
- [80].Pollard C, Rejman J, De Haes W, Verrier B, Van Gulck E, Naessens T, De Smedt S, Bogaert P, Grooten J, Vanham G, Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines, Molecular Therapy, 21 (2013) 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bettinger T, Carlisle RC, Read ML, Ogris M, Seymour LW, Peptide-mediated RNA delivery: a novel approach for enhanced transfection of primary and post-mitotic cells, Nucleic acids research, 29 (2001) 3882–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pardi N, Hogan MJ, Porter FW, Weissman D, mRNA vaccines—a new era in vaccinology, Nature Reviews Drug Discovery, 17 (2018) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, Nonviral delivery of self-amplifying RNA vaccines, Proceedings of the National Academy of Sciences, (2012) 201209367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Magini D, Giovani C, Mangiavacchi S, Maccari S, Cecchi R, Ulmer JB, De Gregorio E, Geall AJ, Brazzoli M, Bertholet S, Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge, PloS one, 11 (2016) e0161193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bogers WM, Oostermeijer H, Mooij P, Koopman G, Verschoor EJ, Davis D, Ulmer JB, Brito LA, Cu Y, Banerjee K, Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion, The Journal of infectious diseases, 211 (2014) 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Maruggi G, Chiarot E, Giovani C, Buccato S, Bonacci S, Frigimelica E, Margarit I, Geall A, Bensi G, Maione D, Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens, Vaccine, 35 (2017) 361–368. [DOI] [PubMed] [Google Scholar]
- [87].Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H, Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses, Molecular Therapy, 26 (2018) 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kreiter S, Vormehr M, Van de Roemer N, Diken M, Löwer M, Diekmann J, Boegel S, Schrörs B, Vascotto F, Castle JC, Mutant MHC class II epitopes drive therapeutic immune responses to cancer, Nature, 520 (2015) 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kariko K, Muramatsu H, Ludwig J, Weissman D, Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA, Nucleic acids research, 39 (2011) e142–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy, Nature, 534 (2016) 396. [DOI] [PubMed] [Google Scholar]
- [91].Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkic-Zrna S, Probst J, Kallen K-J, Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity, Journal of immunotherapy, 34 (2011) 1–15. [DOI] [PubMed] [Google Scholar]
- [92].Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D, Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy, Nano letters, 17 (2016) 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sahin U, Karikó K, Türeci Ö, mRNA-based therapeutics—developing a new class of drugs, Nature reviews Drug discovery, 13 (2014) 759. [DOI] [PubMed] [Google Scholar]
- [94].Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D, Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability, Molecular therapy, 16 (2008) 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Karikó K, Buckstein M, Ni H, Weissman D, Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA, Immunity, 23 (2005) 165–175. [DOI] [PubMed] [Google Scholar]
- [96].Li J, Wang W, He Y, Li Y, Yan EZ, Zhang K, Irvine DJ, Hammond PT, Structurally programmed assembly of translation initiation nanoplex for superior mRNA delivery, ACS nano, 11 (2017) 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].De Haro C, Mendez R, Santoyo J, The eIF-2alpha kinases and the control of protein synthesis, The FASEB Journal, 10 (1996) 1378–1387. [DOI] [PubMed] [Google Scholar]
- [98].Shen X, Corey DR, Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs, Nucleic acids research, 46 (2017) 1584–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shen X, Kilikevicius A, O’Reilly D, Prakash TP, Damha MJ, Rigo F, Corey DR, Activating frataxin expression by single-stranded siRNAs targeting the GAA repeat expansion, Bioorganic & medicinal chemistry letters, 28 (2018) 2850–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Reed SG, Orr MT, Fox CB, Key roles of adjuvants in modern vaccines, Nature medicine, 19 (2013) 1597. [DOI] [PubMed] [Google Scholar]
- [101].Persano S, Guevara ML, Li Z, Mai J, Ferrari M, Pompa PP, Shen H, Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination, Biomaterials, 125 (2017) 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].De Beuckelaer A, Pollard C, Van Lint S, Roose K, Van Hoecke L, Naessens T, Udhayakumar VK, Smet M, Sanders N, Lienenklaus S, Type I interferons interfere with the capacity of mRNA lipoplex vaccines to elicit cytolytic T cell responses, Molecular Therapy, 24 (2016) 2012–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang Y, Zhang L, Xu Z, Miao L, Huang L, mRNA Vaccine with Antigen-Specific Checkpoint Blockade Induces an Enhanced Immune Response against Established Melanoma, Molecular Therapy, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Lipid-like materials for low-dose, in vivo gene silencing, Proceedings of the National Academy of Sciences, 107 (2010) 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yu D, Khan OF, Suvà ML, Dong B, Panek WK, Xiao T, Wu M, Han Y, Ahmed AU, Balyasnikova IV, Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression, Proceedings of the National Academy of Sciences, (2017) 201701911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dahlman JE, Kauffman KJ, Xing Y, Shaw TE, Mir FF, Dlott CC, Langer R, Anderson DG, Wang ET, Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics, Proceedings of the National Academy of Sciences, (2017) 201620874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Adams D, Suhr OB, Dyck PJ, Litchy WJ, Leahy RG, Chen J, Gollob J, Coelho T, Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy, BMC neurology, 17 (2017) 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].ClinicalTrials.Gov, APOLLO: The Study of an Investigational Drug, Patisiran (ALN-TTR02), for the Treatment of Transthyretin (TTR)-Mediated Amyloidosis, NIH U.S. National Library of Medicine, (2018). [Google Scholar]
- [109].Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D, Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes, Journal of Controlled Release, 217 (2015) 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination, Nature, 543 (2017) 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Modified mRNA vaccines protect against Zika virus infection, Cell, 168 (2017) 1114–1125. e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S, Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose, Proceedings of the National Academy of Sciences, 113 (2016) E4133–E4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, Ploegh HL, An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model, Scientific reports, 7 (2017) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ball RL, Hajj KA, Vizelman J, Bajaj P, Whitehead KA, Lipid nanoparticle formulations for enhanced co-delivery of siRNA and mRNA, Nano letters, (2018). [DOI] [PubMed] [Google Scholar]
- [115].Sebastian M, Papachristofilou A, Weiss C, Früh M, Cathomas R, Hilbe W, Wehler T, Rippin G, Koch SD, Scheel B, Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer, BMC cancer, 14 (2014) 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kübler H, Scheel B, Gnad-Vogt U, Miller K, Schultze-Seemann W, Dorp F, Parmiani G, Hampel C, Wedel S, Trojan L, Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study, Journal for immunotherapy of cancer, 3 (2015) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang X-Y, Therapeutic cancer vaccines: past, present, and future, in: Advances in cancer research, Elsevier, 2013, pp. 421–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li W, Joshi MD, Singhania S, Ramsey KH, Murthy AK, Peptide vaccine: progress and challenges, Vaccines, 2 (2014) 515–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cox MM, Recombinant protein vaccines produced in insect cells, Vaccine, 30 (2012) 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Nascimento I, Leite L, Recombinant vaccines and the development of new vaccine strategies, Brazilian Journal of Medical and Biological Research, 45 (2012) 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Slingluff CL Jr, The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination?, Cancer journal (Sudbury, Mass.), 17 (2011) 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chianese-Bullock KA, Lewis ST, Sherman NE, Shannon JD, Slingluff CL Jr, Multi-peptide vaccines vialed as peptide mixtures can be stable reagents for use in peptide-based immune therapies, Vaccine, 27 (2009) 1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, Weaver JC, Dellacherie MO, Shih T-Y, Ali OA, A facile approach to enhance antigen response for personalized cancer vaccination, Nature materials, 17 (2018) 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ, Designer vaccine nanodiscs for personalized cancer immunotherapy, Nature materials, 16 (2017) 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tan ML, Choong PF, Dass CR, Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery, Peptides, 31 (2010) 184–193. [DOI] [PubMed] [Google Scholar]
