Abstract
Objective:
Imaging studies in adults with IBS have shown both morphological and resting state (RS) functional connectivity (FC) alterations related to cortical modulation of sensory processing. Since analogous differences have not been adequately investigated in children, this study compared gray matter volume (GMV) and RS-FC between girls with IBS and healthy controls (HC), and tested the correlation between brain metrics and laboratory-based pain thresholds (Pth).
Methods:
Girls with Rome III criteria IBS (N=32) and matched HCs (N=26) were recruited. In a subset of patients, Pth were determined using a thermode to the forearm. Structural and RS scans were acquired. A voxel-based general linear model, adjusting for age, was applied to compare differences between groups. Seeds were selected from regions with group GMV differences for a seed-to-voxel whole-brain RS-FC analysis. Significance for analyses were considered at p<.05 after controlling for false discovery rate. Significant group differences were correlated with Pth.
Results:
Girls with IBS had lower GMV in the thalamus, caudate nucleus (CaN), nucleus accumbens, anterior mid-cingulate (aMCC), and dorsolateral prefrontal cortex (dlPFC). They also exhibited lower RS-FC between the aMCC and the precuneus, but greater connectivity between the CaN and precentral gyrus. Girls with IBS had higher Pth with a moderate effect size (t(22.81) = 1.63, p = 0.12, d = 0.64) and lower thalamic GMV bilaterally was correlated with higher Pth (left: r=−.62, p(FDR)=.008; right: r=−.51, p(FDR)=.08).
Conclusion:
Girls with IBS had lower GMV in the PFC, basal ganglia, and aMCC, as well as altered functional connectivity between multiple brain networks, suggesting that structural changes related to IBS occur early in brain development. Girls with IBS also showed altered relationships between pain sensitivity and brain structure.
Keywords: children with irritable bowel syndrome, pain sensitivity, sensory processing, cortical modulation, functional and structural MRI
Introduction
Irritable Bowel Syndrome (IBS) is a functional disorder of the brain gut-axis characterized by recurrent abdominal pain and altered bowel habits that are not explained by structural or biochemical abnormalities. Although more frequently diagnosed in adults than in children, IBS is the most common cause of functional Recurrent Abdominal Pain (RAP) in children in the Western Hemisphere (1). A recent study by Hyams et al (2) found in their sample of 507 participants, up to 17% of adolescents with abdominal pain fit criteria for IBS. El-Matery et al (3) found that 36% of their child sample originally presenting with RAP fit the criteria for IBS. IBS symptoms can impair functioning in daily life (4) and can persist into adulthood (5).
The pathophysiology of IBS can be characterized as a bidirectional brain-gut axis disorder. Multiple brain networks, including the salience, sensorimotor and executive-control networks, are thought to mediate the effects of affect, mood and environmental factors on gut function and pain perception, resulting in visceral hypersensitivity and altered bowel habits (6,7). For a review on these networks see (8). Prefrontal brain regions modulate activity in the paralimbic regions, subregions of the anterior cingulate cortex (ACC) and hypothalamus, which then in turn modulate activity of descending pathways through the periaqueductal gray and brainstem nuclei. Activity in these corticolimbic pontine networks mediates the effects of cognitions and emotions on the perception of visceral pain and discomfort (6). The enteric nervous system and the brain are engaged in bi-directional communication in these pathways through vagal and sympathetic fibers. Alterations within this nervous system, whether it be in the gut (bottom-up) or brain (top-down), can result in IBS symptoms (8). These brain network abnormalities are thought to lead to disturbances in the brain in areas related to affect such as the cingulate, amygdala (9) and prefrontal regions (10). Moreover, as many prefrontal regions are involved in central pain inhibition (11), abnormalities in these areas due to chronic IBS may lead to deficiencies in the ability to engage in the endogenous modulation of pain and altered pain sensitivity.
Past research in adult patients with IBS and pain sensitivity have shown mixed results, including hyperalgesia, to mechanical and thermal pain (12–14) and somatic hypoalgesia at non-pain sites such as the hand (15–18). In children, mixed results regarding thermal pain thresholds have also been observed (19–22), including hypoalgesia in response to thermal pain at the hand (20). Studies investigating central mechanisms and changes in pain sensitivity via imaging modalities in IBS have been carried out in adults (23–26). Common brain regions engaged during pain stimuli in adults with IBS relative to controls have been the insula (INS), anterior cingulate cortex (ACC), primary somatosensory cortex (S1), prefrontal cortex (PFC), posterior parietal cortex (PPC), and thalamus (THAL) (27–30). Additionally, patients with IBS have shown activation in the emotional-arousal network (ACC and amygdala) and midbrain in response to visceral pain stimulation, a finding not seen in controls (31). Despite all of the research done in adults, we know of only one study using magnetic resonance imaging (MRI) in children with IBS (32).
Hubbard et al. (32) recently found lower cortical thickness in pediatric IBS patients in the dorso-medial prefrontal cortex (dmPFC), dorso-lateral prefrontal cortex (dlPFC) and PPC, and an increase in the posterior cingulate cortex (PCC). It was found that IBS patients exhibited increased connectivity from the dlPFC to cuneus and from the right PCC to the dlPFC. Controls had greater connectivity from the PCC to the parietal operculum (INS). These atypical connectivity patterns in nodes responsible for cognitive control (33) are thought to lead to disinhibition of descending pain modulation, attention to viscero-sensory processes, and exacerbated visceral hyperalgesia.
The primary aim of the following study was to investigate structural differences in grey matter volume (GMV) in the brain using voxel-based morphometry and its associated resting state (RS) abnormalities in functional connectivity (FC) in girls with IBS compared to healthy, age and sex-matched controls. A secondary aim was to assess if these changes in brain structure and connectivity were associated with differences in state anxiety, trait anxiety, daily pain levels, pain-related anxiety, and pain thresholds. It was hypothesized that, in line with the adult findings, children with IBS would show differences from controls in GMV in regions such as the cingulate, somatosensory cortex, insula, putamen, hippocampus, amygdala, and frontal executive control regions (23). Additionally, we postulated that in an exploratory analysis the brain regions that showed IBS related differences in GMV would show altered resting state functional connectivity (RS-FC) as well.
Materials and Methods
Participants
Study participants included 32 girls; aged 7–17 years, diagnosed with IBS using Rome III criteria (mean age = 11.40, SD = 3.01) and 26 age and sex matched healthy controls (mean age = 10.72, SD = 2.79). See Table 1 for sample breakdown. Participants with IBS were prospectively recruited from three clinical sites: the UCLA Pediatric Pain Clinic and the UCLA-affiliated Whole Child LA (WCLA) pain clinic by LZ, and the Baylor College of Medicine / Texas Children’s Hospital pediatric gastroenterology and primary care clinics by RS. Age-matched healthy controls were recruited via Craigslist, postings around campus, and referrals from other participants. All procedures were approved by the UCLA Medical Institutional Review Board and the Institutional Review Board for Baylor College of Medicine. The parents of all participants provided informed written consent and participants provided assent. Exclusion criteria for both groups included (1) acute illness or injury that may impact lab performance (e.g., fever) or that affects sensitivity of the extremities (e.g., Reynaud’s disease); (2) participants being treated for chronic disease other than pain (e.g., diabetes); (3) daily use of opioids (participants using other analgesics were included, but were asked not to take these analgesics on the day of the lab session); (4) antidepressant use unless the dose had been stable for 3 months or longer; (5) developmental delay, autism, or significant anatomic impairment that may preclude understanding study procedures (e.g. developmental age of less than 7 years, assessed by discussion with parents); (6) significant claustrophobia as assessed by discussion with parents and with children. For the IBS group, diagnosis of an organic GI illness (e.g., celiac disease; inflammatory bowel disease) resulted in exclusion. These exclusion criteria were applied at both sites (UCLA/WCLA & Baylor). Data was collected from April 24, 2013 to November 3, 2015.
