Abstract
Knowing the frequency of positive Lyme disease serology in children without signs of infection facilitates test interpretation. Of 315 asymptomatic children from Lyme disease endemic regions, 32 had positive or equivocal C6 enzyme linked immunoassays, but only 5 had positive IgG or IgM supplemental immunoblots (1.6%, 95% confidence interval 0.7–3.7%).
Keywords: Lyme Disease, Epidemiology, Children
Introduction:
Lyme disease is a tick-borne infection caused by transmission of Borrelia species to humans with a peak incidence in children 5–14 years old. Two-tiered Lyme disease serology includes a first-tier enzyme linked immunoassay (EIA) followed by a supplemental immunoblot for those with a positive or equivocal first tier test. As antibodies persist for many years, patients with either acute or previous Borrelia infection can have positive two-tier Lyme disease serology. In some cases, the previous infection may have been unrecognized or subclinical. Other times the test result may be false positive. In previous studies of children and adults living in disease endemic areas, the background seroprevalence (i.e. the rate of positive two tiered Lyme disease tests in asymptomatic individuals) ranged from 2 to 16%1–7 depending on the specific Lyme disease test performed as well as the geographic region.
There is limited evidence about the prevalence of positive two-tiered tests among children presenting to an emergency department (ED) in a Lyme disease endemic region. We investigated the background seropositivity in children without objective clinical signs of Lyme disease presenting to Pedi Lyme Net EDs. We hypothesized that children have had fewer years to be exposed to Borrelia and would have lower prevalence of positive tests.
Materials and Methods:
Study design/setting:
This was a planned secondary analysis using prospective data collected at Pedi Lyme Net EDs. Pedi Lyme Net is a consortium of six pediatric EDs located in Lyme disease endemic regions of the U.S.: Boston Children’s Hospital (Boston, MA), Nemours/A.I. duPont Hospital for Children (Wilmington, DE), Hasbro Children’s Hospital (Providence, RI), Children’s Hospital of Philadelphia (Philadelphia, PA), Children’s Hospital of Pittsburgh (Pittsburgh, PA) and Children’s Hospital of Wisconsin (Milwaukee, WI). The study protocol was approved by each institution’s Institutional Review Board. Study participation was voluntary and written informed consent was obtained for each participating patient. All sites except Pittsburgh were active in Pedi Lyme Net at the time of data collection for this study.
Participants:
We enrolled a convenience sample of children 1 to 21 years of age presenting to Pedi Lyme Net EDs between July 2015 and February 2018 when research staff was available. Patients were eligible if they were undergoing intravenous cannulation during an ED visit for an injury. We excluded children if they had evidence of infection or if the caregivers did not speak English or Spanish.
Data collection
We collected the following demographic and clinical information: age, gender, race, ethnicity, primary residence zip code and comorbid conditions. Participants, parents and treating clinicians were asked if the enrolled child had previously been diagnosed with Lyme disease (responses included definite previous Lyme disease, possible previous Lyme disease, no previous Lyme disease and unknown). Patients with definite or possible Lyme disease were asked how long ago the diagnosis occurred.
Serum samples were obtained at the time of venous cannulation. Samples were centrifuged at 3,600 RPM for 10–15 minutes immediately after collection. The supernatant was pipetted into Eppendorf tubes, stored at −80˚C and subsequently shipped on dry ice to the Pediatric Lyme Disease Biobank at Boston Children’s Hospital.
Research Lyme disease testing
We performed two-tiered Lyme disease testing on the biobanked serum samples. C6 peptide EIA (Immunetics™; Boston, MA) was used for first tier testing at a single research laboratory (Branda Laboratory; Massachusetts General Hospital; Boston, MA). For samples with a positive (index value ≥ 1.1) or equivocal (index value ≥ 0. 9 and < 1.1) result, we obtained a supplemental IgG and IgM immunoblot performed at a single clinical laboratory (ARUP™, Salt Lake City, Utah).
Outcome measures
Our primary outcome was a positive two-tier Lyme disease test defined as a positive or equivocal C6 EIA followed by either a positive IgG or IgM immunoblot (inclusive definition). Our secondary outcome was a positive or equivocal C6 EIA followed by a positive IgG immunoblot (strict definition), because IgM immunoblots may be falsely positive.8
Data analysis
Our primary analysis was to report the frequency of positive two-tier Lyme disease serology using both the strict and inclusive definition with results reported as point estimates with 95% confidence intervals (CI). To assess the representativeness of the sampled population based we performed geospatial analysis. We obtained 2015 county level Lyme disease case counts from the Centers for Disease Control and Prevention. Case counts for each county were converted to cases per 100,000 population using 2010 United States Census data. Study participants were then assigned to the county containing at least 50% of the residences in the participant’s zip code using a spatial join in ArcGIS 10.5.1 (ESRI, Redlands, CA). For unmapped zip codes, we identified the overlying county using an online zip code locating service.
