Abstract
Objective:
Increased arterial stiffness measured by pulse wave velocity (PWV) has been shown to be an important parameter of cardiovascular risk. Longitudinal development of PWV from youth to early adulthood and its possible sociodemographic, anthropometric, hemodynamic and behavioral moderators will be illustrated.
Methods:
Individual growth curves of carotid–distal PWV across age were created for 559 African American and European American men and women with a maximum of five assessments over an average of 7-year follow-up (mean age at participants’ first assessment, 22.3 ± 3.4).
Results:
African Americans and men had significantly higher PWV than did European Americans and women (Ps < 0.01), respectively. A three-way interaction (P < 0.001) between age, sex and ethnicity was observed with African American men displaying a larger rate of increase in PWV with age than the other three ethnic and sex groups. The ethnicity and sex effects on PWV persisted when controlling for other moderators. Waist circumference was the strongest anthropometric predictor but its effect on PWV was only significant in women. Mean arterial pressure was the strongest hemodynamic predictor, marital status of parents was the strongest socioeconomic predictor and marijuana use was the strongest behavioral predictor of PWV. The best-fitting full model explained in total 59.4% of the between-subject variance in PWV with ethnicity, sex and age explaining 25.6%.
Conclusion:
We observed significant ethnic and sex differences in longitudinal trajectories of PWV in youth and young adults. In addition, individual differences in PWV growth can largely be explained by mean arterial pressure, waist, marital status of parents and marijuana use.
Keywords: African Americans, hemodynamics, longitudinal, pulse wave velocity, socioeconomic factors
INTRODUCTION
Increased arterial stiffness measured by pulse wave velocity (PWV) is a solid index of cardiovascular risk and an independent predictor of cardiovascular events and mortality in patients with hypertension [1], diabetes [2], or end-stage renal disease [3] as well as in general populations [4]. As the pathogenesis of cardiovascular disease has its origin in youth [5], moderators of PWV development need to be evaluated during this period. Moreover, it is important to increase our understanding of PWV development in different ethnicities, especially in African Americans [6], as increased PWV may contribute to the much higher prevalence of cardiovascular events and all-cause mortality in this population [4,7].
PWV is known to increase with age and height in children and adolescents [8,9]. Cross-sectional pediatric and adult studies have shown greater PWV in men and African Americans compared with women and European Americans [10–12], respectively, and our previous studies and other studies have shown positive association of PWV with anthropometric (general and central obesity) and hemodynamic variables (blood pressure and heart rate) in youth and young adults [9,13,14]. There is also some evidence that lower socioeconomic status (SES) [15,16] and use of illicit drugs such as cocaine and marijuana are associated with higher PWV [17,18]. However, it is not clear to what extent these variables account for PWV variability over time, especially in the transition period from youth to young adulthood.
Longitudinal studies on PWV progression in adults and the elderly have been reported [19–26]. The majority of these studies measured PWV at baseline and one follow-up, which does not provide a sufficient basis for studying PWV development with age. In the only study [21] reported with two to nine measurements of PWV collected across 25 years, a steeper increase of PWV with advancing age in men than women was observed. On the other hand, essentially no longitudinal studies on PWV growth and moderators in youth and young adults have been reported.
The Georgia Stress and Heart Study, a prospective cohort study with repeated measures of PWV in youth and young adults, provides an excellent opportunity to examine the pattern and the rate of longitudinal change in PWV within and among individuals. To the best of our knowledge, this is the first study to explore the development of PWV from adolescent through early adulthood in a multiethnic cohort of individuals. Furthermore, this study assesses the effects of social–demographic, anthropometric, hemodynamic, and lifestyle factors on PWV level and changes over time.
METHODS
Participants
The participants were from the Georgia Stress and Heart (GSH) study, an ongoing longitudinal study designed to evaluate the development of cardiovascular risk factors from childhood to adulthood, with evaluations conducted annually from 1989 to 2000 (visits 1–10), every 1.5 years from 2000 to 2006 (visits 11–14), and every 2 years from 2008 to 2012 (visits 15–16). Recruitment and evaluation of participants have been described in detail elsewhere [27]. Briefly, participants who met the following criteria were recruited: aged 5–16 years in 1989, African or European ancestry, normotensive for age and sex based on BP screening, and apparently healthy based on parental reports of the child’s medical history[28]. All participants were recruited using family health history questionnaires obtained from a county-wide (Richmond County, Georgia) public school screening of children in kindergarten through grade 8 whose families were interested in health research. A high participation rate was obtained, with 96.3% of those contacted agreeing to participate.
This is a sub-study of GSH initiated at visit 12 when PWV measurements were started to be performed. Mean ± SD of participants’ characteristics at their first visit with PWV assessment were shown in Table 1. As not all individuals participated each consecutive year, the data set is a complicated one, with not all participants having the same number of visits. As presented in Table 1, in the entire sample 83.9% had at least two visits and 61.4% had at least three visits, making this data set very informative for the study of PWV changes over time. In total, this study involved 559 participants who yielded 1601 PWV measurements. The median follow-up time was 6.78 years with an interquartile range of 4.48 years. The Institutional Review Board at the Medical College of Georgia gave approval for the study. Informed consent was provided by all participants, or by parents if participants were less than 18 years.
TABLE 1.