Table 1:
Clinical variables of both (UCLA and Baylor) study sites.
| UCLA Sample | IBS (N = 10) | HC (N = 14) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | t | p | d |
| Age | 14.54 | 2.91 | 12.05 | 3.13 | 2.05 | 0.06 | 0.82 |
| State-Anxiety (STAI) | 31.45 | 4.70 | 32.57 | 5.26 | −0.56 | 0.58 | −0.22 |
| Trait-Anxiety (STAI) | 43.10 | 8.38 | 35.86 | 8.04 | 2.15 | 0.043 | 0.87 |
| Child Pain Anxiety Symptoms Scale (CPASS) | 39.22 | 18.98 | 31.00 | 13.21 | 1.22 | 0.24 | 0.51 |
| Current Pain (NRS) | 2.82 | 2.64 | 0.86 | 1.35 | 2.24 | 0.041 | 0.97 |
| Anxiety Prior to Pain Task (NRS) | 1.82 | 2.23 | 2.21 | 2.58 | −0.58 | 0.57 | −0.24 |
| Pain Threshold (˚C) | 46.41 | 3.34 | 43.78 | 4.72 | 1.63 | 0.12 | 0.63 |
| Baylor Sample | IBS (N = 22) | HC (N = 12) | |||||
| Variable | Mean | SD | Mean | SD | t | p | d |
| Age | 9.76 | 1.26 | 9.16 | 1.11 | 1.40 | 0.17 | 0.49 |
| Trait-Anxiety (STAI) | 38.50 | 7.29 | 28.71 | 5.47 | 3.16 | 0.007 | 1.48 |
| Number of pain episodes | 10.81 | 8.54 | 0.25 | 0.45 | 5.65 | 1.5e-5 | 1.54 |
Abbreviations: STAI: State-Trait Anxiety Inventory; NRS (Numeric Rating Scale), °C (degrees in Celsius).
Laboratory pain testing – UCLA Sample
Following the collection of psychological and clinical measures, girls at UCLA were connected to a physiological monitoring device for a 10-minute baseline recording. Using a procedure conducted in girls with IBS aged 7–12 years (19), a thermode was placed on the volar surface of the right forearm, and, using a Medoc TSA-II Neurosensory Analyzer (Ramat-Yishai, Israel), the temperature increased from 32˚ C at a rate of 1.5˚ C/sec until the participant indicated that the heat was painful. This assessment of heat pain threshold was conducted four times with 15 sec inter-stimulus intervals (ISI) as a baseline. The average number of seconds of the four stimuli was considered the heat pain threshold for each participant. Volar forearm pain thresholds have been frequently used as a proxy measure for the central sensitization that occurs in patients with IBS (7,34) as administering visceral pain testing can be quite invasive. Laboratory pain testing was not available at the Baylor site at the time of the study.
MRI Acquisition – UCLA and Baylor Samples
For the UCLA sample, imaging was completed on a different day following the lab testing. Imaging at both sites was performed on a Siemens 3 Tesla Trio scanner with a 12-channel head coil. After careful positioning of the participant, padding of the head to reduce movement, and applying noise-reducing headphones, a standard high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) scan was obtained; (TE: 2.98 ms, TR: 2300, flip angle: 9 degrees, FOV: 256 mm × 240 mm, percent phase FOV: 93.75, slice thickness 1.0 mm, voxel size 1 mm3, 240 slices), lasting about 6 minutes. Participants viewed an age appropriate video during structural imaging to help reduce movement. After a short break, participants were instructed to lie quietly with their eyes closed for a 10-minute resting state functional scan, utilizing the following parameters: TE: 30 ms, TR: 2000 ms, flip angle: 77 degrees, FOV 220mm × 220 mm, slice thickness 4.0mm with a 0.5mm skip, acquisition matrix 64 × 64. 31 axial/oblique slices are obtained, with whole-brain coverage. UCLA and Baylor sites utilized the same MRI acquisition parameters.
Psychological and Clinical Measures
At the UCLA site, a Numerical Rating Scale (NRS), a reliable and valid measure in children 8 years and older (35), assessed current levels of abdominal pain and state anticipatory anxiety for the lab testing and imaging procedures. Ratings were made using a 0–10 scale where 0 = no pain/anxiety and 10 = worst pain/anxiety. The State-Trait Anxiety Questionnaire (STAI) was used to assess state (20 items, α = .88) and trait 20 items, α = .91) anxiety (36) in the UCLA and Baylor samples. The Child Pain Anxiety Symptoms Scale (CPASS), a reliable and valid measure of pain-related trait anxiety, was assessed only in the UCLA child sample (20 items, α = .90), ages 8–18 years (37). As these measures were not collected during the brain imaging procedure, results associating these two different procedures should be taken with caution.
Data Analysis
Voxel-Based-Morphometry
Voxel-Based Morphometry analyses were conducted in FSL-VBM which is a part of the FSL software package (38). Brain extraction was first conducted on all participant’s T1 weighted MPRAGE images using the robust version BET command, which calls the command multiple times to move the center-of-gravity closer to the true center. Afterwards, all images were checked manually to determine if the brain was adequately extracted. If the extraction was inadequate, the BET process was manually altered for each brain by changing fractional intensity threshold and/or its vertical gradient until only the whole brain was included. Next, all extracted brain images were segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using FAST in FSL. This method is considered very robust and reliable (39). These images were extensively manually quality controlled to ensure no tissue type was “bleeding” into the other. This procedure has been used to prepare images for templates in previous studies (40). A pediatric study-specific template was created using the Template-O-Matic toolbox (41) toolbox in SPM. The template was made with images from an equal number of controls and girls with IBS necessary to avoid introducing systematic bias. As there were more girls with IBS than the 26 healthy controls, a random subset of 26 girls with IBS were selected. The age of every subject was entered in Template-O-Matic and was modeled as a third order regression. Afterwards, all of the GM images were non-linearly registered to the study specific template, modulated by dividing them by the Jacobian of the warp field to correct for local expansion or contraction and concatenated to create a 4D image (42). The modulated images were then smoothed with at a full-width half maximum (FWHM) of 5 mm. A whole brain mask was used to investigate whole brain differences between girls with IBS and controls. This process also adjusts for total intracranial volume by modifying the volume-preserving Jacobian-modulation step such that the affine component is ignored and only the nonlinear volume changes are preserved (43). Finally, a voxel-based general linear model (GLM) was applied with permutation based testing at 5000 iterations was used to create clusters with the Threshold Free Cluster Enhancement (TFCE) method (44). All analyses were adjusted for age and site. All significance testing was conducted at p < .05, corrected for multiple comparisons using the False-Discovery Rate (FDR) method. Images of results were produced in Mango software (http://ric.uthscsa.edu/mango) and BrainNet (45).
Resting-state fMRI Image Preprocessing
Resting-state preprocessing was performed using Statistical Parametric Mapping 8 (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK). The first three volumes were removed to allow for scanner stabilization. Images were transformed from DICOM to NIFTI format, slice-time corrected, spatially normalized to the MNI template with the MP-RAGE scan, and resampled to a voxel size of 2 × 2 × 2 mm.
