Abstract
Objective:
Both isoniazid preventive therapy (IPT) and antiretroviral therapy (ART) reduce tuberculosis risk in individuals living with HIV. We sought to estimate the broader, population-wide impact of providing a pragmatically-implemented 12-month IPT regimen to ART recipients in a high-burden community.
Design:
Dynamic transmission model of a tuberculosis-HIV epidemic, calibrated to site-specific, historical epidemiologic and clinical trial data from Khayelitsha, South Africa.
Methods:
We projected the five-year impact of delivering a 12-month IPT regimen community-wide to 85% of new ART initiators and 15%/year of those already on ART, accounting for IPT-attributable reductions in TB infection, progression, and transmission. We also evaluated scenarios of continuously-delivered IPT, ongoing ART scale-up, and lower tuberculosis incidence.
Results:
Under historical (early 2010s) ART coverage, this ART-linked IPT intervention prevented one tuberculosis case per 18 (95% credible interval [CrI] 11–29) people treated. It lowered tuberculosis incidence by a projected 23% (95%CrI 14–30%) among people receiving ART, and by 5.2% (95%CrI 2.9–8.7%) in the total population. Continuous IPT reduced the number needed to treat to prevent one case of tuberculosis to 10 (95%CrI 7–16), though it required 74% more person-years of therapy (95%CrI 64–94%) to prevent one TB case, relative to 12-month therapy. Under expanding ART coverage, the tuberculosis incidence reduction achieved by 12-month IPT grew to 7.6% (95%CrI 4.3–12.6%). Effect sizes were similar in a simulated setting of lower tuberculosis incidence.
Conclusions:
IPT in conjunction with ART reduces tuberculosis incidence among those who receive therapy and has additional impact on tuberculosis transmission in the population.
Keywords: tuberculosis, antiretroviral therapy, Africa, latent tuberculosis, tuberculosis prevention & control, transmission, mathematical models
Introduction
Meeting the ambitious targets to reduce tuberculosis (TB) incidence and mortality by 2025 and beyond [1] will require innovative TB control strategies tailored to specific epidemiological settings [2,3]. In areas with a large TB-HIV burden, isoniazid preventive therapy (IPT) and newer regimens [4,5] to prevent TB disease among people living with HIV (PLWH) may play an important role. IPT is known to reduce TB risk for the individual PLWH who receive it [6–8]. Nevertheless, initial efforts at large-scale deployment of IPT have had mixed success [9], and its broader public health impact remains uncertain. Mathematical models can be a useful tool for evaluating how the patient-level effects of preventive therapy, as measured in clinical trials, may translate into population-wide impact.
PLWH on antiretroviral therapy (ART) remain at elevated TB risk compared to people without HIV [10], and two randomized trials have shown additional benefit from IPT among people prescribed ART. In Ivory Coast, a six-month IPT regimen among PLWH (median CD4+ T cell count 463) receiving early or delayed ART lowered incident TB by 56% (31–72%) during the first 2.5 years of follow up and lowered mortality by 37% (3–59%) after 4.9 years (median) of follow up [11,12]. In Khayelitsha, South Africa, a 12-month IPT regimen among PLWH (median CD4+ T cell count 216) showed a 37% (6–59%) reduction in the hazard of TB over a median follow up time of 2.5 years [8].
Here, we investigated the extent to which the individual-level reductions in TB risk achieved by providing IPT to ART recipients are likely to alter TB epidemics at the broader population level. We used detailed site-specific epidemiologic and demographic data and observed effects of IPT treatment from the IPT trial site in Khayelitsha [8] to estimate the potential population-level impact of a pragmatically implemented 12-month IPT regimen for persons on ART in this very-high-burden setting.
Materials and Methods
Study Population
The parent study [8] randomly assigned 1,329 adults attending an HIV clinic and receiving ART to receive 12 months of IPT or placebo and followed participants for a median of 2.5 years (between 2008 and 2011) with a primary endpoint of time to development of incident active TB.
Khayelitsha, an urban community on the outskirts of Cape Town, had a population of approximately 392,000 at the time of the trial [13]. Antenatal HIV prevalence was estimated at 33% [14], and large governmental and NGO programs [15] achieved high ART coverage [16]. Approximately 1,600 cases of active TB per 100,000 residents were detected each year from 2008 to 2010 [15].
TB Transmission Model
We constructed a compartmental model of TB in Khayelitsha, based on a system of ordinary differential equations (Supplemental Digital Content 1). The model is divided into 4 core TB compartments (Figure 1A), representing no current or prior infection (“susceptible”); recent infection with TB (a high-risk period for progression to active TB, through which all newly-infected populations pass and during which their potential to benefit from preventive therapy is greatest); more remote TB infection (with lower risk of progression); and active TB (infectious and with increased mortality). Treatment, with subsequent risk of relapse, is modeled as a return to the remote-infection state. We allow for a parallel series of compartments representing multi-drug resistant (MDR) TB, which in our simplified representation is unaffected by IPT (see Supplemental Digital Content 1 for other details).
Figure 1. Model schematic.

Boxes represent states within the model, and arrows represent flows between states. The model population is compartmentalized across multiple dimensions, including a core TB state, an IPT state (with the effect of any prior IPT being lost if active TB develops or if new infection occurs after IPT ends), an HIV state, and (if TB infected) a TB drug susceptibility state.
*Infection or reinfection with drug-susceptible TB is prevented while on IPT, and progression (rapid and delayed) of drug-susceptible TB is reduced while on IPT and reduced by a weaker factor after IPT has ended. IPT has no effect on drug-resistant TB infection or progression. TB, tuberculosis; IPT, isoniazid preventive therapy; ART, antiretroviral therapy.