We performed data analysis using SPSS software version 23.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY). Spatial analysis and cartographic output were performed using ArcGIS 10.5.1 (ESRI, Redlands, CA).
Results:
We approached 381 eligible children of which 344 (90.3%) agreed to participate. The range of participants per hospital was 31 to 110. The median patient age was 9 years (interquartile range 6–12 years) and 230 (66.9%) were males. Of those enrolled, 23 (7.0%) were of Hispanic ethnicity and 48 (14%) were black. The reason for IV placement was as follows: 335 fracture reductions (97.4%), 1 (0.3%) laceration repair and 8 (2.3%) had blood work obtained for other non-infectious reasons. Twenty-three (6.7%) of the enrolled children had a chronic comorbid condition.
Of 314 children with data about previous clinical history of Lyme disease, 5 (1.6%) reported a definite history and none reported a possible history of previous Lyme disease. In each case of definite Lyme disease, the infection reportedly occurred more than 5 years prior to enrollment.
Of the 344 children enrolled, 315 (91.6%) had serum successfully collected. Of these, 27 had a positive C6 EIA test and 5 had an equivocal C6 EIA test (positive or equivocal first-tier test 10.5%; 95% CI 7.6–14.4%). Five patients had a positive supplemental immunoblot (5/315; 1.6%; 95% CI 0.7%−3.7%). These included 1 positive IgG and IgM immunoblot, 2 positive IgG immunoblots alone and 2 positive IgM immunoblots alone. The seroprevalence was lower using the strict definition of requiring positive IgG (3/315; 1.0%, 95% CI 0.3–2.8%). None of the children with a positive IgG and one child with a positive IgM alone reported a history of previous Lyme disease.
The geographic distribution of seropositive and seronegative study subjects by the county of residence and corresponding Lyme disease incidence is displayed in Figure 1.
Figure 1.
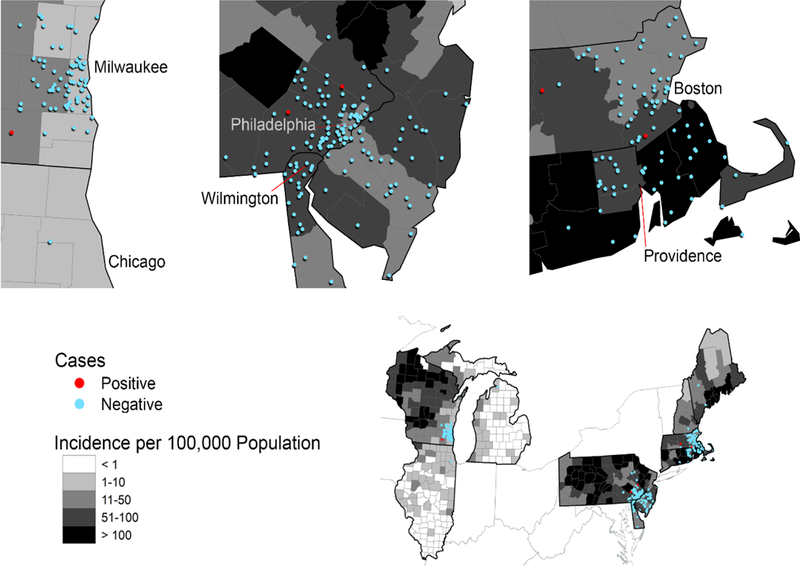
Lyme disease incidence is displayed using a choropleth; an aerial unit map in which a continuous data value is divided into classes and represented using color shades. Counties are classified as < 1, 1–10, 11–50, 51–100 or > 100 Lyme disease cases per 100,000 population. Seropositive and seronegative study subjects are represented using a 1:1 dot density function, which generates a randomly placed dot within the boundaries of each study subject’s county.
Discussion:
Our study evaluates the frequency of Lyme disease seropositivity in children without signs of infection living in Lyme disease endemic regions of the U.S. Less than two percent of asymptomatic children had a positive two-tier Lyme test regardless of whether we applied an inclusive or strict definition. We conclude the background Lyme disease seroprevalence is quite low in children who live in endemic areas.