Seed-Based fMRI Seed-to-Voxel Analysis
Preprocessed and normalized functional images were imported into the CONN-fMRI functional connectivity toolbox version 16 (46) for additional pre-processing and for seed-to-voxel connectivity analysis. Three-dimensional confounds for both white matter and CSF, obtained from segmentation procedures from preprocessing in SPM8, as well as six realignment parameters and first-order temporal derivatives of motion, were removed using regression using the CompCor procedure in CONN. White matter and CSF were confounded out using regression with 5 dimensions each. The effect of motion was regressed out using 12 dimensions. Resting-state images then were bandpass filtered between 0.01 and 0.1Hz in the CONN toolbox. The regions showing differences in the VBM analysis were used as seeds. Based on the peak voxel difference of each significantly different region in the VBM analysis, 4mm spherical seed ROIs at region were used for Seed-to-Voxel whole brain analyses. All spherical seeds were created in MarsBar (http://marsbar.sourforge.net). Voxel-wise analyses were completed for each of these seeds with an initial height threshold of p < .001. Correction for multiple comparisons was done within the CONN toolbox which calculated the number of voxels in a cluster to be deemed significant at an FDR of p < .05. The final significant t-statistic connectivity maps from each seed were projected onto a normalized study-specific template in Mango software.
Subset Analysis
Statistical Analysis for Clinical Variables
Independent t-tests along with Cohen’s d calculations were used to determine differences and effect sizes between IBS patients and controls on clinical and quantitative sensory testing variables. Pearson’s correlations were computed between GM voxel intensity for regions that were different between groups and clinical variables that were different between groups. Eigenvalues for region dyads that were significantly different between groups were extracted using SPM for each participant. Correlations between eigenvalues and clinical variables were then computed to determine associations between differences in RS-FC and clinical variables. Since pain testing was only conducted at the UCLA site, a subset analysis was performed within the UCLA sample to see how differences in GMV and FC relate to pain threshold and pain anxiety. Alpha was set to 0.05, and corrections were made using the FDR method. All statistical analyses for clinical, and quantitative sensory testing variables, as well as correlations, were conducted using R (Version 3.3.0, “Supposedly Educational”) and RStudio (Version 0.99.447).
Results
Clinical Data
The mean age of the UCLA IBS and HC samples did not differ (14.53 ± 2.91 vs 12.05 ± 3.13). The mean age of the Baylor IBS and HC samples also were similar (9.76 ± 1.26 vs 9.16 ± 1.11).
Clinical Data – Combined Sample
Independent sample t-tests revealed that trait anxiety measured via the STAI was significantly higher in girls with IBS in the UCLA sample (t(21.17) = 2.15, p = .043, d = 0.87) and Baylor sample (t(14.87) = 3.16, p = 0.007, d = 1.48) compared to HC. There was no difference in trait anxiety between the UCLA and Baylor samples (t(22.25) = −1.32, p = .20, d = 0.57). See Table 1.
Voxel-Based Morphometry – Combined Sample
IBS patients exhibited lower GMV in limbic, paralimbic, prefrontal, and temporal regions. See Table 2 for a summary of regions showing significant differences and Figure 1 for a visual depiction.
Table 2:
Clusters and regions defined by their MNI coordinates that showed significant differences between IBS patients and HC in the combined sample.
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | Voxels | Hemisphere | t | p(FWE) | X | Y | Z |
| Cluster 1 (IBS < HC) | |||||||
| Caudate Nucleus | 5100 | Left | 3.33 | 0.027 | −6 | 14 | 6 |
| Caudate Nucleus | Right | 4.04 | 0.014 | 8 | 2 | 12 | |
| Nucleus Accumbens (Nacc) | Left | 3.16 | 0.031 | −6 | 14 | −2 | |
| Nucleus Accumbens (Nacc) | Right | 2.89 | 0.041 | 6 | 12 | −4 | |
| Thalamus | Left | 3.57 | 0.022 | −2 | −4 | 8 | |
| Thalamus | Right | 3.87 | 0.017 | 4 | −2 | 8 | |
| Anterior Mid-Cingulate Cortex (aMCC) | Left | 3.54 | 0.029 | 0 | 36 | 12 | |
| Anterior Mid-Cingulate Cortex (aMCC) | Right | 3.54 | 0.029 | 4 | 36 | 12 | |
| Dorsolateral Prefrontal Cortex (dlPFC) | Left | 3.76 | 0.026 | −30 | 40 | 38 | |
| Cluster 2 (IBS < HC) | |||||||
| Dorsolateral Prefrontal Cortex (dlPFC) | 1714 | Right | 4.28 | 0032 | 42 | 38 | 10 |
| Dorsolateral Prefrontal Cortex (dlPFC) | Right | 3.51 | 0.040 | 24 | 14 | 50 | |
| Cluster 3 (IBS < HC) | |||||||
| Superior Temporal Gyrus, Anterior Division | 139 | Right | 4.19 | 0.042 | 54 | 0 | −20 |
Combined sample VBM results show patients with IBS exhibit lower gray matter volume throughout the brain including the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), thalamus, basal ganglia regions, and superior temporal gyrus.
Figure 1: GM differences between IBS and HC in the Combined Sample.
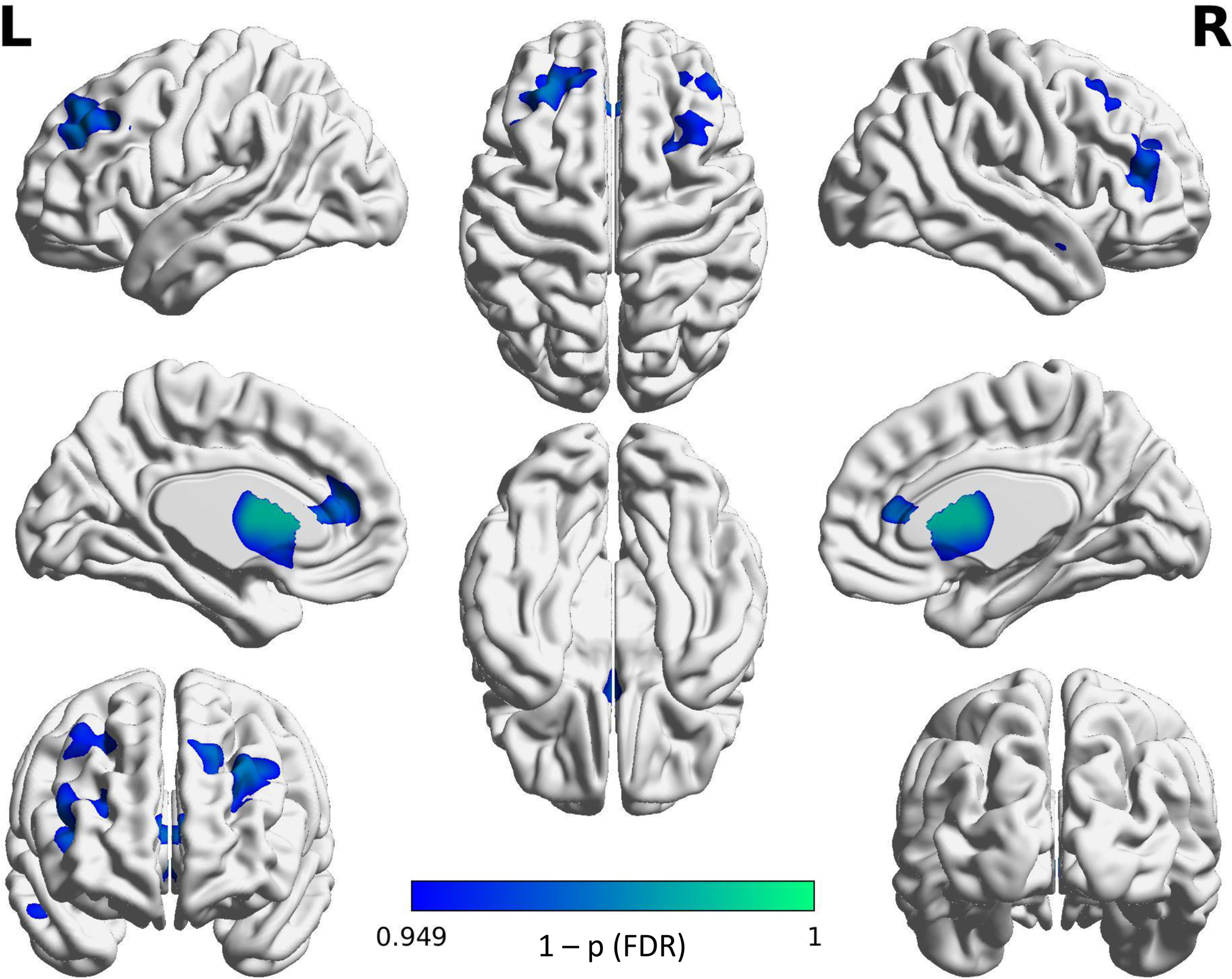
IBS < HC contrast shows regions in IBS that were significantly lower in GMV than in the HC.