- [126].Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z, Yu H, Li Y, Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy, Theranostics, 7 (2017) 2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhang R, Leeper CN, Wang X, White TA, Ulery BD, Immunomodulatory vasoactive intestinal peptide amphiphile micelles, Biomaterials science, (2018). [DOI] [PubMed] [Google Scholar]
- [128].Pol J, Bloy N, Buqué A, Eggermont A, Cremer I, Sautes-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer G, Trial Watch: Peptide-based anticancer vaccines, Oncoimmunology, 4 (2015) e974411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ, MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study, Cancer prevention research, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Reed CM, Cresce ND, Mauldin IS, Slingluff CL, Olson WC, Vaccination with melanoma helper peptides induces antibody responses associated with improved overall survival, Clinical Cancer Research, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Morse MA, Secord AA, Blackwell KL, Hobeika A, Sinnathamby G, Osada T, Hafner J, Philip M, Clay TM, Lyerly HK, MHC class I-presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T cell responses against breast and ovarian cancer, Clinical Cancer Research, (2011) clincanres. 2614.2010. [DOI] [PubMed] [Google Scholar]
- [132].Palucka K, Banchereau J, Cancer immunotherapy via dendritic cells, Nature Reviews Cancer, 12 (2012) 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Palucka K, Banchereau J, Dendritic-cell-based therapeutic cancer vaccines, Immunity, 39 (2013) 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN, Clinical use of dendritic cells for cancer therapy, The lancet oncology, 15 (2014) e257–e267. [DOI] [PubMed] [Google Scholar]
- [135].Mody N, Dubey S, Sharma R, Agrawal U, Vyas SP, Dendritic cell-based vaccine research against cancer, Expert review of clinical immunology, 11 (2015) 213–232. [DOI] [PubMed] [Google Scholar]
- [136].Figdor CG, de Vries IJM, Lesterhuis WJ, Melief CJ, Dendritic cell immunotherapy: mapping the way, Nature medicine, 10 (2004) 475. [DOI] [PubMed] [Google Scholar]
- [137].Jaroslawski S, Toumi M, Sipuleucel-T (Provenge®)-Autopsy of an Innovative Paradigm Change in Cancer Treatment: Why a Single-Product Biotech Company Failed to Capitalize on its Breakthrough Invention, BioDrugs, 29 (2015) 301. [DOI] [PubMed] [Google Scholar]
- [138].Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G, Trial watch: Dendritic cell-based interventions for cancer therapy, Oncoimmunology, 1 (2012) 1111–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, Master VA, Pal SK, Miller WH, Karsh LI, Survival with AGS-003, an autologous dendritic cell–based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results, Journal for immunotherapy of cancer, 3 (2015) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle F, Enk A, Bröcker E-B, Grabbe S, Rittgen W, Edler L, Sucker A, Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG, Annals of Oncology, 17 (2006) 563–570. [DOI] [PubMed] [Google Scholar]
- [141].Sondak VK, Liu P, Tuthill RJ, Kempf RA, Unger JM, Sosman JA, Thompson GRW, Redman BG, Jakowatz JG, Noyes RD, Adjuvant Immunotherapy of Resected, Intermediate-Thickness, Node-Negative Melanoma With an Allogeneic Tumor Vaccine: Overall Results of a Randomized Trial of the Southwest Oncology Group (SWOG-9035), Journal of Clinical Oncology, 20 (2002) 2058–2066. [DOI] [PubMed] [Google Scholar]
- [142].Chiang CL-L, Coukos G, Kandalaft LE, Whole tumor antigen vaccines: where are we?, Vaccines, 3 (2015) 344–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Srivatsan S, Patel JM, Bozeman EN, Imasuen IE, He S, Daniels D, Selvaraj P, Allogeneic tumor cell vaccines: the promise and limitations in clinical trials, Human vaccines & immunotherapeutics, 10 (2014) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chiang CL-L, Benencia F, Coukos G, Whole tumor antigen vaccines, in: Seminars in immunology, Elsevier, 2010, pp. 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW, Adjuvant dendritic cell‐based tumour vaccination for children with malignant brain tumours, Pediatric blood & cancer, 54 (2010) 519–525. [DOI] [PubMed] [Google Scholar]
- [146].Chiang CL-L, Hagemann AR, Leskowitz R, Mick R, Garrabrant T, Czerniecki BJ, Kandalaft LE, Powell DJ Jr, Coukos G, Day-4 myeloid dendritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses, PloS one, 6 (2011) e28732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Shi G-N, Zhang C-N, Xu R, Niu J-F, Song H-J, Zhang X-Y, Wang W-W, Wang Y-M, Li C, Wei X-Q, Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine, Biomaterials, 113 (2017) 191–202. [DOI] [PubMed] [Google Scholar]
- [148].Iranpour S, Nejati V, Delirezh N, Biparva P, Shirian S, Enhanced stimulation of anti-breast cancer T cells responses by dendritic cells loaded with poly lactic-co-glycolic acid (PLGA) nanoparticle encapsulated tumor antigens, Journal of Experimental & Clinical Cancer Research, 35 (2016) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ali OA, Emerich D, Dranoff G, Mooney DJ, In situ regulation of DC subsets and T cells mediates tumor regression in mice, Science translational medicine, 1 (2009) 8ra19–18ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].De Gruijl TD, van den Eertwegh AJ, Pinedo HM, Scheper RJ, Whole-cell cancer vaccination: from autologous to allogeneic tumor-and dendritic cell-based vaccines, Cancer Immunology, Immunotherapy, 57 (2008) 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, Zhou L, Jiao S, Li Y, A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors, Nature communications, 9 (2018) 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S, Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer, Clinical Cancer Research, 12 (2006) 3394–3401. [DOI] [PubMed] [Google Scholar]
- [153].Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M, Lifshitz S, Magee M, Oh J, Mill SW, Phase I trial of “bi-shRNAifurin/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer, Molecular Therapy, 20 (2012) 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Berd D, Sato T, Cohn H, Maguire HC Jr, Mastrangelo MJ, Treatment of metastatic melanoma with autologous, hapten ‐ modified melanoma vaccine: Regression of pulmonary metastases, International journal of cancer, 94 (2001) 531–539. [DOI] [PubMed] [Google Scholar]
- [155].Mak IW, Evaniew N, Ghert M, Lost in translation: animal models and clinical trials in cancer treatment, American journal of translational research, 6 (2014) 114. [PMC free article] [PubMed] [Google Scholar]
- [156].Claesson MH, Why current peptide-based cancer vaccines fail: lessons from the three Es, Immunotherapy, 1 (2009) 513–516. [DOI] [PubMed] [Google Scholar]
- [157].Salem ML, The use of dendritic cells for peptide-based vaccination in cancer immunotherapy, in: Cancer Vaccines, Springer, 2014, pp. 479–503. [DOI] [PubMed] [Google Scholar]
- [158].Gu L, Mooney DJ, Biomaterials and emerging anticancer therapeutics: engineering the microenvironment, Nature Reviews Cancer, 16 (2016) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Brannon-Peppas L, Blanchette JO, Nanoparticle and targeted systems for cancer therapy, Advanced drug delivery reviews, 64 (2012) 206–212. [DOI] [PubMed] [Google Scholar]
- [160].Goldberg MS, Immunoengineering: how nanotechnology can enhance cancer immunotherapy, Cell, 161 (2015) 201–204. [DOI] [PubMed] [Google Scholar]
- [161].Schwendener RA, Liposomes as vaccine delivery systems: a review of the recent advances, Therapeutic advances in vaccines, 2 (2014) 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bulbake U, Doppalapudi S, Kommineni N, Khan W, Liposomal formulations in clinical use: an updated review, Pharmaceutics, 9 (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Campbell PI, Toxicity of some charged lipids used in liposome preparations, Cytobios, 37 (1983) 21–26. [PubMed] [Google Scholar]
- [164].Zucker D, Marcus D, Barenholz Y, Goldblum A, Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties, Journal of Controlled Release, 139 (2009) 73–80. [DOI] [PubMed] [Google Scholar]