General characteristics of participants’ first visit
| Characteristics | Men | Women | ||
|---|---|---|---|---|
| EA | AA | EA | AA 1 | |
| Demographics | ||||
| No. of participants | 148 | 119 | 128 | 164 |
| Age (years) | 21.8 ± 3.5 | 22.8 ± 3.7 | 21.9 ± 3.0 | 22.7 ± 3.5 |
| Participants with at least two visits (%) | 125 (84.5%) | 99 (83.2%) | 105 (82.0%) | 140 (85.4%) |
| Participants with at least three visits (%) | 91 (61.5%) | 73 (61.3%) | 76 (59.4%) | 103 (62.8%) |
| Participants with at least four visits (%) | 53 (35.8%) | 38 (31.9%) | 43 (33.6%) | 43 (26.2%) |
| PWV | 7.83 ± 0.86 | 8.00±1.03 | 7.48 ± 0.84 | 8.10 ± 0.99 |
| Anthropometric measures | ||||
| Height (cm) | 178.3 ± 7.7 | 176.9 ± 5.9 | 164.8 ± 5.9 | 164.7 ± 6.8 |
| Weight (kg) | 81.4 ± 18.7 | 88.8 ± 23.2 | 74.7 ± 21.7 | 82.2 ± 25.4 |
| BMI (kg/m2) | 25.5 ± 5.0 | 28.3 ± 7.2 | 27.5 ± 8.2 | 30.3 ± 9.2 |
| Waist circumference (cm) | 88.6 ± 13.3 | 90.3 ± 17.5 | 86.8 ± 16.5 | 91.1 ± 18.4 |
| SSKF (mm) | 46.8 ± 21.9 | 53.5 ± 29.7 | 69.3 ± 28.9 | 76.3 ± 30.3 |
| Hemodynamic measures | ||||
| SBP (mmHg) | 115.9 ± 11.0 | 119.5 ± 10.9 | 105.8 ± 8.7 | 112.5 ± 11.9 |
| DBP (mmHg) | 61.1 ± 7.4 | 64.2 ± 7.8 | 60.7 ± 5.6 | 66.4 ± 8.7 |
| MAP (mmHg) | 79.4 ± 7.3 | 82.6 ± 7.7 | 75.7 ± 5.9 | 81.8 ± 8.9 |
| PP (mmHg) | 54.9 ± 10.4 | 55.3 ± 9.7 | 45.2 ± 7.3 | 46.1 ± 8.9 |
| Socioeconomic measures | ||||
| Mother’s education level (years)a | 13.9 ± 2.0 | 13.7 ± 1.9 | 13.8 ± 2.0 | 13.4 ± 1.8 |
| Father’s education level (years)b | 14.2 ± 2.5 | 12.7 ± 2.5 | 13.9 ± 2.1 | 13.0 ± 2.1 |
| Single-parent household, n (%)c | 22 (15.0%) | 35 (29.4%) | 15 (11.9%) | 58 (35.4%) |
| Lifestyle factors | ||||
| Smoker, n (%) | 76(51.35%) | 47 (39.50%) | 51 (39.84%) | 34 (20.73%) |
| Marijuana use, n (%) | 46(31.08%) | 29 (24.37%) | 11 (8.59%) | 16 (9.76%) |
| Drinking, n (%) | 60 (40.54%) | 26 (21.85%) | 21 (16.41%) | 17 (10.37%) |
| Physical activityd | ||||
| 1 | 14 (9.66%) | 15 (13.04%) | 40 (32.26%) | 69 (42.59%) |
| 2 | 106 (73.10%) | 84 (73.04%) | 74 (59.68%) | 85 (52.47%) |
| 3 | 25 (17.24%) | 16 (13.91%) | 10 (8.06%) | 8 (4.94%) |
AA, African American; EA, European American; MAP, mean arterial pressure PP, pulse pressure; PWV, pulse wave velocity; SSKF, the sum of the three skinfolds.
A total of 17 participants having missing values in mother’s education level.
A total of seven participants having missing values in father’s education level.
A total of three participants having missing values in single-parent household.
A total of 13 participants having missing values in physical activity (PA), PA for 5–7 days/week coded as 3, PA for 2–4 days/week coded as 2 and PA for 0–1 days/week coded as 1.
On each laboratory visit, demographic information was collected. Evaluation of height, weight, skinfolds (triceps, subscapular, and suprailiac crest), and waist circumference has been described elsewhere [13]. From these primary measures, the sum of the three skinfolds (SSKF) was calculated as a measure of body fat; BMI (weight/height2), as a measure of general adiposity; and waist circumference, as a measure of central adiposity.
Blood pressure (BP) was measured with an automated oscillatory BP system (Dinamap Vital Signs Monitor, Model 1846 SX; Criticon Incorporated, Tampa, Florida, USA), using an appropriately sized BP cuff that was placed on the participant’s right arm. BP measurements were taken at the end of the 11th, 13th, and 15th minute during a 15-min supine relaxation period. The average of the last two readings (at 13 and 15 min) was used to represent resting mean arterial pressure [29], SBP and DBP, respectively. Pulse pressure (PP) was computed as the difference between SBP and DBP.