Projected color map displays FDR corrected “1-p” values.
The combined sample shows IBS patients having lower gray matter volume in multiple regions of the brain including the dorsolateral prefrontal cortex (dlPFC), anterior mid-cingulate cortex (aMCC), thalamus and basal ganglia. Color map represents “1-p” values with lighter values being closer to 1. All results shown are significant at p(FDR) < .05.
Resting State Functional Connectivity (Seed-to-Voxel) – Combined Sample
Girls with IBS exhibited lower connectivity between the bilateral anterior mid-cingulate cortex (aMCC) and right precuneus. They also exhibited greater connectivity between the right CaN and left precentral gyrus compared to HC. Significant differences between groups from the seed-to-voxel analysis is displayed in Table 3, Figure 2 and Figure 3.
Table 3:
Combined Sample Resting-State Functional Connectivity Results.
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Seed | Region | Ke | beta | t | p(FDR) | X | Y | Z |
| Contrast: IBS < HC | ||||||||
| L aMCC | R Precuneus | 127 | .13 | 6.34 | .004 | 6 | −64 | 52 |
| R aMCC | R Precuneus | 87 | .13 | 4.92 | .04 | 6 | −64 | 52 |
| Contrast: IBS > HC | ||||||||
| R CaN | L Precentral Gyrus | 105 | .11 | 5.93 | .008 | −10 | −16 | 56 |
Abbreviations: L: left, R: right, aMCC: anterior mid-cingulate cortex, CaN: Caudate Nucleus, Ke: number of voxels, beta: beta-value, t: t-value, p(FDR): p-value corrected for false-discovery rate
Seeds were chosen based on significant group differences in the VBM analyses. Only dyads that showed significant differences are shown. Locations of clusters are expressed in MNI-space.
Figure 2: RS-FC differences between IBS and HC in the Combined Sample.

The combined sample shows girls with IBS having lower connectivity from the bilateral aMCC to the right precuneus. All results shown are significant at p(FDR) < .05
Abbreviations: t: t-value.
Figure 3: RS-FC differences between IBS and HC in the Combined Sample.
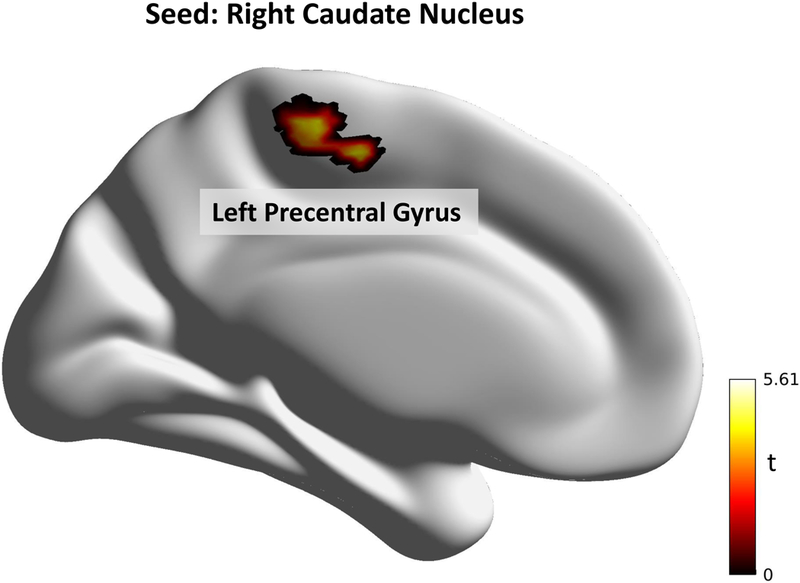
The combined sample shows girls with IBS having greater connectivity from the right caudate nucleus to the left precentral gyrus. All results shown are significant at p(FDR) < .05.
Abbreviations: t: t-value.
Subset Results (UCLA participants only)
Laboratory pain testing
There was no significant difference between IBS (mean = 39.22, SD = 18.98) and HC (mean = 31.00, SD = 13.21) in pain specific anxiety measured using the CPASS (t(17.15) = 1.22, p = .24, d = .51). There was no difference between IBS patients (mean = 1.82, SD = 2.23) and HC (mean = 2.21, SD = 2.58) in current anticipatory anxiety prior to the laboratory pain tasks (t(19.84) = −0.58, p = 0.57, d = −.24). Finally, there was a significant difference between HC (mean = 0.86, SD = 1.35) and IBS (mean = 2.82, SD = 2.64) in current pain rating (t(14.08) = 2.24, p = .041, d = 0.97).
Independent t-tests revealed differences between HC (mean = 43.78˚, SD = 4.72) and girls with IBS (mean = 46.41˚, SD = 3.34), with IBS patients showing higher, but insignificant (perhaps due to low power), baseline pain threshold (t(22.81) = 1.63, p = 0.12, d = 0.63).
Voxel-based Morphometry
To investigate how discrepancies in GMV and FC relate to pain threshold, the UCLA sample was run through the VBM and CONN procedures separately. In this smaller sample, significant differences remained with IBS patients exhibiting lower GMV in the thalamus bilaterally, left CaN and left nucleus accumbens. See Table 4 for a summary. GM intensity values for each participant were then extracted and correlations were run against variables that showed significant differences between groups. Lower GMV in the thalamus bilaterally (Left: r(22) = −.62, p = .001, p(FDR) = .008, Right: r(22) = −.51, p = 0.01, p(FDR) = 0.04) was associated with increased pain thresholds. See Figure 4. No correlations were observed with any other clinical measures.
Table 4:
Regions defined by their MNI coordinates that showed significant GM differences in the UCLA sample between IBS patients and HC.
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Voxels | t | p(FDR) | X | Y | Z |
| CLUSTER (IBS < HC) | 953 | 3.45 | 0.001 | −6 | 16 | −14 |
| L Caudate Nucleus | 5.01 | 0.002 | −10 | 14 | 0 | |
| L Nacc | 5.15 | 0.002 | −10 | −16 | −8 | |
| R Thalamus | 4.56 | 0.002 | 10 | −12 | 18 | |
| L Thalamus | 4 | 3.82 | 0.002 | −16 | −28 | 14 |
Sample show female adolescents with IBS exhibit lower GMV in the bilateral thalamus and left caudate nucleus and left nucleus accumbens.