- [165].Yang T, Cui F-D, Choi M-K, Cho J-W, Chung S-J, Shim C-K, Kim D-D, Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation, International journal of Pharmaceutics, 338 (2007) 317–326. [DOI] [PubMed] [Google Scholar]
- [166].Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A, Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity, Vaccine, 26 (2008) 5046–5057. [DOI] [PubMed] [Google Scholar]
- [167].Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date, Pharmaceutical research, 33 (2016) 2373–2387. [DOI] [PubMed] [Google Scholar]
- [168].Reis CP, Neufeld RJ, Veiga F, Preparation of Drug-Loaded Polymeric Nanoparticles, in: Nanomedicine in Cancer, Pan Stanford, 2017, pp. 197–240. [DOI] [PubMed] [Google Scholar]
- [169].Jass J, Tjärnhage T, Puu G, From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: an atomic force microscopy study, Biophysical journal, 79 (2000) 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Wischke C, Schwendeman SP, Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles, International Journal of pharmaceutics, 364 (2008) 298–327. [DOI] [PubMed] [Google Scholar]
- [171].Manoochehri S, Darvishi B, Kamalinia G, Amini M, Fallah M, Ostad SN, Atyabi F, Dinarvand R, Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel, DARU Journal of Pharmaceutical Sciences, 21 (2013) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Qin Y, Chen H, Zhang Q, Wang X, Yuan W, Kuai R, Tang J, Zhang L, Zhang Z, Zhang Q, Liposome formulated with TAT-modified cholesterol for improving brain delivery and therapeutic efficacy on brain glioma in animals, International journal of pharmaceutics, 420 (2011) 304–312. [DOI] [PubMed] [Google Scholar]
- [173].Qiu F, Becker KW, Knight FC, Baljon JJ, Sevimli S, Shae D, Gilchuk P, Joyce S, Wilson JT, Poly (propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines, Biomaterials, 182 (2018) 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Cui L, Osada K, Imaizumi A, Kataoka K, Nakano K, Feasibility of a subcutaneously administered block/homo-mixed polyplex micelle as a carrier for DNA vaccination in a mouse tumor model, Journal of Controlled Release, 206 (2015) 220–231. [DOI] [PubMed] [Google Scholar]
- [175].Keller S, Wilson JT, Patilea GI, Kern HB, Convertine AJ, Stayton PS, Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8+ T cell responses, Journal of Controlled Release, 191 (2014) 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chiu Y-C, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM, Assembly and immunological processing of polyelectrolyte multilayers composed of antigens and adjuvants, ACS applied materials & interfaces, 8 (2016) 18722–18731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Tibbitt MW, Dahlman JE, Langer R, Emerging frontiers in drug delivery, Journal of the American Chemical Society, 138 (2016) 704–717. [DOI] [PubMed] [Google Scholar]
- [178].Silva JM, Videira M, Gaspar R, Préat V, Florindo HF, Immune system targeting by biodegradable nanoparticles for cancer vaccines, Journal of Controlled Release, 168 (2013) 179–199. [DOI] [PubMed] [Google Scholar]
- [179].Bolhassani A, Safaiyan S, Rafati S, Improvement of different vaccine delivery systems for cancer therapy, Molecular cancer, 10 (2011) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Bencherif SA, Sands RW, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-Y, Verbeke CS, Bhatta D, Dranoff G, Injectable cryogel-based whole-cell cancer vaccines, Nature communications, 6 (2015) 7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ, Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy, Nature biotechnology, 33 (2015) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Wang H, Mooney DJ, Biomaterial-assisted targeted modulation of immune cells in cancer treatment, Nature materials, (2018) 1. [DOI] [PubMed] [Google Scholar]
- [183].Bharti C, Nagaich U, Pal AK, Gulati N, Mesoporous silica nanoparticles in target drug delivery system: a review, International journal of pharmaceutical investigation, 5 (2015) 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Shih TY, Blacklow SO, Li AW, Freedman BR, Bencherif S, Koshy ST, Darnell MC, Mooney DJ, Injectable, Tough Alginate Cryogels as Cancer Vaccines, Advanced healthcare materials, (2018) 1701469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Nicodemus GD, Bryant SJ, Cell encapsulation in biodegradable hydrogels for tissue engineering applications, Tissue Engineering Part B: Reviews, 14 (2008) 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wang C, Ye Y, Hu Q, Bellotti A, Gu Z, Tailoring biomaterials for cancer immunotherapy: Emerging trends and future outlook, Advanced Materials, 29 (2017) 1606036. [DOI] [PubMed] [Google Scholar]
- [187].Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity, Advanced Materials, 29 (2017) 1703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Jiang W, Gupta RK, Deshpande MC, Schwendeman SP, Biodegradable poly (lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens, Advanced drug delivery reviews, 57 (2005) 391–410. [DOI] [PubMed] [Google Scholar]
- [189].Card CM, Shann SY, Swartz MA, Emerging roles of lymphatic endothelium in regulating adaptive immunity, The Journal of clinical investigation, 124 (2014) 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lallas A, Kyrgidis A, Ferrara G, Kittler H, Apalla Z, Castagnetti F, Longo C, Moscarella E, Piana S, Zalaudek I, Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review, The lancet oncology, 15 (2014) e178–e183. [DOI] [PubMed] [Google Scholar]
- [191].Kuai R, Sun X, Yuan W, Xu Y, Schwendeman A, Moon JJ, Subcutaneous Nanodisc Vaccination with Neoantigens for Combination Cancer Immunotherapy, Bioconjugate chemistry, 29 (2018) 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Andorko JI, Tostanoski LH, Solano E, Mukhamedova M, Jewell CM, Intra-lymph node injection of biodegradable polymer particles, Journal of visualized experiments: JoVE, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Johansen P, Senti G, Martínez Gómez JM, Wüthrich B, Bot A, Kündig TM, Heat denaturation, a simple method to improve the immunotherapeutic potential of allergens, European journal of immunology, 35 (2005) 3591–3598. [DOI] [PubMed] [Google Scholar]
- [194].Johansen P, Häffner A, Koch F, Zepter K, Erdmann I, Maloy K, Simard J, Storni T, Senti G, Bot A, Direct intralymphatic injection of peptide vaccines enhances immunogenicity, European journal of immunology, 35 (2005) 568–574. [DOI] [PubMed] [Google Scholar]
- [195].Filippelli M, Lionetti E, Gennaro A, Lanzafame A, Arrigo T, Salpietro C, La Rosa M, Leonardi S, Hepatitis B vaccine by intradermal route in non responder patients: an update, World Journal of Gastroenterology: WJG, 20 (2014) 10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Herzog C, Influence of parenteral administration routes and additional factors on vaccine safety and immunogenicity: a review of recent literature, Expert review of vaccines, 13 (2014) 399–415. [DOI] [PubMed] [Google Scholar]
- [197].Cook IF, Best vaccination practice and medically attended injection site events following deltoid intramuscular injection, Human vaccines & immunotherapeutics, 11 (2015) 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Charest AF, McDougall J, Goldstein MB, A randomized comparison of intradermal and intramuscular vaccination against hepatitis B virus in incident chronic hemodialysis patients, American journal of kidney diseases, 36 (2000) 976–982. [DOI] [PubMed] [Google Scholar]
- [199].Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, Schlereth B, Handley A, King L, Hülsemann V, Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine, Vaccine, 24 (2006) 2065–2070. [DOI] [PubMed] [Google Scholar]
- [200].Klinge J, Lugauer S, Korn K, Heininger U, Stehr K, Comparison of immunogenicity and reactogenicity of a measles, mumps and rubella (MMR) vaccine in German children vaccinated at 9–11, 12–14 or 15–17 months of age☆, Vaccine, 18 (2000) 3134–3140. [DOI] [PubMed] [Google Scholar]
- [201].Zhang R, Smith JD, Allen BN, Kramer JS, Schauflinger M, Ulery BD, Peptide Amphiphile Micelle Vaccine Size and Charge Influence the Host Antibody Response, ACS Biomaterials Science & Engineering, (2018). [DOI] [PubMed] [Google Scholar]
- [202].Chang TZ, Stadmiller SS, Staskevicius E, Champion JA, Effects of ovalbumin protein nanoparticle vaccine size and coating on dendritic cell processing, Biomaterials science, 5 (2017) 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Trevaskis NL, Kaminskas LM, Porter CJ, From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity, Nature Reviews Drug Discovery, 14 (2015) 781. [DOI] [PubMed] [Google Scholar]