The model incorporates HIV through a series of compartments (Figure 1C) representing HIV infection, natural history (conceptualized in relation to CD4+ T-cell count), and uptake of ART. ART is assumed to immediately confer partial protection against progression to active TB (represented as a reduction in risk within a given CD4-count stratum), followed by increasing protection over time (as populations on ART cross into higher CD4-count strata). PLWH may initiate ART either at the time of TB treatment, or at CD4-dependent rates outside of TB diagnosis. When active TB is not the reason for ART initiation, ART initiators are screened for active TB, with the sensitivity of the Xpert MTB/RIF assay [17]. Those found to have active TB are provided with a course of IPT after TB treatment completion, affording them the potential benefits of more-prolonged isoniazid therapy [18]. To reflect treatment guidelines at the time of the trial [19], ART initiation in the absence of active TB occurred only among populations with CD4<500 (mostly at CD4<200 [20]) during model calibration, although expanded ART eligibility is considered in some projections of IPT’s future impact.
We assumed that IPT has three individual-level effects, as illustrated in Figure 1B: (1) temporary high-level protection against progression of previously-acquired latent non-MDR TB to active disease while taking IPT; (2) long-term lower-level protection against progression of previously-acquired latent non-MDR TB to active disease after completing a course of IPT; and (3) complete protection against non-MDR TB infection or reinfection while taking IPT. IPT was assumed to have no effect on underlying active TB that was missed by pre-IPT screening and no effect on active TB that developed during preventive therapy.
Model Calibration
We used an approximate Bayesian method [21] to calibrate steady-state models that reflected the TB-HIV epidemic in Khayelitsha at the time of the parent trial. We first drew 120,000 samples of model parameters (Table 1), from distributions based on published literature, using Latin Hypercube sampling. For each sampled parameter set, we ran the corresponding model to steady state and estimated a pseudo-likelihood by comparing six key population-level outputs of the model to log-normal or logit-normal distributions that corresponded to published data. The six calibration targets (Table 2) included TB notifications, TB-associated mortality, MDR prevalence among TB cases initiating curative treatment, HIV prevalence in the population, proportion of ART initiators with CD4 T-cell count <200 cells/mm3, and active TB prevalence among those who would have enrolled in the parent trial; each was treated as independent and equally weighted.
Table 1.
Model input parameters.
| Parameter | Mean of Sampled Distribution | Sampled Range* | References |
|---|---|---|---|
| TB transmission | |||
| Number of effective contacts for TB transmission per infectious person-year | 8 | 4–12 | [36] |
| Relative transmissibility of TB in HIV+ (reflecting propensity to be smear negative) |
0.6 | 0.36–0.84 | [37] |
| Fraction of new TB infections that are multi-drug resistant (MDR) | 0.05 | 0.03–0.07 | Assumed, targeting 4% among notified TB |
| Relative risk of reinfection, latently-infected versus uninfected# | 0.5 | 0.2–0.8 | [38–41] |
| Percentage of 15-year-olds with latent TB infection | 57% | 30–84% | [42] |
| TB natural history | |||
| Duration of “recently-infected” period with elevated risk for fast progression to active TB after initial infection | 2 years | 1.4–3.3 (rate of progression sampled uniformly) | [43] |
| Rate of rapid progression of latent TB after recent infection (HIV-) | 0.05 years−1 | 0.02–0.08 | [43] |
| Rate of rapid progression of latent TB after recent infection (HIV+, CD4<200#, not on ART) | 0.6 years−1 | 0.3–0.9 | [44] |
| Rate of slow progression of latent TB to active (HIV-) |
0.001 years−1 | 0.0005–0.0015 | [45,46] |
| Rate of slow progression of latent TB to active (HIV+, CD4<200#, not on ART) |
0.20 years−1 | 0.05–0.35 | [10,47,48] |
| Relative risk of TB progression and of HIV mortality, HIV+ on ART versus HIV+ not on ART, within the same CD4 count stratum | 0.6 | 0.3–0.9 | [49–51] |
| Relative risk of TB progression, CD4 200–500 versus CD4 <200 | 0.65 | 0.3–1.0 | [50] |
| Relative risk of TB progression, CD4 >500 versus CD4 <200 | 0.3 | 0.18–0.42 | [50] |
| TB treatment | |||
| Rate of resolution or cure of active drug-susceptible TB (HIV-)§ | 0.7 years−1 | 0.3–1.1 | [52], accounting for mortality |
| Rate of resolution or cure of active drug-susceptible TB (HIV+ CD4<200)§ | 1.1 years−1 | 0.5–1.7 | [52], accounting for mortality |
| Rate of resolution or cure of active MDR TB§ | 0.45 years−1 | 0.30–0.60 | Assumed 6- to 9-month delay |
| TB specific excess mortality rate (HIV-) | 0.10 years−1 | 0.05–0.15 | [53] |
| TB specific excess mortality rate (HIV+, CD4<200#, not on ART) | 0.25 years−1 | 0.10–0.40 | [54] |
| Sensitivity of Xpert MTB/RIF screening for active TB | 73% | 65–81% | [17] |
| Proportion of patients not initiating TB treatment after TB diagnosis | 18% | 11–25% | [55] |
| Proportion not initiating and adhering to ART during TB treatment, of TB patients not already on ART | 60% | 40–80% | Reflecting local practice, 2008–2012 |
| HIV and other | |||
| Annual rate of new HIV infection | 0.023 years−1 | 0.015–0.031 | [56] |
| HIV specific excess mortality rate (CD4<200), exclusive of TB | 0.14 years−1 | 0.080–0.200 | [57–59] |
| HIV specific excess mortality rate (200<CD4<500), exclusive of TB | 0.05 years−1 | 0.030–0.070 | [57–60] |
| HIV specific excess mortality rate (CD4>500, exclusive of TB | 0.01 years−1 | 0.006–0.014 | [57–59] |
| Rate of HIV progression from [CD4>500] to [200<CD4<500] | 0.11 years−1 | 0.04–0.18 | [61–63] |
| Rate of HIV progression from [200<CD4<500] to [CD4<200] | 0.20 years−1 | 0.05–0.35 | [61,62] |
| Net rate of ART initiation (initiation minus discontinuation),† 200<CD4<500 | 0.055 years−1 | 0.01–0.1 | [20,64] |
| Net rate of ART initiation (initiation minus discontinuation),† CD4<200 | 0.70 years−1 | 0.20–1.20 | [56,65,66] |
| CD4+ reconstitution after ART initiation: rate of transitioning from CD4<200 to 200<CD4<500 | 1.50 years−1 | 0.50–2.50 | [50] |
| CD4+ reconstitution after ART initiation: rate of transitioning from 200<CD4<500 to CD4 >500 | 0.20 years−1 | 0.05–0.35 | [50] |
| Background mortality rate | 0.03 years−1 | 0.025–0.035 | [67] |
Priors were set by default to a symmetric beta distribution with shape parameter of 3.