Abbreviations: Nacc: nucleus accumbens. L: left, R: right, t : t-value, p(FDR): corrected p-value using the false discovery rate.
Figure 4:
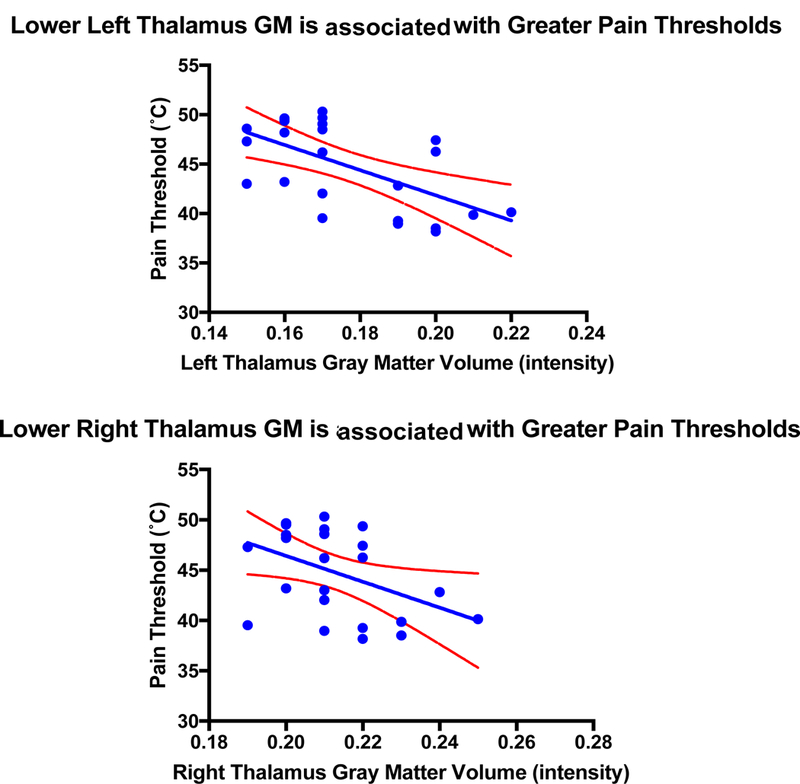
UCLA Sample – Lower GMV in the Thalamus bilaterally (Left: r = −.62, p = .001, p(FDR) = 0.008; Right: r = −.51, p = .01, p(FDR) = 0.04) is associated with increased pain thresholds. Blue lines represent line of best fit and red dotted lines represent 95% confidence intervals.
Resting State Functional Connectivity (Seed-to-Voxel)
Based on the results of the UCLA subsample VBM analysis, regions showing differences were used as seeds in a seed-voxel RS-FC analysis. IBS patients exhibited lower connectivity from the left CaN to the left middle frontal gyrus (dlPFC), compared to connectivity in those regions in HC (Ke (cluster size in voxels) = 53, beta = −0.15, t = −6.45, p(FDR) < 0.001, MNI Coordinates: X = −42, Y = 26, Z = 34). IBS patients also exhibited greater connectivity between the left thalamus and left posterior parietal cortex, a key node of the default-mode network (Ke = 48, beta = 0.14, t = 6.66, p(FDR) < 0.001, MNI Coordinates: X = −40, Y = −52, Z = 36). A visual depiction can be seen in Figure 5. No connectivity correlations were observed with clinical or behavioral variables.
Figure 5:
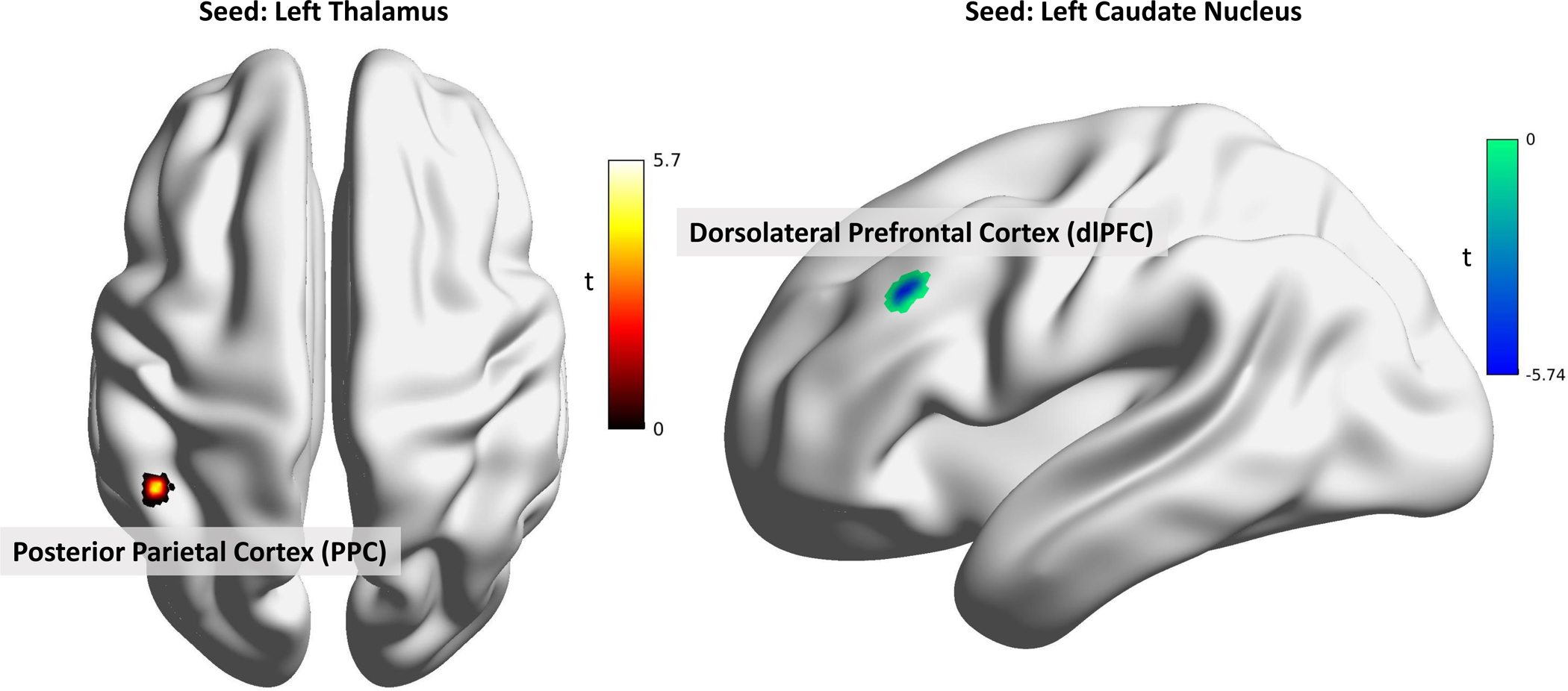
In the UCLA Sample, patients with IBS exhibited greater connectivity between the left thalamus and left posterior parietal cortex and lower connectivity between the left caudate nucleus and the left middle frontal gyrus (dlPFC) compared to HC. All results shown are significant at p(FDR) < .05.
Abbreviations: t: t-value.