- [204].Irvine DJ, Swartz MA, Szeto GL, Engineering synthetic vaccines using cues from natural immunity, Nature materials, 12 (2013) 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Strauss O, Dunbar PR, Bartlett A, Phillips A, The immunophenotype of antigen presenting cells of the mononuclear phagocyte system in normal human liver–A systematic review, Journal of hepatology, 62 (2015) 458–468. [DOI] [PubMed] [Google Scholar]
- [206].Bachmann MF, Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns, Nature Reviews Immunology, 10 (2010) 787. [DOI] [PubMed] [Google Scholar]
- [207].Stylianopoulos T, Poh M-Z, Insin N, Bawendi MG, Fukumura D, Munn LL, Jain RK, Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions, Biophysical journal, 99 (2010) 1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zhang P, Chiu Y-C, Tostanoski LH, Jewell CM, Polyelectrolyte multilayers assembled entirely from immune signals on gold nanoparticle templates promote antigen-specific T cell response, Acs Nano, 9 (2015) 6465–6477. [DOI] [PubMed] [Google Scholar]
- [209].Varypataki EM, van der Maaden K, Bouwstra J, Ossendorp F, Jiskoot W, Cationic liposomes loaded with a synthetic long peptide and poly (I: C): a defined adjuvanted vaccine for induction of antigen-specific T cell cytotoxicity, The AAPS journal, 17 (2015) 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Zeng Q, Jiang H, Wang T, Zhang Z, Gong T, Sun X, Cationic micelle delivery of Trp2 peptide for efficient lymphatic draining and enhanced cytotoxic T-lymphocyte responses, Journal of controlled release, 200 (2015) 1–12. [DOI] [PubMed] [Google Scholar]
- [211].Liu L, Ma P, Wang H, Zhang C, Sun H, Wang C, Song C, Leng X, Kong D, Ma G, Immune responses to vaccines delivered by encapsulation into and/or adsorption onto cationic lipid-PLGA hybrid nanoparticles, Journal of Controlled Release, 225 (2016) 230–239. [DOI] [PubMed] [Google Scholar]
- [212].Zeng Q, Li H, Jiang H, Yu J, Wang Y, Ke H, Gong T, Zhang Z, Sun X, Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines, Biomaterials, 122 (2017) 105–113. [DOI] [PubMed] [Google Scholar]
- [213].Pozzi D, Colapicchioni V, Caracciolo G, Piovesana S, Capriotti AL, Palchetti S, De Grossi S, Riccioli A, Amenitsch H, Laganà A, Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells, Nanoscale, 6 (2014) 2782–2792. [DOI] [PubMed] [Google Scholar]
- [214].Mishra S, Webster P, Davis ME, PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles, European journal of cell biology, 83 (2004) 97–111. [DOI] [PubMed] [Google Scholar]
- [215].Kourtis IC, Hirosue S, De Titta A, Kontos S, Stegmann T, Hubbell JA, Swartz MA, Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice, PloS one, 8 (2013) e61646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Irvine DJ, Hanson MC, Rakhra K, Tokatlian T, Synthetic nanoparticles for vaccines and immunotherapy, Chemical reviews, 115 (2015) 11109–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Bolhassani A, Jafarzade BS, Mardani G, In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides, Peptides, 87 (2017) 50–63. [DOI] [PubMed] [Google Scholar]
- [218].Kreutz M, Tacken PJ, Figdor CG, Targeting dendritic cells—why bother?, Blood, 121 (2013) 2836–2844. [DOI] [PubMed] [Google Scholar]
- [219].Bernhard CA, Ried C, Kochanek S, Brocker T, CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells, Proceedings of the National Academy of Sciences, 112 (2015) 5461–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].van Dinther D, Veninga H, Iborra S, Borg EG, Hoogterp L, Olesek K, Beijer MR, Schetters ST, Kalay H, Garcia-Vallejo JJ, Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8+ T Cell Cross-Priming, Cell Reports, 22 (2018) 1484–1495. [DOI] [PubMed] [Google Scholar]
- [221].Heath WR, Belz GT, Behrens G, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA, Cross‐presentation, dendritic cell subsets, and the generation of immunity to cellular antigens, Immunological reviews, 199 (2004) 9–26. [DOI] [PubMed] [Google Scholar]
- [222].Joffre OP, Segura E, Savina A, Amigorena S, Cross-presentation by dendritic cells, Nature Reviews Immunology, 12 (2012) 557. [DOI] [PubMed] [Google Scholar]
- [223].Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee H-W, Park CG, Differential antigen processing by dendritic cell subsets in vivo, Science, 315 (2007) 107–111. [DOI] [PubMed] [Google Scholar]
- [224].Den Haan JM, Lehar SM, Bevan MJ, CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo, Journal of Experimental Medicine, 192 (2000) 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Zhang W, An M, Xi J, Liu H, Targeting CpG Adjuvant to Lymph Node via Dextran Conjugate Enhances Antitumor Immunotherapy, Bioconjugate chemistry, 28 (2017) 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Qian Y, Jin H, Qiao S, Dai Y, Huang C, Lu L, Luo Q, Zhang Z, Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy, Biomaterials, 98 (2016) 171–183. [DOI] [PubMed] [Google Scholar]
- [227].De Koker S, Cui J, Vanparijs N, Albertazzi L, Grooten J, Caruso F, De Geest BG, Engineering Polymer Hydrogel Nanoparticles for Lymph Node‐Targeted Delivery, Angewandte Chemie International Edition, 55 (2016) 1334–1339. [DOI] [PubMed] [Google Scholar]
- [228].Cruje C, Chithrani D, Polyethylene glycol density and length affects nanoparticle uptake by cancer cells, J Nanomed Res, 1 (2014) 00006. [Google Scholar]
- [229].Leleux J, Atalis A, Roy K, Engineering immunity: Modulating dendritic cell subsets and lymph node response to direct immune-polarization and vaccine efficacy, Journal of Controlled Release, 219 (2015) 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Macri C, Dumont C, Johnston AP, Mintern JD, Targeting dendritic cells: a promising strategy to improve vaccine effectiveness, Clinical & translational immunology, 5 (2016) e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Löwik CW, Ossendorp F, Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: a comparative study, Journal of Controlled Release, 192 (2014) 209–218. [DOI] [PubMed] [Google Scholar]
- [232].Raghuwanshi D, Mishra V, Suresh MR, Kaur K, A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles, Vaccine, 30 (2012) 7292–7299. [DOI] [PubMed] [Google Scholar]
- [233].Tacken PJ, Figdor CG, Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines, in: Seminars in immunology, Elsevier, 2011, pp. 12–20. [DOI] [PubMed] [Google Scholar]
- [234].Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W, de Gruijl T, Löwik C, Oostendorp J, van der Burg SH, CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses, Biomaterials, 40 (2015) 88–97. [DOI] [PubMed] [Google Scholar]
- [235].Saluja SS, Hanlon DJ, Sharp FA, Hong E, Khalil D, Robinson E, Tigelaar R, Fahmy TM, Edelson RL, Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen, International journal of nanomedicine, 9 (2014) 5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Chatterjee B, Smed-Sörensen A, Cohn L, Chalouni C, Vandlen R, Lee B-C, Widger J, Keler T, Delamarre L, Mellman I, Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells, Blood, 120 (2012) 2011–2020. [DOI] [PubMed] [Google Scholar]
- [237].Liu JK, The history of monoclonal antibody development–Progress, remaining challenges and future innovations, Annals of Medicine and Surgery, 3 (2014) 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Yan Z, Wu Y, Du J, Li G, Wang S, Cao W, Zhou X, Wu C, Zhang D, Jing X, A novel peptide targeting Clec9a on dendritic cell for cancer immunotherapy, Oncotarget, 7 (2016) 40437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Sioud M, Skorstad G, Mobergslien A, Sæbøe-Larssen S, A novel peptide carrier for efficient targeting of antigens and nucleic acids to dendritic cells, The FASEB Journal, 27 (2013) 3272–3283. [DOI] [PubMed] [Google Scholar]
- [240].Kreutz M, Tacken PJ, Figdor CG, Targeting dendritic cells: why bother?, Blood, (2013) blood-2012–2009-452078. [DOI] [PubMed] [Google Scholar]
- [241].Yang G, Jiang Y, Tong P, Li C, Yang W, Hu J, Ye L, Gu W, Shi C, Shan B, Alleviation of enterotoxigenic Escherichia coli challenge by recombinant Lactobacillus plantarum expressing a FaeG-and DC-targeting peptide fusion protein, Beneficial microbes, 8 (2017) 379–391. [DOI] [PubMed] [Google Scholar]
- [242].HogenEsch H, Mechanism of immunopotentiation and safety of aluminum adjuvants, Frontiers in immunology, 3 (2013) 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Oleszycka E, Lavelle EC, Immunomodulatory properties of the vaccine adjuvant alum, Current opinion in immunology, 28 (2014) 1–5. [DOI] [PubMed] [Google Scholar]