Socioeconomic status (SES) was represented by marital status of the parents, that is, single household (single, divorced, widowed, separated) versus two-parent households (married), and parental education level, that is, mother’s or father’s education level. As these measures remained highly stable across the years of the study, marital status and parental education as measured at the midpoint of the study were taken as representatives for the whole study period. Parental education level was measured on a seven-point scale, ranging from less than high school to postgraduate education. Several individuals had missing values for marital status (n = 3), father’s education level (n = 7), or mother’s education level (n = 17). These individuals were omitted from analyses in which these respective variables were included.
At each visit, the participant’s smoking status was assessed by the self-reported number of days smoked during the past 30 days and the number of cigarettes smoked per day. Individuals who smoked at least five cigarettes in the past 30 days at any visit were considered smokers. In addition, the participants were asked whether they used marijuana in the past 30 days. Individuals who used marijuana at least five times in the past 30 days at any visit were considered as marijuana users. Alcohol use was also assessed using the self-reported number of days at least one drink of alcohol was consumed during the past 30 days. Individuals who used alcohol at least 10 days in the past 30 days at any visit were defined as alcohol user. Physical activity was assessed by the self-reported number of days per week, inside or outside of school, during which physical activity that was sufficient to ‘work up a sweat’ was performed. The average of physical activity days across all the visits was used to represent the participant’s regular physical activity.
Assessment of pulse wave velocity
Carotid–distal PWV was measured noninvasively with applanation tonometry and the SphygmoCor CPV analysis software (SphygmoCor, AtCor Medical, Sydney, Australia). Pressure waves were recorded at the common carotid and dorsalis-pedis arteries for the carotid–distal PWV. The SphygmoCor system calculated PWV from measurements of pulse transit time and the distance traveled by the pulse between the two recording sites: PWV = distance (m)/transit time (s). The carotid–distal rather than carotid–femoral was used because of its ease of access. Carotid–distal PWV measures arterial stiffness in both elastic and muscular artery and studies from our own and others have shown that PWV measures covering both elastic and muscular artery not only showed reasonable stability over time [13] but also were comparable to carotid–femoral PWV regarding to their correlations with other CVD risk factors [13,30–32] as well as their predictive value of CVD morbidity and mortality [2,33–35].
Statistical analyses
The development of PWV was explored by use of individual growth curve modeling within a multilevel framework [36], which is a data analysis technique especially designed to explore longitudinal data. Repeated PWV measurement was regarded as a two-level hierarchy, with subjects at level 2 (between-subject level) and repeated measurements (or visits) at level 1 (within-subject level). We first specified the unconditional growth model in which fixed and random linear and quadratic were fitted by the addition of age and age2 (age × age), to the intercept-only model. Age was expressed as a deviation from its mean of 24.8 years. Ethnicity and sex were then added to the unconditional growth model, the latter modeled as interaction with age, the interaction between ethnicity and sex, and the three-way interaction of age, ethnicity and sex were also tested, which constituted the full ethnicity, sex and age model. As we observed a significant three-way interaction among ethnicity, sex and age on PWV, stratified analyses by sex were further conducted to better illustrate the findings. Next, anthropometric variables (i.e. height, BMI, waist and SSKF), hemodynamic variables (i.e. SBP, DBP, MAP and PP), SES variables (father’s education level and single-parent household) and lifestyle variables (smoking, marijuana use, drinking and physical activity) were added to the model, respectively, to estimate the effect on PWV development. In addition to the main effect, we tested whether interactions of each of the variables with age, ethnicity and sex affected the growth curve. In the final step, all variables that had significant effects on PWV development in the previous models were entered simultaneously as a full model. To judge the significance of parameters in the full model, each parameter was removed from the model, and a likelihood ratio test with one degree of freedom was used to examine whether its effect was significant in this full model. Multilevel modeling was performed using the program SAS9.4 Mixed model.
RESULTS
Ethnicity, sex and age model
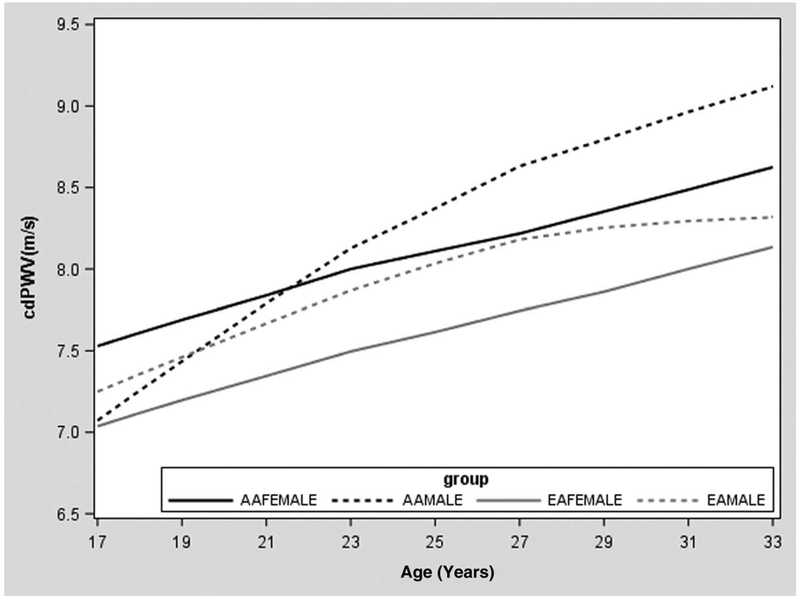
The unconditional growth model with a fixed and random linear (age) effect provided the best fit (Table 2). Table 2 displays the results of growth curve modeling of PWV with demographic variables. Both sex (β = 0.21, P < 0.01) and ethnicity (β = 0.377, P < 0.01) had a significant effect on PWV level, indicating that men and African Americans had higher PWV levels than women and European Americans, respectively (Table 2, models 4 and 6). A significant three-way interaction (P < 0.001) among age, sex and ethnicity on PWV was observed (Table 2, Model 9). As shown in Fig. 1, the three-way interaction was driven by the fact that African American men display a larger rate of increase in PWV with age than the other three ethnic and sex groups. This was confirmed by the stratified analyses by sex (Table 2, models 6 and 7 in men and women), in which the interaction between ethnicity and age on PWV was only significant (β = 0.06, P < 0.01) in men.
