Abstract
In pregnancy, opioids may be used medically and also misused. We hypothesized that the umbilical cord (UC) could be a good screening tool for determining opioid exposure and improving medical care. One hundred and one UC, each with 50 associated ICD9/ICD10 codes were used. Using predictive pharmacokinetic analysis we determined that opioids could be detected since last ingestion prior to birth. The UC were lysed and screened using ELISA detecting multiple opioids and their metabolites. Statistical comparisons to obstetric and neonatal outcomes were performed. Although the commercial ELISA was less sensitive in UC than blood or urine, there was perfect method selectivity as compared to a subset of cords designated positive or negative by clinical diagnostics, so our results are accurate and reliable. Absolute quantitation was not possible because the antibody cross reacts with multiple compounds, but “low” or “high” levels of exposure were assigned. Prevalence of opioids was 11%, which reduced to 7% when caesarean-section births were eliminated. For non-caesarean-section infants adjusted for preterm birth, advanced maternal age and smoking (independent risk factors), opioids were significantly associated with intra-uterine growth restriction (p = 0.017) and admission to neonatal intensive care (p = 0.002). UC can be collected non-invasively and rapidly providing a reliable tools for semi-quantitative opioid screening using ELISA. Moreover, as UC are usually discarded collection presents few technical or safety concerns for staff or patients. Further development of this methodology may provide a rapid, non-invasive clinical screening tool to identify NAS and/or opioid use in late pregnancy.
Keywords: opioid misuse disorder, ELISA, neonatal abstinence syndrome, screening
Introduction
Management of pain is especially problematic during pregnancy (Kallen and Reis 2016). Because medications commonly pass from the maternal circulation to the fetus, prescription analgesics should only be given to pregnant women in cases where the therapeutic benefits outweigh the risk of neonatal withdrawal and other theoretical risks to the fetus. Despite this, opioid analgesic medications are prescribed frequently, mainly because there are few other safe options for the treatment of pain in pregnancy.
For pregnant women, the safest medication for pain is acetaminophen. Unfortunately, in many cases acetaminophen does not give adequate relief, resulting in use of other over-the-counter or prescription analgesics (Babb et al. 2010). This is particularly relevant in the context of common and painful conditions affecting pregnant patients such as headaches and migraine (Sances et al. 2003), back pain (Casagrande et al. 2015), and dental infections (Tocaciu et al. 2016). Additionally, pain and fatigue crest in the third trimester when physiological changes of pregnancy and fetal neurologic development peak, placing additional strain on the mother (Price and Collier 2017). The problem of choosing appropriate medications for pain management in pregnant women has been an ongoing concern in dentistry, orthopedics, and chronic pain where no real guidelines or evidence based appraisals exist to guide practice.
The use of NSAIDs in the third trimester of pregnancy is contraindicated due to their tendency to prolong labor, the potential for premature closure of the ductus arteriosus in the fetus, and/or fetal pulmonary hypertension (Venuto et al. 1975; Van Marter et al. 1996). Opioids are a viable alternative for strong pain, but in clinical dose ranges, they can cross the placenta due to their low molecular weight and lipophilic nature. Opioid safety in pregnancy has not been well established, but associations with fetal abnormalities exist in some, but not all studies; in the case of long term, and/or high dose users, neonatal abstinence syndrome (NAS), also known as neonatal opioid withdrawal syndrome (NOWS) can occur (Kocherlakota 2014). The occurrence of NAS is common with repeated use and misuse of opioids during pregnancy, although not with single usage such as during labor. Additionally, there are few data on the rates of medically-supervised opioid use in pregnancy and outcomes relating to dosage.
Although clinically-managed use of opioids is beneficial for pain, the world is currently facing an epidemic of opioid misuse that cuts across socio-economic, educational, and geographical boundaries. This problem has escalated in the last 5 years, and also affects pregnant women and their babies (Patrick et al. 2015; Wilder and Winhusen 2015). Between 2000 and 2009 opioid misuse among women giving birth in the United States, increased almost 5-fold (Smith 2017). With this increase, in addition to adverse effects on the mother, there is growing concern about neonatal abstinence syndrome (Noormohammadi et al. 2016). NAS occurs when the fetus is exposed to drugs during pregnancy, and experiences withdrawal-like symptoms following birth (Kocherlakota 2014). Concurrent to the recent opioid epidemic, a four-fold rise in NAS has been reported between 2004 and 2013 in the USA (Tolia et al. 2015).