- [244].Rudra JS, Banasik BN, Milligan GN, A combined carrier-adjuvant system of peptide nanofibers and toll-like receptor agonists potentiates robust CD8+ T cell responses, Vaccine, 36 (2018) 438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Bachmann MF, Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns, Nature Reviews Immunology, 10 (2010) 787–796. [DOI] [PubMed] [Google Scholar]
- [246].de Groot AM, Du G, Mönkäre J, Platteel AC, Broere F, Bouwstra JA, Sijts AJ, Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium, Journal of Controlled Release, 266 (2017) 27–35. [DOI] [PubMed] [Google Scholar]
- [247].Rahimian S, Fransen MF, Kleinovink JW, Christensen JR, Amidi M, Hennink WE, Ossendorp F, Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation, Journal of Controlled Release, 203 (2015) 16–22. [DOI] [PubMed] [Google Scholar]
- [248].Du G, Hathout RM, Nasr M, Nejadnik MR, Tu J, Koning RI, Koster AJ, Slütter B, Kros A, Jiskoot W, Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles, Journal of Controlled Release, 266 (2017) 109–118. [DOI] [PubMed] [Google Scholar]
- [249].Chiu Y-C, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM, Modular vaccine design using carrier-free capsules assembled from polyionic immune signals, ACS biomaterials science & engineering, 1 (2015) 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Molino NM, Neek M, Tucker JA, Nelson EL, Wang S-W, Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses, Biomaterials, 86 (2016) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, Peng R, Liu Z, Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination, ACS nano, (2018). [DOI] [PubMed] [Google Scholar]
- [252].Fan Y, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ, Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy, Nano letters, 17 (2017) 7387–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity, Advanced Materials, 29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Mohsen MO, Gomes AC, Cabral-Miranda G, Krueger CC, Leoratti FM, Stein JV, Bachmann MF, Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination, Journal of Controlled Release, 251 (2017) 92–100. [DOI] [PubMed] [Google Scholar]
- [255].Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release, Vaccine, 32 (2014) 2882–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Programming the magnitude and persistence of antibody responses with innate immunity, Nature, 470 (2011) 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Lee BL, Barton GM, Trafficking of endosomal Toll-like receptors, Trends in cell biology, 24 (2014) 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Neefjes J, Jongsma ML, Paul P, Bakke O, Towards a systems understanding of MHC class I and MHC class II antigen presentation, Nature Reviews Immunology, 11 (2011) 823. [DOI] [PubMed] [Google Scholar]
- [259].Armstrong L, Medford A, Hunter K, Uppington K, Millar A, Differential expression of Toll‐like receptor (TLR)‐2 and TLR‐4 on monocytes in human sepsis, Clinical & Experimental Immunology, 136 (2004) 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Zhang R, Kramer JS, Smith JD, Allen BN, Leeper CN, Li X, Morton LD, Gallazzi F, Ulery BD, Vaccine Adjuvant Incorporation Strategy Dictates Peptide Amphiphile Micelle Immunostimulatory Capacity, The AAPS journal, 20 (2018) 73. [DOI] [PubMed] [Google Scholar]
- [261].Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S, Antigen presentation and T cell stimulation by dendritic cells, Annual review of immunology, 20 (2002) 621–667. [DOI] [PubMed] [Google Scholar]
- [262].Yang L, Seki E, Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms, Frontiers in physiology, 3 (2012) 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Daftarian P, Sharan R, Haq W, Ali S, Longmate J, Termini J, Diamond DJ, Novel conjugates of epitope fusion peptides with CpG-ODN display enhanced immunogenicity and HIV recognition, Vaccine, 23 (2005) 3453–3468. [DOI] [PubMed] [Google Scholar]
- [264].Azmi F, Fuaad AAHA, Giddam AK, Batzloff MR, Good MF, Skwarczynski M, Toth I, Self-adjuvanting vaccine against group A streptococcus: application of fibrillized peptide and immunostimulatory lipid as adjuvant, Bioorganic & medicinal chemistry, 22 (2014) 6401–6408. [DOI] [PubMed] [Google Scholar]
- [265].De Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, Van Der Vlies AJ, Swartz MA, Hubbell JA, Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose, Proceedings of the National Academy of Sciences, 110 (2013) 19902–19907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Palmer CR, Jacobson ME, Fedorova O, Pyle AM, Wilson JT, Environmentally Triggerable Retinoic Acid-Inducible Gene I Agonists Using Synthetic Polymer Overhangs, Bioconjugate chemistry, 29 (2018) 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Swee LK, Guimaraes CP, Sehrawat S, Spooner E, Barrasa MI, Ploegh HL, Sortase-mediated modification of αDEC205 affords optimization of antigen presentation and immunization against a set of viral epitopes, Proceedings of the National Academy of Sciences, 110 (2013) 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Putta MR, Bhagat L, Wang D, Zhu F-G, Kandimalla ER, Agrawal S, Immune-Stimulatory Dinucleotide at the 5′-End of Oligodeoxynucleotides Is Critical for TLR9-Mediated Immune Responses, ACS medicinal chemistry letters, 4 (2013) 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Kandimalla ER, Bhagat L, Yu D, Cong Y, Tang J, Agrawal S, Conjugation of ligands at the 5 ‘-end of CpG DNA affects immunostimulatory activity, Bioconjugate chemistry, 13 (2002) 966–974. [DOI] [PubMed] [Google Scholar]
- [270].Putta MR, Zhu F-G, Wang D, Bhagat L, Dai M, Kandimalla ER, Agrawal S, Peptide conjugation at the 5′-end of oligodeoxynucleotides abrogates toll-like receptor 9-mediated immune stimulatory activity, Bioconjugate chemistry, 21 (2009) 39–45. [DOI] [PubMed] [Google Scholar]
- [271].Yu C, An M, Li M, Liu H, Immunostimulatory Properties of Lipid Modified CpG Oligonucleotides, Molecular pharmaceutics, 14 (2017) 2815–2823. [DOI] [PubMed] [Google Scholar]
- [272].Skakuj K, Wang S, Qin L, Lee A, Zhang B, Mirkin CA, Conjugation Chemistry-Dependent T-Cell Activation with Spherical Nucleic Acids, Journal of the American Chemical Society, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Kang JY, Nan X, Jin MS, Youn S-J, Ryu YH, Mah S, Han SH, Lee H, Paik S-G, Lee J-O, Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer, Immunity, 31 (2009) 873–884. [DOI] [PubMed] [Google Scholar]
- [274].Agnihotri G, Crall BM, Lewis TC, Day TP, Balakrishna R, Warshakoon HJ, Malladi SS, David SA, Structure–activity relationships in Toll-like receptor 2-agonists leading to simplified monoacyl lipopeptides, Journal of medicinal chemistry, 54 (2011) 8148–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Azuma M, Sawahata R, Akao Y, Ebihara T, Yamazaki S, Matsumoto M, Hashimoto M, Fukase K, Fujimoto Y, Seya T, The peptide sequence of diacyl lipopeptides determines dendritic cell TLR2-mediated NK activation, PLoS One, 5 (2010) e12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Fujimoto Y, Hashimoto M, Furuyashiki M, Katsumoto M, Seya T, Suda Y, Fukase K, Lipopeptides from Staphylococcus aureus as Tlr2 Ligands: prediction with mrna expression, chemical synthesis, and immunostimulatory activities, ChemBioChem, 10 (2009) 2311–2315. [DOI] [PubMed] [Google Scholar]
- [277].Steinman RM, Some interfaces of dendritic cell biology, Apmis, 111 (2003) 675–697. [DOI] [PubMed] [Google Scholar]
- [278].Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K, Development of monocytes, macrophages, and dendritic cells, Science, 327 (2010) 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Oppenheim JJ, Yang D, Alarmins: chemotactic activators of immune responses, Current opinion in immunology, 17 (2005) 359–365. [DOI] [PubMed] [Google Scholar]
- [280].Khajah M, Millen B, Cara DC, Waterhouse C, McCafferty DM, Granulocyte‐macrophage colony-stimulating factor (GM‐CSF): a chemoattractive agent for murine leukocytes in vivo, Journal of leukocyte biology, 89 (2011) 945–953. [DOI] [PubMed] [Google Scholar]
- [281].Randolph GJ, Angeli V, Swartz MA, Dendritic-cell trafficking to lymph nodes through lymphatic vessels, Nature Reviews Immunology, 5 (2005) 617. [DOI] [PubMed] [Google Scholar]
- [282].McKee AS, Marrack P, Old and new adjuvants, Current opinion in immunology, 47 (2017) 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM, Antigen depot is not required for alum adjuvanticity, The FASEB Journal, 26 (2012) 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E, Molecular and cellular signatures of human vaccine adjuvants, Proceedings of the National Academy of Sciences, 105 (2008) 10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, Van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J, Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome, The Journal of Immunology, 181 (2008) 3755–3759. [DOI] [PubMed] [Google Scholar]