Our observed background seroprevalence was lower than previously reported in studies of adults from endemic regions.1–6 However, adults have more years to be exposed to Borrelia compared with children. Adults may also have increased exposures to other infections or autoimmune diseases that result in cross-reactivity with other Borrelia antigens. Our seroprevalence was similar to that found in a study of 410 Connecticut school children.7 That study, however, included symptomatic children and all but one child with a positive two-tiered Lyme disease serology had a history of previous confirmed Lyme disease. In our study, only one asymptomatic control patient with a positive two-tiered Lyme disease serology had a history of previous Lyme disease.
The C6 EIA test is a newer first-tier Lyme disease test that has similar sensitivity but higher specificity in children than older generation first-tier Lyme disease tests.9 However, the C6 EIA does not have sufficient specificity to be used as a stand-alone test.10 This was evident in this study as 10% of our control patients had a positive C6 EIA but only five had a positive supplemental immunoblot.
Our study has several limitations. First, children presenting with injuries to pediatric hospitals may not have the same risk for Lyme disease as all children because these children often come from urban and suburban areas clustered around the hospital where they are less frequently exposed to ticks. Our geospatial analysis showed that while children did cluster around the enrolling hospitals, many of these areas had moderate to high Lyme disease incidence similar to other areas of the respective states. Second, we enrolled a convenience sample of ED patients based on staff availability and slightly more children were male. This reflects the higher proportion of male patients that present with injuries. Third, not all children with a history of clinical Lyme disease had a positive serology. This was likely attributable to either waning seropositivity (all reported previous Lyme disease occurred more than 5 years before enrollment) or to an unconfirmed history of previous Lyme disease. Last, we had a small number of children with positive two-tier Lyme disease test results, limiting our power to measure differences by geography or age.
Footnotes
Conflicts of Interest and Funding Support: There are no conflicts of interest to declare. This work was supported by the following research grants: Boston Children’s Hospital Research Faculty Council Grant, Harvard Catalyst Pilot Grant and Bay Area Lyme Disease Foundation Research Grant. Dr. Balamuth received career development support from NICHD K23-HD082368
References:
- 1.Hvidsten D, Mortensen L, Straume B, et al. Blood donor Borrelia burgdorferi sensu lato seroprevalence and history of tick bites at a northern limit of the vector distribution. APMIS 2017; 125:717–24. [DOI] [PubMed] [Google Scholar]
- 2.Zákutná Ľ, Dorko E, Rimárová K, et al. Pilot Cross-Sectional Study of Three Zoonoses (Lyme Disease, Tularaemia, Leptospirosis) among Healthy Blood Donors in Eastern Slovakia. Cent Eur J Public Health 2015; 23:100–6. [DOI] [PubMed] [Google Scholar]
- 3.Munro H, Mavin S, Duffy K, et al. Seroprevalence of lyme borreliosis in Scottish blood donors. Transfus Med 2015; 25:284–6. [DOI] [PubMed] [Google Scholar]
- 4.Christiann F, Rayet P, Ngueodjibaye DB, et al. Endemic level of Lyme borreliosis in a region of central France: a sero-epidemiologic examination involving blood donors. Eur J Epidemiol 1997; 13: 361–2. [DOI] [PubMed] [Google Scholar]
- 5.Smith PF, Benach JL, White DJ, et al. Occupational risk of Lyme disease in endemic areas of New York State. Ann N Y Acad Sci 1988; 539: 289–301. [DOI] [PubMed] [Google Scholar]
- 6.Steere AC, Sikand VK, Meurice F, et al. Vaccination against Lyme disease with Recombinant Borrelia burgdorferi Outer-Surface Lipoprotein A with Adjuvant. NEJM 1998; 339: 209–15. [DOI] [PubMed] [Google Scholar]
- 7.Feder HM, Gerber MA, Cartter ML, et al. Prospective assessment of Lyme disease in a school-aged population in Connecticut. J Infect Dis 1995;171:1371–4. [DOI] [PubMed] [Google Scholar]
- 8.Lantos PM, Lipsett SC, Nigrovic LE. False positive Lyme disease IgM immunoblots in children. J Pediatr 2016; 174:267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsett SC, Branda JA, McAdam AJ, et al. Evaluation of the C6 Lyme enzyme immunoassay for the diagnosis of Lyme disease in children and adolescents. Clin Infect Dis 2016; 63:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diag Microbiol Infect Dis 2013; 75: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