Rate at which people with active TB recover or are cured. This parameter together with mortality rates determine the duration of active TB.
For HIV stages with higher CD4, TB progression and TB mortality rates are an average of the HIV- and the HIV+, CD4<200 rates, and protection if latently infected is an average of the HIV- value and the 0 that is assumed for advanced HIV.
Estimated indirectly from proportion of ART initiators with CD4>200 and with CD4>100, accounting for competing rates of mortality, CD4 depletion, and ART initiation. No ART initiation modeled for CD4>500, except in the expanding-ART scenario.
Table 2.
Baseline Model Calibration (without IPT)
| Target distribution | Model Output | Target Source | ||||
|---|---|---|---|---|---|---|
| Median | Inner 95% | Shape | Median | 95% Credible interval | ||
| Outputs used for calibration of baseline epidemic | ||||||
| TB Notifications (per 100,000 population/yr) | 1630 | 1481–1794 | Log-normal | 1657 | 1544–1830 | [15] |
| Mortality in those with active TB (including HIV+) (per 100,000 population/yr) | 500 | 298–826 | Log-normal | 490 | 345–517 | [68], South Africa, scaled for higher local TB incidence |
| Percentage of cured TB cases that are MDR-TB (ineffective treatment attempts are not modeled) | 4.0% | 2.97–5.37 | Logit-normal | 4.4% | 3.4–5.5 | [68,69]¶ |
| Adult HIV Prevalence (%) | 26.5% | 22.2–31.2 | Logit-normal | 24.3% | 21.4–27.7 | Antenatal prevalence survey [70] adjusted per [71] |
| Percentage of ART initiators with CD4>200 (%) | 39% | 30–48 | Log-normal | 42% | 36–50 | [20] and Cape Town programmatic data, 2010–2011 |
| Percentage of potential IPT initiators with active TB† | 11.7% | 10.4–13.1 | Logit-normal | 11.1% | 9.9–12.1 | [8] |
weighted average of MDR proportions among local new and previously treated cases, weighted based on the ratio of new:retreatment notifications in South Africa-wide data in WHO Global TB Report 2013.
weighted average of new ART initiators and those already on ART, based on the relative proportions of each who enrolled in the IPT trial.
The different components of IPT’s overall protective effect, whose mechanisms our model distinguishes, will contribute to different degrees under different conditions of IPT duration, follow-up period, and TB exposure frequency. To parameterize the protective effects against TB progression during and after IPT, we performed a simulated trial designed to recapitulate the parent trial (see Supplemental Digital Content 1). We compared hazard ratios for TB among IPT recipients versus controls in the actual and simulated trials as an additional calibration step, giving this fit equal weight as that to the six epidemiological outputs above.
Assigning each set of parameters a probability weight (pseudo-likelihood) based on its ability to fit both epidemiological data and results of the parent trial, we re-sampled by weights to create an approximate posterior distribution of 100,000 sets of model parameters. Except where otherwise specified, we present numerical results as the median from this approximate posterior distribution, with 95% credible intervals reflecting the 2.5 and 97.5 percent quantiles.
Interventions
In each of the 100,000 selected simulations, we modeled the implementation of a pragmatic, ART-linked IPT intervention. Specifically, we first assumed that of all individuals initiating ART, 85% also initiated and were sufficiently adherent to the IPT regimen prescribed; the remaining 15% of ART initiators (the proportion lost to follow up in the parent study [8]) did not receive sufficient IPT to derive benefit. We also included a catch-up component of the intervention, in which, each year, IPT was also provided to 15% of those who were on ART but not already benefitting from current or past IPT. Where not otherwise specified, we modeled a 12-month IPT regimen, delivered in association with the historic levels of ART coverage at which the model was calibrated. In secondary analyses, we also projected (1) the impact of continuous IPT (continued throughout the remainder of the five-year analysis period) [22] and (2) the impact of expanding levels of ART coverage. ART was expanded by allowing those with CD4>500 to start ART at the same rate as those with CD4 200–500, gradually increasing the ART initiation rates for all groups, and decreasing HIV transmission in proportion to the decreasing prevalence of untreated HIV infection (details in Supplemental Digital Content 1).
In a sensitivity analysis, we considered the 5-year impact of our IPT intervention in settings of more moderate TB incidence (details in Supplemental Digital Content 1). We also used partial rank correlation coefficients to explore the dependence of key results on individual parameters after adjustment for all other model parameters.
Results
Population-level Impact of IPT
The calibrated model reproduced key features of the TB and HIV epidemics of Khayelitsha (Table 2, and text sections S4.1-S4.2 of Supplemental Digital Content 1). Over a five-year period in absence of IPT, the resulting model projected 34,000 TB cases, with 7,000 TB-related deaths, among adults in Khayelitsha (population 392,000). Of the projected cases, 28,000 were among PLWH, and 10,000 were among people receiving ART.