TABLE 2.
Results of growth curve modeling of age, ethnicity and sex for pulse wave velocity
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % Explained variance | |||||||||
| Model | Variables | β | P | Within | Between | β | P | β | P |
| 1 | Age fixed | 0.074 | <0.01 | 0.100 | <0.01 | 0.050 | <0.01 | ||
| 2 | Model 1 + age random | 0.076 | <0.01 | 0.100 | <0.01 | 0.050 | <0.01 | ||
| 3 | Model 2 + age2 fixed | - | NS | - | NS | - | NS | ||
| Unconditional growth model: fixed and random age | 11.1 | 16.1 | |||||||
| 4 | Model 2 + sex | 0.210 | <0.01 | ||||||
| 5 | Model 4 + sex × age | 0.060 | <0.01 | ||||||
| Sex model | 11.6 | 17.3 | |||||||
| 6 | Model 2 + ethnicity | 0.377 | <0.01 | 0.200 | <0.05 | 0.530 | <0.01 | ||
| 7 | Model 6 + ethnicity × age | 0.022 | <0.05 | 0.060 | <0.01 | −0.012 | 0.45 | ||
| Ethnicity model | 11.8 | 25.6 | |||||||
| 8 | Model 7 + sex + sex × age + ethnicity × sexa | 0.330 | 0.01 | ||||||
| 9 | Model 8 + ethnicity × sex × age | −0.070 | <0.01 | ||||||
| Full ethnicity, sex and age model+ | 12.2 | 25.6 | |||||||
Test of interest of model 8.
FIGURE 1.
Mean values of raw pulse wave velocity data across age for European American males, European American females, African American males and African American females.
Anthropometric model
Supplement Table 1, http://links.lww.com/HJH/B9, lists the results of growth curve modeling of PWV with anthropometric variables. Height, BMI, waist and SSKF (models 10, 14, 18, 22) each had a significant effect on PWV level, reflecting that PWV increases with increasing height and obesity. In the stratified analyses by sex, the effects of BMI, waist and SSKF (models 14, 18, 22 in women) on PWV level were significant in women, but not in men. This sex difference regarding the effect of obesity on PWV was borderline significant for BMI (P = 0.07 for BMI × sex interaction model 16) and SSKF (P = 0.06 for SSKF × sex interaction in model 24) and significant for waist (P < 0.05 for waist × sex interaction in model 20). Height, BMI, waist circumference, SSKF and their interactions were simultaneously entered to obtain an anthropometric model that explained the most between-subjects’ variance in PWV. The model in which waist circumference and its interaction with sex were entered turned out to be the anthropometric model that explained most of the between-subject variance in PWV (Supplement table 1 model 20, http://links.lww.com/HJH/B9). The contribution of height, BMI and SSKF to PWV was no longer significant.
Hemodynamic model
Supplement table 1, http://links.lww.com/HJH/B9, also lists the results of growth curve modeling of PWV with hemodynamic predictor variables. SBP, DBP, MAP and PP each had significant effects on PWV (Ps < 0.01), indicating that PWV increased with increasing SBP, DPP, MAP and PP (model 26, model 30, model 34 and model 38). The effect existed in both the total sample and sex subgroups. SBP, DBP, MAP and PP showed no significant interactions with age, ethnicity, or sex. As SBP, DBP and MAP were highly correlated, only MAP and PP were simultaneously entered into the hemodynamic model to test their independent effect. The contribution of PP to PWV was not significant. Thus, the model in which only MAP was entered turned out to be the best hemodynamic model (Supplement Table 1, model 34, http://links.lww.com/HJH/B9).
Socioeconomic status model
Supplement Table 2, http://links.lww.com/HJH/B9 shows the results of growth curve modeling of PWV with SES variables. Father’s education level (model 42) showed a significant negative effect and single-parent household (model 46) showed a significant positive effect on PWV, indicating that participants whose fathers have lower education levels or participants living in a single-parent household have higher PWV levels. In the stratified analyses by sex, the effect of single-parent household on PWV was only significant in men, with the sex × single-parent household interaction in the overall samples showing a P value of 0.09. When father’s education level and single-parent household were entered into the model together, only single-parent household remained significant in the SES model and the ethnicity and sex effects remained significant.
