Abstract
Objective:
Examine prevalence of dysphagia at the population level in head and neck cancer (HNC) survivors.
Methods:
Surveillance, Epidemiology and End Results (SEER)-Medicare claims among 16,194 HNC patients (2002–2011) were analyzed to estimate 2-year prevalence of dysphagia, stricture, and aspiration pneumonia, and derive treatment- and site-specific estimates.
Results:
Prevalence of dysphagia, stricture, pneumonia, and aspiration pneumonia was 45.3% (95% CI: 44.5–46.1), 10.2% (95% CI: 9.7–10.7), 26.3% (95% CI: 25.6–26.9), 8.6% (95% CI: 8.2–9.1), respectively. Dysphagia increased by 11.7% over the 10-year period (p<0.001). Prevalence was highest after chemoradiation and multimodality therapy.
Conclusions:
Comparing to published rates using similar methodology the preceding decade (1992–1999), prevalence of dysphagia based on claims data was similar in 2002 to 2011 in this study. These results suggest persistence of dysphagia as a highly prevalent morbidity, even in the decade in which highly conformal radiotherapy and minimally invasive surgeries were popularized.
Keywords: SEER-Medicare, Dysphagia, Aspiration pneumonia, Stricture, Head and neck cancer
INTRODUCTION
Dysphagia is a high impact morbidity of head and neck cancer (HNC). Swallowing difficulty can result from tumor or treatment and adversely impacts both the health1,2 and quality of life3,4 of survivors. Prevalence estimates for dysphagia vary widely in published literature and depend on tumor stage, subsite of disease, age, and treatment modality. Much of the published work in the area of dysphagia derives from single-institution, clinical datasets. While clinical data offer far superior detail about the nature of dysphagia, small numbers of patients and institutional biases may limit generalizability for inferences about the frequency of dysphagia in the broad population of HNC survivors as well as in clinically relevant subgroups of patients. Population-level data are critical to examine broad outcomes outside of academic institutions. Prior population-level analyses from the United States often use SEER-Medicare; these include analyses of feeding tube duration as a function of radiotherapy modality5, case-control comparisons of aspiration pneumonia risk after chemoradiation2, examination of dysphagia as a predictor of morbidity6, as well as quality studies suggesting positive impact of early speech pathology utilization7.
Perhaps the most comprehensive assessment of national HNC trends in dysphagia using the SEER-Medicare database was published by Francis and colleagues (2010)8 reporting 3-year prevalence of dysphagia, stricture, and pneumonia of 40%, 7%, and 10%, respectively, among 8,002 patients treated between the years 1992 and 1999. Prevalence of all endpoints was higher among patients requiring multimodality therapy presumably for advanced stages of disease, and also among those with pharyngeal primary tumors relative to oral cavity or laryngeal sites. Among all modalities, prevalence of swallowing-related endpoints was highest in patients who received primary chemoradiation. This comprehensive analysis led to insights about higher risk subgroups of patients, and also suggested increasing prevalence of dysphagia-related events over the decade of study (1990’s) in an era when organ preservation regimens were intensifying. Since the period of their analysis (1992–1999), the field of head and neck oncology has refined the delivery of surgery and radiotherapy by rapid adoption of both minimally invasive surgical methods (e.g., transoral robotic surgery) and highly conformal radiotherapy planning methods (e.g., IMRT). Refinements in treatment delivery and other notable shifts including more widespread adoption of proactive supportive care alongside shifting epidemiology of HNC (i.e., human papilloma virus associated HNC) could conceivably impact population level trends. Therefore, the purpose of this analysis was: 1) to re-examine prevalence of dysphagia-related endpoints in HNC survivors at the population-level in a more current decade (2002–2011), and 2) to compare prevalence by treatment modalities and site of disease.
MATERIALS AND METHODS
Data source
The Surveillance, Epidemiology, and End Results (SEER) data were linked to Medicare claims to allow HNC patients aged 65 or older to be followed longitudinally for information about both the initial diagnosis and downstream medical care. Our linked dataset includes cases diagnosed 2002 through 2011 with Medicare claims through 2013, allowing estimation of 2-year prevalence for all eligible cases.
Cohort selection
This retrospective cohort analysis of SEER-Medicare included patients with: 1) histologically confirmed carcinoma of the oral cavity, oropharynx, hypopharynx, nasopharynx, or larynx diagnosed between 2002 and 2011, 2) age equal to or greater than 66 years (allowing calculation of a comorbidity index), 3) enrollment in both Medicare A and B (to ensure inclusion of inpatient and outpatient medical utilization), 4) known diagnosis date, and 5) recorded initial treatment modality. The following Site Recode ICD-O-3 codes captured eligible disease sites: cancers of the lip (20010), tongue (20020), floor of mouth (20040), gum and other mouth (20050), nasopharynx (20060), tonsil (20070), oropharynx (20080), hypopharynx (20090), and larynx (22020). Patients were excluded for: 1) any prior cancer diagnosis; 2) synchronous HNCs; and/or 3) death within the first surveillance year. A total of 16,194 patients met these criteria.
Selection of Non-Cancer Controls
Non-cancer controls for the head and neck cancer cases were selected from the Medicare 5% sample by using 1:1 match based on cancer patients’ age, gender, and race/ethnicity. For each matched control, we used the diagnosis date of its matched cases as its index date; each control was fully covered by Medicare Part A & B from the index date till 28 months after the index date or till the end of the study period if the control was followed up for less than 28 months. Prevalence was computed using the period of 4 months after the index date till 28 months or till the end of the study if the patient followed up less than 28 months. 15,439 controls with full Medicare coverage till the end of the 2-year study period were identified to match the 16,194 cases (95.3%).
Dysphagia-related Outcomes
Swallowing problems manifest in various ways with clinical observations including oral and/or pharyngeal dysfunction, pharyngoesophageal stenosis or stricture, aspiration, and/or aspiration pneumonia. To account for multiple (often co-existing) dysphagia presentations, 4 primary outcomes assessed in this study included dysphagia, stricture, pneumonia, and aspiration pneumonia over the two years following completion of treatment for HNC. Oropharyngeal dysphagia was identified by ICD-9-CM codes, 787.20 – 787.24. Similarly, pneumonia was defined on the basis of ICD-9- CM codes (481, 485–486, 482.0–482.9, 507.0–507.8) and aspiration pneumonia was coded only using the ICD-9 diagnosis code of 507.0. Stricture was defined on the basis of ICD-9-CM codes for pharyngeal and esophageal strictures (478.29, 530.3), ICD-9 procedure codes (29.91, 42.01, 42.92), and CPT-4 codes for esophageal dilation (43220, 43226, 43248, 43249, 43450, 43453, 43456, 74360).
Treatment
Four treatment categories were defined including: surgery alone, surgery with adjuvant therapy, radiation alone, and RT with chemotherapy. Only index treatment was considered (not salvage surgery or re-irradiation). Treatment groups were identified using Current Procedural Terminology (CPT)/Healthcare Common Procedure Coding System (HCPCS) and International Classification of Disease (ICD-9) codes for surgery, radiation, and chemotherapy occurring in the 4 months after cancer diagnosis (supplementary Table 1).
Covariates
Age at diagnosis, sex (male, female), race, stage at diagnosis, and sub-site of disease (oral cavity, oropharynx, hypopharynx, and larynx) were included as covariates in the analysis. Age was categorized into four groups (<69, 69–74, 74–79, >79). Race/ethnicity/origin was classified as non-Hispanic white, Spanish-Hispanic-Latino, black, or other (American Indian/AK Native, Asian/Pacific Islander). Stage at diagnosis was coded as available in SEER for the duration of the study period: in situ (no invasion of basement membrane)/localized (confined to organ of origin), regional (extends beyond organ of origin, regional lymph nodes), distant (spread remote from primary tumor), and unstaged. Comorbidities (0 to 2) were coded by searching inpatient and outpatient claims for diagnostic billing codes for various conditions during the year prior to the diagnosis of cancer, as suggested by Klabunde9, and by using the Deyo implementation of the Charlson comorbidity score10. Comorbidity index was categorized into none, one, and two or more.
Statistical Methods
Two-year period prevalence and 95% confidence intervals were first calculated for each dysphagia-related outcome (dysphagia, stricture, pneumonia, aspiration-pneumonia) among all patients. Prevalence of dysphagia, stricture, pneumonia and aspiration pneumonia was then estimated for subgroups of patients by tumor site, stage, and treatment modality and compared using the chi-square test. A full model including all HNC patients plus four treatment subgroup multivariable logistic regression models were then fit regressing treatment modality on each dysphagia endpoint to estimate independent impact of the following treatment scenarios after adjustment for clinicodemographic covariates using a theoretical model building approach retaining all a priori clinically relevant covariates regardless of statistical significance11. Acknowledging the diversity of HNC as a clinical population, treatment subgroup models were evaluated to derive the most clinically plausible effect estimates among more homogeneous groups of patients than the full model combining all HNC cases. Primary treatment modality comparisons from the four treatment subgroup multivariable regression models included: 1) single modality treatment options (RT alone versus surgery alone), 2) multimodality treatment options (chemoradiation versus surgery + RT), 3) adjuvant radiotherapy (surgery versus surgery + RT), and 4) adjuvant chemotherapy (RT alone versus RT with chemotherapy). Period estimates are reported as adjusted odds ratios (AORs) with 95% confidence intervals (CIs). Final models included treatment modality, race/ethnicity, age at diagnosis, disease sub-site, sex, comorbidity, and disease stage. All p values are two-sided (α=0.05). Statistical analyses were performed using SAS software (Version 9.3, SAS Institute, Cary, NC).