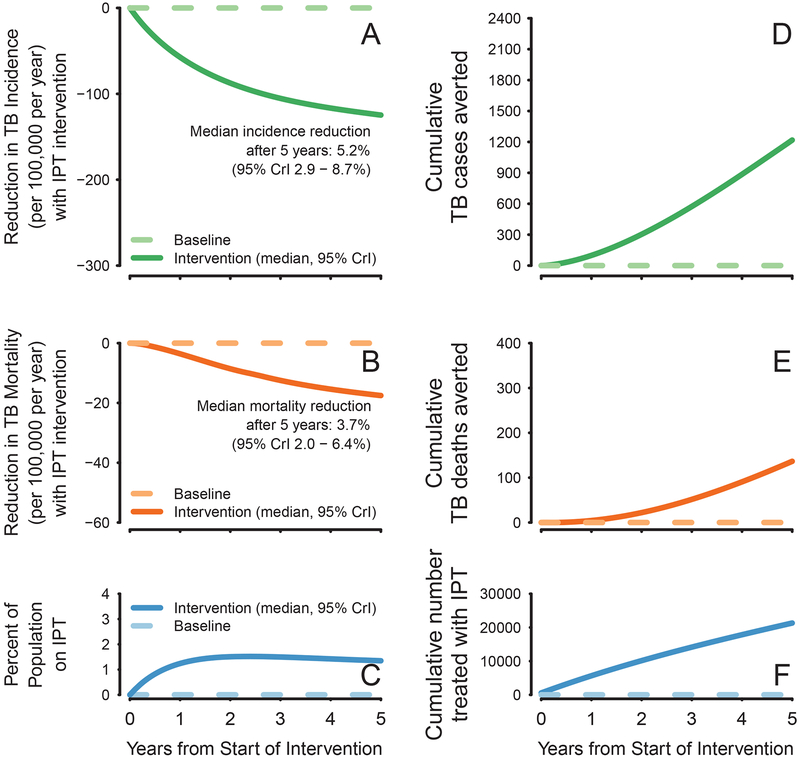
IPT was projected to reach 5.4% (95% credible interval [CrI] 4.6–6.6%) of the total population after five years, with less than 2% of the total population (but up to 7% of all PLWH and 14% of those currently on ART) taking IPT at any single time (Figure 2C). Treating this small subpopulation with a 12-month IPT regimen averted 1,219 TB cases (95%CrI 732–1,925) and 137 TB-related deaths (95%CrI 70–245) over 5 years (Figure 2A–2B). Thus, IPT was projected to avert one case of active TB for every 18 (95%CrI 11–29) individuals treated, and one TB death for every 158 (95%CrI 87–314) individuals treated. By the end of year 5, we projected a 5.2% (95%CrI 2.9–8.7%) reduction in the TB incidence rate and a 3.7% (95%CrI 2.0–6.4%) reduction in the TB mortality rate within the general population. Among individuals on ART, we projected a 23% (95%CrI 14–30%) reduction in TB incidence by the end of year 5. Over a longer time horizon, the projected impact of continuing to provide 12-month IPT to eligible individuals grew modestly, reaching median reductions in population-level TB incidence and mortality of 6.1% and 5.0%, respectively, after 15 years.
Figure 2. Population Level Impact of IPT.
Panels A and B illustrate the median and 95% credible interval of reductions in incidence (green), reductions in TB mortality (orange), and people treated with IPT (blue) in the IPT intervention scenario, both at any given time (A-C) and cumulatively over 5 years of IPT program implementation (D-F), in the Khayelitsha population of 392,000 with TB incidence and TB mortality of 2408 (95\% CrI 2170–2622) and 490 (95%CrI 345–517), respectively.
Relative impact and efficiency of continuous IPT
While the effectiveness of a 12-month isoniazid regimen was estimated in the parent trial [8], it may be feasible in an ART delivery setting to provide IPT indefinitely (“continuous IPT”) [23], with a larger impact on TB incidence and mortality in the community. We found that continuous IPT could avert 2,050 cases (95%CrI 1,319–2,898) and 217 deaths (95%CrI 113–364) over a five-year period. Continuous IPT was less efficient, however: over a five-year period of implementation, continuous delivery required 74% (95%CrI 64–94%) more person-years of IPT per case of active TB prevented, relative to 12-month courses of IPT (median 26.3 versus 15.1, Table 3).
Table 3.
Projected epidemiological impact of IPT in Khayelitsha, cumulative over five years
| 12-month IPT regimen (95%CrI) |
Continuous IPT regimen (95%CrI) |
|
|---|---|---|
| Total number of people treated with IPT, thousands | 21.3 (17.9–26.0) | 20.8 (17.3–25.5) |
| Cumulative person-years on IPT, thousands | 18.2 (15.7–22.4) | 53.4 (45.3–66.4) |
| Cumulative TB cases averted, thousands | 1.2 (0.7–1.9) | 2.0 (1.3–2.8) |
| Cumulative percentage of TB cases averted over 5 years | 3.6% (2.2–5.7%) | 6.1% (3.9–8.6%) |
| Reduction in TB incidence after 5 years | 5,2% (2.9–8.7%) | 10.5% (6.9–14.8%) |
| Number needed to treat to prevent a TB case | 17.6 (11.3–28.9) | 10.1 (7.2–15.8) |
| Number IPT person-years to prevent a TB case | 15.1 (9.8–24.7) | 26.3 (19.0–40.6) |
| Cumulative TB deaths averted, thousands* | 0.14 (0.07–0.24) | 0.22 (0.11–0.36) |
| Cumulative percentage of TB deaths averted over 5 years* | 2.0% (1.1–3.5%) | 3.2% (1.7–5.1%) |
| Reduction in TB mortality after 5 years* | 3.7% (2.0–6.4%) | 6.7% (3.8–10.7%) |
| Number needed to treat to prevent a TB death* | 158 (87–314) | 96 (55–188) |
| Number IPT person-years to prevent a TB death* | 135 (74–167) | 249 (145–490) |
includes TB-HIV deaths
IPT impact in context of expanding ART coverage
Given changes in guidelines and practices with respect to ART, we also considered the impact of the IPT intervention as ART coverage increased. Increasing the rates of ART initiation as described above (which caused the median ART coverage among all PLWH to increase from 48% to 61% over five years) resulted, in absence of IPT, in a 6% (95% CrI 2–10%) reduction in TB incidence due to better HIV management alone. Compared to this improved baseline projection, the use of IPT (versus no IPT) resulted in a further 7.6% (95% CrI 4.3 −12.6%) reduction in TB incidence and a 5.4% (95% CrI 2.8–9.6%) reduction in TB mortality after five years – compared to 5.2% and 3.7%, respectively, under the fixed ART coverage scenario.