Lifestyle model
Smoking showed a borderline significant effect on PWV levels (model 50 in supplement Table 2, http://links.lww.com/HJH/B9), whereas the use of marijuana was significantly associated with PWV levels (model 54 in supplement Table 2, http://links.lww.com/HJH/B9). In the stratified analyses by sex, the effect of marijuana use on PWV was more significant in men than in women (P < 0.01 vs. P = 0.50), although the sex × marijuana use interaction term in the overall sample was not significant. Physical activity and use of alcohol had no significant impact on PWV in our study. In the model including both smoking and marijuana use, only marijuana use remained significant.
Full model
In the full model, ethnicity, sex, anthropometric, hemodynamic, SES and lifestyle models were combined, this is, age, ethnicity, sex and their two-way and three-way interactions, waist circumference and its interaction with sex, MAP, single-parent household and marijuana use were entered simultaneously. All the variables remained significant and were kept in the final model. This model (Table 3) in total explained 14.6% of the within-subject and 59.4% of the between-subject variance in PWV (at age 24.8). For men, the full model included age, ethnicity and their interaction, MAP, single-parent household and marijuana use; whereas for women, the full model included age, ethnicity, waist and MAP with the age-related PWV increase explained by waist and MAP.
TABLE 3.
Full model of growth curve for pulse wave velocity
| Variables | β | P |
|---|---|---|
| Full model of growth curve for PWV in the overall sample | ||
| Age | 0.049 | <0.001 |
| Sex | −0.190 | 0.015 |
| Ethnicity | 0.091 | 0.247 |
| Sex × age | −0.034 | 0.030 |
| Ethnicity × age | 0.044 | 0.003 |
| Sex × ethnicity | 0.135 | 0.208 |
| Sex × ethnicity × age | −0.057 | 0.006 |
| Waist | 0.003 | 0.294 |
| Waist × sex | 0.010 | 0.001 |
| MAP | 0.038 | <0.001 |
| Single-parent household | 0.130 | 0.041 |
| Marijuana use | 0.149 | 0.036 |
| Full model of growth curve of PWV in men | ||
| Age | 0.049 | <0.001 |
| Ethnicity | 0.032 | 0.682 |
| Ethnicity × age | 0.043 | 0.003 |
| MAP | 0.046 | <0.001 |
| Single-parent household | 0.240 | 0.009 |
| Marijuana use | 0.173 | 0.037 |
| Full model of growth curve of PWV in women | ||
| Age | 0.010 | 0.17 |
| Ethnicity | 0.267 | <0.001 |
| Waist | 0.014 | <0.001 |
| MAP | 0.039 | <0.001 |
MAP, mean arterial pressure; PWV, pulse wave velocity.
DISCUSSION
The aim of this study was to assess the potential moderating influences of a variety of anthropometric, hemodynamic, SES and lifestyle variables on PWV level and growth from youth to early adulthood. This is the first longitudinal study that involves multiple PWV measurements over an average of 7-year follow-up from late adolescent into young adulthood, in both African Americans and European American men and women.
As expected, our results showed that PWV increased with age, and age was the major factor explaining the within-subject variance of PWV growth. Different from BP and BMI, which show a linear increase in adolescence and a nonlinear leveling off in young adulthood with age [27,37,38], PWV showed a linear increase with age from late adolescent to young adulthood. Similar to previous studies [12,39], higher PWV level was observed in men than in women. The present study also found that African Americans had higher PWV levels than did European Americans from late childhood through early adulthood. This is in line with previous cross-sectional findings. The study by Snijder et al. [40] observed that African descendant has higher PWV than do other ethnic groups across the entire age range from 18 to 70 years. In addition, our study for the first time showed that African American men displayed a larger rate of increase in PWV with age than the other three ethnic and gender groups, suggesting that African Americans men are at particular risk to develop higher PWV in adulthood. These ethnic differences in PWV levels and growth cannot be explained by obesity-related variables and BP, which have been shown to be higher in African Americans than in European Americans, indicating that higher PWV may contribute to the higher prevalence of cardiovascular events and all-cause mortality in this population independent of obesity and higher BP.
There is controversy in the literature regarding the effects of obesity on arterial stiffness. Recently, a large-scale cross-sectional study [41] involving over 6000 participants across the age range of 14–102 observed that the association between adiposity and PWV was steeper in women than in men, in younger than in older individuals, and stronger if using waist circumference as an index rather than BMI. This is in consistent with the current longitudinal study in which we observed that obesity-related variables only showed effects on PWV in women with waist circumference being the strongest predictor. This is also in line with the findings from the Baltimore Longitudinal Study of Aging [21], the only longitudinal study with multiple PWV measurements in adults, that waist circumference predicts longitudinal PWV increase in women but not in men. Although the mechanisms explaining the sex differences in terms of the effect of obesity on PWV are yet to be examined, we strongly suggest all further studies in this area to adopt a more critical approach to not only distinguish different body composition measures but also test the potential sex difference.