RESULTS
Cohort characteristics
16,194 patients were included in the analysis. The median age was 74 years, and 65.4% were male. The most common tumor subsites were oral cavity (39.3%) and laryngeal/hypopharyngeal (34.1%) followed by oropharyngeal cancers (8%). Treatment modalities were fairly evenly distributed; approximately half (51.1%) underwent single modality therapy (surgery or RT alone) and the remaining 48.9% underwent multimodality therapy (surgery + adjuvant or chemoradiation). Cohort characteristics are detailed in Table 1.
Table 1.
Patient characteristics (n=16,194)
| No (%) | |
|---|---|
| Sex | |
| Female | 5,606 (34.62) |
| Male | 10,588 (65.38) |
| Race/Ethnicity | |
| Caucasian | 13,709 (84.65) |
| Black | 1,001 (6.18) |
| Hispanic | 763 (4.71) |
| Other | 721 (4.45) |
| Age quartiles | |
| <69 | 3,111 (19.21) |
| 69–74 | 4,674 (28.86) |
| 74–79 | 3,722 (22.98) |
| >79 | 4,687 (28.94) |
| Disease sub-site | |
| Oral cavity | 6,371 (39.34) |
| Larynx | 5,521 (34.09) |
| Oropharynx | 1,308 (8.08) |
| Nasopharynx | 360 (2.22) |
| Missing | 2,634 (16.27) |
| SEER stage | |
| In situ/Localized | 5,349 (33.03) |
| Regional | 5,131 (31.68) |
| Distant | 1,372 (8.47) |
| Unstaged/Missing | 4,342 (26.81) |
| Comorbidity | |
| 0 | 9,340 (57.68) |
| 1 | 4,123 (25.46) |
| 2 | 2,731 (16.86) |
| Treatment Modality | |
| Surgery alone | 4,147 (25.61) |
| Surgery + adjuvant therapy | 4,430 (27.36) |
| Radiation alone | 4,130 (25.50) |
| Chemoradiation | 3,487 (21.53) |
| 2-Year Prevalence (95% CI) | |
| Dysphagia | 7,335 (45.29) |
| Stricture | 1,649 (10.18) |
| Pneumonia | 4,252 (26.26) |
| Aspiration pneumonia | 1,400 (8.65) |
| Total | 16,194 |
Prevalence of Dysphagia-Related Outcomes among All HNC Survivors
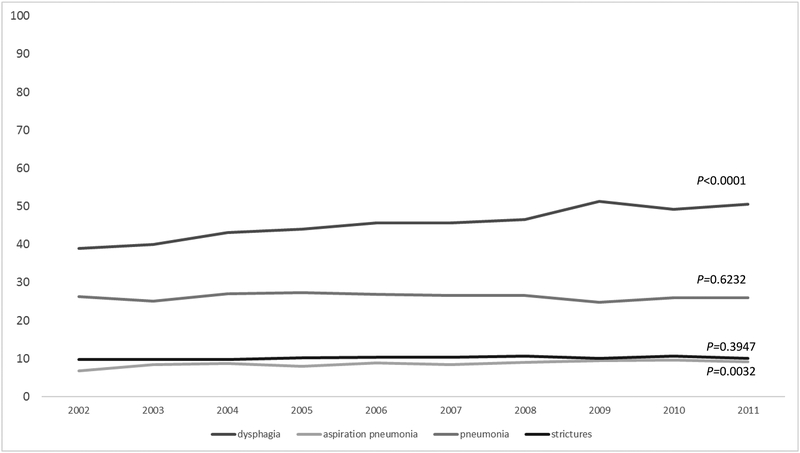
Dysphagia, stricture, and aspiration pneumonia were highly prevalent, occurring among 45.3% (95% CI: 44.5–46.1), 10.2% (95% CI: 9.7–10.7), 8.7% (95% CI: 8.2–9.1) of all patients, respectively. Prevalence of aspiration pneumonia and stricture remained stable over the decade, but dysphagia increased by 11.7% (Figure 1). Prevalence of all dysphagia-related endpoints was significantly higher in HNC cases relative to matched non-cancer controls: dysphagia (OR: 7.8, 95% CI: 7.2–8.3), stricture (OR: 4.6, 95% CI: 4.1–5.2), pneumonia (OR: 2.1, 95% CI: 2.0–2.2), and aspiration pneumonia (OR: 3.7, 95% CI: 3.3–4.1).
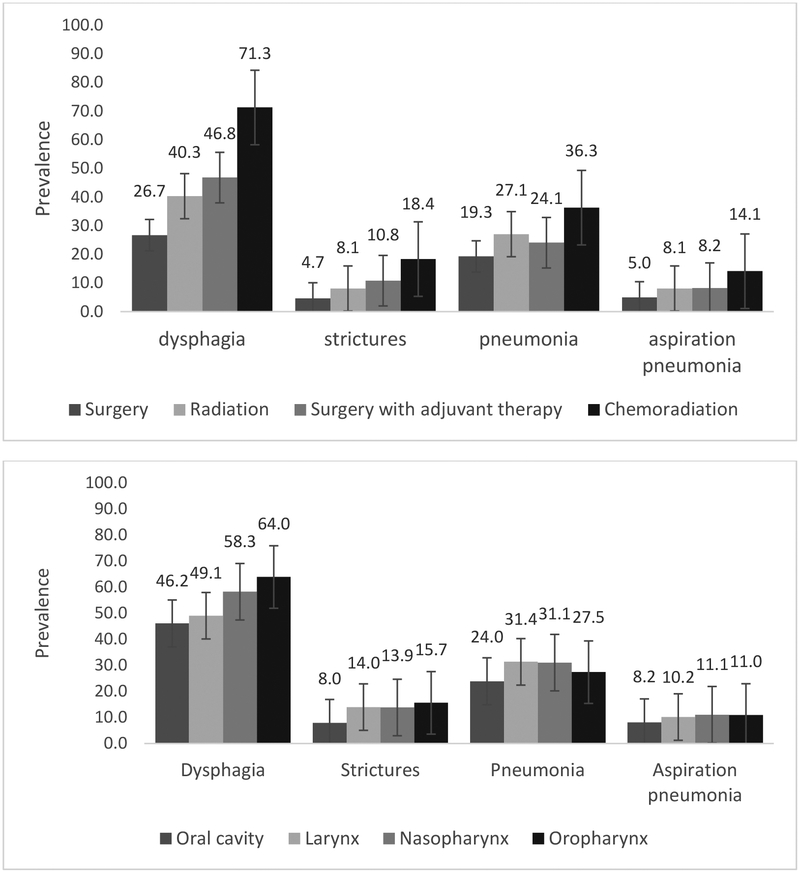
FIGURE 1.
Two year prevalence of swallowing-related events by treatment modality (top) and disease sub-site (bottom), n=16,194.
Prevalence of Dysphagia-Related Outcomes among All HNC Survivors by Site, Stage, and Treatment
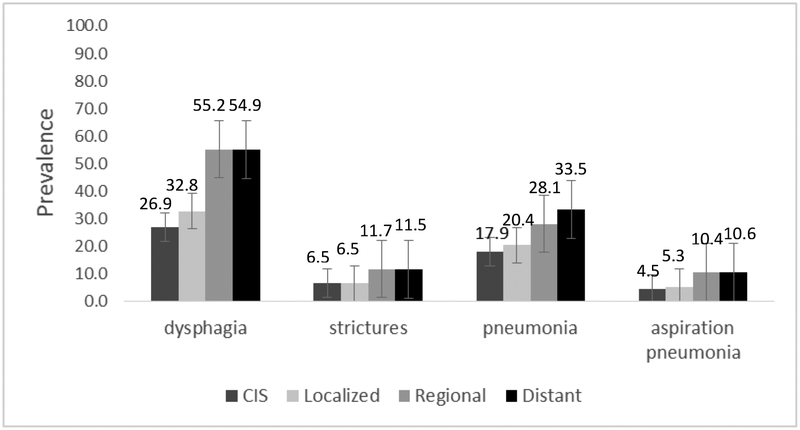
Site of disease (p≤0.0001), stage (p<0.0001), and treatment modality (p<0.0001) significantly influenced prevalence of all endpoints in univariate analysis, as illustrated in Figure 2. Combining all sites of HNC, prevalence of all swallowing-related outcomes was highest among those treated with chemoradiation relative to all other treatment modalities. Combining all treatment modalities, prevalence of dysphagia and stricture was highest among patients treated for oropharyngeal cancers whereas pneumonia was most prevalent among laryngeal/hypopharyngeal and nasopharyngeal subgroups. Oral cavity cases had the lowest prevalence of all dysphagia-related endpoints. Site by treatment prevalence is detailed in Table 2. Treatment modality and stage retained statistical significant for all endpoints in full multivariate models among all patients, whereas site of disease remained significant for stricture, pneumonia, and aspiration pneumonia but not for dysphagia (Table 3).