- [286].Lu F, HogenEsch H, Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant, Vaccine, 31 (2013) 3979–3986. [DOI] [PubMed] [Google Scholar]
- [287].Rambe DS, Del Giudice G, Rossi S, Sanicas M, Safety and mechanism of action of licensed vaccine adjuvants, Int Curr Pharm J, 4 (2015) 420–431. [Google Scholar]
- [288].Coffman RL, Sher A, Seder RA, Vaccine adjuvants: putting innate immunity to work, Immunity, 33 (2010) 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Petrovsky N, Aguilar JC, Vaccine adjuvants: current state and future trends, Immunology and cell biology, 82 (2004) 488–496. [DOI] [PubMed] [Google Scholar]
- [290].van Doorn E, Liu H, Huckriede A, Hak E, Safety and tolerability evaluation of the use of Montanide ISA™ 51 as vaccine adjuvant: A systematic review, Human vaccines & immunotherapeutics, 12 (2016) 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].van Aalst S, Ludwig IS, van Kooten PJS, van der Zee R, van Eden W, Broere F, Dynamics of APC recruitment at the site of injection following injection of vaccine adjuvants, Vaccine, 35 (2017) 1622–1629. [DOI] [PubMed] [Google Scholar]
- [292].Shirota Y, Shirota H, Klinman DM, Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells, The Journal of Immunology, (2012) 101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Song H, Lu Y, Qu Z, Mossine VV, Martin MB, Hou J, Cui J, Peculis BA, Mawhinney TP, Cheng J, Effects of aged garlic extract and FruArg on gene expression and signaling pathways in lipopolysaccharide-activated microglial cells, Scientific reports, 6 (2016) 35323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Casares N, Lasarte JJ, Cerio A.L.D.d., Sarobe P, Ruiz M, Melero I, Prieto J, Borrás‐Cuesta F, Immunization with a tumor‐associated CTL epitope plus a tumor‐related or unrelated Th1 helper peptide elicits protective CTL immunity, European journal of immunology, 31 (2001) 1780–1789. [DOI] [PubMed] [Google Scholar]
- [295].Reed SG, Bertholet S, Coler RN, Friede M, New horizons in adjuvants for vaccine development, Trends in immunology, 30 (2009) 23–32. [DOI] [PubMed] [Google Scholar]
- [296].Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S, Chemokine and chemotactic signals in dendritic cell migration, Cellular & molecular immunology, (2018) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Chen R, Ma H, Zhang L, Bryers JD, Precision‐porous templated scaffolds of varying pore size drive dendritic cell activation, Biotechnology and bioengineering, 115 (2018) 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Liu Y, Xiao L, Joo K-I, Hu B, Fang J, Wang P, In situ modulation of dendritic cells by injectable thermosensitive hydrogels for cancer vaccines in mice, Biomacromolecules, 15 (2014) 3836–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Kumamoto T, Huang EK, Paek HJ, Morita A, Matsue H, Valentini RF, Takashima A, Induction of tumor-specific protective immunity by in situ Langerhans cell vaccine, Nature biotechnology, 20 (2002) 64. [DOI] [PubMed] [Google Scholar]
- [300].Harris LD, Kim B-S, Mooney DJ, Open pore biodegradable matrices formed with gas foaming, (1998). [DOI] [PubMed]
- [301].Ali OA, Verbeke C, Johnson C, Sands RW, Lewin SA, White D, Doherty E, Dranoff G, Mooney DJ, Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants, Cancer research, 74 (2014) 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ, Infection-mimicking materials to program dendritic cells in situ, Nature materials, 8 (2009) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Abkenari LD, Theuns DA, Valk SD, Van Belle Y, de Groot NM, Haitsma D, Muskens-Heemskerk A, Szili-Torok T, Jordaens L, Clinical experience with a novel subcutaneous implantable defibrillator system in a single center, Clinical Research in Cardiology, 100 (2011) 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, Mooney DJ, Injectable preformed scaffolds with shape-memory properties, Proceedings of the National Academy of Sciences, 109 (2012) 19590–19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Koshy ST, Zhang DK, Grolman JM, Stafford AG, Mooney DJ, Injectable nanocomposite cryogels for versatile protein drug delivery, Acta biomaterialia, 65 (2018) 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Burdick JA, Prestwich GD, Hyaluronic acid hydrogels for biomedical applications, Advanced materials, 23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R, Transdermal photopolymerization of poly (ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage, Plastic and reconstructive surgery, 104 (1999) 1014–1022. [DOI] [PubMed] [Google Scholar]
- [308].Klouda L, Mikos AG, Thermoresponsive hydrogels in biomedical applications, European Journal of Pharmaceutics and Biopharmaceutics, 68 (2008) 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Ward MA, Georgiou TK, Thermoresponsive polymers for biomedical applications, Polymers, 3 (2011) 1215–1242. [Google Scholar]
- [310].Hyun H, Kim YH, Song IB, Lee JW, Kim MS, Khang G, Park K, Lee HB, In vitro and in vivo release of albumin using a biodegradable MPEG-PCL diblock copolymer as an in situ gel-forming carrier, Biomacromolecules, 8 (2007) 1093–1100. [DOI] [PubMed] [Google Scholar]
- [311].Sun Z, Liang J, Dong X, Wang C, Kong D, Lv F, Injectable hydrogels coencapsulating granulocyte-macrophage colony-stimulating factor and ovalbumin nanoparticles to enhance antigen uptake efficiency, ACS applied materials & interfaces, (2018). [DOI] [PubMed] [Google Scholar]
- [312].Li WA, Lu BY, Gu L, Choi Y, Kim J, Mooney DJ, The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration, Biomaterials, 83 (2016) 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Young S, Koshy S, Lei W, Golfman L, Melville J, Shum J, Sikora A, Wong M, Mooney D, Development of Mesoporous Silica Rod-Based Immunotherapies for Head and Neck Squamous Cell Carcinoma, Journal of Oral and Maxillofacial Surgery, 74 (2016) e5. [Google Scholar]
- [314].Dellacherie MO, Li AW, Lu BY, Mooney DJ, Covalent Conjugation of Peptide Antigen to Mesoporous Silica Rods to Enhance Cellular Responses, Bioconjugate chemistry, (2018). [DOI] [PubMed] [Google Scholar]
- [315].Tang F, Li L, Chen D, Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery, Advanced materials, 24 (2012) 1504–1534. [DOI] [PubMed] [Google Scholar]
- [316].Virginio VG, Bandeira NC, dos Anjos Leal FM, Lancellotti M, Zaha A, Ferreira HB, Assessment of the adjuvant activity of mesoporous silica nanoparticles in recombinant Mycoplasma hyopneumoniae antigen vaccines, Heliyon, 3 (2017) e00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Wang X, Li X, Ito A, Watanabe Y, Sogo Y, Tsuji NM, Ohno T, Stimulation of in vivo antitumor immunity with hollow mesoporous silica nanospheres, Angewandte Chemie International Edition, 55 (2016) 1899–1903. [DOI] [PubMed] [Google Scholar]
- [318].Chen T, Li D, Song Y, Yang X, Liu Q, Jin X, Zhou D, Huang Z, A heterologous prime-boost Ebola virus vaccine regimen induces durable neutralizing antibody response and prevents Ebola virus-like particle entry in mice, Antiviral research, 145 (2017) 54–59. [DOI] [PubMed] [Google Scholar]
- [319].Radtke AJ, Anderson CF, Riteau N, Rausch K, Scaria P, Kelnhofer ER, Howard RF, Sher A, Germain RN, Duffy P, Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate, Scientific reports, 7 (2017) 40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, de Silva AM, Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection, Emerging infectious diseases, 23 (2017) 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Sheppard NC, Brinckmann SA, Gartlan KH, Puthia M, Svanborg C, Krashias G, Eisenbarth SC, Flavell RA, Sattentau QJ, Wegmann F, Polyethyleneimine is a potent systemic adjuvant for glycoprotein antigens, International immunology, 26 (2014) 531–538. [DOI] [PubMed] [Google Scholar]
- [322].He W, Liang P, Guo G, Huang Z, Niu Y, Dong L, Wang C, Zhang J, Re-polarizing myeloid-derived suppressor cells (MDSCs) with cationic polymers for cancer immunotherapy, Scientific reports, 6 (2016) 24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Coley WB, THE TREATMENT OF MALIGNANT TUMORS BY REPEATED INOCULATIONS OF ERYSIPELAS: WITH A REPORT OF TEN ORIGINAL CASES. 1, The American Journal of the Medical Sciences (1827–1924), 105 (1893) 487. [PubMed] [Google Scholar]
- [324].Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, Furutani Y, Yano O, Kataoka T, Sudo T, Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity, Journal of the National Cancer Institute, 72 (1984) 955–962. [PubMed] [Google Scholar]