Discussion
The alterations in brain structure described here in girls with IBS show striking similarities to prior studies in adults with IBS (23). Girls with IBS have reduced gray matter volume in key brain regions involved in sensory, salience and default-mode networks. Previous studies investigating children with chronic pain have also observed decreases in GMV in regions such as the caudate nucleus, nucleus accumbens, putamen, dlPFC, aMCC, and PCC (32,47). Unlike adults with IBS and other pain disorders, in whom increases in volume or cortical thickness have been noted in the somatosensory cortex, no gray matter increases were identified in this study (23,48,49). Girls with IBS also demonstrated lower resting state neural connectivity between the salience (aMCC) and default-mode (precuneus) networks, and between the sensorimotor (caudate nucleus) and central-executive network (dlPFC). Girls with IBS exhibited greater neural connectivity within the sensorimotor network (caudate nucleus and motor cortex). This suggests an alteration in the intraregional brain communication required for normal sensory processing. Furthermore, thalamus brain volume in girls with IBS was associated with pain thresholds, suggesting that this impaired communication in the sensory relay center may be related to the interpretation of pain stimuli in IBS.
Structural and functional brain alterations in girls with IBS
Our analyses found that GMV differences were prominent in regions involved in determining salience from external and internal stimuli and regulating appropriate physiological responses, (50–52) such as the aMCC, dlPFC, and ventral striatum. IBS patients also had lower GMV in the sensory regions including the thalamus, along with the dlPFC. Furthermore, patients with IBS exhibited lower RS-FC between aMCC and the precuneus, suggesting that the salience network has less integration with the default-mode network in girls with IBS. The aMCC within the salience network is involved in modulating responses in the sensorimotor and association cortices, and is considered to generate the hypervigilance to body sensations found in IBS (53–55). In neuroimaging of a visceral pain task in adolescents with IBS, the IBS group was noted to have increased connectivity of the salience network with executive control network but not sensory regions, suggesting that even in the setting of active visceral stimulation, the most prominent abnormality in IBS is in determining signal salience (54). Girls with IBS also exhibited lower RS-FC between the caudate nucleus and dlPFC, suggesting lower integration between the sensorimotor and central-executive networks, or less inhibitory control over sensory stimuli. The greater connectivity observed between the caudate and precentral gyrus and between the bilateral thalamus and PPC suggest greater communication within the sensorimotor network, and to a region in the default-mode network responsible for self-centered mental imagery and self-referential processing (56). We hypothesize that a combination of these processes could increase painful communication and interoceptive rumination in girls with IBS.
Pain sensitivity in IBS
While pain is the cardinal symptom distinguishing IBS from other functional disorders involving bowel habits, the experimental data on pain sensitivity is highly variable and the degree of sensitivity often does not correlate well with clinical symptoms. Visceral sensitivity, measured by balloon distension in the gut, was initially thought to be a biomarker of the disorder in adults, however it is now well established that only a portion of IBS patients are hypersensitive, some are normosensitive and some are even hyposensitive (57). Somatic sensitivity in adults and children with IBS has been similarly variable (13,15–17). In this study, girls with IBS tended toward higher somatic pain thresholds compared to HC, as has been observed in children and adult females with IBS (15,20). More importantly, the girls with IBS show alterations in the brain structure in regions that have a key role in pain processing and rumination (58), such as the thalamus, basal ganglia, precuneus and aMCC, as well as lower functional connectivity of pain regions with the dlPFC, suggesting that patients with IBS may have a suppressed endogenous analgesic (pain modulation) system (58,59). Correlations between experimental pain sensitivity and brain structure (thalamic GMV) in girls with IBS also differed.
Limitations
This study is limited in that it views only a cross-sectional state in children who have already developed IBS symptoms. This makes it impossible to determine whether the brain differences identified are predisposing factors to developing IBS or the result of the symptoms. While longitudinal and symptom intervention studies are required to better answer this question, the presence of these findings in this young group of children is suggestive that the brain in IBS is developing its salience and sensory circuitry differently at an early age. This study is also limited by the exclusion of boys. With the known sex differences in pain processing and brain development, these results cannot be extrapolated to male IBS patients. The size of the current sample must also be taken into consideration, with larger studies needed for reliable replication.
Conclusions
As has been shown in adults with IBS, girls with IBS have significant brain structural and functional differences compared to healthy peers. Disruption of the salience network and its communication with other brain regions should be a target of additional studies across the lifespan. Insight into the clinical relevance of these differences will be elucidated via studies incorporating interventions aimed at brain-gut neural signaling to capture those brain changes most associated with symptom improvement. Children, with rapidly developing brains and a risk for lifelong vulnerability to chronic pain (60,61), should be a focus of such studies.
Acknowledgments
Sources of Funding: The research described was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124 (CTRC Seed Grant; PI’s: Jennie Tsao and Kirsten Tillisch), NIH Grant Number R01 NR013497 (PI: Robert J. Shulman), the Daffy’s Foundation (PI: Robert J. Shulman), the Pediatric Cancer Research Foundation, Arthur Wallace Pediatric Cancer Research Fund, and the UCLA Division of Pediatric Hematology Oncology (UCLA Pediatric Hematology Oncology Seed Grant; PI: Lonnie Zeltzer).
Footnotes
Conflicts of Interest:
The authors report no conflicts of interest
References
- 1.Kaur Sandhu B, Prosad Paul S. Irritable bowel syndrome in children: Pathogenesis, diagnosis and evidence-based treatment. World Journal of Gastroenterology [Internet] 2014. [cited 2017 Mar 16];20:6013–23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4033441/pdf/WJG-20-6013.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable syndrome in adolescents: A community-based study bowel. The Journal of Pediatrics [Internet] 1996. [cited 2017 Mar 13];129:220–26. Available from: http://www.jpeds.com/article/S0022-3476(96)70246-9/pdf [DOI] [PubMed] [Google Scholar]
- 3.El-Matary W, Spray C, Sandhu B. Irritable bowel syndrome: the commonest cause of recurrent abdominal pain in children. European Journal of Pediatrics [Internet] 2004. [cited 2017 Mar 13];163:584–88. Available from: http://download.springer.com/static/pdf/530/art%253A10.1007%252Fs00431-004-1503-0.pdf?originUrl=http%3A%2F%2Flink.springer.com%2Farticle%2F10.1007%2Fs00431-004-1503-0&token2=exp=1489442860~acl=%2Fstatic%2Fpdf%2F530%2Fart%25253A10.1007%25252Fs00431-004-150 [DOI] [PubMed] [Google Scholar]