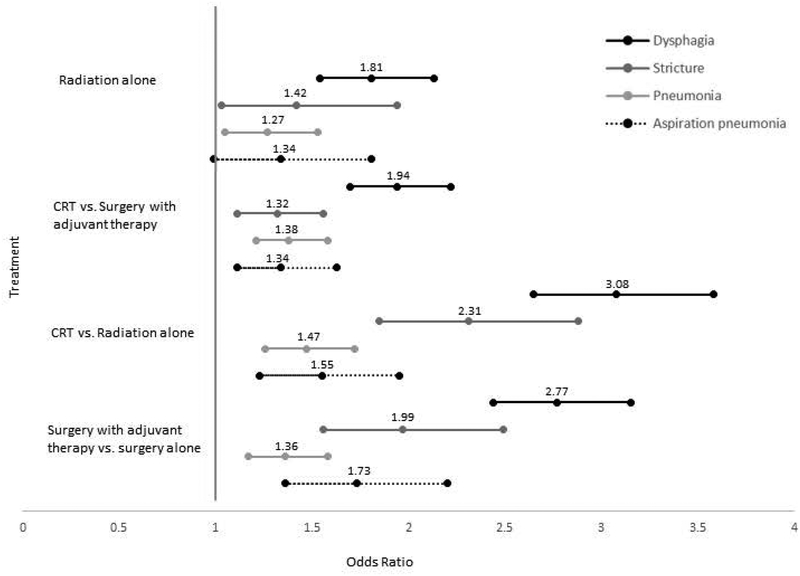
FIGURE 2.
Adjusted odds of 2-year swallowing-related events by treatment modality, n=16,194. Adjusted for age, sex, race/ethnicity, tumor site, comorbidity stage, SEER stage. CRT: chemoradiation
Table 2.
Two year prevalence of swallowing-related events by site and treatment modality, n=16,194
| Single modality No. pts (%) |
Multimodality No. pts (%) |
p value | Surgery alone No. pts (%) |
Surgery + adjuvant No. pts (%) |
RT alone No. pts (%) |
CRT No. pts (%) |
p value | |
|---|---|---|---|---|---|---|---|---|
| NPC | ||||||||
| Dysphagia | 35/81 (43.2) | 175/279 (62.7) | 0.002 | NR | 26/46 (56.5) | 34/74 (46.0) | 149/233 (64.0) | 0.004 |
| Stricture | NR | 42/279 (15.1) | 0.236 | NR | NR | NR | 33/233 (14.2) | 0.472 |
| Pneumonia | 18/81 (22.2) | 94/279 (33.7) | 0.050 | NR | 12/46 (26.1) | 17/74 (23.0) | 82/233 (35.2) | 0.157 |
| Asp Pneumonia | NR | 31/279 (11.1) | 1.0 | NR | NR | NR | 26/233 (11.2) | 0.986 |
| OPC | ||||||||
| Dysphagia | 170/336 (50.6) | 667/972 (68.6) | <0.001 | 39/87 (44.8) | 198/310 (63.9) | 131/249 (52.6) | 469/662 (70.9) | <0.001 |
| Stricture | 36/336 (10.7) | 169/972 (17.4) | 0.004 | NR | 49/310 (15.9) | 25/249 (10.0) | 120/662 (18.1) | 0.022 |
| Pneumonia | 77/336 (22.9) | 282/972 (29.0) | 0.031 | 13/87 (14.9) | 77/310 (24.8) | 64/249 (25.7) | 205/662 (31.0) | 0.006 |
| Asp Pneumonia | 26/336 (7.7) | 118/972 (12.1) | 0.026 | NR | 29/310 (9.4 | 23/249 (9.2) | 89/662 (13.4) | 0.013 |
| Laryngeal cancer | ||||||||
| Dysphagia | 1,283/3,333 (38.5) | 1,429/2,188 (65.3) | <0.001 | 148/362 (40.9) | 449/855 (52.5) | 1135/2,971 (38.2) | 980/1,333 (73.5) | <0.001 |
| Stricture | 288/3,333 (8.6) | 487/2,188 (22.3) | <0.001 | 37/362 (10.2) | 192/855 (22.5) | 251/2,971 (8.5) | 295/1,333 (22.1) | <0.001 |
| Pneumonia | 897/3,333 (26.9) | 837/2,188 (38.3) | <0.001 | 100/362 (27.6) | 273/855 (31.9) | 797/2,971 (26.8) | 564/1,333 (42.3) | <0.001 |
| Asp Pneumonia | 261/3,333 (7.8) | 303/2,188 (13.9) | <0.001 | 26/362 (7.2) | 87/855 (10.2) | 235/2,971 (7.9) | 216/1,333 (16.2) | <0.001 |
| Oral cavity cancer | ||||||||
| Dysphagia | 1,110/3,622 (30.7) | 1,830/2,749 (66.6) | <0.001 | 822/3,047 (27.0) | 1027/1,679 (61.2) | 288/575 (50.1) | 803/1,070 (75.1) | <0.001 |
| Stricture | 174/3,622 (4.8) | 333/2,749 (12.1) | <0.001 | 131/3,047 (4.3) | 162/1,679 (9.7) | 43/575 (7.5) | 171/1,070 (16.0) | <0.001 |
| Pneumonia | 720/3,622 (19.9) | 806/2,749 (29.3) | <0.001 | 565/3,047 (18.5) | 439/1,679 (26.2) | 155/575 (27.0) | 367/1,070 (34.3) | <0.001 |
| Asp Pneumonia | 203/3,622 (5.6) | 318/2,749 (11.6) | <0.001 | 155/3,047 (5.1) | 170/1,679 (10.1) | 48/575 (8.4) | 148/1,070 (13.8) | <0.001 |
| Missing | ||||||||
| Dysphagia | 177/905 (19.6) | 459/1,729 (26.6) | <0.001 | 99/644 (15.4) | 374/1,540 (24.3) | 78/261 (29.9) | 85/189 (45.0) | <0.001 |
| Stricture | 22/905 (2.4) | 90/1,729 (5.2) | <0.001 | 14/644 (2.2) | 68/1,540 (4.4) | NR | 22/189 (11.6) | <0.001 |
| Pneumonia | 206/905 (22.8) | 315/1,729 (18.2) | 0.005 | 121/644 (18.8) | 266/1,540 (17.3) | 85/261 (32.6) | 49/189 (25.9) | <0.001 |
| Asp Pneumonia | 44/905 (4.9) | 87/1,729 (5.0) | 0.849 | 24/644 (3.7) | 73/1,540 (4.7) | 20/261 (7.7) | 14/189 (7.4) | <0.034 |
| All sites | ||||||||
| Dysphagia | 2775/8277 (33.5) | 4560/7917 (57.6) | <0.001 | 1109/4147 (26.7) | 2074/4430 (46.8) | 1666/4130 (40.3) | 2486/3487 (71.3) | <0.001 |
| Stricture | 528/8277 (6.4) | 1121/7917 (14.2) | <0.001 | 193/4147 (4.7) | 480/4430 (10.8) | 335/4130 (8.1) | 641/3487 (18.4) | <0.001 |
| Pneumonia | 1918/8277 (23.2) | 2334/7917 (29.5) | <0.001 | 800/4147 (19.3) | 1067/4430 (24.1) | 1118/4130 (27.1) | 1267/3487 (36.3) | <0.001 |
| Asp Pneumonia | 543/8277 (6.6) | 857/7917 (10.8) | <0.001 | 208/4147 (5.0) | 364/4430 (8.2) | 335/4130 (8.1) | 493/3487 (14.1) | <0.001 |
| All patients | 8,277 (51.11) | 7,917 (48.89) | 4,147 (25.61) | 4,430 (27.36) | 4,130 (25.50) | 3,487 (21.53) | ||
NR, numbers of patients not reported for cells in which n≤11 observations contributed to the estimate.
Table 3.