Mechanisms of epidemiologic impact
After fitting the model to results of the clinical trial, we estimated that the risk of progression from latent to active drug-susceptible TB was reduced by 44% (interquartile range, 38–50%, 95%CrI 26–60%) while taking IPT (relative to ART alone), and by 21% (IQR 12–31%, 95%CrI 2–43%) after IPT had ended. This model also assumed that new infection with drug-susceptible TB was prevented while taking IPT.
Within the overall population, we estimated that 82% of all cases averted by IPT (95%CrI 74–88%) were averted in IPT recipients themselves, as opposed to people who had never taken IPT (i.e., averted secondary transmission). Of this 82%, a median 51% of averted cases occurred during the time of IPT administration and 31% after completion of therapy (median post-IPT follow up time 1.6 years over the five-year analysis frame).
If the effect of IPT was restricted only to the prevention of infection (or reinfection) during treatment, the epidemiological impact of IPT was reduced by more than 80% (details described in Supplemental Digital Content 1) – suggesting that the majority of IPT impact, even in this high-transmission setting, stems from preventing progression from infection to TB.
Additional Sensitivity Analyses
As anticipated, the projected impact of IPT was strongly associated with the estimated efficacy of IPT in preventing TB progression during therapy and with the persistence of this effect after the end of therapy (Figure 3). Continuous provision of IPT was most beneficial in simulations where the shorter-term effects on infection and progression while taking IPT were relatively more important. Other influential parameters were related to the ART-linked nature of the modeled IPT intervention: the projected epidemiological impact of IPT was higher when taking ART alone provided less protection against TB progression, when a high rate of HIV progression to CD4 <500 allowed individuals to become eligible for ART and IPT more quickly, and when the rate of ART initiation during treatment of active TB was high (Figure 3).
Figure 3. Sensitivity of Main Results to Model Parameters.
Intensity of shading indicates the strength of association (value of partial rank correlation coefficient, PRCC) between a parameter’s value and a measure of IPT impact, after adjusting for other model parameters, and color shows the direction of association. Purple indicates that the outcome measure increases with an increase in the parameter value, and orange indicates that a reduction in parameter value is associated with an increase in the outcome measure. PRCCs are also shown numerically within the colored boxes. Epidemiologic outcomes shown are the 5-year percentage reductions in incidence and mortality rates (comparing a 12-month IPT regimen to no IPT), the cumulative number of person-years of IPT provided (using a 12-month regimen) per TB case averted, the 5-year TB incidence reduction achieved when IPT is provided continuously compared to the reduction achieved with the 12-month regimen (expressed as a ratio), and the 5-year percentage reduction in incidence rate achieved by the 12-month regimen in an alternative model scenario of expanding ART coverage. Parameters displayed are those which were among the 5 most strongly correlated with at least one of the three epidemiologic outcomes. The interquartile range of values for each parameter among calibrated models (posterior distribution) is shown to the right; the starting estimates and associated references for these parameters are provided in Table 1. All rates are annual.
When the model was recalibrated to a setting with a TB incidence 67% lower than that of Khayelitsha (593 (95%CrI 510–699) notifications per 100,000 person-years), the 5-year population impact of the IPT intervention using a 12-month regimen remained proportionally similar, with TB incidence reduced by 5.7% (95%CrI 2.6–9.3%) and TB mortality reduced by 3.6% (95%CrI 1.6–6.7%) after 5 years. The efficiency of IPT, however, was considerably lower in this moderate-incidence setting, with a number needed to treat of 40 (95%CrI 25–73) individuals to prevent one TB case.
Discussion
In this modeling study using data from Khayelitsha, South Africa, a 12-month regimen of IPT linked to ART initiation had important clinical impact, with 1 case of TB averted for every 18 patients completing IPT. Although less than 2% of the population of 392,000 would receive IPT at any point in time when delivered in this fashion, such an intervention could avert 1,200 cases and 140 deaths over a five-year period – with even greater impact in a context of ongoing ART scale-up. Thus, while IPT alone is unlikely to transform the TB epidemic in settings like Khayelitsha unless expanded greatly beyond ART initiators, it could have an important role as part of a comprehensive package of TB prevention and treatment.
Compared to the number needed to treat of 25 (of whom 22 completed therapy) to prevent one case among IPT recipients in the parent trial [8], our lower estimate of 18 people treated per TB case prevented primarily reflects additional downstream prevention of transmitted TB in the wider population. This number needed to treat to prevent a TB case is also lower than has been estimated in analyses arguing for continuous over 6-month IPT (50 needed to treat) [22], and is comparable to the number needed to screen to detect a case of active TB using the highest-yield screening approaches in high-burden settings [24]. From an economic standpoint, preventive therapy in high-burden settings has consistently been shown to be a highly cost effective intervention [25,26], but a separate question is its affordability under existing budget constraints. Providing IPT within the context of ART delivery as modeled here may reduce overhead costs and enhance cost-effectiveness and affordability.
