Introduction
Vincristine is a widely used anticancer drug for the treatment of leukemia, lymphoma and many solid tumors in children and adults. A major adverse effect of vincristine is peripheral neuropathy, which can be either sensory or motor in nature. The current issue of CPT reports new research that extends prior work identifying an inherited germline polymorphism that predisposes to vincristine-induced neuropathy.
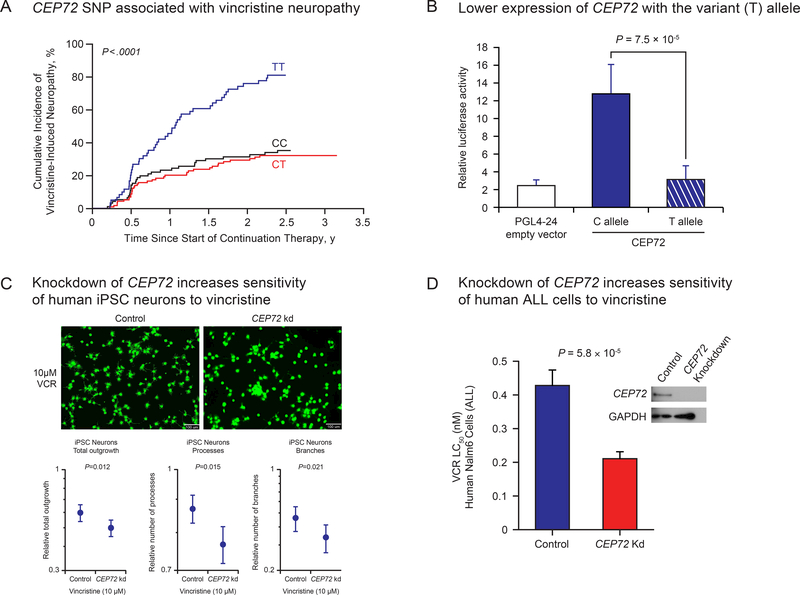
There are many patient and treatment related variables that have been reported to influence the incidence and severity of vincristine-induced peripheral neuropathy, including the dosage given (more with 2 mg/m2 than 1.5 mg/m2), total cumulative dosage given (more neuropathy at higher cumulative dosage), concomitant therapy (CYP3A4/5 inhibitors decrease clearance and increase neuropathy), and ancestry (less common in patients of African ancestry than in those of European ancestry)1. The latter suggest there may be genetic determinants of vincristine neuropathy, and indeed there have been prior studies indicating that CYP3A4/5 genotype can influence vincristine neuropathy2, but these have not been consistently reproduced3. The current issue of CPT contains an important report from the Canadian Pharmacogenomics Consortium4, confirming an earlier report from our group that an inherited genetic polymorphism in the promoter of the CEP72 gene increases the incidence of vincristine-induced peripheral neuropathy (Figure panel A). In the studies by Diouf et al and Wright et al, vincristine neuropathy was assessed and graded prospectively by treating clinicians according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Figure 1.
Association of genetic polymorphism in CEP72 and vincristine-induced peripheral neuropathy. (A) The cumulative incidence of grade 2–4 vincristine-induced peripheral neuropathy as originally discovered in two cohorts of children treated for acute lymphoblastic leukemia (ALL). (B) The CEP72 risk-allele (T at rs924607) leads to low expression of CEP72 as shown by the luciferase activity of the of the CEP72 promoter containing the T allele compared to the C allele. (C) Reduction of CEP72 expression by shRNA in human iPSC neurons increased their sensitivity to vincristine, and (D) reduction in the expression of CEP72 in human ALL cells also increased their sensitivity to vincristine.
Panel A and the images in Panel C appear as they were originally published in Diouf et al., JAMA 20158. Reproduced with permission from JAMA. 2015. 313(8):815–823. Copyright©2015 American Medical Association. All rights reserved.
Although the association of this genetic polymorphism with vincristine neuropathy was replicated in a case-control study of adults treated for leukemia at the University of Chicago5, with genotyping performed in a blinded fashion at St. Jude Children’s Research Hospital, it was technically not a fully independent validation of the original discovery. The work by Wright et al is thus the first fully independent replication of the association of the CEP72 promoter polymorphism with vincristine-induced neuropathy. The current paper also includes a meta-analysis of all of the prior studies assessing neuropathy after chronic vincristine treatment, each of which had a similar odds ratio (OR) for the incidence of peripheral neuropathy in those homozygous for the CEP72 risk allele (T/T at rs924607), with an overall OR of 2.77 (meta p-value =8.02×10−11). A fourth study from the Geisinger Clinic also found an association of the CEP72 polymorphism with vincristine-induced neuropathy in adults, but this has only been reported in abstract form to date6. It is worth noting that the CEP72 polymorphism has only been associated with the incidence of peripheral neuropathy after chronic treatment with vincristine (20–40 doses over 1–2 years), whereas this association is not evidence when one assesses peripheral neuropathy after only four doses of vincristine7. Also, prior studies have shown that this association is only evident when the conventional dose of vincristine is given (1.5 mg/m2), and not when a higher dose of vincristine is prescribed (e.g., 2 mg/m2), since the higher dosage produces peripheral neuropathy in the great majority of patients (>80%), regardless of CEP72 genotype8.
It is interesting that the CEP72 risk allele (T at rs924607) has a lower allele frequency in people of African ancestry than people of European ancestry, which is concordant with the reported lower frequency of vincristine-induced peripheral neuropathy in black patients1. It is also true that people of African ancestry are more likely to express functional CYP3A5, due to a lower frequency of the CYP3A5 splice variant that inactivates CYP3A5 in most people of European ancestry2,9; CYP3A4/5 are primarily responsible for vincristine metabolism. Thus, while the CEP72 polymorphism may contribute to the ancestry differences in vincristine-induced neuropathy, it is likely multi-factorial.