Dysphagia, pneumonia, and stricture regressed in multivariable models in full sample (n=16,194)
| Dysphagia No. pts (%), OR (95% CI) |
Stricture No. pts (%), OR (95% CI) |
Pneumonia No. pts (%), OR (95% CI) |
Aspiration pneumonia No. pts (%), OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment Modality | ||||||||
| Chemoradiation | 2,486/3,487 (71.3) | 5.58 (4.84–6.44) | 641/3,487 (18.4) | 2.95 (2.35–3.72) | 1,267/3,487 (36.3) | 1.90 (1.63–2.21) | 493/3,487 (14.1) | 2.24 (1.77–2.84) |
| Surgery + Adjuvant | 2,074/4,430 (46.8) | 2.01 (1.74–2.32) | 480/4,430 (10.8) | 1.29 (0.99–1.67) | 1,067/4,430 (24.1) | 1.28 (1.09–1.51) | 364/4,430 (8.2) | 1.44 (1.11–1.87) |
| Radiation | 1,666/4,130 (40.3) | 2.93 (2.59–3.31) | 335/4,130 (8.1) | 2.22 (1.78–2.76) | 1,118/4,130 (27.1) | 1.36 (1.18–1.56) | 335/4,130 (8.1) | 1.67 (1.33–2.09) |
| Surgery | 1,109/4,147 (26.7) | -Ref- | 193/4,147 (4.7) | -Ref- | 800/4,147 (19.3) | -Ref- | 208/4,147 (5.0) | -Ref- |
| Race/Ethnicity | ||||||||
| Other | 329/721 (45.6) | 1.21 (0.97–1.51) | 38/721 (5.3) | 0.44 (0.27–0.70) | 203/721 (28.2) | 1.12 (0.88–1.41) | 71/721 (9.9) | 1.25 (0.89–1.75) |
| Hispanic | 377/763 (49.4) | 1.30 (1.05–1.62) | 95/763 (12.5) | 1.23 (0.91–1.66) | 230/763 (30.1) | 1.02 (0.81–1.28) | 74/763 (9.7) | 0.94 (0.66–1.33) |
| Black | 569/1,001 (56.8) | 1.33 (1.10–1.61) | 131/1,001 (13.1) | 1.04 (0.80–1.35) | 302/1,001 (30.2) | 1.17 (0.96–1.42) | 101/1,001 (10.1) | 1.08 (0.81–1.45) |
| Caucasian | 6,060/13,709 (44.2) | -Ref- | 1,385/13,709 (10.1) | -Ref- | 3,517/13,709 (25.7) | -Ref- | 1,154/13,709 (8.4) | -Ref- |
| Age quartiles | ||||||||
| >79 | 1,494/3,111 (48.0) | 1.02 (0.89–1.16) | 383/3,111 (12.3) | 0.78 (0.64–0.96) | 749/3,111 (24.1) | 1.58 (1.37–1.82) | 237/3,111 (7.6) | 1.69 (1.35–2.12) |
| 74–79 | 2,227/4,674 (47.7) | 1.08 (0.94–1.23) | 542/4,674 (11.6) | 0.88 (0.72–1.08) | 1,163/4,674 (24.9) | 1.35 (1.16–1.56) | 363/4,674 (7.8) | 1.53 (1.22–1.91) |
| 69–74 | 1,695/3,722 (45.5) | 1.03 (0.90–1.17) | 380/3,722 (10.2) | 0.91 (0.76–1.09) | 993/3,722 (26.7) | 1.13 (0.98–1.30) | 345/3,722 (9.3) | 1.16 (0.93–1.44) |
| <69 | 1,919/4,687 (40.9) | -Ref- | 344/4,687 (7.3) | -Ref- | 1,387/4,687 (28.7) | -Ref- | 455/4,687 (9.7) | -Ref- |
| Disease sub-site | ||||||||
| Nasopharynx | 210/360 (58.3) | 0.74 (0.48–1.13) | 50/360 (13.9) | 1.29 (0.69–2.41) | 112/360 (31.1) | 0.95 (0.60–1.52) | 40/360 (11.1) | 0.85 (0.42–1.72) |
| Oropharynx | 837/1,308 (64.0) | 0.99 (0.86–1.14) | 205/1,308 (15.7) | 1.45 (1.20–1.75) | 359/1,308 (27.5) | 0.88 (0.76–1.02) | 144/1,308 (11.0) | 0.95 (0.77–1.18) |
| Larynx | 2,712/5,521 (49.1) | 0.92 (0.81–1.04) | 775/5,521 (14.0) | 1.81 (1.53–2.15) | 1,734/5,521 (31.4) | 1.39 (1.23–1.58) | 564/5,521 (10.2) | 1.03 (0.86–1.25) |
| Oral cavity | 2,940/6,371 (46.2) | -Ref- | 507/6,371 (8.0) | -Ref- | 1,526/6,371 (23.9) | -Ref- | 521/6,371 (8.2) | -Ref- |
| Missing | 101 | 90 | 315 | 87 | ||||
| Sex | ||||||||
| Female | 2,401/5,606 (42.8) | 1.11 (1.01–1.22) | 503/5,606 (9.0) | 1.03 (0.88–1.19) | 1306/5,606 (23.3) | 0.90 (0.82–1.00) | 382/5,606 (6.8) | 0.77 (0.65–0.90) |
| Male | 4,934/10,588 (46.6) | -Ref- | 1,146/10,588 (10.8) | -Ref- | 2,946/10,588 (27.8) | -Ref- | 1,018/10,588 (9.6) | -Ref- |
| Comorbidity | ||||||||
| 2 | 844/1,653 (51.1) | 1.46 (1.25–1.69) | 166/1,653 (10.0) | 0.96 (0.76–1.22) | 665/1,653 (40.2) | 2.32(2.01–2.69) | 226/1,653 (13.7) | 1.96 (1.59–2.41) |
| 1 | 1,746/3,469 (50.3) | 1.35 (1.21–1.51) | 406/3,469 (11.7) | 1.21 (1.03–1.42) | 1,114/3,469 (32.1) | 1.49 (1.33–1.68) | 367/3,469 (10.6) | 1.37 (1.15–1.63) |
| 0 | 4,745/11,072 (42.9) | -Ref- | 1,077/11,072 (9.7) | -Ref- | 2,473/11,072 (22.3) | -Ref- | 807/11,072 (7.3) | -Ref- |
| SEER stage | ||||||||
| Distant | 774/1,762 (43.9) | 1.81 (1.56–2.11) | 132/938 (14.1) | 1.31 (1.05–1.64) | 335/938 (35.7) | 1.81 (1.54–2.11) | 116/938 (12.4) | 1.75 (1.37–2.23) |
| Regional | 2,153/3,590 (60.0) | 1.81 (1.63–2.00) | 491/3,590 (13.7) | 1.37 (1.17–1.61) | 1,007/3,590 (28.1) | 1.42 (1.27–1.58) | 383/3,590 (10.7) | 1.76 (1.47–2.10) |
| In situ/Localized | 580/938 (61.8) | -Ref- | 172/1,762 (9.8) | -Ref- | 392/1,762 (22.3) | -Ref- | 118/1,762 (6.7) | -Ref- |
| Unstaged/missing | 1,053 | 326 | 600 | 240 | ||||
| Total | 7335/16,194 (45.3) | 1,649/16,194 (10.2) | 4252/16,194 (26.3) | 1400/16,194 (8.7) | ||||
Bold cells denote statistically significant (p<0.05) adjusted odds ratios.
Multivariable Models Comparing Prevalence of Dysphagia-Related Outcomes between Single Modality Treatments (n=8,277)
Results of multivariable regression models among the subgroup of 8,277 patients treated with single modality therapy (surgery or RT alone) are reported in supplementary Table 2 and in Figure 3. Adjusted odds of all dysphagia-related outcomes were higher with single modality RT relative to surgery. Relative to single modality surgery, single modality radiation was associated with 1.8 (95% CI: 1.5–2.1), 1.4 (95% CI: 1.0–1.9), and 1.3 (95% CI: 0.99–1.8) greater odds of dysphagia, stricture, and aspiration pneumonia respectively.
FIGURE 3.
Adjusted odds of dysphagia, stricture, pneumonia and aspiration pneumonia by treatment modality.
Multivariable Models Comparing Prevalence of Dysphagia-Related Outcomes between Multi-Modality Treatments (n=7,917)
Results of multivariable regression models among the subgroup of 7,917 patients treated with multi-modality therapy (surgery + adjuvant or chemoradiation) are reported in supplementary Table 3 and in Figure 3. Adjusted odds of all dysphagia-related outcomes were higher with chemoradiation relative to surgery + adjuvant therapy. Relative to multi-modality surgery+RT, CRT was associated with 1.9 (95% CI: 1.7–2.2), 1.3 (95% CI: 1.1–1.6), and 1.3 (95% CI: 1.1–1.6) greater odds of dysphagia, stricture, and aspiration pneumonia respectively.
Multivariable Models Estimating Impact of Adjuvant Chemotherapy on Prevalence of Dysphagia-Related Outcomes in Nonsurgically Treated Patients (n=7,617)
Results of multivariable regression models among the subgroup of 7,617 patients treated with nonsurgical therapy (RT alone or chemoradiation) are reported in supplementary Table 4 and in Figure 3. Adjusted odds of all dysphagia-related outcomes were higher among patients who received adjuvant chemotherapy with RT relative to RT alone. Relative to single modality RT, CRT was associated with 3.1 (95% CI: 2.7–3.6), 2.3 (95% CI: 1.9–2.9), and 1.6 (95% CI: 1.2–2.0) greater odds of dysphagia, stricture, and aspiration pneumonia respectively.
Multivariable Models Estimating Impact of Adjuvant Treatment after Primary Surgery on Prevalence of Dysphagia-Related Outcomes (n=8,577)
Results of multivariable regression models among the subgroup of 8,577 patients treated with primary surgical therapy (surgery alone or surgery + adjuvant) are reported in supplementary Table 5 and in Figure 3. Adjusted odds of all dysphagia-related outcomes were higher among patients who received adjuvant therapy with surgery relative to surgery alone. Relative to single modality surgery, surgery + adjuvant therapy was associated with 2.8 (95% CI: 2.4–3.2), 2.0 (95% CI: 1.6–2.5), and 1.7 (95% CI: 1.4–2.2) greater odds of dysphagia, stricture, and aspiration pneumonia respectively.
DISCUSSION
Trends in population-level prevalence of dysphagia in HNC survivorship in the United States were last comprehensively explored in a cohort comprised of patients diagnosed in the 1990’s (1992–1999)8. Using similar methodology and the SEER-Medicare administrative datasets, we report persistently high prevalence estimates for dysphagia (45%), stricture (10%), and pneumonia (9%) among patients diagnosed in the 2000’s despite notable advancements in minimally invasive surgical technique and highly conformal radiotherapy in this decade. Using the adjacent boundary of the 95% CI of our estimates (2000’s) against published prevalence by Francis et al8 (1990’s) as reference, prevalence of dysphagia increased by 4.7%, stricture increased by 2.6%, and pneumonia decreased by 0.9% in our SEER-Medicare cohort (2002–2011) relative to estimates published by Francis et al from the 1990’s cohort. We present 2-year prevalence estimates whereas Francis et al reported 3-year prevalence. This methodological difference, if anything, would be expected to attenuate the magnitude of the differences in prevalence between these reports, suggesting that dysphagia and stricture are more commonly coded in administrative data in the 2000’s than they were in the 1990’s. It is not possible to know whether this reflects greater numbers of survivors affected by dysphagia, or greater reporting or coding of this outcome with growing awareness in the clinical community. Nonetheless, the persistently high prevalence of dysphagia at the population level in modern survivors is noteworthy as swallowing difficulty is a top driver of decisional regret4 in survivorship and is a leading source of non-cancer mortality in survivorship12.