Diagnosing NAS can be difficult in the absence of co-operation from the mother (Kocherlakota 2014). Clinical signs and symptoms in both mother and neonate must be clearly read, and differentiated from other causes of neonatal distress. At present, a differential diagnosis of NAS in neonates is made based on urine or first bowel movement, which usually takes 7–9 hours and 12–24 hours, respectively (Montgomery et al. 2006; Tunc et al. 2008). Currently, there is a critical need for rapid, non-invasive diagnostic tests for opioids, supporting rapid clinical diagnosis. The umbilical cord (UC) has excellent potential to provide the basis for such a screen due to the fact cords that would otherwise be discarded can be used. Moreover, it is rapidly available, easily handled, and can be processed and used within 3–5 minutes of receipt in a properly equipped laboratory.
In 2006, Montgomery et al. described a mass spectrometry (MS) method to detect illicit drugs, including opioids, in UC and showed that the levels detected correlated well with those detected in meconium. Lendoiero et al. (2012), and Jones et al. (2015), have shown that UC tissues can be used to quantify methadone and heroin exposure, respectively. These researchers used MS because of its extremely high sensitivity and low limits of detection, allowing the detection of drugs and their metabolites for a long period following the last dose. However, due to the expense and highly technical nature of the methods, MS does not lend itself to rapid, high-throughput, inexpensive detection in clinical laboratories. Antibody-based technologies similar to ELISA, may present an avenue for such screening. ELISA-based tests can be used to assess fetal exposure to drugs of abuse, with extremely high negative predictive values (>98%) as compared with MS, although quantitation can be problematic (Montgomery et al. 2008).
It is useful to note that umbilical cords can be used for drugs other than opioids and we have previously demonstrated that methamphetamine can be detected and quantitated in umbilical cords by ELISA and correlated to changes in norepinephrine levels in cord tissues (Collier et al. 2015). Recently, the umbilical cord was demonstrated to be a sensitive and specific screening tissue for cannabis use (Kim et al. 2018). Excellent concordance with meconium was shoen, and absolutely quantitation of tetrahydrocannabinol (THC) as well as several of its hydroxyl and glucuronide metabolites were acheived. Umbilical cords are one of many maternal and fetal tissues that may be used for screening, including maternal and fetal blood, urine and hair, and neonatal meconium, as well as placenta. The use of these different tissues for screening varies and is dependent on several considerations including that drug physicochemical characteristics and pharmacokinetic profiles affect the suitability of different matrices (e.g. blood vs. meconium), the speed with which tissues can be collected and invasiveness (or not) of collection, as well as the specificity and sensitivity of the analytical method being used also affects outcomes.
We hypothesized that by using a commercial ELISA that is able to detect multiple opioids and metabolites, it would be possible to rapidly detect and semi-quantitate opioids in UC tissue as a measure of fetal exposure. Additionally, we wished to determine if UC is a sensitive and specific matrix for screening for opioids, and if it can be used to accurately report the prevalence of opioid use in the third trimester of pregnancy.
Materials and Methods
All chemicals were obtained from VWR International Ltd (Mississauga, ON, Canada) unless otherwise stated.
Human umbilical sample collection and processing
The human umbilical cords used in this project were collected between 2012 – 2014. The UCs were collected at birth, with informed consent from mothers for inclusion of their tissues into the Hawaii Biorepository, including consent for future investigation. These tissues are de-identified. This specific study was approved by the Review Ethics Board at the University of British Columbia (H14–00092) and tissues transferred under an executed material transfer agreement M17–00402.
The UCs were collected, snap-frozen in liquid nitrogen and stored at −80°C in the Biorepository until release. Upon request, individual pieces of umbilical cord tissue (0.2 – 0.5 g) were cut from frozen cords and shipped on dry ice to the University of British Columbia, where they were stored at −80°C until processing to lysates.
UCs (n=101) were processed to lysates as previously described (Wright et al. 2011). Briefly, tissues were thawed and mechanically homogenized using a Tissue Tearor™ (Daigger Scientific, Vernon Hills, IL, USA) in 1:3 (w:v) Tris-HCl buffer containing 5 mM MgCl2 and 2 mM PMSF. Lysates were separated into 50 μL aliquots and frozen at −80°C until use.