- [325].Krieg AM, Yi A-K, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM, CpG motifs in bacterial DNA trigger direct B-cell activation, Nature, 374 (1995) 546. [DOI] [PubMed] [Google Scholar]
- [326].Shirota Y, Shirota H, Klinman DM, Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells, The Journal of Immunology, 188 (2012) 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, Guiducci C, Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells, Proceedings of the National Academy of Sciences, 113 (2016) E7240–E7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Charlebois R, Allard B, Allard D, Buisseret L, Turcotte M, Pommey S, Chrobak P, Stagg J, PolyI: C and CpG synergize with anti-ErbB2 mAb for treatment of breast tumors resistant to immune checkpoint inhibitors, Cancer research, 77 (2017) 312–319. [DOI] [PubMed] [Google Scholar]
- [329].Ma Q, DeLyria ES, Wen X, Lu W, Thapa P, Liu C, Li D, Bassett RL Jr, Overwijk WW, Hwu P, Synthetic poly (L-glutamic acid)-conjugated CpG exhibits antitumor efficacy with increased retention in tumor and draining lymph nodes after intratumoral injection in a mouse model of melanoma, Journal of immunotherapy (hagerstown, Md.: 1997), 40 (2017) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].An M, Yu C, Xi J, Reyes J, Mao G, Wei W-Z, Liu H, Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity, Nanoscale, 10 (2018) 9311–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ, Localized immunotherapy via liposome-anchored Anti-CD137+ IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity, Cancer research, 73 (2013) 1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Mehta NK, Moynihan KD, Irvine DJ, Engineering new approaches to cancer vaccines, Cancer immunology research, 3 (2015) 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Sallets A, Kardosh A, Robinson S, Levy R, In-Situ Vaccination Using Sting Agonists Combined with Immune-Modulating Antibodies to Treat Lymphoma, in, Am Soc Hematology, 2017. [Google Scholar]
- [334].Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z, Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy, Nature communications, 7 (2016) 13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, Dong Z, Zhu W, Peng R, Liu Z, Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer, ACS nano, 11 (2017) 4463–4474. [DOI] [PubMed] [Google Scholar]
- [336].Rowshanravan B, Halliday N, Sansom DM, CTLA-4: a moving target in immunotherapy, Blood, (2017) blood-2017–2006-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Sun C, Mezzadra R, Schumacher TN, Regulation and function of the PD-L1 checkpoint, Immunity, 48 (2018) 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Rauschenberg R, Garzarolli M, Dietrich U, Beissert S, Meier F, Systemic therapy of metastatic melanoma, JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 13 (2015) 1223–1237. [DOI] [PubMed] [Google Scholar]
- [339].Morton DL, Eilber FR, Holmes EC, Hunt JS, Ketcham AS, Silverstein MJ, Sparks FC, BCG immunotherapy of malignant melanoma: summary of a seven-year experience, Annals of surgery, 180 (1974) 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity, Proceedings of the National Academy of Sciences, 112 (2015) 15408–15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Son C-H, Bae J-H, Shin D-Y, Lee H-R, Choi Y-J, Jo W-S, Jung MH, Kang C-D, Yang K, Park Y-S, CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model, Journal of Immunotherapy, 37 (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [342].Mitchell MJ, Jain RK, Langer R, Engineering and physical sciences in oncology: challenges and opportunities, Nature Reviews Cancer, 17 (2017) 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Le IM, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J, Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment, Cancer research, 73 (2013) 4629–4640. [DOI] [PubMed] [Google Scholar]
- [344].Kwong B, Liu H, Irvine DJ, Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy, Biomaterials, 32 (2011) 5134–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Brigger I, Dubernet C, Couvreur P, Nanoparticles in cancer therapy and diagnosis, Advanced drug delivery reviews, 64 (2012) 24–36. [DOI] [PubMed] [Google Scholar]
- [346].Kim DH, Moon C, Oh S-S, Park S, Jeong J-W, Kim S, Lee HG, Kwon H-J, Kim KD, Liposome-encapsulated CpG enhances antitumor activity accompanying the changing of lymphocyte populations in tumor via intratumoral administration, nucleic acid therapeutics, 25 (2015) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Zabielska-Koczywąs K, Lechowski R, The Use of Liposomes and Nanoparticles as Drug Delivery Systems to Improve Cancer Treatment in Dogs and Cats, Molecules, 22 (2017) 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Naik E, Ying C, Milley R, Calacsan C, Chipman S, Tüting T, Coffman R, Guiducci C, Intratumoral administration of a TLR9-adjuvanted nanoparticle cancer vaccine stimulates more effective immunity in both injected and un-injected tumor sites compared to subcutaneous administration, in, AACR, 2017. [Google Scholar]
- [349].Makkouk A, Joshi VB, Wongrakpanich A, Lemke CD, Gross BP, Salem AK, Weiner GJ, Biodegradable microparticles loaded with doxorubicin and CpG ODN for in situ immunization against cancer, The AAPS journal, 17 (2015) 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Kwon DY, Kwon JS, Park JH, Park SH, Oh HJ, Kim JH, Min BH, Park K, Kim MS, Synergistic anti-tumor activity through combinational intratumoral injection of an in-situ injectable drug depot, Biomaterials, 85 (2016) 232–245. [DOI] [PubMed] [Google Scholar]
- [351].Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, Imahori H, Umeki Y, Shiomi T, Takakura Y, DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy, Biomaterials, 146 (2017) 136–145. [DOI] [PubMed] [Google Scholar]
- [352].Fan H, Zhang IY, Chen X, Zhang L, Wang H, da Fonseca ACC, Manuel ER, Diamond DJ, Raubitschek AA, Badie B, Intracerebral CpG immunotherapy with carbon nanotubes abrogates growth of subcutaneous melanomas in mice, Clinical Cancer Research, (2012) clincanres. 1911.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Umeki Y, Mohri K, Kawasaki Y, Watanabe H, Takahashi R, Takahashi Y, Takakura Y, Nishikawa M, Induction of Potent Antitumor Immunity by Sustained Release of Cationic Antigen from a DNA‐Based Hydrogel with Adjuvant Activity, Advanced Functional Materials, 25 (2015) 5758–5767. [Google Scholar]
- [354].Ishii-Mizuno Y, Umeki Y, Onuki Y, Watanabe H, Takahashi Y, Takakura Y, Nishikawa M, Improved sustained release of antigen from immunostimulatory DNA hydrogel by electrostatic interaction with chitosan, International journal of pharmaceutics, 516 (2017) 392–400. [DOI] [PubMed] [Google Scholar]
- [355].Brandl F, Kastner F, Gschwind RM, Blunk T, Teßmar J, Göpferich A, Hydrogel-based drug delivery systems: comparison of drug diffusivity and release kinetics, Journal of Controlled Release, 142 (2010) 221–228. [DOI] [PubMed] [Google Scholar]
- [356].Guvendiren M, Lu HD, Burdick JA, Shear-thinning hydrogels for biomedical applications, Soft matter, 8 (2012) 260–272. [Google Scholar]
- [357].Rosales AM, Anseth KS, The design of reversible hydrogels to capture extracellular matrix dynamics, Nature Reviews Materials, 1 (2016) 15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1, Science, (2015) aad0779. [DOI] [PubMed] [Google Scholar]
- [359].Böhm S, Montfort A, Pearce OM, Topping J, Chakravarty P, Everitt GL, Clear A, McDermott JR, Ennis D, Dowe T, Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma, Clinical Cancer Research, 22 (2016) 3025–3036. [DOI] [PubMed] [Google Scholar]
- [360].Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy, Immunity, 44 (2016) 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Danhier F, Feron O, Préat V, To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, Journal of controlled release, 148 (2010) 135–146. [DOI] [PubMed] [Google Scholar]
- [362].Perry JL, Reuter KG, Luft JC, Pecot CV, Zamboni W, DeSimone JM, Mediating passive tumor accumulation through particle size, tumor type, and location, Nano letters, 17 (2017) 2879–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Albanese A, Tang PS, Chan WC, The effect of nanoparticle size, shape, and surface chemistry on biological systems, Annual review of biomedical engineering, 14 (2012) 1–16. [DOI] [PubMed] [Google Scholar]
- [364].Guimarães PP, Gaglione S, Sewastianik T, Carrasco RD, Langer R, Mitchell MJ, Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy, ACS nano, 12 (2018) 912–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Mikhail AS, Allen C, Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels, Journal of Controlled Release, 138 (2009) 214–223. [DOI] [PubMed] [Google Scholar]
