Abstract
The cost of prescription drugs in the U.S. continues to be a source of concern for patients, caregivers, and policymakers. Drug prices typically decline rapidly once generic drugs receive U.S. Food and Drug Administration (FDA) approval and enter the market, but the past decade has witnessed rising costs and shortages of generic drugs. We describe the strategies employed by brand-name manufacturers to undermine generic competition and the reasons underlying the price increases of off-patent drugs, some of which continue to lack any competition from generic versions, and others that have increased in price despite having generic versions. We discuss the FDA’s role in addressing drug prices and promoting competition, including recent agency policies to modify its process of reviewing generic drug applications and prioritize applications for off-patent drugs with few competitors. We also examine proposed policy solutions and research areas that could help address the price increases of off-patent drugs.
The cost of prescription drugs in the U.S. continues to be a source of concern for patients, caregivers, and policymakers. In a recent poll of U.S. adults, 77% of respondents with varying political affiliations said that prescription drug costs were “unreasonable” (1). In 2016, the U.S. spent $450 billion on prescription medicines, accounting for 14% of total health care spending and projected to increase to $610 billion by 2021 (2). Much of this increase in drug spending is due to brand-name drugs that are protected from generic competition by patents and regulatory exclusivity (3). Though they constitute only 10% of prescriptions dispensed in the U.S., brand-name drugs account for 74% of drug spending (4). During the market exclusivity period, the brand-name manufacturer can earn sizable profits, which can help to drive further pharmaceutical innovation and investment in drug development.
In the U.S., drug prices typically decline rapidly once generic drugs receive U.S. Food and Drug Administration (FDA) approval and begin to enter the market. The greater the number of generic manufacturers’ versions in a market, the steeper the price decline, with prices decreasing to less than 20% of the original drug’s price (5, 6). In 2016, generic drugs accounted for only 27% of overall U.S. drug spending yet constituted 89% of drug prescriptions in the U.S. (7), a dramatic increase from just 19% of prescriptions in 1984 (8). Low-cost generic drugs generated $253 billion in savings to the U.S. health care system in 2017 and more than $1 trillion in the past decade (4, 9). Appropriate use of low-cost generic drugs is associated with improved patient medication adherence (10, 11) and health outcomes (12).
In the past decade, however, there has been growing concern about the rapid rise in costs and shortages of generic drugs, despite their substantially lower prices when compared to brand-name drugs. A recent U.S. Government Accountability Office report found that 315 of 1,441 (22%) generic drugs sold in the U.S. experienced price increases of 100% or more from 2010 to 2015, many of which were older, small-market medicines (13). Shortages of generic drugs in the U.S. have also risen, quadrupling between 2005 and 2011, from 61 to 250 drugs (14, 15). Large price increases of generic drugs have been associated with decreases in physician prescribing and drug utilization (16). Despite no longer being protected by patents and regulatory exclusivity, these older drugs experiencing price increases and shortages often lack robust competition. In some cases, individual companies have drawn sharp public condemnation for dramatically raising the price of single-source drugs that have been off-patent for decades. The most notable example was that of Turing Pharmaceutical’s acquisition of pyrimethamine (Daraprim), wherein pharmaceutical entrepreneurs acquired the rights to the drug and raised its price from $13.50 to $750 a tablet due to the absence of any competition (17). To address this issue, the FDA recently published lists of drugs that are off-patent yet still lack any generic versions, with the aim of attracting additional competitors to enter specific drugs’ markets (18).
Though competition from multiple independent competitors exists for most large-market and easy-to-manufacture generic drugs, helping drive down prices, many drugs have increased in price despite facing competition from generic versions. These specific drug markets often continue to lack sufficient generic competition, face supply limitations due to concentration of manufacturers, or experience market exit of individual manufacturers due to low profits. Complicated arrangements between drug manufacturers, pharmaceutical benefit managers, and insurance companies can also result in the use of brand-name drugs even though generic alternatives are available. A recent report from the Office of the Assistant Secretary for Planning and Evaluation found that Medicare Part D plans spent almost $9 billion on brand-name drugs when therapeutically equivalent generics were available, and the program could have saved $3 billion had generic versions been dispensed instead (19).
Here, we examine the reasons underlying the price increases of off-patent drugs, some of which continue to lack any competition from generic versions, and others that have increased in price despite having generic versions. We discuss the role of the FDA in promoting competition through prioritization of approval applications for off-patent drugs with few generic competitors. We also examine policy solutions and areas for further research that could help address the price increases of off-patent drugs.
FDA DRUG APPROVAL AND PRICE IMPACT OF GENERIC DRUGS
The Drug Price Competition and Patent Term Restoration Act of 1984 – commonly known as the Hatch-Waxman Act – instigated the growth of generic drugs in the United States by allowing for their earlier and less costly FDA approval. The new system aimed to accelerate patient access to affordable prescription drugs while also protecting pharmaceutical innovation. In the first phase of this system, to spur continued innovation, manufacturers of brand-name drugs approved by the FDA are awarded a monopoly consisting of patent protection and 5 to 7 years of market exclusivity. During this time, no other competitor can enter the market, and the manufacturer of the sole-source drug can set any price. In the second phase, once the patent protection and exclusivity extensions have ended, the FDA approves additional drugs that are proven to be bioequivalent and considered interchangeable to the original drug product.
Whereas a new drug product that has never before been approved by the FDA submits a New Drug Application to obtain approval, manufacturers of generic drugs submit Abbreviated New Drug Applications. Prior to the 1984 Hatch-Waxman Act, generic drug manufacturers were required to conduct the same lengthy and expensive clinical trials as their brand-name counterparts in order to demonstrate their version’s safety and efficacy. As a result, few generic drugs made it to market. To introduce greater competition, the Hatch-Waxman Act established a new system in which instead of repeating clinical trials, manufacturers of generic drugs must demonstrate bioequivalence to their brand-name counterparts, meaning that the drug must contain the same active ingredient in the same dosage form and route of administration and have the same availability of the active ingredient at the site of action. Comparisons of generic drugs with the original brand-name drug have largely demonstrated clinical equivalence (20–23). The equivalence of some drugs, such as levothyroxine (24) and lamotrigine (25, 26), has been called into question, though evidence from comparisons of brand-name and generic versions confirm their equivalence (27, 28).