- 4.Varni JW, Lane MM, Burwinkle TM, Fontaine EN, Youssef NN, Schwimmer JB, Pardee PE, Pohl JF, Easley DJ. Health-related quality of life in pediatric patients with irritable bowel syndrome: a comparative analysis. Journal of developmental and behavioral pediatrics : JDBP 2006;27:451–58. [DOI] [PubMed] [Google Scholar]
- 5.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain [Internet] 2010. [cited 2018 Mar 12];150:568–72. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3040563/pdf/nihms270095.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer EA, Tillisch K. The Brain-Gut Axis in Abdominal Pain Syndromes. Annual Review of Medicine [Internet] 2011. [cited 2017 Mar 13];62:381–96. Available from: http://www.annualreviews.org/doi/pdf/10.1146/annurev-med-012309-103958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsenbruch S Abdominal pain in Irritable Bowel Syndrome: A review of putative psychological, neural and neuro-immune mechanisms. Brain Behavior and Immunity [Internet] 2011. [cited 2017 Mar 13];25:386–94. Available from: http://ac.els-cdn.com/S0889159110005659/1-s2.0-S0889159110005659-main.pdf?_tid=64521064-082a-11e7-949d-00000aab0f26&acdnat=1489436562_b39aa0db891f4d902c6e677f3b1d0c3a [DOI] [PubMed] [Google Scholar]
- 8.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nature reviews. Gastroenterology & Hepatology [Internet] 2015;12:592–605. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26303675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt BA. Pain and Emotion Interactions in Subregions of the Cingulate Gyrus. Nature Reviews Neuroscience [Internet] 2005. [cited 2017 Mar 13];6:533–44. Available from: http://www.nature.com/nrn/journal/v6/n7/pdf/nrn1704.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients With Irritable Bowel Syndrome Have Altered Emotional Modulation of Neural Responses to Visceral Stimuli. Gastroenterology [Internet] 2010. [cited 2017 Mar 13];139:1310–19. Available from: http://www.gastrojournal.org/article/S0016-5085(10)00963-7/pdf [DOI] [PubMed] [Google Scholar]
- 11.Bogdanov VB, Viganò A, Noirhomme Q, Bogdanova OV, Guy N, Laureys S, Renshaw PF, Dallel R, Phillips C, Schoenen J. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behavioural Brain Research [Internet] 2015. [cited 2016 Dec 18];281:187–98. Available from: 10.1016/j.bbr.2014.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moshiree B, Price DD, Robinson ME, Gaible R, Verne GN. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. The Clinical Journal of Pain [Internet] 2007;23:323–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17449993 [DOI] [PubMed] [Google Scholar]
- 13.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain 2001;93:7–14. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues AC, Verne GN, Schmidt S, Mauderli AP. Hypersensitivity to cutaneous thermal nociceptive stimuli in irritable bowel syndrome. Pain 2005;115:5–11. [DOI] [PubMed] [Google Scholar]
- 15.Chang L, Mayer EA, Johnson T, Fitzgerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain 2000;84:297–307. [DOI] [PubMed] [Google Scholar]
- 16.Cook I, van Eeden A, Collins S. Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. Gastroenterology 1987;93:727–33. [DOI] [PubMed] [Google Scholar]
- 17.Accarino AM, Azpiroz F, Malagelada J-R. Selective Dysfunction of Mechanosensitive Intestinal Afferents in Irritable Bowel Syndrome. Gastroenterology [Internet] 1995. [cited 2017 Apr 19];108:636–43. Available from: http://www.gastrojournal.org/article/0016-5085(95)90434-4/pdf [DOI] [PubMed] [Google Scholar]
- 18.Iovino P, Tremolaterra F, Consalvo D, Sabbatini F, Mazzacca G, Ciacci C. Perception of electrocutaneous stimuli in irritable bowel syndrome. American Journal of Gastroenterology 2006;101:596–603. [DOI] [PubMed] [Google Scholar]
- 19.Williams AE, Heitkemper M, Self MM, Czyzewski DI, Shulman RJ. Endogenous Inhibition of Somatic Pain is Impaired in Girls with Irritable Bowel Syndrome Compared with Healthy Girls. Journal of Pain 2013;14:921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zohsel K, Hohmeister J, Flor H, Hermann C. Somatic Pain Sensitivity in Children With Recurrent Abdominal Pain. American Journal of Gastroenterology 2008;103:1517–23. [DOI] [PubMed] [Google Scholar]
- 21.Hermann C, Zohsel K, Hohmeister J, Flor H. Cortical correlates of an attentional bias to painful and innocuous somatic stimuli in children with recurrent abdominal pain. Pain 2008;136:397–406. [DOI] [PubMed] [Google Scholar]
- 22.Feuerstein M, Barr RG, Francoeur TE, Houle M, Rafman S, Stairs GW. Potential Biobehavioral Mechanisms of Recurrent Abdominal Pain in Children. Pain [Internet] 1982. [cited 2017 Apr 19];13:287–98. Available from: https://www.researchgate.net/profile/Michael_Feuerstein2/publication/16077188_Potential_biobehavioral_mechanisms_of_recurrent_abdominal_pain_in_children/links/56a3d54608aeef24c589d0c7.pdf [DOI] [PubMed] [Google Scholar]
- 23.Labus J, Dinov I, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong J-Y, Gupta A, Tillisch K, Gutman B, Ebrat B, Hobel S, Joshi S, Thompson P, Toga A, Mayer E. Irritable Bowel Syndrome in female patients is associated with alterations in structural brain networks. Pain 2014;155:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut [Internet] 2010. [cited 2017 Apr 20];59:489–95. Available from: http://gut.bmj.com/content/gutjnl/59/4/489.full.pdf [DOI] [PubMed] [Google Scholar]
- 25.Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology [Internet] 2005;65:1268–77. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed7&NEWS=N&AN=2005479696 [DOI] [PubMed] [Google Scholar]
- 26.Mayer EA, Gupta A, Kilpatrick LA, Hong J-Y. Brain mechanisms in chronic visceral pain. Pain [Internet] 2015;156 Suppl:S50–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25789437%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4428597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: A Rome Working Team Report. Neurogastroenterology and Motility 2009;21:579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blankstein U, Chen J, Diamant NE, Davis KD. Altered Brain Structure in Irritable Bowel Syndrome: Potential Contributions of Pre-Existing and Disease-Driven Factors. Gastroenterology [Internet] 2010. [cited 2018 Apr 16];138:1783–89. Available from: https://ac.els-cdn.com/S0016508509022409/1-s2.0-S0016508509022409-main.pdf?_tid=862a536c-7f0f-4990-a12f-f6db6c49ae5c&acdnat=1523903171_8f616c51b28a48b1b2a79d3e276ae1d6 [DOI] [PubMed] [Google Scholar]
- 29.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical Thinning in IBS: Implications for Homeostatic, Attention, and Pain Processing. Neurology 2008;70:153–54. [DOI] [PubMed] [Google Scholar]
- 30.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology 2010;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tillisch K, Mayer EA, Labus JS. Quantitative Meta-analysis Identifies Brain Regions Activated During Rectal Distension in Irritable Bowel Syndrome. Gastroenterology [Internet] 2011. [cited 2017 Apr 20];140:91–100. Available from: http://ac.els-cdn.com/S0016508510011510/1-s2.0-S0016508510011510-main.pdf?_tid=5fe06906-2600-11e7-bb6a-00000aacb361&acdnat=1492717050_419e90e2d44c72c071831e10c4489084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubbard CS, Becerra L, Heinz N, Ludwick A, Rasooly T, Wu R, Johnson A, Schechter NL, Borsook D, Nurko S. Abdominal pain, the adolescent and altered brain structure and function. PLoS ONE [Internet] 2016;11:1–30. Available from: 10.1371/journal.pone.0156545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker KS, Gibson S, Georgiou-Karistianis N, Roth RM, Giummarra MJ. Everyday Executive Functioning in Chronic Pain. The Clinical Journal of Pain [Internet] 2016;32:673–80. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00002508-900000000-99220 [DOI] [PubMed] [Google Scholar]