These results confirm the excess burden of dysphagia and dysphagia-related conditions (e.g., pneumonia) in HNC survivors. Relative to non-cancer controls, HNC cases were 4.6- to 7.8-times more likely to have stricture or dysphagia related claims and 2.1- to 3.7-times more likely to have pneumonia or aspiration pneumonia related claims. These data suggest that the high prevalence of dysphagia observed in HNC cases in this sample do not simply reflect age- or comorbidity-related swallowing dysfunction, as controls were matched on these factors. Similar to comparisons to non-cancer controls previously reported by Xu et al in a SEER-Medicare analysis focused solely on aspiration pneumonia after chemoradiation for mixed sites of HNC2, these estimates appear to largely reflect the impact of HNC and its treatment on dysphagia-related outcomes.
Frequency of dysphagia is influenced by many factors, most notably tumor site and treatment modality. In four treatment subgroups, multivariable regression models (adjusted for clinicodemographic confounders) were fit regressing each dysphagia outcome on treatment modality to examine two primary questions - first, the impact of surgical versus nonsurgical modalities (1: RT alone versus surgery alone, and 2: CRT versus Surgery + adjuvant), and second, the impact of adjuvant therapy (3: RT versus CRT, i.e., the added impact of chemotherapy; and 4: surgery alone versus surgery + adjuvant, i.e., the added impact of adjuvant RT or CRT). Two expected themes emerged exploring the results of treatment subgroup models. First, odds of dysphagia-related events were higher after primary nonsurgical methods when compared to primary surgery. This finding aligns with the trends observed in the similar 1990’s SEER-Medicare analysis of these endpoints in dysphagia8. It is noteworthy that this trend persisted in the decade (2000s) when IMRT and other highly conformal methods of radiotherapy were popularized with the goal of toxicity reduction. Second, subgroup analyses confirmed that odds of dysphagia-related events were higher when adjuvant therapy was required (i.e., comparing single versus multi-modality strategies). That is, both the addition of chemotherapy to radiation and the addition of adjuvant therapy after primary surgery were associated with greater prevalence and odds of dysphagia. These trends align with clinical observations, and are supported by hosts of clinical and population data supporting excess burden of dysphagia with combined modality (compared to single modality) treatment8,13.
Differences in observed effects sizes are perhaps the most notable results from treatment subgroup multivariable analyses. Specifically, as depicted in Figure 3, effect sizes were largest for addition adjuvant therapy (CRT versus RT alone or surgery + adjuvant versus surgery alone) suggesting 2- to 3-fold increased odds of dysphagia and stricture associated with addition of adjuvant therapy to either surgery or RT. Effect sizes were much smaller when comparing primary surgical or nonsurgical options for single modality (RT v surgery alone) or multimodality therapy (CRT v surgery + adjuvant) suggesting 30% to 90% increased odds (<2-fold) of dysphagia or stricture when primary nonsurgical therapy was delivered relative to primary surgical options (adjusted odds ratios: 1.27 to 1.94, see Figure 3). These observations suggest, at least with treatment practices in the 2000’s, greater impact of the number of modalities rather than selection of the primary treatment strategy (surgery or radiation) on the prevalence of dysphagia-related outcomes after treatment. This is particularly important when considering strategies to reduce dysphagia burden among patients with low- or intermediate risk disease, as these population-level data support the notion that the largest impact may be seen with de-escalation strategies that promote a single treatment modality when possible rather than those that simply trade one form of multimodality therapy for another.
Herein, we report updated trends in population-level prevalence of dysphagia in more than 16,000 patients treated for HNC in the U.S. between 2002 and 2011. Strengths of this analysis include large numbers offering the ability to yield narrow confidence intervals from estimates of multivariable models derived from clinically relevant subgroups of patients. We must acknowledge a number of caveats inherent to use of the SEER-Medicare database for these analyses. First, prevalence estimates may be inflated because we analyzed an older cohort of Medicare beneficiaries (age >65) as clinical series report age as the most discriminant demographic factor predicting poor swallowing outcomes14. Rising prevalence as shown in Figure 1 occurred in an era where treatment optimization (such as more conformal RT planning like IMRT and rising utilization of minimally invasive surgery). This is counter-intuitive and may in part reflect better coding over the study period rather than true rise in prevalence.
Use of administrative claims to code binary dysphagia outcomes over a 2-year period of observation (i.e., ever/never present) also lacks specificity with regard to the nature, severity, or duration of dysphagia events. Likewise, events must be coded on a claim to be coded in the analysis and claims data likely are not sensitive to milder toxicity events. Finally, cancer stage is a major driver of dysphagia outcomes, particularly primary tumor stage (or T-classification) but analysis of stage as a relevant covariate is limited using SEER-Medicare as their cancer staging (localized, regional, distant) is not compatible with the clinical standard of the AJCC system. Limiting the sample to 2004 onward (when full staging is available in SEER) would have allowed more relevant analysis of this covariate, but would also have limited sufficient sample size to examine all subgroup trends. This should be considered in future work.
It is also noteworthy that inferences regarding effect sizes, statistical significance, and direction of effects were most consistent between related endpoints of dysphagia and stricture. Pneumonia and aspiration pneumonia performed less consistently, and subgroup trends were not always compatible between these outcomes. As an outcome, aspiration pneumonia trended more similarly with stricture and dysphagia whereas general pneumonia diverged in subgroup analyses both by tumor site and treatment modality. These observations support the use of the aspiration pneumonia codes as most specific to dysphagia-related endpoints.
CONCLUSION
Updated population-level analysis using SEER-Medicare data find that prevalence of dysphagia, stricture, and aspiration pneumonia remains high in the decade studied (2002 to 2011) when comparing to published rates using similar methodology in an earlier decade (1992–1999). These results suggest persistence of this morbidity in the decade in which highly conformal radiotherapy methods and minimally invasive surgery were popularized. Prevalence of each dysphagia related endpoints was highest among those treated with combined modality RT with chemotherapy. As expected, multivariable models also indicated that odds of dysphagia related endpoints were higher among patients treated with multimodality therapy compared to single modality whether primary treatment was surgical or nonsurgical. Finally, larger effect sizes for the impact of adjuvant therapy (i.e., single versus multi-modality regimens) rather than modality (non-surgical versus surgical modalities) supports the notion that de-escalation strategies that promote a single treatment modality rather than those that simply trade one form of multimodality therapy for another could have largest impact on toxicity reduction for swallowing.
ACKNOWLEDGEMENT
(Funding): This work was supported by the MD Anderson Institutional Research Grant fund and the Duncan Family Institute. Dr. Hutcheson receives funding support from the National Cancer Institute (R03CA188162; R01CA218148) and the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248). The authors also acknowledge infrastructure support from NCI Cancer Center Support Grant (P30CA016672).
Acknowledgments: Portions of this work were presented at the American Society for Therapeutic Radiation Oncology Meeting in San Diego, California, September, 2017.
The authors gratefully acknowledge administrative support of Ms. Janet Hampton.
Appendix Table 1.
Medicare claims codes.
| Variable | Codes |
|---|---|
| Radiotherapy | ICD-9 procedure codes:
92.21–92.27, 92.29. ICD-9 diagnosis codes: V58.0, V66.1-V67.1. CPT/HCPCS codes: 77401–77525, 77761–77799. Revenue Center codes: 0330, 0333. |
| Surgery | ICD-9 procedure codes: 24.31,
25.1–25.4, 27.3, 27.32, 27.4, 27.42–43, 27.49,
27.72, 28.92, 29.33, 29.39, 30.0, 30.09, 30.1,
30.21–30.22, 30.29, 30.3–30.5, 76.2, 76.31,
76.39–76.42, 76.44–45. CPT/HCPCS codes: 21044–21045, 31365, 31367–31368, 31370, 31375, 31380, 31382, 31390, 31395, 31420, 38700, 38720, 38724, 40810, 40812, 40814, 40816, 40819, 41110, 41112–41116, 41120, 41130, 41135, 41140, 41145, 41150, 41153, 41155, 42104, 42106–42107, 42120, 42140, 42842, 42844–42845, 42890. |
| Chemotherapy | ICD-9 procedure code:
99.25. ICD-9 diagnosis codes: V58.1, V58.11, V58.12, V66.2, or V67.2. CPT/HCPCS codes: 96400 to 96599, J8999 to J9999, J8520, J8521, or Q0083 to Q0085. Revenue Center codes: 0331, 0332, or 0335. |
Abbreviations: CPT, Current Procedural Terminology; ICD, International Classification of Diseases; HCPCS, Healthcare Common Procedure Coding System.
Appendix Table 2.