The Hatch-Waxman Act allows manufacturers of generic drugs to begin conducting bioequivalence tests and to apply for FDA approval prior to the expiration of brand-name patent protection and exclusivity extensions. A generic manufacturer must either wait for the expiration of any patents held by the original drug before marketing its drug, or it can certify (Paragraph IV certification) that its drug does not infringe the patents, that the patents are invalid, or both (29). The first generic manufacturer to file a substantially complete abbreviated new drug application and a successful Paragraph IV certification is awarded 180 days of exclusivity, during which time a second generic manufacturer cannot sell their drug. The 180-day exclusivity period was created to incentivize the entry of generic manufacturers into individual drug markets. However, during this duopoly period, prices do not appreciably decline given the lack of competitors (30), leading some observers to question the continued utility of this exclusivity period (31).
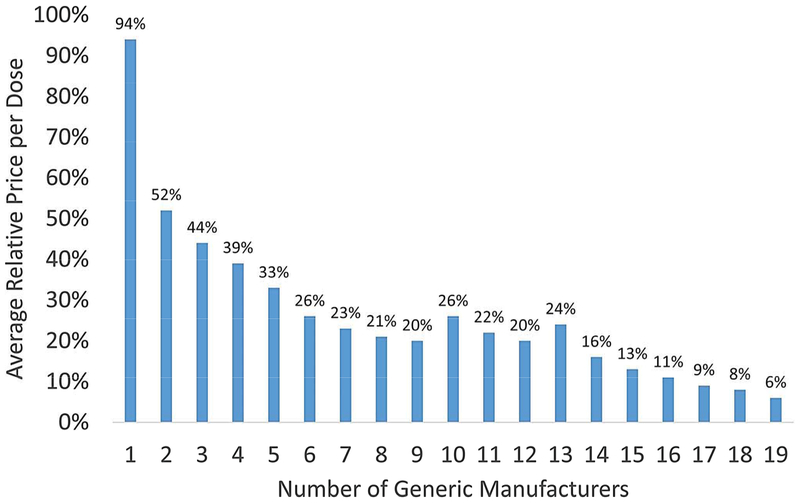
Prices typically decrease rapidly with the entry of subsequent generic manufacturers. Generic drugs that entered the market between 2002 and 2014 reduced drug prices by 51% in the first year, and after a plateau in drug prices during the 180-day exclusivity when only the first generic drug manufacturer can market its drug, nearly all reductions in the price of oral medications occurred in the first eight months after generic entry (32). As the number of generic manufacturers within specific drug markets increases, drug prices continue to decline. An 2005 FDA analysis found that after patent and exclusivity expiration, the introduction of one generic manufacturer into the market reduced the price of the drug by only 6% (5) (Figure 1). With two generic manufacturers, the price reached 52% of the brand-name drug’s price. Additional studies have found that at least four generic manufacturers are required to achieve substantial price reductions (30, 33). A more recent analysis corroborated these results, finding that the impact on price was most evident with the entry of three to four generic manufacturers (6), though more than one-third of off-patent drugs have three or fewer generic versions (34). The spread of laws throughout every state that authorize, and in some states require, automatic substitution of generic drugs for their brand-name counterparts at the pharmacy has helped contribute to the rapid market penetration of generic drugs (35).
Figure 1.
Generic Competition and Drug Prices
DELAYED AVAILABILITY OF GENERIC DRUGS
Given the impending loss of profits of brand-name manufacturers’ products with expiring patents and exclusivity, the period of patent and exclusivity expiration or invalidation typically generates substantial litigation as manufacturers of brand-name drugs challenge the generic manufacturers’ certifications. Brand-name manufacturers often engage in various strategies to delay and prevent the entry of generic drugs into the market during this period. Such strategies increase overall spending on drugs and may delay patient access to low-cost generic drug alternatives.
One way in which brand-name manufacturers achieve this is by modifying their initial drug, such as by developing an extended-release version that can reduce the number of times a patient takes the medication in a day, thereby helping improve adherence. In many cases, however, in a strategy called “product-hopping,” the brand-name manufacturer precludes automatic switching of its drug for a generic version by launching a branded reformulation at the time of generic entry and simultaneously discontinuing its original version. In the case of fenofibrate, for example, Abbott Laboratories engaged in a serial switching strategy in which at the time of generic entry it launched multiple sequential branded reformulations without demonstrated superiority to the original product (36), which prevented their substitution with generics of the older products. Abbott also simultaneously filed patent litigation to delay the approval of generic versions.
Similarly, brand-name manufacturers may acquire “secondary patents” that cover peripheral aspects of the drug product, such as modified forms of the active ingredient, salt moieties, and various methods of administration. These additional patents make it difficult for successful challenges by generic competitors once the patent period has ended. One analysis found that among drugs approved by the FDA between 1988 and 2005, secondary patents extended the original drug’s patent life by an average of 6.7 years (37). Features covered by secondary patents sometimes offer improvements that are beneficial for patients, but in many cases they do not represent a therapeutic advance (38).
Brand-name drug manufacturers have also used “reverse-payment” or “pay-for-delay” settlements to delay generic entry. In these settlements, the brand-name drug manufacturer and the first generic drug manufacturer come to an agreement in which the latter withdraws its legal challenge to the brand-name drug manufacturers’ patent and delays the generic drug’s market entry in exchange for monetary compensation. In other situations, brand-name manufacturers release a branded generic, called an “authorized generic,” to compete with the newly approved generic versions. There is some evidence suggesting that authorized generics may prevent multiple generic drugs from entering the market and limit potential competition (39).