There are also rare variants in the coding region of CEP72 that are predicted to be damaging to the encoded protein, and hence could increase the risk of vincristine-induced neuropathy in a manner analogous to the CEP72 promoter variant. It will be important for future studies to assess whether these rare variants can be incorporated along with the common promoter variant in CEP72 to identify additional patients at high-risk for vincristine neuropathy.
This independent replication is important for several reasons, one of which is that there are no clear intermediate phenotypes that can be linked to the effect of the CEP72 polymorphism, because there is no evidence that CEP72 alters the pharmacokinetics of vincristine. Rather, the promoter SNP alters the expression of CEP72 (lower with the risk allele, Figure panel B), and because CEP72 is a centrosomal protein essential for microtubule formation, the lower expression is presumed to increase the sensitivity of cells to a microtubule inhibitor (vincristine) by compromising microtubule formation via a mechanism distinct from vincristine. In essence, the genetic polymorphism is resetting the “rheostat” (resistance) of cells to vincristine by compromising microtubule formation by a second mechanism, thereby increasing the effects of the microtubule inhibitor. Thus, this constitutes a pharmacodynamic mechanism for a pharmacogenomic trait, making it impossible to substantiate the effect by an intermediate phenotype such as perturbations in drug concentrations. However, knocking down the expression of CEP72 in human iPSC neurons was shown to increase their sensitivity to vincristine8, and the same effect was documented when CEP72 was knocked down in human ALL cells8 (Figure panels C and D). The latter finding, coupled with greater ex vivo sensitivity of primary ALL cells in patients with the high-risk CEP72 T/T promoter genotype, suggests that it may be possible to successfully treat these “high-risk” patients with a lower dosage of vincristine (since both their leukemia cells and their neurons are more sensitive), a question that is currently being investigated in a prospective randomized clinical trial(https://www.clinicaltrials.gov/ct2/show/NCT03117751?term=total+XVII&rank=1). Without a pharmacokinetic intermediary endpoint, discoveries of genetic predisposition due to alterations in the pharmacodynamics of a drug rely predominantly on the association of genetic variants with drug-induced phenotypes. For this reason, robust and consistent assessment of drug-induced phenotypes becomes of increasing importance to move the field forward. With the constantly improving technologies for genome interrogation and computational algorithms for assessing genome variation, drug-induced phenotypes are typically much more difficult to robustly measure than are genotypes, especially in the routine clinical setting. This remains a challenge for extending pharmacogenomic discoveries, but it is reassuring when such data can be reliably captured in multi-institutional settings such as reported in this issue by the Canadian Pharmacogenomic Consortium.
Wright et al have also found that polymorphisms in ABCC1, SLC5A7 and TTPA genes were associated with an increased risk of vincristine-induced peripheral neuropathy in their patient cohort. These are plausible findings, as ABCC1 has been previously associated with vincristine transport and vincristine-induced peripheral neuropathy, whereas SLC5A7 and TTPA have been previously associated with inherited neuropathies4. Like any new genes found to be statistically associated with a pharmacogenomic phenotype, these results need to be independently validated in additional cohorts of patients.
It has also been shown that a small percentage of patients treated with chronic vincristine develop neuropathy that persists for 10-plus years after completion of curative treatment10, and it is plausible that the CEP72 polymorphism may predispose to the persistent neuropathy phenotype as well. This will be important to assess, as there is increasing importance on the quality of life of patients who are cured of childhood ALL, now that the cure rates have surpassed 90% at major cancer centers worldwide. Indeed, pharmacogenomics has the potential to identify patients who are genetically predisposed to both acute and persistent adverse effects of cancer treatment, and may offer a strategy for modifying treatment to mitigate side effects that can compromise quality of life during and after successful treatment.
Acknowledgments
Funding
The study was supported by NIH Grants R37 CA36401, U01GM92666, P50 GM115279 and by the American Lebanese Syrian Associated Charities
Footnotes
Conflict of Interest statement
The authors declared no competing interests for this work.
References
- 1.Renbarger JL, McCammack KC, Rouse CE & Hall SD Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatric blood & cancer 50, 769–771, doi: 10.1002/pbc.21435 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Egbelakin A et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatric blood & cancer 56, 361–367, doi: 10.1002/pbc.22845 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aplenc R et al. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. British journal of haematology 122, 240–244 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Wright GEB et al. Pharmacogenomics of vincristine-induced peripheral neuropathy implicates pharmacokinetic and inherited neuropathy genes. Clinical pharmacology and therapeutics (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stock W et al. An Inherited Genetic Variant in CEP72 Promoter Predisposes to Vincristine-Induced Peripheral Neuropathy in Adults With Acute Lymphoblastic Leukemia. Clinical pharmacology and therapeutics 101, 391–395, doi: 10.1002/cpt.506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal V, Smelser DT, Carey DJ, Khan SS & Vadakara J Association of CEP72 genotype with chemotherapy-induced neuropathy. Journal of Clinical Oncology 34, e14107–e14107, doi: 10.1200/JCO.2016.34.15_suppl.e14107 (2016). [DOI] [Google Scholar]
- 7.Diouf B, Crews KR & Evans WE Vincristine pharmacogenomics: ‘winner’s curse’ or a different phenotype? Pharmacogenetics and genomics 26, 51–52, doi: 10.1097/fpc.0000000000000192 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Diouf B et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. Jama 313, 815–823, doi: 10.1001/jama.2015.0894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehl P et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature genetics 27, 383–391, doi: 10.1038/86882 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Tay CG et al. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatric blood & cancer 64, doi: 10.1002/pbc.26471 (2017). [DOI] [PubMed] [Google Scholar]