Dysphagia, pneumonia, and stricture regressed on single modality treatments in multivariable models.
| Dysphagia No. pts (%), OR (95% CI) |
Stricture No. pts (%), OR (95% CI) |
Pneumonia No. pts (%), OR (95% CI) |
Aspiration
pneumonia No. pts (%), OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment Modality | ||||||||
| Radiation alone | 1,666/4,130 (40.3) | 1.81 (1.54–2.13) | 335/4,130 (8.1) | 1.42 (1.03–1.94) | 1,118/4,130 (27.1) | 1.27 (1.05–1.53) | 335/4,130 (8.1) | 1.34 (0.99–1.81) |
| Surgery alone | 1,109/4,147 (26.7) | -Ref- | 193/4,147 (4.7) | -Ref- | 800/4,147 (19.3) | -Ref- | 208/4,147 (5.0) | -Ref- |
| Race/Ethnicity | ||||||||
| Other | 111/365 (30.4) | 1.25 (0.93–1.68) | NR | 1.20 (0.87–1.67) | 83/365 (22.7) | 1.10 (0.79–1.55) | 22/365 (6.0) | 1.20 (0.69–2.09) |
| Hispanic | 124/361 (34.4) | 1.21 (0.87–1.67) | NR | 1.41 (1.05–1.91) | 92/361 (25.5) | 1.03 (0.71–1.50) | 31/361 (8.6) | 1.58 (0.93–2.68) |
| Black | 197/435 (45.3) | 1.47 (1.11–1.95) | NR | 1.21 (0.94–1.56) | 117/435 (26.9) | 1.22 (0.89–1.67) | 37/435 (8.5) | 1.24 (0.75–2.07) |
| Caucasian | 2,343/7,116 (32.9) | -Ref- | NR | -Ref- | 1,626/7,116 (22.9) | -Ref- | 453/7,116 (6.4) | -Ref- |
| Age quartiles | ||||||||
| >79 | 1,006/2,887 (34.9) | 1.12 (0.92–1.35) | 158/2,887 (5.5) | 0.66 (0.47–0.94) | 796/2,887 (27.6) | 1.87 (1.49–2.35) | 245/2,887 (8.5) | 2.23 (1.48–3.35) |
| 74–79 | 637/1,919 (33.2) | 1.05 (0.86–1.29) | 138/1,919 (7.2) | 0.72 (0.50–1.04) | 430/1,919 (22.4) | 1.36 (1.06–1.73) | 127/1,919 (6.6) | 1.41 (0.90–2.20) |
| 69–74 | 694/2,141 (32.4) | 1.05 (0.86–1.28) | 125/2,141 (5.8) | 0.76 (0.53–1.08) | 454/2,141 (21.2) | 1.30 (1.02–1.65) | 117/2,141 (5.5) | 1.54 (1.00–2.38) |
| <69 | 438/1,330 (32.9) | -Ref- | 107/1,330 (8.1) | -Ref- | 238/1,330 (17.9) | -Ref- | 54/1,330 (4.1) | -Ref- |
| Disease sub-site | ||||||||
| Nasopharynx | 35/81 (43.2) | 0.96 (0.46–1.97) | NR | 1.10 (0.25–4.76) | 18/81 (22.2) | 0.70 (0.28–1.74) | NR | 1.28 (0.37–4.41) |
| Oropharynx | 170/336 (50.6) | 1.46 (1.13–1.88) | NR | 1.82 (1.18–2.80) | 77/336 (22.9) | 0.90 (0.66–1.21) | NR | 1.00 (0.62–1.61) |
| Larynx | 1,283/3,333 (38.5) | 1.08 (0.90–1.30) | NR | 1.59 (1.13–2.24) | 897/3,333 (26.9) | 1.38 (1.12–1.70) | NR | 1.14 (0.82–1.60) |
| Oral cavity | 1,110/3,622 (30.7) | -Ref- | NR | -Ref- | 720/3,622 (19.9) | -Ref- | NR | -Ref- |
| Missing | 177 | NR | 206 | NR | ||||
| Sex | ||||||||
| Female | 1,002/3,103 (32.3) | 1.16 (1.02–1.33) | 181/3,103 (5.8) | 1.07 (0.93–1.23) | 624/3,103 (20.1) | 0.80 (0.68–0.92) | 155/3,103 (5.0) | 0.70 (0.54–0.91) |
| Male | 1,773/5,174 (34.3) | -Ref- | 347/5,174 (6.7) | -Ref- | 1,294/5,174 (25.0) | -Ref- | 388/5,174 (7.5) | -Ref- |
| Comorbidity | ||||||||
| 2 | 599/1,475 (40.6) | 1.51 (1.27–1.79) | 99/1,475 (6.7) | 0.97 (0.69–1.36) | 525/1,475 (35.6) | 2.26 (1.88–2.71) | 144/1,475 (9.8) | 1.77 (1.31–2.39) |
| 1 | 804/2,152 (37.4) | 1.31 (1.13–1.52) | 160/2,152 (7.4) | 1.09 (0.82–1.45) | 582/2,152 (27.0) | 1.64 (1.39–1.93) | 173/2,152 (8.0) | 1.52 (1.15–2.01) |
| 0 | 1,372/4,650 (29.5) | -Ref- | 269/4,650 (5.8) | -Ref- | 811/4,650 (17.4) | -Ref- | 226/4,650 (4.9) | -Ref- |
| SEER stage | ||||||||
| Distant | 173/434 (39.9) | 1.97 (1.71–2.27) | 26/434 (6.00) | 2.00 (1.62–2.48) | 124/434 (28.6) | 1.55 (1.33–1.82) | 29/434 (6.7) | 2.13 (1.65–2.75) |
| Regional | 677/1,541 (43.9) | 1.46 (1.15–1.84) | 109/1,541 (7.1) | 1.66 (1.44–1.92) | 433/1,541 (28.1) | 1.46 (1.12–1.89) | 152/1,541 (9.9) | 1.24 (0.78–1.96) |
| In situ/Localized | 955/3,587 (26.6) | -Ref- | 173/3,587 (4.8) | -Ref- | 687/3,587 (19.2) | -Ref- | 160/3,587 (4.5) | -Ref- |
| Unstaged/Missing | 970 | 220 | 674 | 202 | ||||
| Total | 2,775/8277 (33.5) | 528/8277 (6.4) | 1,918/8,277 (23.2) | 543/8,277 (6.6) | ||||
NR, numbers of patients not reported for cells in which n≤11 observations contributed to the estimate.
Appendix Table 3.
Dysphagia, pneumonia, and stricture regressed on multi-modality treatments in multivariable models.
| Dysphagia No. pts (%), OR (95% CI) |
Stricture No. pts (%), OR (95% CI) |
Pneumonia No. pts (%), OR (95% CI) |
Aspiration
pneumonia No. pts (%), OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment Modality | ||||||||
| Chemoradiation | 2,486/3,487 (71.3) | 1.94 (1.7–2.22) | 641/3,487 (18.4) | 1.32 (1.11–1.56) | 1,267/3,487 (36.3) | 1.38 (1.21–1.58) | 493/3,487 (14.1) | 1.34 (1.11–1.63) |
| Surgery + Adjuvant | 2,074/4,430 (46.8) | -Ref- | 480/4,430 (10.8) | -Ref- | 1,067/4,430 (24.1) | -Ref- | 364/4,430 (8.2) | -Ref- |
| Race/Ethnicity | ||||||||
| Other | 218/356 (61.2) | 0.32 (0.12–0.86) | 29/356 (8.2) | 0.49 (0.29–0.84) | 120/356 (33.7) | 1.15 (0.83–1.59) | 49/356 (13.7) | 1.30 (0.85–2.00) |
| Hispanic | 253/402 (62.9) | 1.03 (0.55–1.93) | 74/402 (18.4) | 1.32 (0.93–1.85) | 138/402 (34.3) | 1.02 (0.76–1.37) | 43/402 (10.7) | 0.69 (0.43–1.11) |
| Black | 372/566 (65.7) | 0.93 (0.55–1.59) | 97/566 (17.1) | 1.08 (0.80–1.46) | 185/566 (32.7) | 1.12 (0.88–1.43) | 64/566 (11.3) | 0.99 (0.70–1.42) |
| Caucasian | 3,717/6,593 (56.4) | -Ref- | 921/6,593 (14.0) | -Ref- | 1,891/6,593 (28.7) | -Ref- | 701/6,593 (10.6) | -Ref- |
| Age quartiles | ||||||||
| >79 | 913/1,800 (50.7) | 0.89 (0.73–1.07) | 186/1,800 (10.3) | 0.82 (0.64–1.06) | 551/1,800 (30.6) | 1.32 (1.09–1.61) | 210/1,800 (11.7) | 1.42 (1.07–1.88) |
| 74–79 | 1,058/1,803 (58.7) | 1.11 (0.92–1.34) | 242/1,803 (13.4) | 0.94 (0.75–1.20) | 563/1,803 (31.2) | 1.36 (1.13–1.64) | 218/1,803 (12.1) | 1.63 (1.25–2.12) |
| 69–74 | 1,533/2,533 (60.5) | 1.02 (0.86–1.20) | 417/2,533 (16.5) | 0.97 (0.79–1.20) | 709/2,533 (28.0) | 1.05 (0.88–1.24) | 246/2,533 (9.7) | 1.04 (0.80–1.35) |
| <69 | 1,056/1,781 (59.3) | -Ref- | 276/1,781 (15.5) | -Ref- | 511/1,781 (28.7) | -Ref- | 183/1,781 (10.3) | -Ref- |
| Disease sub-site | ||||||||
| Nasopharynx | 175/279 (62.7) | 0.64 (0.38–1.08) | 42/279 (15.1) | 1.35 (0.67–2.71) | 94/279 (33.7) | 1.08 (0.63–1.85) | 31/279 (11.1) | 0.74 (0.31–1.75) |