Similar to the literature [42], we observed that BP was another major contributor to PWV in addition to age. The relationship between BP and PWV is probably bidirectional, based on vascular biology and hemodynamics. The concept that arterial stiffness increases SBP, leading to an increase in PP because of alterations of the ‘buffering’ function of the conduit arteries in older individuals, has been well established and PP has been used as a surrogate index of arterial stiffness in the elderly [43,44]. However, existing data also suggest that elevated BP accelerates arterial stiffening during childhood and early adulthood because of increased ‘wear and tear’ of the artery walls, subsequently leading to an increase in arterial stiffness [45,46]. Chen et al. [25] explored the temporal relationship between elevated BP and arterial stiffness in a cohort of middle-aged adults aged 32–51 years and concluded that elevated BP preceded increased arterial stiffness during young adulthood, suggesting the mechanisms underlying the development of hypertension are different during younger and older age periods. In addition to decreased elastin content contributes to the progression of arterial stiffness, the hypertrophy and increased elastic stiffness of vascular smooth muscle cells (VSMCs) also contributes to arterial stiffness [47,48], and DBP might be relatively more dependent on basal tone of VSMCs and less dependent on cardiac contractility and vascular compliance as determined by changes in extracellular matrix than SBP and PP [49]. Our current longitudinal study of youth and young adults supports this concept. We observed that DBP and MAP explain more variance in PWV than SBP and the effect of PP on PWV was not independent of MAP. This is also supported by previous cross-sectional studies [50,51] which found that PWV correlated more closely with DBP in young individuals. Different from the Baltimore Longitudinal Study of Aging, which observed that the impact of SBP on PWV increased over time in men [21], we did not observe that BP’s effect on PWV changed with age, this is, we found no significant interaction between BP and age on PWV. This discrepancy might be because of the young age of our population and the relatively short follow-up time (median follow-up time = ~7 years).
Low SES is associated with cardiovascular mortality and morbidity, and increased vascular aging might be one of the potential mechanisms. Although limited studies have been conducted on this aspect, the findings in general supported this hypothesis. Thurston and Matthews [52] observed that lower parental SES including low education, family income and neighborhood deprivation was associated with high PWV in adolescence in a small sample of African Americans. Trudel et al. [15] showed that PWV increase over 5 years was higher among participants with lower employment grade, household income and education in the elderly (mean age of 65 years). Interestingly, in this study, the effect of father’s social class, an indicator of SES in childhood, was associated with baseline PWV but not PWV changes over 5 years. Our current longitudinal study in youth and young adults provided further support to this concept. We observed that lower father’s education level and single-parental household increased PWV levels across the period of youth and young adulthood, although the effect of father’s education level was not independent of single-parental household. The lack of parental SES–age interaction on PWV is also consistent with Trudel et al. s [15] findings on father’s social class, suggesting that the alteration to arteries attributable to adverse social conditions have already occurred at an earlier stage of life and the effect might last a lifetime. Given the overrepresentation of African Americans in low SES positions, we further checked whether the ethnic difference in PWV can be explained by the ethnic difference in SES and found that ethnic differences persisted after the adjustment of SES.
Illicit drugs are frequently used across a wide range of demographic groups and its acute cardiovascular effects are well known. However, the chronic effects of illicit drug use are difficult to study, especially in the general population. One study [17] exploring the effect of cocaine use on aortic stiffness showed that regular use was associated with increased PWV in a small sample of young healthy individuals. Another study [18] observed that the current use of marijuana was associated with increased PWV in 86 healthy participants aged 8–25 years. Our study measured marijuana use at multiple visits and confirmed that use of marijuana was linked with higher PWV across youth and young adulthood, suggesting a cumulative effect of marijuana exposure on the vessel wall. The previously reported findings [53–55] of marijuana’s effect on immune modulation, endothelial function and sympathetic nervous system activity might be the underlying mechanisms explaining the link between marijuana use and increased arterial stiffness, although direct evidence from animal studies is still missing. In addition, we observed that the contribution of marijuana use to PWV was larger than the contribution of smoking, a known factor impacting vascular stiffness. In the lifestyle model, the borderline significant effect of smoking on PWV disappeared, which might be because of the high co-occurence of smoking and marijuana use.
Our study has some limitations that should be considered with interpreting the results. First, ethnicity and sex differences in PWV may be because of differences in genetic background. In the present study, we addressed anthropometric variables, hemodynamic variables and SES variables, the differences in ethnicity and sex remained significant, suggesting genetics or interaction between genetic and environmental factors should be considered in the future studies. Second, self-reported ethnicity was used in this study, which is neither purely biological nor measured with precision. It represents a mixture of genetic, social, economic, behavioral, psychological and other environmental factors. Despite this, use of self-reported ethnicity has been advocated in biomedical and genetic research [56]. Third, we used the dorsalis-pedis as an alternative to the femoral site for the measurement of PWV. As stated, distal PWV represents a mixture of both proximal elastic arteries and distal, more muscular arteries. However, based on its strong correlation with age, other CVD risk factors as well as its predictive value of CVD morbidity and mortality, we believe that distal PWV mostly represents stiffness of the elastic component, and can be considered a reasonable proxy of aortic stiffness. Fourth, supine posture BP rather than sitting BP was measured in this study. BP differs according to posture [57], however, BP measurement of supine posture was widely used in clinical and population studies [57,58]. More importantly, supine BP was measured consistently across all the visits in this study. Finally, information on marijuana use only relied on self-reported data. Future studies with measurements on marijuana metabolites in blood and urine are warranted.