Another way that brand-name drug manufacturers can delay the entry of generic competitors is by using citizen petitions, which were intended to allow an average citizen to voice their concerns regarding a drug product. A recent analysis found, however, that brand-name drug manufacturers were increasingly filing citizen petitions with trivial claims just prior to generic entry, thereby delaying approval of generic versions and extending their monopoly period (40). Filing of citizen petitions increased between 2001 and 2010, with half filed by brand-name companies targeting generic drugs (41). Though the FDA is required to rule on citizen petitions within 180 days, the number of citizen petitions does not seem to have declined (41). Finally, brand-name manufacturers can delay generic entry by invoking risk evaluation and mitigation strategies (REMS) requirements (42, 43). In doing so, the brand-name manufacturers refuse to provide generic drug manufacturers with samples of their drugs in order for the generic manufacturers to conduct bioequivalence studies for FDA approval of their application. Brand-name manufacturers can also delay negotiations with generic drug manufacturers to meet the FDA requirement of developing a single shared REMS program, thus preventing the final approval of the generic drug.
CAUSES OF HIGH PRICES FOR GENERIC DRUGS
Once brand-name drug patent protections and regulatory exclusivities expire, in most cases, particularly for widely-used drugs, multiple generic competitors enter the market and prices quickly decline. The number of generic drugs that enter markets is driven by a variety of manufacturer-specific factors, including the availability and cost of raw materials, fixed start-up costs, synchronization with other product lines manufactured by the company, and experience with similar drug products. The time to drug approval and the attractiveness of specific drug markets, based on size of the patient population being treated and projected profits, also dictate the competitiveness of the market. Previous studies have demonstrated that the number of generic manufacturers entering a market is greater for drugs with higher sales prior to expiration of patents and exclusivity (44, 45). Drugs that treat smaller patient populations, such as many older orphan drugs, have fewer generic competitors (34).
Some generic markets, however, have faced price escalations in part due to increasing concentration and reduced competitiveness of the generic drug industry. One reason for increased concentration is that while the number of mergers and acquisitions among generic companies has remained steady over the past several years, the transactions have increased in value and a greater number have involved large companies (46), suggesting growing consolidation among larger companies. In 2016, for example, Teva, one of the largest generic drug companies in the world, acquired Allergen’s generic business, prompting the Federal Trade Commission (FTC) to require Teva to divest 79 pharmaceutical products given the anticompetitive nature of the merger (47). Large generic drug companies that own more extensive drug portfolios can more easily increase prices in noncompetitive markets to balance lower profits in more competitive ones.
Another reason for recent price escalations pertains to the number of generic manufacturers entering and exiting in the industry. Historically, the number of generic manufacturers entering the drug industry was greater than the number exiting. A recent analysis found, however, that in the last five years the number of manufacturers exiting the industry has begun to outpace the number entering (48). One explanation for decreased entry to specific markets is that the generic drug industry is composed primarily of relatively small companies with limited drug portfolios, each of which on average brings modest revenue (48). New manufacturers may find it difficult to enter a market given the modest revenue associated with each drug product, in addition to the start-up time and cost for approval. In certain scenarios, particularly for drugs in mature markets with a large number of competitors, as the price of the drug product approaches its marginal cost, generic manufacturers make a calculated decision to exit the market (49). As the competition decreases in these markets, the remaining manufacturers can dramatically raise the price of drugs.
The most notable examples of price increases have been among drugs in monopolistic or duopolistic markets, including pyrimethamine (17), nitroprusside (16), isoproterenol (16), and albendazole (50). Drugs with such limited competition are vulnerable to being acquired by a new company that can then dramatically raise the price of the drug. The price of repository corticotropin, for example, used to treat infantile spasms, multiple sclerosis exacerbations, and various autoimmune states, increased from $1650 to over $24,000 per 5-mL vial when it was acquired by Questcor in 2001, and increased again to $34,034 after being acquired a second time by Mallinckrodt in 2014 (51). There is evidence to suggest that this practice may be more widespread (52) and that companies may be targeting old, essential medicines that are particularly susceptible (53). In most cases, acquisitions do not offer valuable drug modifications and provide no additional benefit to patients. Additional entrants into suddenly more lucrative markets may be dissuaded by the threat that the initial manufacturer will drop the price of its product. Drug products with few competitors are also more susceptible to shortages, which can lead to abrupt price increases, particularly among injectable drugs (54, 55).
Some drugs remain expensive despite generic competition, often due to insufficient generic substitution for the brand-name drug. For example, after the patent expiration of the cholesterol-lowering drug Lipitor (atorvastatin) and the availability of a generic version, continued prescription of Lipitor resulted in $2.1 billion in extra spending (56), likely due to both brand-name drug coupon promotion and arrangements made between pharmacy benefit managers and the brand-name drug manufacturer (57). Recent analyses found that Medicare Part D could realize sizeable savings through greater promotion of generic substitution (19, 58). Another report found that Medicare could have saved nearly $1 billion in 2016 had generic constituents of brand-name combination drug products been substituted (59). Even when generics are prescribed, until recently pharmacy contracts with insurers and pharmacy benefit managers sometimes included a “gag clause” prohibiting pharmacists from telling patients that a drug might be cheaper if paid for directly, without insurance, as opposed to through the insurance co-pay. For example, despite being offered through Medicare prescription drug plans, many generic drug products are cheaper through commercial generic drug discount programs like Walmart’s $4 drug program (60).
As noted above, brand-name drug manufacturers also produce drug coupons that lower patient cost-sharing for brand-name drug products beyond that of generic alternatives, thereby undermining the tiered-formulary system used by insurers to promote the use of cheaper generic versions (61, 62). Though coupons make drug products more affordable for patients in the short-term through lower out-of-pocket costs, system-wide spending increases because insurers must still pay the cost of the brand-name drug to the manufacturer. Moreover, insurers may ultimately pass on the increased costs to the patients through higher premiums.