| Oropharynx | 667/972 (68.6) | 0.84 (0.71–0.99) | 169/972 (17.4) | 1.38 (1.12–1.71) | 282/972 (29.0) | 0.88 (0.74–1.04) | 118/972 (12.1) | 0.94 (0.74–1.20) |
| Larynx | 1,429/2,188 (65.3) | 0.84 (0.72–0.98) | 487/2,188 (22.3) | 1.93 (1.59–2.35) | 837/2,188 (38.3) | 1.39 (1.18–1.62) | 303/2,188 (13.9) | 0.98 (0.77–1.23) |
| Oral cavity | 1,830/2,749 (66.6) | -Ref- | 333/2,749 (12.1) | -Ref- | 806/2,749 (29.3) | -Ref- | 318/2,749 (11.6) | -Ref- |
| Missing | 101 | 90 | 315 | 87 | ||||
| Sex | ||||||||
| Female | 1,399/2,503 (55.9) | 1.12 (0.86–1.45) | 322/2,503 (12.9) | 0.90 (0.75–1.08) | 682/2,503 (27.3) | 1.02 (0.88–1.17) | 227/2,503 (9.1) | 0.82 (0.67–1.01) |
| Male | 3,161/5,414 (58.4) | -Ref- | 799/5,414 (14.8) | -Ref- | 1,652/5,414 (30.5) | -Ref- | 630/5,414 (11.6) | -Ref- |
| Comorbidity | ||||||||
| 2 | 790/1,256 (62.9) | 1.41 (1.17–1.69) | 191/1,256 (15.2) | 1.04 (0.83–1.32) | 522/1,256 (41.6) | 2.06 (1.74–2.45) | 193/1,256 (15.4) | 1.77 (1.40–2.22) |
| 1 | 1,206/1,971 (61.2) | 1.25 (1.08–1.46) | 309/1,971 (15.7) | 1.21 (1.00–1.47) | 670/1,971 (34.0) | 1.41 (1.21–1.64) | 237/1,971 (12.0) | 1.17 (0.94–1.46) |
| 0 | 2,564/4,690 (54.7) | -Ref- | 621/4,690 (13.2) | -Ref- | 1,142/4,690 (24.4) | -Ref- | 27/4,690 (9.1) | -Ref- |
| SEER stage | ||||||||
| Distant | 774/1,762 (43.9) | 0.98 (0.62–1.56) | 132/938 (14.1) | 1.45 (1.11–1.89) | 335/938 (35.7) | 1.98 (1.61–2.43) | 116/938 (12.4) | 1.82 (1.35–2.47) |
| Regional | 2,153/3,590 (60.0) | 1.37 (1.05–1.79) | 491/3,590 (13.7) | 1.42 (1.15–1.74) | 1,007/3,590 (28.1) | 1.35 (1.15–1.58) | 383/3,590 (10.7) | 1.54 (1.21–1.96 |
| In situ/Localized | 580/938 (61.8) | -Ref- | 172/1,762 (9.8) | -Ref- | 392/1,762 (22.3) | -Ref- | 118/1,762 (6.7) | -Ref- |
| Unstaged/missing | 1,053 | 326 | 600 | 240 | ||||
| Total | 4,560/7,917 (57.6) | 1,121/7,917 (14.2) | 2,334/7,917 (29.5) | 857/7,917 (10.8) | ||||
Appendix Table 4.
Dysphagia, pneumonia, and stricture regressed on non-surgical treatments in multivariable models.
| Dysphagia No. pts (%), OR (95% CI) |
Stricture No. pts (%), OR (95% CI) |
Pneumonia No. pts (%), OR (95% CI) |
Aspiration
pneumonia No. pts (%), OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment Modality | ||||||||
| Chemoradiation | 2,486/3,487 (71.3) | 3.08 (2.65–3.58) | 641/3,487 (18.4) | 2.31 (1.85–2.88) | 1,267/3,487 (36.3) | 1.47 (1.26–1.72) | 493/3,487 (14.1) | 1.55 (1.23–1.95) |
| Radiation alone | 1,666/4,130 (40.3) | -Ref- | 335/4,130 (8.1) | -Ref- | 1,118/4,130 (27.1) | -Ref- | 335/4,130 (8.1) | -Ref- |
| Race/Ethnicity | ||||||||
| Other | 181/332(54.5) | 1.10 (0.79–1.55) | 23/332 (6.9) | 1.15 (0.83–1.59) | 111/332 (33.4) | 1.24 (0.86–1.80) | 38/332 (11.5) | 1.31 (0.79–2.16) |
| Hispanic | 207/364 (56.9) | 1.03 (0.71–1.50) | 56/364 (15.4) | 1.02 (0.76–1.37) | 140/364 (38.5) | 1.26 (0.90–1.76) | 47/364 (12.9) | 0.95 (0.58–1.57) |
| Black | 388/611 (63.5) | 1.22 (0.89–1.67) | 79/611 (12.9) | 1.12 (0.88–1.43) | 206/611 (33.7) | 1.21 (0.94–1.55) | 73/611 (12.0) | 1.12 (0.78–1.61) |
| Caucasian | 3,376/6,310 (53.5) | -Ref- | 818/6,310 (13.0) | -Ref- | 1,928/6,310 (30.6) | -Ref- | 670/6,310 (10.6) | -Ref- |
| Age quartiles | ||||||||
| >79 | 1,005/1,982 (50.7) | 1.04 (0.85–1.28) | 192/1,982 (9.7) | 0.85 (0.64–1.12) | 674/1,982 (34.0) | 1.49 (1.21–1.83) | 255/1,982 (12.9) | 1.90 (1.39–2.59) |
| 74–79 | 953/1,737 (54.9) | 1.10 (0.90–1.35) | 229/1,737 (13.2) | 0.87 (0.67–1.14) | 574/1,737 (33.1) | 1.45 (1.18–1.78) | 215/1,737 (12.4) | 1.77 (1.30–2.41) |
| 69–74 | 1,294/2,309 (56.1) | 1.05 (0.87–1.28) | 326/2,307 (14.1) | 0.89 (0.70–1.14) | 690/2,307 (29.9) | 1.11 (0.91–1.35) | 226/2,307 (9.8) | 1.24 (0.92–1.67) |
| <69 | 900/1,591 (56.6) | -Ref- | 229/1,591 (14.4) | -Ref- | 447/1,591 (28.1) | -Ref- | 132/1,591 (8.3) | -Ref- |
| Disease sub-site | ||||||||
| Nasopharynx | 183/307 (59.6) | 0.63 (0.39–1.03) | 41/307 (13.4) | 0.96 (0.46–1.97) | 94/279 (33.7) | 0.81 (0.48–1.36) | 35/307 (11.4) | 0.54 (0.21–1.36) |
| Oropharynx | 600/911 (65.9) | 0.89 (0.74–1.07) | 145/911 (15.9) | 1.23 (0.97–1.56) | 282/972 (29.0) | 0.86 (0.71–1.04) | 112/911 (12.3) | 1.00 (0.77–1.30) |
| Larynx | 2,115/4,304 (49.1) | 0.95 (0.81–1.12) | 546/4,304 (12.7) | 1.44 (1.15–1.79) | 837/2,188 (38.3) | 1.38 (1.17–1.62) | 451/4,304 (10.5) | 1.09 (0.86–1.39) |
| Oral cavity | 1,091/1,645 (66.3) | -Ref- | 214/1,645 (13.0) | -Ref- | 806/2,749 (29.3) | -Ref- | 196/1,645 (11.9) | -Ref- |
| Missing | 163 | 30 | 366 | 34 | ||||
| Sex | ||||||||
| Female | 1,118/1,930 (57.9) | 0.80 (0.68–0.92) | 249/1,930 (12.9) | 1.02 (0.88–1.17) | 597/1,930 (30.9) | 0.99 (0.84–1.15) | 191/1,930 (9.9) | 0.88 (0.70–1.11) |
| Male | 3,034/5,687 (53.4) | -Ref- | 727/5,687 (12.8) | -Ref- | 1,788/5,687 (31.4) | -Ref- | 637/5,687 (11.2) | -Ref- |
| Comorbidity | ||||||||
| 2 | 858/1,450 (59.2) | 1.65 (1.37–2.00) | 177/1,450 (12.2) | 0.95 (0.74–1.23) | 614/1,450 (42.3) | 2.03 (1.69–2.43) | 205/1,450 (14.1) | 1.73 (1.34–2.22) |
| 1 | 1,194/2,056 (58.1) | 1.32 (1.12–1.56) | 294/2,056 (14.3) | 1.15 (0.93–1.42) | 732/2,056 (35.6) | 1.48 (1.26–1.74) | 258/2,056 (12.6) | 1.31 (1.04–1.67) |
| 0 | 2,100/4,111 (51.1) | -Ref- | 505/4,111 (12.3) | -Ref- | 1,039/4,111 (25.3) | -Ref- | 365/4,111 (8.9) | -Ref- |
| SEER stage | ||||||||
| Distant | 458/712 (64.3) | 1.55 (1.33–1.82) | 108/712 (15.2) | 1.98 (1.61–2.43) | 273/712 (38.3) | 1.68 (1.36–2.09) | 92/712 (12.9) | 1.97 (1.50–2.58) |
| Regional | 1,477/2,262 (65.3) | 1.46 (1.12–1.89) | 332/2,262 (14.7) | 1.35 (1.15–1.58) | 752/2,262 (33.2) | 1.42 (1.20–1.69) | 297/2,262 (13.1) | 1.88 (1.35–2.62) |
| In situ/Localized | 654/1,331 (49.1) | -Ref- | 140/1,331 (10.5) | -Ref- | 365/1,331 (27.4) | -Ref- | 90/1,331 (6.8) | -Ref- |
| Unstaged/missing | 1,563 | 396 | 995 | 349 | ||||
| Total | 4,152/7,617 (54.5) | 976/7,617 (12.8) | 2,385/7,617 (31.3) | 828/7,617 (10.9) | ||||
Appendix Table 5.