In conclusion, our results showed that ethnicity and sex differences in PWV become apparent in early adolescence and remain relatively stable through early adulthood, with African Americans and men having higher PWV levels than European Americans and women, respectively. These ethnic and sex differences in PWV persisted even after adjusting for anthropometric, hemodynamic, SES and lifestyle variables. Apart from these effects of ethnicity and sex, waist circumference, MAP, single-parent household and marijuana use appeared to be the strongest predictors of PWV in the present study. Our study emphasizes the importance of both BP control and development of novel prevention strategies to limit the progress of arterial stiffness such as by reducing or controlling adiposity, emphasizing the importance of two-parent household and reducing or preventing the use of illicit drugs.
Supplementary Material
ACKNOWLEDGEMENT
We thank the GSH participants for their invaluable contributions to the study. The GSH study was supported by grant HL69999 from the National Heart Lung and Blood Institute. X.L. has been supported by China Scholarship Council. X.W. has been supported in part by grants from the National Heart Lung and Blood Institute (HL104125 and HL105689).
Abbreviations:
- BP
blood pressure
- GSH
Georgia Stress and Heart
- MAP
mean arterial pressure
- PP
pulse pressure
- PWV
pulse wave velocity
- SES
socioeconomic status
- SSKF
the sum of the three skinfolds
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33:1111–1117. [DOI] [PubMed] [Google Scholar]
- 2.Maeda Y, Inoguchi T, Etoh E, Kodama Y, Sasaki S, Sonoda N, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care 2014; 37:2383–2390. [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 1998; 32: 570–574. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998; 338:1650–1656. [DOI] [PubMed] [Google Scholar]
- 6.Santos PC, Alvim Rde O, Ferreira NE, de Sa Cunha R, Krieger JE, Mill JG, et al. Ethnicity and arterial stiffness in Brazil. Am J Hypertens 2011; 24:278–284. [DOI] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55: 1318–1327. [DOI] [PubMed] [Google Scholar]
- 8.Kozakova M, Morizzo C, Guarino D, Federico G, Miccoli M, Giannattasio C, et al. The impact of age and risk factors on carotid and carotid-femoral pulse wave velocity. J Hypertens 2015; 33:1446–1451. [DOI] [PubMed] [Google Scholar]
- 9.Thurn D, Doyon A, Sozeri B, Bayazit AK, Canpolat N, Duzova A, et al. Aortic pulse wave velocity in healthy children and adolescents: reference values for the Vicorder device and modifying factors. Am J Hypertens 2015; 28:1480–1488. [DOI] [PubMed] [Google Scholar]
- 10.Saeed S, Waje-Andreassen U, Fromm A, Oygarden H, Kokorina MV, Naess H, et al. Early vascular aging in young and middle-aged ischemic stroke patients: the Norwegian Stroke in the Young Study. PloS One 2014; 9:e112814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmenhorst J, Hulpke-Wette M, Barta C, Dalla Pozza R, Springer S, Oberhoffer R. Percentiles for central blood pressure and pulse wave velocity in children and adolescents recorded with an oscillometric device. Atherosclerosis 2015; 238:9–16. [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, Park JB, Kim DS, Kim KS, Jeong JW, Park JC, et al. Gender difference in arterial stiffness in a multicenter cross-sectional study: the Korean Arterial Aging Study (KAAS). Pulse (Basel, Switzerland) 2014; 2:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye C, Pan Y, Xu X, Su S, Snieder H, Treiber F, et al. Pulse wave velocity in elastic and muscular arteries: tracking stability and association with anthropometric and hemodynamic measurements. Hypertens Res 2016; 39:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canepa M, AlGhatrif M, Pestelli G, Kankaria R, Makrogiannis S, Strait JB, et al. Impact of central obesity on the estimation of carotid-femoral pulse wave velocity. Am J Hypertens 2014; 27:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trudel X, Shipley MJ, McEniery CM, Wilkinson IB, Brunner EJ. Socioeconomic status, education, and aortic stiffness progression over 5 years: the Whitehall II prospective cohort study. J Hypertens 2016; 34:2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balakumar P, Kaur J. Is nicotine a key player or spectator in the induction and progression of cardiovascular disorders? Pharmacol Res 2009; 60:361–368. [DOI] [PubMed] [Google Scholar]
- 17.Kozor R, Grieve SM, Buchholz S, Kaye S, Darke S, Bhindi R, et al. Regular cocaine use is associated with increased systolic blood pressure, aortic stiffness and left ventricular mass in young otherwise healthy individuals. PloS One 2014; 9:e89710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckard AR, Raggi P, Ruff JH, O’Riordan MA, Rosebush JC, Labbato D, et al. Arterial stiffness in HIV-infected youth and associations with HIV-related variables. Virulence 2017; 8:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zachariah JP, Rong J, Larson MG, Hamburg NM, Benjamin EJ, Vasan RS, et al. Metabolic predictors of change in vascular function: prospective associations from a community-based cohort. Hypertension 2018; 71:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birru MS, Matthews KA, Thurston RC, Brooks MM, Ibrahim S, Barinas-Mitchell E, et al. , SWAN Heart Study. African-American ethnicity and cardiovascular risk factors are related to aortic pulse-wave velocity progression. Am J Hypertens 2011; 24:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 2013; 62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohyama Y, Teixido-Tura G, Ambale-Venkatesh B, Noda C, Chugh AR, Liu CY, et al. Ten-year longitudinal change in aortic stiffness assessed by cardiac MRI in the second half of the human lifespan: the multiethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2016; 17:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomiyama H, Shiina K, Matsumoto-Nakano C, Ninomiya T, Komatsu S, Kimura K, et al. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J Am Heart Assoc 2017; 6:; e005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neill D, Britton A, Brunner EJ, Bell S. Twenty-five-year alcohol consumption trajectories and their association with arterial aging: a prospective cohort study. J Am Heart Assoc 2017; 6:; pii: e005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Li S, Fernandez C, Sun D, Lai CC, Zhang T, et al. Temporal relationship between elevated blood pressure and arterial stiffening among middle-aged black and white adults: the Bogalusa Heart Study. Am J Epidemiol 2016; 183:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Khoudary SR, Barinas-Mitchell E, White J, Sutton-Tyrrell K, Kuller LH, Curb JD, et al. , ERA JUMP Study Group. Adiponectin, systolic blood pressure, and alcohol consumption are associated with more aortic stiffness progression among apparently healthy men. Atherosclerosis 2012; 225:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation 2006; 114:2780–2787. [DOI] [PubMed] [Google Scholar]
- 28.Online Mendelian inheritance in man OM-NIoGM, Johns Hopkins University; (Baltimore, MD: ). 5/November/2018. Available at: http://omim.org/. [Google Scholar]
- 29.Roadmap Epigenomics C, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature 2015; 518:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, et al. Correlates of segmental pulse wave velocity in older adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2016; 29:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo J, Shin C, Barinas-Mitchell E, Masaki K, Willcox BJ, Seto TB, et al. Regional pulse wave velocities and their cardiovascular risk factors among healthy middle-aged men: a cross-sectional population-based study. BMC Cardiovasc Disord 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng XA, Qie LY, Wang YY, Zhong M, Li L. Assessment of arterial stiffness affected by atorvastatin in coronary artery disease using pulse wave velocity. Clin Invest Med 2009; 32:E238. [DOI] [PubMed] [Google Scholar]
- 33.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension 2014; 64:240–246. [DOI] [PubMed] [Google Scholar]
- 35.Leng GC, Fowkes FGR, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. Brit Med J 1996; 313:1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein HG. Multilevel statistical models. New York: Wiley; 1995. [Google Scholar]
- 37.Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: a 10-year longitudinal study. J Pediatr 2002; 141:770–779. [DOI] [PubMed] [Google Scholar]
- 38.Dekkers JC, Podolsky RH, Treiber FA, Barbeau P, Gutin B, Snieder H. Development of general and central obesity from childhood into early adulthood in African American and European American males and females with a family history of cardiovascular disease. Am J Clin Nutr 2004; 79:661–668. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Longitudinal and cross-sectional studies in a cohort of healthy children and adolescents. Atherosclerosis 2012; 220:269–274. [DOI] [PubMed] [Google Scholar]
- 40.Snijder MB, Stronks K, Agyemang C, Busschers WB, Peters RJ, van den Born BJ. Ethnic differences in arterial stiffness the Helius study. Int J Cardiol 2015; 191:28–33. [DOI] [PubMed] [Google Scholar]
- 41.Scuteri A, Orru M, Morrell CH, Tarasov K, Schlessinger D, Uda M, et al. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis 2012; 221:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009; 54:1328–1336. [DOI] [PubMed] [Google Scholar]
- 43.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315. [DOI] [PubMed] [Google Scholar]
- 44.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874. [DOI] [PubMed] [Google Scholar]
- 45.Franklin SS. Arterial stiffness and hypertension: a two-way street? Hypertension 2005; 45:349–351. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 2014; 64:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension 2005; 45:1050–1055. [DOI] [PubMed] [Google Scholar]
- 48.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, et al. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol 2013; 305:H1281–H1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bautista Nino PK, Durik M, Danser AH, de Vries R, Musterd-Bhaggoe UM, Meima ME, et al. Phosphodiesterase 1 regulation is a key mechanism in vascular aging. Clin Sci (Lond) 2015; 129:1061–1075. [DOI] [PubMed] [Google Scholar]
- 50.Nurnberger J, Dammer S, Opazo Saez A, Philipp T, Schafers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hyper-tens 2003; 17:153–158. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension 2001; 38:1461–1466. [DOI] [PubMed] [Google Scholar]
- 52.Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med 2009; 68:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol 2006; 1:50–64. [DOI] [PubMed] [Google Scholar]
- 54.Wang XY, Derakhshandeh R, Liu JT, Narayan S, Nabavizadeh P, Le S, et al. One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc 2016; 5:; pii: e003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franz CA, Frishman WH. Marijuana use and cardiovascular disease. Cardiol Rev 2016; 24:158–162. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA 2003; 289:2709–2716. [DOI] [PubMed] [Google Scholar]
- 57.Vrachatis D, Papaioannou TG, Konstantopoulou A, Nasothimiou EG, Millasseau S, Blacher J, et al. Effect of supine versus sitting position on noninvasive assessment of aortic pressure waveform: a randomized cross-over study. J Hum Hypertens 2014; 28: 236–241. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.