ROLE OF FDA AND REVIEW PRIORITIZATION
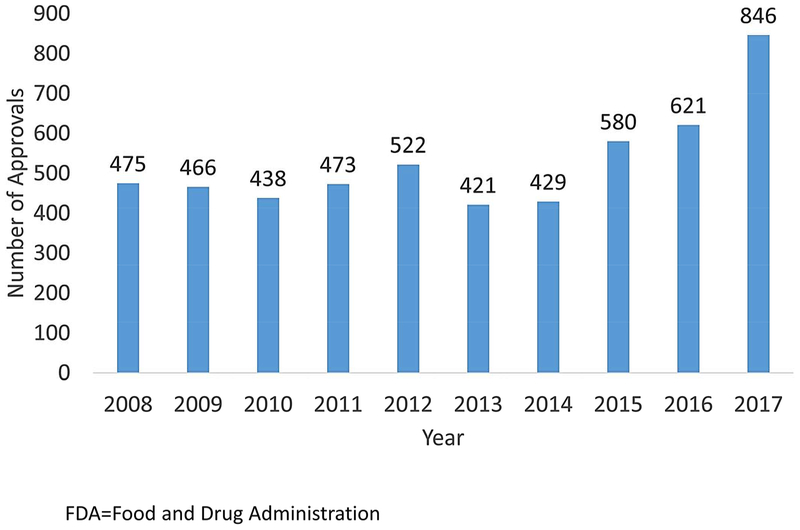
The time and cost of FDA approval of generic drug applications can be an additional barrier to generic entry into drug markets. Due to under resourcing (63), prior to 2013 the FDA accumulated a large backlog of generic drug applications and review times increased to over 3 years, a long delay in drug approval. In 2012, Congress passed the Generic Drug User Fee Amendments, also known as GDUFA I, as part of the Food and Drug Administration Safety and Innovation Act, requiring the generic drug industry to pay $300 million per year for five years to the FDA. This legislation helped to nearly eliminate the majority of the backlog by the end of 2017 and decrease application review times to less than one year. Moreover, 2017 saw the largest number of generic drug approvals, of which 80 were first generics for brand-name drugs (64, 65) (Figure 2).
Figure 2.
FDA Generic Drug Approvals by Year
Though part of the delay in generic drug approval is due to the speed of the FDA, inadequate applications submitted by generic companies are also part of the problem. According to the FDA’s estimate, less than 10% of generic drug applications are approved in the first review cycle, compared to 90% of new drug applications for brand-name drugs (66). On average, in late 2017 generic drug applications required four review cycles for approval, often due to the applicant providing insufficient scientific evidence to support approval. Multiple review cycles further delay the availability of generic drugs, though the number of cycles needed for approval seems to be declining (67).
In 2017, as part of the Food and Drug Administration Reauthorization Act, GDUFA was reauthorized for an additional 5 years, known as GDUFA II. Instead of requiring flat fees for all generic manufacturers, which favored large companies with multiple drug portfolios, GDUFA II introduced a tiered system in which the fees differed based on the number of drug portfolios held by the generic company, with the aim of reducing the barrier to entry for smaller companies. GDUFA II also set a goal of completing application reviews within eight months if no preapproval facility inspection was required and ten months if an inspection was deemed necessary. In 2017 the FDA also announced a Drug Competition Action Plan to streamline the generic drug approval process to address the issue of multiple review cycles and to prevent “gaming” of the system by brand-name manufacturers that impedes generic competition, such as by making it difficult for generic manufacturers to purchase drug samples to conduct bioequivalence studies (68, 69). The Action Plan also specifically aims to update the FDA’s scientific and regulatory capabilities to assess the bioequivalence of complex drugs, such as products that are difficult to measure in the blood or act locally on an organ (69).
The Food and Drug Administration Reauthorization Act also authorized the Office of Generic Drugs to expedite the review of generic drug applications for products with fewer than three competitors to help attract additional manufacturers to non-competitive drug markets (70, 71). In addition, the act created a new pathway for generic drug approval called the Competitive Generic Therapy (CGT) designation (72). A drug is eligible for this designation if the FDA determines there is “inadequate generic competition,” meaning that there is only one other approved, marketed drug product. As part of the pathway, the FDA will provide additional resources and advising to the applicant throughout the approval process. If approved, these CGT-designated drugs are awarded 180 days of exclusivity, a period in which no other drugs can be marketed, if the applicant begins to market the drug within 75 days of approval. Furthermore, in an effort to improve transparency and promote the competitiveness of monopolistic markets, the FDA published a publicly available list of off-patent drugs that have been approved for more than one year but still lack any generic competition (18). The FDA plans to update this list every six months.
These measures are encouraging though their effect is not yet clear. The FDA continues to approve generic drug applications at a higher rate than before, but whether this is due to the additional resources now available to the agency or because of incentives such as prioritization of review remains uncertain and requires additional investigation. The larger reason that certain markets contain few generic competitors is because of limited demand for the drug product and thus lower potential profits. Increasing the speed with which generic drug applications are approved by the FDA is a necessary step in promoting generic competition but it is not enough. Additional solutions are needed to help spur competition and thereby reduce prices of and shortages among generic drugs.
ADDITIONAL POLICY SOLUTIONS AND AREAS FOR RESEARCH
For drug markets with few competitors and limited demand to attract greater competition, one strategy could be the development of a non-profit generic drug manufacturer with the clear aim of providing a stable supply of affordable medicines (73). For example, a collaboration of Intermountain Healthcare, Trinity Health, SSM Health, and Ascension, together with the Department of Veteran Affairs, is forming a non-profit generic drug company called Project Rx that will either manufacturer generic drugs or sub-contract with other organizations (74). Such a non-profit manufacturer could rely on purchasing agreements that set a predetermined price and minimum volume to ensure stable demand and to prevent being driven out of the market by existing for-profit manufacturers that suddenly decrease the drug’s price. A similar arrangement could be led by the federal government through bulk purchasing of single-source drugs at a negotiated price in situations where drugs face dramatic price increases. Long-term contracts with the government ensuring stable demand could also be used to incentivize additional manufacturers to enter these markets.
Another strategy to increase competition for off-patent drugs with few U.S. versions is to import additional generic versions that have not been approved in the U.S. but have been approved in other countries with comparable regulatory approval standards and requirements. The FDA recently announced plans to create a working group to examine how to safely import drugs when there is a sharp price increase of an off-patent, single-source drug (75). Imported drugs are already widely used in the U.S., with drugs manufactured outside the U.S. constituting approximately one-quarter of the U.S. pharmaceutical market (76). One way that the FDA could accelerate the importation of generic drugs is through a mechanism known as reciprocal approval, in which the FDA would issue its approval of a drug based on the evidence of the drug’s prior approval by another stringent national regulatory authority. Such a system could incentivize the entry of previously non-FDA-approved generic versions of drugs manufactured by companies that may have otherwise been deterred by the cost of additional approval by the FDA (77). A recent analysis suggests that almost half of off-patent drugs approved by the FDA since 1939 with limited generic competition in the U.S. could reach four or more generic competitors through a system of reciprocal approval (78). Such a system would preclude the need for a manufacturer to newly enter the market by obtaining FDA approval and building new manufacturing capabilities. Further research is needed to better understand the ability of non-U.S. manufacturers to handle the increased demand that such a system would create, along with the price points of the drugs outside the U.S.