Attempts to achieve population-level impact on TB epidemics through IPT have had mixed results. In the 1960’s, IPT was an important component of a highly successful example of population-level TB control in Alaska [27], but a more recent trial of community-wide IPT in South African mines (Thibela) showed no significant decrease in TB incidence [9], due at least in part to very high incidence of reinfection after treatment ended [28]. The current analysis is consistent with previous modeling analyses of IPT impact, but augments them by directly incorporating empirical results of a clinical trial in a high-burden setting. Previously, Dowdy and colleagues [29] estimated that a 6-month IPT regimen among PLWH in Rio de Janeiro could reduce incidence in the general population by 3% over five years, and Knight and colleagues [30] estimated that IPT for PLWH from 2014 to 2032 could reduce TB incidence in South Africa by 14%. Ragonnet and colleagues [31] identified a U-shaped relationship between TB incidence and IPT impact in HIV-naïve populations, with maximal effect in a middle range of TB incidence (between 500 and 900 cases per 100,000 persons per year) that balances sufficiently high prevalence of recent infections that can benefit from IPT with sufficiently low frequency of reinfection after IPT ends. Our finding of comparable IPT impact at incidence of 850/100,000 persons/year and 2400/100,000 persons/year suggests that a high HIV prevalence extends this optimal incidence range for IPT impact.
A strength of the current analysis is its use of setting-specific data to estimate the population-level impact of IPT. We were able to use surveillance data, notification data, and baseline data from trial subjects to model the TB and HIV epidemics in Khayelitsha at the time of a clinical trial of IPT, then link observed clinical effect sizes (TB incidence reductions in the trial’s treatment versus control groups) to mechanistic parameters describing IPT effects, and finally translate those to projections of epidemiologic impact. Our analyses depend on future trends in epidemiologic variables, however. For example, expansion of ART will initially increase the reach and impact of ART-linked IPT, but over time may make IPT less efficient as TB incidence declines in the population.
Relative to 12 months of IPT, we found that continuous IPT offered additional benefit but was less efficient in terms of person-years of treatment necessary to achieve the same population-level impact. If resources to deliver and monitor therapy are limited (and are not greatly outweighed by the difficulty of recruiting and enrolling new patients onto IPT), then it may be preferable to focus on covering more at-risk individuals with up to a 12-month course rather than providing longer courses to a smaller number of individuals. Future adoption of shorter preventive therapy regimens [4,5] may shift this balance further, improving the ease and efficiency of delivering preventive therapy to more of the at-risk population. LTBI screening is another consideration for improving IPT efficiency; however, LTBI screening has not been associated with benefit from IPT among people on ART in high prevalence settings [8,12], and in very-high-TB-burden settings like Khayelitsha, using tuberculin skin tests or interferon-gamma release assays to select continuous IPT recipients would reduce IPT prescriptions by only a small amount (and would prevent TB-uninfected individuals from receiving protection against new infection during IPT), while the imperfect sensitivity of LTBI testing in advanced HIV [32] would falsely screen out many whom IPT could otherwise benefit.
Other models [30,33] have suggested a larger benefit from continuous IPT relative to short-term IPT. This may reflect, in part, differences in the modeled persistence of IPT effects after therapy has ended. Our calibrated estimate of the post-treatment effect of 12 months of IPT in latently infected individuals – a permanent 21% (95%CrI 16–43%) reduction in the risk that preexisting infections will progress to active disease, added to the persistent benefit of having prevented new infections or reinfections during therapy – is consistent with the 35% (2–76%) probability of LTBI cure estimated by Sumner and colleagues [34] using data from the post-treatment period of the same trial.
Our results have several limitations. We simplify HIV and TB natural history into a limited number of discrete states. Because we model an adult population with a homogenous risk structure, we cannot explore potential targeting of high-risk groups based on age, location, etc. To keep the model simple and maximize the use of clinical trial data about IPT effects, we modeled HIV incidence and treatment coverage as they were at the time of that trial. We intentionally focused on a shorter (five-year) time horizon that matches funding cycles and budgetary decisions; longer-term projections would require more complexity in terms of HIV transmission assumptions and ART scale-up over time. We did not model acquisition of drug resistance, limiting our inference about the potential for IPT to generate new isoniazid resistance – though a meta-analysis has suggested that this effect may be low [35]. Our model also did not allow for a possibility that undetected active TB might be cured by IPT alone, an effect which might offset any negative effects of inadvertent isoniazid monotherapy in the short term. Finally, our model represents detailed data from one community in South Africa and may not generalize to other settings, particularly those whose TB epidemics have much lower annual risk of infection or are not driven by HIV.
In summary, achieving the ambitious targets set for TB control will require comprehensive packages of interventions tailored to local epidemiology. In settings like Khayelitsha where TB incidence is very high, most TB is associated with HIV, and ART coverage is high, we show that co-provision of IPT and ART can serve as a key component of such a package of interventions, with important – if not transformative – impact on TB transmission and mortality in the community.
Supplementary Material
Acknowledgements
Funding: EAK was supported by the National Institution of Allergy and Infectious Diseases of the National Institutes of Health under award number K08AI127908. MXR was supported by an Excellence Fellowship administered by the University College of London. RJW was supported by Francis Crick Institute (which receives support from CRUK [10218], MRC [10218], and Wellcome), Wellcome [104803, 203135], NIH U19 AI 111276, and Medical Research Council of South Africa via its strategic health innovation partnerships. The ART-IPT RCT was supported in part by the Department of Health of South Africa, the Wellcome Trust and Médecins sans Frontières.