- [366].Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, Napier M, Bear JE, DeSimone JM, PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics, Nano letters, 12 (2012) 5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Zhang Y, Li N, Suh H, Irvine DJ, Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity, Nature communications, 9 (2018) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Lila ASA, Kiwada H, Ishida T, The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage, Journal of Controlled Release, 172 (2013) 38–47. [DOI] [PubMed] [Google Scholar]
- [369].Zhang C, Yuan J, Lu J, Hou Y, Xiong W, Lu H, From neutral to zwitterionic poly (α-amino acid) nonfouling surfaces: Effects of helical conformation and anchoring orientation, Biomaterials, (2018). [DOI] [PubMed] [Google Scholar]
- [370].Zhang P, Sun F, Tsao C, Liu S, Jain P, Sinclair A, Hung H-C, Bai T, Wu K, Jiang S, Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity, Proceedings of the National Academy of Sciences, 112 (2015) 12046–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Lau KHA, Sileika TS, Park SH, Sousa AM, Burch P, Szleifer I, Messersmith PB, Molecular Design of Antifouling Polymer Brushes Using Sequence‐Specific Peptoids, Advanced materials interfaces, 2 (2015) 1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Zhang R, Morton LD, Smith JD, Gallazzi F, White TA, Ulery BD, Instructive Design of Triblock Peptide Amphiphiles for Structurally Complex Micelle Fabrication, ACS Biomaterials Science & Engineering, (2018). [DOI] [PubMed] [Google Scholar]
- [373].Hung HC, Jain P, Zhang P, Sun F, Sinclair A, Bai T, Li B, Wu K, Tsao C, Liu EJ, A Coating‐Free Nonfouling Polymeric Elastomer, Advanced Materials, 29 (2017) 1700617. [DOI] [PubMed] [Google Scholar]
- [374].Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Sen Gupta A, Mitragotri S, Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries, ACS nano, 8 (2014) 11243–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE, Shape effects of filaments versus spherical particles in flow and drug delivery, Nature nanotechnology, 2 (2007) 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Toy R, Peiris PM, Ghaghada KB, Karathanasis E, Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles, Nanomedicine, 9 (2014) 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Barua S, Yoo J-W, Kolhar P, Wakankar A, Gokarn YR, Mitragotri S, Particle shape enhances specificity of antibody-displaying nanoparticles, Proceedings of the National Academy of Sciences, 110 (2013) 3270–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, Dai H, Sinclair R, Gambhir SS, Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation, Nano letters, 12 (2012) 3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Sykes EA, Chen J, Zheng G, Chan WC, Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency, ACS nano, 8 (2014) 5696–5706. [DOI] [PubMed] [Google Scholar]
- [380].Zhong Y, Meng F, Deng C, Zhong Z, Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy, Biomacromolecules, 15 (2014) 1955–1969. [DOI] [PubMed] [Google Scholar]
- [381].Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy, Cancer cell, 25 (2014) 846–859. [DOI] [PubMed] [Google Scholar]
- [382].Esposito M, Kang Y, Targeting tumor–stromal interactions in bone metastasis, Pharmacology & therapeutics, 141 (2014) 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Yu M, Zheng J, Clearance pathways and tumor targeting of imaging nanoparticles, ACS nano, 9 (2015) 6655–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Smith JD, Cardwell LN, Porciani D, Nguyen JA, Gallazzi F, Tata RR, Burke DH, Daniels MA, Ulery B, Aptamer-displaying peptide amphiphile micelles as a cell-targeted delivery vehicle of peptide cargoes, Physical biology, (2018). [DOI] [PubMed] [Google Scholar]
- [385].Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L, Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery, Nano letters, 14 (2014) 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy, Science, 348 (2015) 69–74. [DOI] [PubMed] [Google Scholar]
- [387].Kessler J, Melief C, Identification of T-cell epitopes for cancer immunotherapy, Leukemia, 21 (2007) 1859. [DOI] [PubMed] [Google Scholar]
- [388].Gjerstorff MF, Andersen MH, Ditzel HJ, Oncogenic cancer/testis antigens: prime candidates for immunotherapy, Oncotarget, 6 (2015) 15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Cebon J, Knights A, Ebert L, Jackson H, Chen W, Evaluation of cellular immune respo nses in cancer vaccine recipients: lessons from NY-ESO-1, Expert review of vaccines, 9 (2010) 617–629. [DOI] [PubMed] [Google Scholar]
- [390].Ladjemi MZ, Jacot W, Chardès T, Pèlegrin A, Navarro-Teulon I, Anti-HER2 vaccines: new prospects for breast cancer therapy, Cancer Immunology, Immunotherapy, 59 (2010) 1295–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Hoffman M, Rajapakse A, Shen X, Gates KS, Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically relevant conditions: chemistry relevant to both the genotoxic and cell signaling properties of H2S, Chemical research in toxicology, 25 (2012) 1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Li L, Shen X, Liu Z, Norrbom M, Prakash TP, O’Reilly D, Sharma VK, Damha MJ, Watts JK, Rigo F, Activation of frataxin protein expression by antisense oligonucleotides targeting the mutant expanded repeat, nucleic acid therapeutics, 28 (2018) 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Mittal D, Gubin MM, Schreiber RD, Smyth MJ, New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape, Current opinion in immunology, 27 (2014) 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Goodwin S, McPherson JD, McCombie WR, Coming of age: ten years of next-generation sequencing technologies, Nature Reviews Genetics, 17 (2016) 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Fang L, Hu J, Wang D, Wang K, NextSV: a meta-caller for structural variants from low-coverage long-read sequencing data, BMC bioinformatics, 19 (2018) 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Sahin U, Türeci Ö, Personalized vaccines for cancer immunotherapy, Science, 359 (2018) 1355–1360. [DOI] [PubMed] [Google Scholar]
- [397].Pan L, Wang Z, Li Y, Xu F, Zhang Q, Zhang C, Nicking enzyme-controlled toehold regulation for DNA logic circuits, Nanoscale, 9 (2017) 18223–18228. [DOI] [PubMed] [Google Scholar]
- [398].Chen D, Fan F, Zhao X, Xu F, Chen P, Wang J, Ban L, Liu Z, Feng X, Zhang Y, Single Cell Chemical Proteomics with Membrane-Permeable Activity-Based Probe for Identification of Functional Proteins in Lysosome of Tumors, Analytical chemistry, 88 (2016) 2466–2471. [DOI] [PubMed] [Google Scholar]
- [399].Guo Y, Lei K, Tang L, Neoantigen vaccine delivery for personalized anticancer immunotherapy, Frontiers in immunology, 9 (2018) 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Mehlen P, Puisieux A, Metastasis: a question of life or death, Nature Reviews Cancer, 6 (2006) 449. [DOI] [PubMed] [Google Scholar]
- [401].Goldman B, DeFrancesco L, The cancer vaccine roller coaster, Nature biotechnology, 27 (2009) 129. [DOI] [PubMed] [Google Scholar]
- [402].Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Development and function of human innate immune cells in a humanized mouse model, Nature biotechnology, 32 (2014) 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Mittapalli RK, Adkins CE, Bohn KA, Mohammad AS, Lockman JA, Lockman PR, Quantitative fluorescence microscopy measures vascular pore size in primary and metastatic brain tumors, Cancer research, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Panchalingam KM, Jung S, Rosenberg L, Behie LA, Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: a review, Stem cell research & therapy, 6 (2015) 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Kepplinger EE, FDA’s expedited approval mechanisms for new drug products, Biotechnology law report, 34 (2015) 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Havel HA, Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials, The AAPS journal, 18 (2016) 1351–1353. [DOI] [PubMed] [Google Scholar]
- [407].Ventola CL, Progress in nanomedicine: approved and investigational nanodrugs, Pharmacy and Therapeutics, 42 (2017) 742. [PMC free article] [PubMed] [Google Scholar]
- [408].ClinicalTrials.Gov, Dendritic Cell Activating Scaffold in Melanoma, NIH U.S. National Library of Medicine, (2018). [Google Scholar]
- [409].Trusheim MR, Berndt ER, Douglas FL, Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers, Nature reviews Drug discovery, 6 (2007) 287. [DOI] [PubMed] [Google Scholar]