The FDA will also need to continue to work closely with the FTC to address anticompetitive mergers and acquisitions that lead to increasing industry concentration, as it did when Teva acquired Allergen’s generic business (47). The two agencies will also have to carefully monitor the acquisitions of small generic companies and individual off-patent drugs in monopolistic or duopolistic markets that face sudden price increases. The FDA and FTC recently held a joint public meeting to discuss shared efforts in addressing rising generic drug prices (79).
Furthermore, when there are no approved generic versions for off-patent drugs, promoting the substitution of in-class generic drugs, known as therapeutic substitution, could be one potential solution. One study found that between 2010 and 2012, more than $73 billion was spent on brand-name drugs when there was no FDA-approved generic, but a within-class generic was available (80). Where clinically appropriate, such therapeutic substitution could result in substantial savings for patients and for the health system. Moreover, allowing for therapeutic substitution of the generic constituents of brand-name combination drugs could also generate savings. As most states have enacted pharmacy-level generic substitution laws to promote generic drug use when available, additional research is needed to further understand when therapeutic substitutions are safe, beneficial, and feasibly implementable, based on patient and clinician preferences, as well as outcomes.
Addressing the anticompetitive strategies utilized by brand-name manufacturers to hamper the approval of generic competitors is also essential. An initial step is to require manufacturers to provide drug samples to generic manufacturers to conduct bioequivalence steps. More efficient mechanisms are also needed to address trivial citizen petitions filed by brand-name manufacturers that exhaust resources and also delay generic approval. Finally, given the rise of inconsequential secondary patents filed by brand-name manufacturers, the use of inter partes review could help allow for quicker and less expensive patent challenges by providing an alternative to court litigation (81). Inter partes review is a mechanism through which any U.S. citizen can challenge a patent’s validity through an administrative body called the Patent Trial and Appeal Board.
States have increasingly crafted legislation to control drug prices through various mechanisms, including greater drug price transparency, reform of pharmacy benefit managers’ practices, drug importation from outside the U.S., and Medicaid spending caps. For example, California, Connecticut, and Vermont have passed legislation requiring companies to justify price increases beyond a certain threshold (82–84). California also prohibits the use of drug manufacturer drug coupons if it makes a drug cheaper than another drug version covered by the patient’s insurance (85). Maryland passed a similar transparency law that required price increase justifications and also allowed for the prosecution of companies that engaged in “price gouging” (86), though the law was ultimately overturned based on the commerce clause. Vermont has also passed a law allowing for the wholesale importation of drugs from Canada (87). New York’s Fiscal Year 2018 budget includes a cap provision on Medicaid’s drug spending (88). If the spending cap is surpassed, the state can negotiate supplemental rebates with drug manufacturers to lower the spending.
Furthermore, the Know the Lowest Price Act of 2018 was recently signed into law, which prohibits the pharmacy “gag clause” that restricts pharmacists from informing patients if a drug is cheaper if not paid for through insurance. However, this applies only if the patient directly asks the pharmacist about pricing. Many states are continuing to work on their own legislation pertaining to the pharmacy “gag clause”. The recently released blueprint to lower drug prices by the current administration also calls for greater price transparency and for considering changes to the regulation of drug copay discount cards (89), though some observers report that the plan offers incomplete solutions to rising generic drug prices (90).
CONCLUSION
To ensure continued patient access to affordable medications, particularly once the period of patent and exclusivity protections has ended, a deeper understanding of the strategies used by brand-name manufacturers to undermine generic competition and the reasons underlying the price increases of off-patent drugs is needed. Ensuring the availability of affordable medications is a complex problem requiring multiple solutions and the joint efforts of multiple stakeholders. The FDA in particular continues to play a unique role in guaranteeing the timely approval of life-saving brand-name and generic medications, especially in markets that lack sufficient competition. Building on initiatives currently being led by the FDA, other branches of the federal government, and multiple states, policymakers must utilize a variety of policy solutions to directly address continued price increases and shortages of generic drugs.
Acknowledgments
Funding: No funding was received for this work.
Potential conflicts of interest: Dr. Gupta was previously supported by the Yale University-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI). In the past 36 months, Dr. Shah has received research support through Mayo Clinic from the Food and Drug Administration to establish Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Agency for Healthcare Research and Quality (R01HS025164; R01HS025402; R03HS025517; U19HS024075), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R56HL130496; R01HL131535), National Science Foundation, and from the Patient Centered Outcomes Research Institute (PCORI) to develop a Clinical Data Research Network (LHSNet). In the past 36 months, Dr. Ross has received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from Medtronic, Inc. and the Food and Drug Administration (FDA) to develop methods for postmarket surveillance of medical devices (U01FD004585), from the Food and Drug Administration to establish Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Blue Cross Blue Shield Association to better understand medical technology evaluation, from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, from the Agency for Healthcare Research and Quality to examine community predictors of healthcare quality (R01HS022882), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International and to establish the Collaboration for Research Integrity and Transparency (CRIT) at Yale.
REFERENCES
- (1).Kirzinger A, Wu B & Brodie M Kaiser Health Tracking Poll: September 2016. <https://www.kff.org/health-costs/report/kaiser-health-tracking-poll-september-2016/> (2016). Accessed July 1, 2018.
- (2).Iqvia Institute for Human Data Science. Medicines Use and Spending in the U.S <https://www.iqvia.com/institute/reports/medicines-use-and-spending-in-the-us-a-review-of-2016/> (2017). Accessed July 1, 2018.
- (3).Papanicolas I, Woskie LR & Jha AK Health Care Spending in the United States and Other High-Income Countries. JAMA 319, 1024–39 (2018). [DOI] [PubMed] [Google Scholar]
- (4).Association for Accessible Medications. Generic drug access & savings in the U.S (2017). Accessed July 1, 2018.