Dysphagia, pneumonia, and stricture regressed on surgical treatments in multivariable models.
| Dysphagia No. pts (%), OR (95% CI) |
Stricture No. pts (%), OR (95% CI) |
Pneumonia No. pts (%), OR (95% CI) |
Aspiration
pneumonia No. pts (%), OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment Modality | ||||||||
| Surgery + adjuvant therapy | 2,074/4,430 (46.8) | 2.77 (2.44–3.15) | 480/4,430 (10.8) | 1.97 (1.56–2.49) | 1,067/4,430 (24.1) | 1.36 (1.17–1.58) | 364/4,430 (8.2) | 1.73 (1.36–2.20) |
| Surgery alone | 1,109/4,147 (26.7) | -Ref- | 193/4,147 (4.7) | -Ref- | 800/4,147 (19.3) | -Ref- | 208/4,147 (5.0) | -Ref- |
| Race/Ethnicity | ||||||||
| Other | 148/389 (38.1) | 1.20 (0.69–2.09) | 15/389 (3.9) | 1.30 (0.85–2.00) | 92/389(23.7) | 1.05 (0.77–1.43) | 33/389 (8.5) | 1.22 (0.77–1.93) |
| Hispanic | 170/399 (42.6) | 1.58 (0.93–2.68) | 39/399 (9.8) | 0.69 (0.43–1.11) | 90/399 (22.6) | 0.86 (0.62–1.18) | 27/399 (6.8) | 0.93 (0.56–1.53) |
| Black | 181/390 (46.4) | 1.24 (0.75–2.07) | 52/390 (13.3) | 0.99 (0.70–1.42) | 96/390 (24.6) | 1.10 (0.81–1.50) | 28/390 (7.2) | 0.99 (0.60–1.64) |
| Caucasian | 2,684/7,399 (36.3) | -Ref- | 567/7,399 (7.7) | -Ref- | 1,589/7,399 (21.5) | -Ref- | 484/7,399 (6.5) | -Ref- |
| Age quartiles | ||||||||
| >79 | 914/2,705 (33.8) | 1.01 (0.85–1.20) | 152/2,705 (5.6) | 0.73 (0.54–0.99) | 673/2,705 (24.9) | 1.59 (1.30–1.95) | 200/2,705 (7.4) | 1.45 (1.05–1.99) |
| 74–79 | 742/1,985 (37.4) | 1.06 (0.88–1.27) | 151/1,985 (7.6) | 0.90 (0.67–1.22) | 419/1,985 (21.1) | 1.23 (0.99–1.52) | 130/1,985 (6.6) | 1.25 (0.89–1.74) |
| 69–74 | 933/2,367 (39.4) | 1.01 (0.85–1.20) | 216/2,367 (9.1) | 0.95 (0.72–1.25) | 473/2,367 (19.9) | 1.13 (0.93–1.39) | 137/2,367 (5.8) | 1.04 (0.75–1.44) |
| <69 | 594/1,520 (39.1) | -Ref- | 154/1,520 (10.1) | -Ref- | 302/1,520 (19.9) | -Ref- | 105/1,520 (6.9) | -Ref- |
| Disease sub-site | ||||||||
| Nasopharynx | 27/53 (50.9) | 0.94 (0.35–2.51) | NR | 2.55 (0.72–9.12) | 99/307 (32.3) | 1.71 (0.62–4.71) | NR | 3.23 (1.03–10.15) |
| Oropharynx | 237/397 (59.7) | 1.19 (0.95–1.50) | NR | 1.76 (1.28–2.43) | 269/911 (29.5) | 0.88 (0.68–1.15) | NR | 0.81 (0.54–1.21) |
| Larynx | 597/1,217 (49.1) | 0.82 (0.69–0.98) | NR | 2.42 (1.88–3.12) | 1,361/4,304 (31.6) | 1.36 (1.12–1.66) | NR | 0.93 (0.68–1.27) |
| Oral cavity | 1,849/4,726 (39.1) | -Ref- | NR | -Ref- | 522/1,645 (31.7) | -Ref- | NR | -Ref- |
| Missing | 473 | 387 | ||||||
| Sex | ||||||||
| Female | 1,283/3,676 (34.9) | 0.70 (0.54–0.91) | 254/3,676 (6.9) | 0.82 (0.67–1.01) | 709/3,676 (19.3) | 0.85 (0.75–0.98) | 191/3,676 (5.2) | 0.68 (0.54–0.85) |
| Male | 1,900/4,901 (38.8) | -Ref- | 419/4,901 (8.6) | -Ref- | 1,158/4,901 (23.6) | -Ref- | 381/4,901 (7.8) | -Ref- |
| Comorbidity | ||||||||
| 2 | 531/1,281 (41.5) | 1.34 (1.14–1.58) | 113/1,281 (8.8) | 1.11 (0.83–1.47) | 433/1,281 (33.8) | 2.28 (1.92–2.70) | 132/1,281 (10.3) | 1.84 (1.42–2.40) |
| 1 | 816/2,067 (39.5) | 1.26 (1.09–1.44) | 175/2,067 (8.5) | 1.20 (0.95–1.52) | 520/2,067 (25.2) | 1.54 (1.32–1.79) | 152/2,067 (7.4) | 1.26 (0.98–1.63) |
| 0 | 1,836/5,229 (35.1) | -Ref- | 385/5,229 (7.4) | -Ref- | 914/5,229 (17.5) | -Ref- | 288/5,229 (5.5) | -Ref- |
| SEER stage | ||||||||
| Distant | 295/660 (44.7) | 2.13 (1.65–2.75) | 50/660 (7.6) | 1.82 (1.35–2.47) | 186/660 (28.2) | 2.00 (1.57–2.54) | 53/660 (8.0) | 1.69 (1.16–2.46) |
| Regional | 1,353/2,869 (47.2) | 1.24 (0.78–1.96) | 268/2,869 (9.3) | 1.54 (1.21–1.96) | 688/2,869 (24.0) | 1.42 (1.22–1.65) | 238/2,869 (8.3) | 1.61 (1.27–2.05) |
| In situ/Localized | 1,075/4,018 (26.8) | -Ref- | 205/4,018 (5.1) | -Ref- | 714/4,018 (17.8) | -Ref- | 188/4,018 (4.7) | -Ref- |
| Unstaged/missing | 460 | 82 | 279 | 93 | ||||
| Total | 3,183/8,577 (37.1) | 673/8,577 (7.9) | 1,867/8,577 (21.8) | 572/8,577 (6.7) | ||||
NR, numbers of patients not reported for cells in which n≤11 observations contributed to the estimate.
Footnotes
CONFLICT OF INTEREST: The authors made no disclosures.
REFERENCES
- 1.Hunter KU, Lee OE, Lyden TH, et al. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head Neck 2014;36(1):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu B, Boero IJ, Hwang L, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer 2015;121(8):1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter KU, Schipper M, Feng FY, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys 2013;85(4):935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goepfert RP, Fuller CD, Gunn GB, et al. Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck 2017;39(11):2151–2158. [DOI] [PubMed] [Google Scholar]
- 5.Beadle BM, Liao KP, Giordano SH, et al. Reduced feeding tube duration with intensity-modulated radiation therapy for head and neck cancer: A Surveillance, Epidemiology, and End Results-Medicare Analysis. Cancer 2017;123:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary H, Stewart CM, Webster K, et al. Readmission following primary surgery for larynx and oropharynx cancer in the elderly. Laryngoscope 2017;127:631–641. [DOI] [PubMed] [Google Scholar]
- 7.Starmer HM, Quon H, Simpson M, et al. Speech-language pathology care and short- and long-term outcomes of laryngeal cancer treatment in the elderly. Laryngoscope 2015;125:2756–2763. [DOI] [PubMed] [Google Scholar]
- 8.Francis DO, Weymuller EA Jr., Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol 2010;119:391–397. [DOI] [PubMed] [Google Scholar]
- 9.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe KE. How to construct regression models for observational studies (and how NOT to do it!). Can J Anaesth 2017;64:461–470. [DOI] [PubMed] [Google Scholar]
- 12.Szczesniak MM, Maclean J, Zhang T, Graham PH, Cook IJ. Persistent dysphagia after head and neck radiotherapy: a common and under-reported complication with significant effect on non-cancer-related mortality. Clin Oncol (R Coll Radiol) 2014;26:697–703. [DOI] [PubMed] [Google Scholar]
- 13.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009;73:410–415. [DOI] [PubMed] [Google Scholar]
- 14.Anderson MD Head Neck Cancer Symptom Working Group. Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: Dose-volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiother Oncol 2016;118:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]