- 34.Naliboff BD, Munakata J, Chang L, Mayer EA. Towards a Biobehavioral Model of Visceral Hypersensitivity In Irritable Bowel Syndrome. Journal of Psychosomatic Research [Internet] 1998. [cited 2017 Nov 16];45:485–92. Available from: https://ac.els-cdn.com/S002239999800049X/1-s2.0-S002239999800049X-main.pdf?_tid=37734ce2-cb58-11e7-a094-00000aab0f01&acdnat=1510896729_0c2446afca534ab01761d5b182b5247c [DOI] [PubMed] [Google Scholar]
- 35.Miró J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. European Journal of Pain [Internet] 2009;13:1089–95. Available from: 10.1016/j.ejpain.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 36.Spielberger CD. State-Trait Anxiety Inventory (STAI). Mind Garden 1983;94061:261–3500. [Google Scholar]
- 37.Pagé GM, Fuss S, Martin AL, Escobar MRE, Katz J. Development and Preliminary Validation of the Child Pain Anxiety Symptoms Scale in a Community Sample. Journal of Pediatric Psychology [Internet] 2010. [cited 2017 Apr 17];35:1071–82. Available from: https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jpepsy/35/10/10.1093/jpepsy/jsq034/2/jsq034.pdf?Expires=1492821896&Signature=IEUMeD-pidSUc9ydkl7iT~Vf-bf4gtcUvwuIYxaNbBOY-t5ykoV6Vz3~lxhmUSIruIjSxDDr2LGeeF-s4-YoRdgwMKvLqk1Nx00Wto7cLHMU2e [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage [Internet] 2004. [cited 2017 Apr 17];23:S208–19. Available from: http://ac.els-cdn.com/S1053811904003933/1-s2.0-S1053811904003933-main.pdf?_tid=8cdb3f60-23d2-11e7-bdeb-00000aacb35e&acdnat=1492477467_8ef9c01f6f7b09ad2f888e25b1fca9ee [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging [Internet] 2001;20:45–57. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-0034745001&partnerID=tZOtx3y1 [DOI] [PubMed] [Google Scholar]
- 40.Desouza DD, Moayedi M, Chen DQ, Davis KD, Hodaie M, Binshtok A. Sensorimotor and Pain Modulation Brain Abnormalities in Trigeminal Neuralgia: A Paroxysmal, Sensory-Triggered Neuropathic Pain. PLoS ONE [Internet] 2013. [cited 2018 Jun 3];8:e66340 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3688879/pdf/pone.0066340.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: A toolbox for creating customized pediatric templates. NeuroImage [Internet] 2008. [cited 2017 Sep 26];41:903–13. Available from: http://dbm.neuro.uni-jena.de/pdf-files/wilke-ni08.pdf [DOI] [PubMed] [Google Scholar]
- 42.Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage [Internet] 2001. [cited 2017 Apr 17];14:21–36. Available from: http://ac.els-cdn.com/S1053811901907864/1-s2.0-S1053811901907864-main.pdf?_tid=21b8344e-23d3-11e7-9560-00000aacb361&acdnat=1492477717_0caf99a28fa0de6dd86a0538f5b6b13f [DOI] [PubMed] [Google Scholar]
- 43.Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, Fox NC, Ridgway GR. Accurate automatic estimation of total intracranial volume: A nuisance variable with less nuisance. NeuroImage 2015;104:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage [Internet] 2008. [cited 2017 Apr 17];44:83–98. Available from: http://ac.els-cdn.com/S1053811908002978/1-s2.0-S1053811908002978-main.pdf?_tid=786a6528-23d3-11e7-8e5e-00000aacb35f&acdnat=1492477862_0112451637ccfc1d10898095f5688bde [DOI] [PubMed] [Google Scholar]
- 45.Xia M, Wang J, He Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity 2012;2:125–41. [DOI] [PubMed] [Google Scholar]
- 47.Erpelding N, Simons L, Lebel A, Serrano P, Pielech M, Prabhu S, Becerra L, Borsook D. Rapid treatment-induced brain changes in pediatric CRPS. Brain Structure and Function 2016;221:1095–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, Farmer MA, Ness TJ, Deutsch G, Mayer EA, Mackey S, Vania Apkarian A, Maravilla K, Clauw DJ, Harris RE. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Patients with Interstitial Cystitis/Painful Bladder Syndrome. Journal of Urology [Internet] 2015. [cited 2017 Mar 9];193:131–37. Available from: http://ac.els-cdn.com/S002253471404213X/1-s2.0-S002253471404213X-main.pdf?_tid=a6983480-04fb-11e7-9866-00000aab0f6c&acdnat=1489086633_3674362bd0c14a0f617089f2a4cc1b06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong J, Spaeth RB, Wey H-Y, Cheetham A, Cook AH, Jensen K, Tan Y, Liu H, Wang D, Loggia ML, Napadow V, Smoller JW, Wasan AD, Gollub RL. S1 is associated with chronic low back pain: a functional and structural MRI study. Molecular Pain [Internet] 2013. [cited 2017 May 14];9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3765748/pdf/1744-8069-9-43.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menon V Salience Network. Brain Mapping: An Encyclopedia Reference [Internet] 2015. [cited 2017 May 4];2:597–611. Available from: https://med.stanford.edu/content/dam/sm/scsnl/documents/Menon_Salience_Network_15.pdf [Google Scholar]
- 51.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: The value of salience circuits. Progress in Neurobiology [Internet] 2013. [cited 2017 Apr 9];104:93–105. Available from: http://ac.els-cdn.com/S0301008213000130/1-s2.0-S0301008213000130-main.pdf?_tid=041c104a-1d75-11e7-8ca2-00000aab0f01&acdnat=1491777587_ae7ba34f26f669f46d6b645fe9bf2db6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: A salience detection system for the body. Progress in Neurobiology [Internet] 2011. [cited 2017 Apr 9];93:111–24. Available from: http://ac.els-cdn.com/S0301008210001759/1-s2.0-S0301008210001759-main.pdf?_tid=c4b94d5c-1d77-11e7-8c6b-00000aab0f02&acdnat=1491778769_fda3f13d92e558f23ac349e545e8da64 [DOI] [PubMed] [Google Scholar]
- 53.Legrain V, Iannetti GD, On Plaghki L, Mouraux A. The pain matrix reloaded: A salience detection system for the body. Progress in Neurobiology [Internet] 2011. [cited 2017 Mar 18];93:111–24. Available from: http://ac.els-cdn.com/S0301008210001759/1-s2.0-S0301008210001759-main.pdf?_tid=803aac7e-0c3e-11e7-ac8e-00000aab0f02&acdnat=1489885003_ade4729b0536f42f6cb38a5713faf026 [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Silverman A, Kern M, Ward BD, Li SJ, Shaker R, Sood MR. Excessive coupling of the salience network with intrinsic neurocognitive brain networks during rectal distension in adolescents with irritable bowel syndrome: A preliminary report. Neurogastroenterology and Motility 2016;28:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function [Internet] 2010. [cited 2018 Mar 22];214:655–67. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2899886/pdf/nihms212978.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–83. [DOI] [PubMed] [Google Scholar]
- 57.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Vol. 41, Pain 1990. p. 167–234. [DOI] [PubMed] [Google Scholar]
- 58.Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia - insights gained through human functional imaging. Molecular Pain [Internet] 2010;6:27 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20465845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seminowicz DA, Moayedi M. The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. The Journal of Pain [Internet] 2017. [cited 2017 Aug 14];18:1027–35. Available from: http://ac.els-cdn.com/S152659001730531X/1-s2.0-S152659001730531X-main.pdf?_tid=8d9d71f2-8183-11e7-9c50-00000aab0f26&acdnat=1502778947_36c232e80235d8c15c9c3e4d58b7a032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Supekar K, Musen M, Menon V. Development of Large-Scale Functional Brain Networks in Children. PLoS Biology [Internet] 2009. [cited 2017 May 7];7:e1000157 Available from: http://journals.plos.org/plosbiology/article/file?id=10.1371/journal.pbio.1000157&type=printable [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menon V Developmental pathways to functional brain networks: emerging principles. Trends in Cognitive Sciences 2013;17:627–40. [DOI] [PubMed] [Google Scholar]