References
- 1.Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO’s new end TB strategy. Lancet 2015; 385:1799–1801. [DOI] [PubMed] [Google Scholar]
- 2.Houben RMGJ Menzies NA, Sumner T Huynh GH, Arinaminpathy N Goldhaber-Fiebert JD, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob Health 2016; 4:e806–e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowdy DW, Azman AS, Kendall EA, Mathema B. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin Infect Dis 2014; 59:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterling TR, Scott NA, Miro JM, Calvet G, La Rosa A, Infante R, et al. Three months of weekly rifapentine plus isoniazid for treatment of M. tuberculosis infection in HIV co-infected persons. AIDS Published Online First: 17 March 2016. doi: 10.1097/QAD.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz JT, Omoz-Oarhe A, et al. One Month of Rifapentine/Isoniazid to prevent TB in people with HIV: BRIEF-TB/A5279. Boston, MA:; 2018. http://www.croiconference.org/sessions/one-month-rifapentineisoniazid-prevent-tb-people-hiv-brief-tba5279 [Google Scholar]
- 6.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377:1588–1598. [DOI] [PubMed] [Google Scholar]
- 7.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS 2009; 23:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet 2014; 384:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, Godfrey-Faussett P, et al. A Trial of Mass Isoniazid Preventive Therapy for Tuberculosis Control. N Engl J Med 2014; 370:301–310. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Wood R, Cock KMD, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 2010; 10:489–498. [DOI] [PubMed] [Google Scholar]
- 11.TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 12.Badje A, Moh R, Gabillard D, Guéhi C, Kabran M, Ntakpé J-B, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5:e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 13.2011. Census Suburb Khayelitsha. Strategic Development Information and GIS Department, City of Cape Town. City of Cape Town: https://www.capetown.gov.za/en/stats/2011CensusSuburbs/2011_Census_CT_Suburb_Khayelitsha_Profile.pdf [Google Scholar]
- 14.Draper B, Pienaar D, Parker W, Rehle T. Recommendations for Policy in the Western Cape Province for the prevention of Major Infectious Diseases, including HIV/AIDS and Tuberculosis. Cape Town, South Africa: Human Sciences Research Council; 2007. https://vula.uct.ac.za/access/content/group/91e9e9d8-39b6-4654-00ae-f4d74cba085f/CD%20Volume%203%20%20Major%20Infectious%20Diseases%20HIV%20T.doc (accessed 1 Jun2017). [Google Scholar]
- 15.Médecins Sans Frontiéres. Khayelitsha Activity Report 2001–2011: 10 Years of HIV/TB Care at Primary Health Care Level. South Africa:; 2011. https://www.msf.org.za/about-us/publications/reports/khayelitsha-activity-report-2001-2011-10-years-hivtb-care-primary (accessed 31 Oct2016). [Google Scholar]
- 16.Stinson K, Goemaere E, Coetzee D, van Cutsem G, Hilderbrand K, Osler M, et al. Cohort Profile: The Khayelitsha antiretroviral programme, Cape Town, South Africa. Int J Epidemiol 2017; 46:e21. [DOI] [PubMed] [Google Scholar]
- 17.Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013; 13:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management. Geneva:; 2018. http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ (accessed 18 Jun2018). [PubMed] [Google Scholar]
- 19.Meintjes G, Maartens G, Boulle A, Conradie F, Goemaere E, Hefer E, et al. Guidelines for antiretroviral therapy in adults. Southern African Journal of HIV Medicine 2012; 13:114–131. [Google Scholar]
- 20.Patten GE, Cox V, Stinson K, Boulle AM, Wilkinson LS. Advanced HIV disease at antiretroviral therapy (ART) initiation despite implementation of expanded ART eligibility guidelines during 2007–2012 in Khayelitsha, South Africa. Clin Infect Dis 2014; 59:456–457. [DOI] [PubMed] [Google Scholar]
- 21.Lopes JS, Beaumont MA. ABC: A useful Bayesian tool for the analysis of population data. Infection, Genetics and Evolution 2010; 10:825–832. [DOI] [PubMed] [Google Scholar]
- 22.Den Boon S, Matteelli A, Ford N, Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV. AIDS 2016; 30:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Recommendation on 36 Months Isoniazid Preventive Therapy to Adults and Adolescents Living with HIV in Resource-Constrained and High TB- and HIV-Prevalence Settings: 2015 Update. Geneva: World Health Organization; 2015. http://www.ncbi.nlm.nih.gov/books/NBK305262/ (accessed 9 Apr2016). [PubMed] [Google Scholar]
- 24.Shapiro AE, Chakravorty R, Akande T, Lonnroth K, Golub JE. A systematic review of the number needed to screen to detect a case of active tuberculosis in different risk groups; 2013. www.who.int/tb/Review3NNS_case_active_TB_riskgroups.pdf (accessed 7 Dec2017). [Google Scholar]
- 25.Shayo GA, Chitama D, Moshiro C, Aboud S, Bakari M, Mugusi F. Cost-Effectiveness of isoniazid preventive therapy among HIV-infected patients clinicaly screened for latent tuberculosis infection in Dar es Salaam, Tanzania: A prospective Cohort study. BMC Public Health 2017; 18:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azadi M, Bishai DM, Dowdy DW, Moulton LH, Cavalcante S, Saraceni V, et al. Cost-effectiveness of tuberculosis screening and isoniazid treatment in the TB/HIV in Rio (THRio) Study. Int J Tuberc Lung Dis 2014; 18:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comstock GW, Philip RN. Decline of the tuberculosis epidemic in Alaska. Public Health Rep 1961; 76:19–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Hermans SM, Grant AD, Chihota V, Lewis JJ, Vynnycky E, Churchyard GJ, et al. The timing of tuberculosis after isoniazid preventive therapy among gold miners in South Africa: a prospective cohort study. BMC Med 2016; 14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowdy DW, Golub JE, Saraceni V, Moulton LH, Cavalcante SC, Cohn S, et al. Impact of isoniazid preventive therapy for HIV-infected adults in Rio de Janeiro, Brazil: an epidemiological model. J Acquir Immune Defic Syndr 2014; 66:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight GM, Dodd PJ, Grant AD, Fielding KL, Churchyard GJ, White RG. Tuberculosis prevention in South Africa. PLoS ONE 2015; 10:e0122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragonnet R, Trauer JM, McBryde ES, Houben RMGJ, Denholm JT, Handel A, et al. Is IPT more effective in high-burden settings? Modelling the effect of tuberculosis incidence on IPT impact. Int J Tuberc Lung Dis 2017; 21:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 Infection on T-Cell–based and Skin Test Detection of Tuberculosis Infection. Am J Respir Crit Care Med 2007; 175:514–520. [DOI] [PubMed] [Google Scholar]