- (5).US Food and Drug Administration. Generic competition and drug prices. <https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm129385.htm/>. Accessed July 1, 2018.
- (6).Dave CV, Hartzema A & Kesselheim AS Prices of Generic Drugs Associated with Numbers of Manufacturers. N Engl J Med 377, 2597–8 (2017). [DOI] [PubMed] [Google Scholar]
- (7).Generic Pharmaceutical Association. Generic Drugs Continue to Deliver Billions in Savings to the U.S. Healthcare System, New Report Finds 2016. <http://www.prnewswire.com/news-releases/generic-drugs-continue-to-deliver-billions-in-savings-to-the-us-healthcare-system-new-report-finds-300347698.html/>. Accessed July 1, 2018.
- (8).Frank RG The ongoing regulation of generic drugs. N Engl J Med 357, 1993–6 (2007). [DOI] [PubMed] [Google Scholar]
- (9).U.S. Government Accountability Office. Drug Pricing: Research on Savings from Generic Drug Use. <https://www.gao.gov/assets/590/588064.pdf> (2012). Accessed July 1, 2018.
- (10).Shrank WH et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med 166, 332–7 (2006). [DOI] [PubMed] [Google Scholar]
- (11).Barbui C & Conti V Adherence to generic v. brand antidepressant treatment and the key role of health system factors. Epidemiol Psychiatr Sci 24, 23–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gagne JJ et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med 161, 400–7 (2014). [DOI] [PubMed] [Google Scholar]
- (13).U.S. Government Accountability Office. Part D Generic Drug Prices Declined Overall But Some Had Extraordinary Price Increases 2016. <http://www.gao.gov/assets/680/679022.pdf> (2016). Accessed July 1, 2018.
- (14).U.S. Government Accountability Office. Drug shortages: public health threat continues, despite efforts to help ensure product availability. <https://www.gao.gov/assets/670/660785.pdf> (2014). Accessed July 1, 2018.
- (15).U.S. Food and Drug Administration. Report on Drug Shortages for Calendar Year 2016 <https://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM561290.pdfJ> (2017). Accessed July 1, 2018.
- (16).Khot UN, Vogan ED & Militello MA Nitroprusside and Isoproterenol Use after Major Price Increases. N Engl J Med 377, 594–5 (2017). [DOI] [PubMed] [Google Scholar]
- (17).Pollack A Drug Goes From $13.50 a Tablet to $750, Overnight. <https://www.nytimes.com/2015/09/21/business/a-huge-overnight-increase-in-a-drugs-price-raises-protests.html/> (2015). Accessed July 1, 2018.
- (18).U.S. Food and Drug Administration. List of Off-Patent, Off-Exclusivity Drugs without an Approved Generic. <https://www.fda.gov/downloads/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingGenericDrugs/UCM564441.pdf> (2018). Accessed July 1, 2018.
- (19).Office of the Assistance Secretary for Planning and Evaluation. Data point: Savings available under full generic substitution of multiple source brand drugs in Medicare Part D. <https://aspe.hhs.gov/system/files/pdf/259326/DP-Multisource-Brands-in-Part-D.pdf> (2018). Accessed August 1, 2018.
- (20).Manzoli L et al. Generic versus brand-name drugs used in cardiovascular diseases. Eur J Epidemiol 31, 351–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kesselheim AS et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs 70, 605–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kesselheim AS et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA 300, 2514–26 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gagne JJ et al. Comparative effectiveness of generic versus brand-name antiepileptic medications. Epilepsy Behav 52, 14–8 (2015). [DOI] [PubMed] [Google Scholar]
- (24).Gagne JJ et al. Patterns and predictors of generic narrow therapeutic index drug use among older adults. J Am Geriatr Soc 61, 1586–91 (2013). [DOI] [PubMed] [Google Scholar]
- (25).Shaw SJ & Hartman AL The Controversy over Generic Antiepileptic Drugs. J Pediatr Pharmacol Ther 15, 81–93 (2010). [PMC free article] [PubMed] [Google Scholar]
- (26).Makus KG & McCormick J Identification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsy. Clin Ther 29, 334–41 (2007). [DOI] [PubMed] [Google Scholar]
- (27).Berg M et al. Bioequivalence Between Generic and Branded Lamotrigine in People With Epilepsy: The EQUIGEN Randomized Clinical Trial. JAMA Neurol 74, 919–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dong BJ et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA 277, 1205–13 (1997). [PubMed] [Google Scholar]
- (29).Panattoni LE The effect of Paragraph IV decisions and generic entry before patent expiration on brand pharmaceutical firms. J Health Econ 30, 126–45 (2011). [DOI] [PubMed] [Google Scholar]
- (30).Reiffen D & Ward MR Generic drug industry dynamics. The Review of Economics and Statistics 87, 37–49 (2005). [Google Scholar]
- (31).Luo J, Seeger JD, Donneyong M, Gagne JJ, Avorn J & Kesselheim AS Effect of Generic Competition on Atorvastatin Prescribing and Patients’ Out-of-Pocket Spending. JAMA Intern Med 176, 1317–23 (2016). [DOI] [PubMed] [Google Scholar]
- (32).IMS Institute for Healthcare Informatics. Price Declines after Branded Medicines Lose Exclusivity in the U.S <https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/price-declines-after-branded-medicines-lose-exclusivity-in-the-us.pdf?la=en&hash=642B9A40F3F176CE93E8E9F791EE2BE4975C8580/> (2016). Accessed July 1, 2018.
- (33).Berndt ER, Mortimer R, Bhattacharjya A, Parece A & Tuttle E Authorized generic drugs, price competition, and consumers’ welfare. Health Aff (Millwood) 26, 790–9 (2007). [DOI] [PubMed] [Google Scholar]
- (34).Gupta R, Kesselheim AS, Downing N, Greene J & Ross JS Generic Drug Approvals Since the 1984 Hatch-Waxman Act. JAMA Intern Med 176, 1391–3 (2016). [DOI] [PubMed] [Google Scholar]
- (35).Greene J Generic: The Unbranding of Modern Medicine (Johns Hopkins University Press: 2014). [Google Scholar]
- (36).Downing NS, Ross JS, Jackevicius CA & Krumholz HM Avoidance of generic competition by Abbott Laboratories’ fenofibrate franchise. Arch Intern Med 172, 724–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kapczynski A, Park C & Sampat B Polymorphs and prodrugs and salts (oh my!): an empirical analysis of “secondary” pharmaceutical patents. PLoS One 7, e49470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hemphill CS & Sampat BN Evergreening, patent challenges, and effective market life in pharmaceuticals. J Health Econ 31, 327–39 (2012). [DOI] [PubMed] [Google Scholar]
- (39).Shcherbakova N, Shepherd M, Lawson K & Richards K The role of authorized generics in the prescription drug marketplace. Journal of Generic Medicines 8, 28–40 (2011). [Google Scholar]
- (40).Feldman R & Wang C A Citizen’s Pathway Gone Astray - Delaying Competition from Generic Drugs. N Engl J Med 376, 1499–501 (2017). [DOI] [PubMed] [Google Scholar]
- (41).Carrier MA & Wander D Citizen Petitions: An Empirical Study. <https://www.fdanews.com/ext/resources/files/archives/0/06/06-18-12-CitizenPetitionsStudy.pdf> (2012). Accessed July 1, 2018.
- (42).U.S. Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on new policies to reduce the ability of brand drug makers to use REMS programs as a way to block timely generic drug entry, helping promote competition and access. <https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm609365.htm/> (2018). Accessed July 1, 2018.
- (43).Sarpatwari A, Avorn J & Kesselheim AS Using a drug-safety tool to prevent competition. N Engl J Med 370, 1476–8 (2014). [DOI] [PubMed] [Google Scholar]
- (44).Grabowski HG, Kyle M, Mortimer R, Long G & Kirson N Evolving brand-name and generic drug competition may warrant a revision of the Hatch-Waxman Act. Health Aff (Millwood) 30, 2157–66 (2011). [DOI] [PubMed] [Google Scholar]
- (45).Morton FM Entry decisions in the generic pharmaceutical industry. Rand J Econ 30, 421–40 (1999). [PubMed] [Google Scholar]
- (46).U.S. Government Accountability Office. Profits, Research and Development Spending, and Merger and Acquisition Deals. <https://www.gao.gov/assets/690/688472.pdf> (2017). Accessed July 1, 2018.
- (47).Federal Trade Commission. FTC Requires Teva to Divest Over 75 Generic Drugs to Settle Competition Concerns Related to its Acquisition of Allergan’s Generic Business. <https://www.ftc.gov/news-events/press-releases/2016/07/ftc-requires-teva-divest-over-75-generic-drugs-rival-firms-settle/> (2016). Accessed July 1, 2018.
- (48).Berndt ER, Conti RM & Murphy SJ The Landscape of US Generic Prescription Drug Markets, 2004–2016. <http://www.nber.org/papers/w23640/> (2017). Accessed July 1, 2018.
- (49).Wiske CP, Ogbechie OA & Schulman KA Options to Promote Competitive Generics Markets in the United States. JAMA 314, 2129–30 (2015). [DOI] [PubMed] [Google Scholar]
- (50).Kesselheim AS, Alpern JD & Stauffer WM High-cost generic drugs--implications for patients and policymakers. N Engl J Med 372, 686 (2015). [DOI] [PubMed] [Google Scholar]
- (51).Shakil S & Redberg RF New (Very High) Prices on Old Drugs. JAMA Intern Med 177, 1568 (2017). [DOI] [PubMed] [Google Scholar]
- (52).Gupta R, Henkel A, Forman HP & Ross JS The Impact of Off-Patent Drug Acquisitions on Prices. J Gen Intern Med 33, 1007–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Alpern JD, Song J & Stauffer WM Essential Medicines in the United States--Why Access Is Diminishing. N Engl J Med 374, 1904–7 (2016). [DOI] [PubMed] [Google Scholar]
- (54).Davies BJ, Hwang TJ & Kesselheim AS Ensuring Access to Injectable Generic Drugs - The Case of Intravesical BCG for Bladder Cancer. N Engl J Med 376, 1401–3 (2017). [DOI] [PubMed] [Google Scholar]
- (55).Woodcock J & Wosinska M Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther 93, 170–6 (2013). [DOI] [PubMed] [Google Scholar]
- (56).Warraich HJ, Salami JA, Khera R, Valero-Elizondo J, Okunrintemi V & Nasir K Trends in Use and Expenditures of Brand-name Atorvastatin After Introduction of Generic Atorvastatin. JAMA Intern Med 178, 719–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Jackevicius CA, Chou MM, Ross JS, Shah ND & Krumholz HM Generic atorvastatin and health care costs. N Engl J Med 366, 201–4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Egilman AC, Wallach JD, Ross JS & Dhruva SS Medicare Spending and Potential Savings on Brand-Name Drugs With Available Generic Substitutes Excluded by 2 Large Pharmacy Benefit Managers, 2012 Through 2015. JAMA Intern Med 178, 567–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Sacks CA, Lee CC, Kesselheim AS & Avorn J Medicare Spending on Brand-name Combination Medications vs Their Generic Constituents. JAMA 320, 650–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Liu P, Dhruva SS, Shah ND & Ross JS Medicare Beneficiary Out-of-Pocket Costs for Generic Cardiovascular Medications Available Through $4 Generic Drug Discount Programs. Ann Intern Med, (2018). [DOI] [PubMed] [Google Scholar]
- (61).Ross JS & Kesselheim AS Prescription-drug coupons--no such thing as a free lunch. N Engl J Med 369, 1188–9 (2013). [DOI] [PubMed] [Google Scholar]
- (62).U.S. Government Accountability Office. Data on Coupon Discounts Needed to Evaluate Methodology for Setting Drug Payment Rates. <https://www.gao.gov/assets/680/678690.pdf> (2016). Accessed July 1, 2018.
- (63).U.S. Food and Drug Administration. Implementation of the Generic Drug User Fee Amendments of 2012 (GDUFA). <https://www.fda.gov/newsevents/testimony/ucm484304.htm/> (2016). Accessed July 1, 2018.