- 33.Vynnycky E, Sumner T, Fielding KL, Lewis JJ, Cox AP, Hayes RJ, et al. Tuberculosis control in South African gold mines: mathematical modeling of a trial of community-wide isoniazid preventive therapy. Am J Epidemiol 2015; 181:619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumner T, Houben RMGJ, Rangaka MX, Maartens G, Boulle A, Wilkinson RJ, et al. Post-treatment effect of isoniazid preventive therapy on tuberculosis incidence in HIV-infected individuals on antiretroviral therapy. AIDS 2016; 30:1279–1286. [DOI] [PubMed] [Google Scholar]
- 35.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerging Infect Dis 2006; 12:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Leth F, van der Werf MJ, Borgdorff MW. Prevalence of tuberculous infection and incidence of tuberculosis: a re-assessment of the Styblo rule. Bull World Health Organ 2008; 86:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: Global trends and interactions with the hiv epidemic. Arch Intern Med 2003; 163:1009–1021. [DOI] [PubMed] [Google Scholar]
- 38.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of Progression to Active Tuberculosis Following Reinfection With Mycobacterium tuberculosis. Clin Infect Dis 2012; 54:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houben RMGJ, Glynn JR, Mallard K, Sichali L, Malema S, Fine PEM, et al. Human immunodeficiency virus increases the risk of tuberculosis due to recent re-infection in individuals with latent infection. Int J Tuberc Lung Dis 2010; 14:909–915. [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert M-L, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis 2003; 3:282–287. [DOI] [PubMed] [Google Scholar]
- 41.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358:1687–1693. [DOI] [PubMed] [Google Scholar]
- 42.Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams D-A, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis 2011; 15:331–336. [PubMed] [Google Scholar]
- 43.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 1997; 119:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med 1992; 326:231–235. [DOI] [PubMed] [Google Scholar]
- 45.Horsburgh CR, O’Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med 2010; 182:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raviglione MC, Harries AD, Msiska R, Wilkinson D, Nunn P. Tuberculosis and HIV: current status in Africa. AIDS 1997; 11 Suppl B:S115–123. [PubMed] [Google Scholar]
- 48.Aaron L, Saadoun D, Calatroni I, Launay O, Mémain N, Vincent V, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect 2004; 10:388–398. [DOI] [PubMed] [Google Scholar]
- 49.Low A, Gavriilidis G, Larke N, B-Lajoie M-R, Drouin O, Stover J, et al. Incidence of Opportunistic Infections and the Impact of Antiretroviral Therapy Among HIV-Infected Adults in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clin Infect Dis 2016; 62:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn SD, Myer L, Bekker L-G, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 2006; 20:1605–1612. [DOI] [PubMed] [Google Scholar]
- 51.Suthar AB, Lawn SD, del Amo J, Getahun H, Dye C, Sculier D, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glaziou P, Sismanidis C, Zignol M, Floyd K. Methods used by WHO to estimate the global burden of TB disease. 2016http://www.who.int/tb/publications/global_report/en/
- 53.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJD. Natural History of Tuberculosis: Duration and Fatality of Untreated Pulmonary Tuberculosis in HIV Negative Patients: A Systematic Review. PLoS ONE 2011; 6:e17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS One 2011; 6:e20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacPherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K, MacPherson P, et al. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ 2014; 92:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.UNAIDS. South Africa HIV Epidemic Profile.; 2014. http://www.unaidsrstesa.org/wp-content/uploads/2015/05/UNAids-Profile-South-Africa.pdf-18-Feb.pdf (accessed 27 Jan2017).
- 57.Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis 2012; 54:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet 2006; 368:1254–1259. [DOI] [PubMed] [Google Scholar]
- 59.Phillips A, Pezzotti P, CASCADE Collaboration. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS 2004; 18:51–58. [DOI] [PubMed] [Google Scholar]
- 60.Brinkhof MWG, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med 2009; 6:e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinson NA, Gupte N, Msandiwa R, Moulton LH, Barnes GL, Ram M, et al. CD4 and viral load dynamics in antiretroviral-naïve HIV-infected adults from Soweto, South Africa: a prospective cohort. PLoS ONE 2014; 9:e96369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr 2006; 42:464–469. [DOI] [PubMed] [Google Scholar]
- 63.Malaza A, Mossong J, Bärnighausen T, Viljoen J, Newell M-L. Population-based CD4 counts in a rural area in South Africa with high HIV prevalence and high antiretroviral treatment coverage. PLoS ONE 2013; 8:e70126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson LF. Access to antiretroviral treatment in South Africa, 2004 – 2011. Southern African Journal of HIV Medicine 2012; 13:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Republic of South Africa. Global AIDS Response Progress Report 2012. http://files.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_ZA_Narrative_Report.pdf (accessed 23 May2017).
- 66.Poorolajal J, Hooshmand E, Mahjub H, Esmailnasab N, Jenabi E. Survival rate of AIDS disease and mortality in HIV-infected patients: a meta-analysis. Public Health 2016; 139:3–12. [DOI] [PubMed] [Google Scholar]
- 67.Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013; 10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Global Tuberculosis Report 2013. Geneva: World Health Organization; www.who.int/tb/publications/global_report/en/ (accessed 26 Aug2014). [Google Scholar]
- 69.Cox HS, McDermid C, Azevedo V, Muller O, Coetzee D, Simpson J, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS ONE 2010; 5:e13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Essel V HIV surveillance: A 12 year analysis of HIV prevalence trends and comparing HIV prevalence from sentinel antenatal clinic surveys and Prevention of mother-to-child programmes. 2014https://open.uct.ac.za/handle/11427/13804 (accessed 11 May2017).
- 71.Gouws E, Mishra V, Fowler TB. Comparison of adult HIV prevalence from national population-based surveys and antenatal clinic surveillance in countries with generalised epidemics: implications for calibrating surveillance data. Sex Transm Infect 2008; 84 Suppl 1:i17–i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.