- (64).Uhl KC 2017 Was Another Record-Setting Year for Generic Drugs. <https://blogs.fda.gov/fdavoice/index.php/2018/02/2017-was-another-record-setting-year-for-generic-drugs/> (2018). Accessed July 1, 2018.
- (65).U.S. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. <https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm/> (2018). Accessed July 1, 2018.
- (66).Brennan Z FDA Targets Multiple Review Cycles With New Draft Guidance, MAPP. <https://www.raps.org/regulatory-focus%E2%84%A2/news-articles/2018/1/fda-targets-multiple-review-cycles-with-new-draft-guidance,-mapp/> (2018). Accessed July 1, 2018.
- (67).U.S. Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D. on new steps to facilitate efficient generic drug review to enhance competition, promote access and lower drug prices. <https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm591184.htm/> (2018). Accessed July 1, 2018.
- (68).Office of Generic Drugs and Office of Pharmaceutical Quality. Good Abbreviated New Drug Application Assessment Practices. <https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ManualofPoliciesProcedures/UCM591143.pdf> (2018). Accessed July 1, 2018.
- (69).Gottlieb S Opening Remarks for Part 15 Public Meeting on Generic Drug Competition. <https://www.fda.gov/newsevents/speeches/ucm567323.htm> (2017). Accessed July 1, 2018.
- (70).Gupta R, Kesselheim AS & Ross JS Prioritization of Generic Drug Review-Reply. JAMA Intern Med 177, 141–2 (2017). [DOI] [PubMed] [Google Scholar]
- (71).U.S. Food and Drug Administration. Prioritization of the Review of Original ANDAs, Amendments, and Supplements. <https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ManualofPoliciesProcedures/UCM407849.pdf> (2017). Accessed July 1, 2018.
- (72).House - Energy and Commerce. H.R.2430 - FDA Reauthorization Act of 2017. <https://www.congress.gov/bill/115th-congress/house-bill/2430/> (2017). Accessed July 1, 2018.
- (73).Liljenquist D, Bai G & Anderson GF Addressing Generic-Drug Market Failures - The Case for Establishing a Nonprofit Manufacturer. N Engl J Med 378, 1857–9 (2018). [DOI] [PubMed] [Google Scholar]
- (74).Kodjak A Hospitals Prepare To Launch Their Own Drug Company To Fight High Prices And Shortages. <https://www.npr.org/sections/health-shots/2018/09/06/644935958/hospitals-prepare-to-launch-their-own-drug-company-to-fight-high-prices-and-shor/> (2018). Accessed 10/19/18.
- (75).Gottlieb S Statement by FDA Commissioner Scott Gottlieb, M.D., on the formation of a new work group to develop focused drug importation policy options to address access challenges related to certain sole-source medicines with limited patient availability, but no blocking patents or exclusivities. <https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm613931.htm/> (2018). Accessed August 1, 2018.
- (76).International Trade Administration U.S. Department of Service. 2016 Top Markets Report Pharmaceuticals. <https://www.trade.gov/topmarkets/pdf/Pharmaceuticals_Executive_Summary.pdf> (2016). Accessed July 1, 2018.
- (77).Bollyky TJ & Kesselheim AS. Can drug importation address high generic drug prices? <www.brookings.edu/research/can-drug-importation-address-high-generic-drug-prices/> (2017). Accessed July 1, 2018.
- (78).Gupta R, Bollyky TJ, Cohen M, Ross JS & Kesselheim AS Affordability and availability of off-patent drugs in the United States-the case for importing from abroad: observational study. BMJ 360, k831 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Federal Trade Commission. Understanding Competition in Prescription Drug Markets: Entry and Supply Chain Dynamics. <https://www.ftc.gov/news-events/events-calendar/2017/11/understanding-competition-prescription-drug-markets-entry-supply/> (2017). Accessed July 1, 2018.
- (80).Johansen ME & Richardson C Estimation of Potential Savings Through Therapeutic Substitution. JAMA Intern Med 176, 769–75 (2016). [DOI] [PubMed] [Google Scholar]
- (81).Darrow JJ, Beall RF & Kesselheim AS Will inter partes review speed US generic drug entry? Nat Biotechnol 35, 1139–41 (2017). [DOI] [PubMed] [Google Scholar]
- (82).Armental M California Governor Signs Bill Requiring Greater Drug Price Transparency. <https://www.wsj.com/articles/california-governor-signs-bill-requiring-greater-drug-price-transparency-1507588211/> (2017). Accessed July 1, 2018.
- (83).Silverman E Vermont becomes first state to require drug makers to justify price hikes. <https://www.statnews.com/pharmalot/2016/06/06/vermont-drug-prices-transparency/> (2016). Accessed July 1, 2018.
- (84).Kirschenbaum AM Connecticut Becomes Seventh State to Enact Drug Price Transparency Law. <http://www.fdalawblog.net/2018/06/connecticut-becomes-seventh-state-to-enact-drug-price-transparency-law/> (2018). Accessed July 1, 2018.
- (85).AB-265 Prescription drugs: prohibition on price discount. <https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201720180AB265/> (2017). Accessed July 1, 2018.
- (86).Greene JA & Padula WV Targeting Unconscionable Prescription-Drug Prices - Maryland’s Anti-Price-Gouging Law. N Engl J Med 377, 101–3 (2017). [DOI] [PubMed] [Google Scholar]
- (87).Schlanger SJ & Kirschenbaum AM New Vermont Law Seeks to Allow Wholesale Importation of Drugs from Canada. <http://www.fdalawblog.net/2018/05/new-vermont-law-seeks-to-allow-wholesale-importation-of-drugs-from-canada/> (2018). Accessed July 1, 2018.
- (88).State of New York. Senate Assembly. <https://legislation.nysenate.gov/pdf/bills/2017/S2007B/> (2017). Accessed July 1, 2018.
- (89).Department of Health and Human Services. American Patients First. <https://www.hhs.gov/sites/default/files/AmericanPatientsFirst.pdf> (2018). Accessed July 1, 2018.
- (90).Sarpatwari A, Avorn J & Kesselheim AS An Incomplete Prescription: President Trump’s Plan to Address High Drug Prices. JAMA 319, 2373–4 (2018). [DOI] [PubMed] [Google Scholar]