Inclusion criteria: race self-reported back to grandparents’ generation; age >18 years; singleton birth; no infection; no repeat donation from the same woman. The characteristics of the cohort are as follows: average age 28.62 years ± 6.26, (n = 101), BMI 26.5 ± 6.04, (n = 97), ethnicity of the cohort: 17.32% ± 12.98 of Caucasian descent, 4.30% (±3.77) of Chinese descent, 20.87% ±12.36 of Filipino descent, 11.81% ± 9.25 of Japanese descent, 26.41% ±17.90 of Native Hawaiian descent, and 17.31% ± 12.98 of other descent. The average gestational age of the infants at birth was 38.63 weeks ± 2.39 (n = 101). For these participants, 50 clinical ICD9/ICD10 chart fields as well as other clinical data were analyzed for maternal and neonatal outcomes, comparing opioid positive and opioid negative results.
Validation of commercial ELISA for detection of opioids and their metabolites in umbilical cords.
Commercial ELISAs were from Randox Corporation (Crumlin, County Antrim, UK) and performed as per the manufacturer’s instructions. These ELISAs detect most opioids containing the natural opiate backbone structure, but not synthetic opioid structures. As a matter on nomenclature, opiate refers to natural products that bind the opioid receptor. Whereas opioids may be natural or synthetic, opiates are opioids, but not all opioids are opiates. This commercial ELISA detects opiates and opioids, but from here on, this manuscript we will use the term “opioid”.
To validate these ELISAs for quantitative/qualitative use in human umbilical cord lysates, a pooled sample of n =110 charcoal-stripped umbilical cord lysates was generated. Charcoal stripping was performed according to the method of Carter (1978), which does not contain dextran, and is considered to be less damaging to the matrix, removing fewer endogenous compounds (Carter 1978). Briefly, 140 mg/mL of activated charcoal was added to the pooled lysate. The sample was vortexed 3 X 30 sec and then incubated for 8 h at room temperature on a rotating platform. After 8h, the mixture was centrifuged at 2,000 x g for 20 min. The supernatant was decanted, 83.3 mg/mL of kaolin added and vortexed 3 X 30 sec. This mixture was centrifuged at 2,000 x g for 15 min, the supernatant filtered through 0.2 μm syringe filters, aliquoted, and frozen at −80 °C until use. Charcoal stripping was necessary for spiking and validation due to the possibility that the samples already contained opioids, because as no clinical data was available for these 110 control tissues.
Validation occurred over 3 separate days where the manufacturer’s standard curve was used as supplied, or spiked into aliquots of charcoal stripped UC lysate. Additional samples of charcoal stripped UC lysate were spiked with identical concentrations of codeine solution, excluding highest concentration points (Sigma Aldrich, ON, Canada). The limit of sensitivity and lower limit of quantitation were determined for UC lysates as compared to the manufacturer’s pure standards. Sensitivity (comparison of manufacturer supplied standard alone and spiked into UC lysate) and specificity (concentration of known codeine spiked into pooled lysate as compared to manufacturer’s pure standards) were obtained.
Screening cords for opioid detection
For screening of individual umbilical cord lysates, samples were blinded by a second investigator and included spiked positives (assay positive control), as well as three samples where maternal blood was known to be positive for opioids and eight samples that were known negatives (screening control, previously performed by clinical testing and noted in chart). The bench investigator did not receive the key for analysis until all samples had been screened.
Pharmacokinetic (PK) Analysis
Predictive pharmacokinetic analysis was undertaken for morphine, codeine, ethylmorphine, hydrocodone, hydromorphone, dihydrocodeine, oxymorphone, and oxycodone. The following drugs and metabolites can also be detected by this ELISA, however no PK analysis was possible due to lack of parameters in the literature: 6-acetylmorphine, 6-acetyl-codeine, thebaine, desomorphine, morphine 3β glucuronide, morphine 6β glucuronide, norcodeine, normorphine, noroxycodone, and noroxymorphone. Fentanyl, buprenorphine, and methadone were not detectable with this assay.
Standard dosing intervals and drug/metabolite half-lives were used to generate predicted maximum concentrations (Cmax) of opioids after a single oral dose, or a single IV dose. Mean steady state plasma concentrations were predicted for multiple oral doses and multiple IV bolus doses of the drugs. The elimination half-lives were used to predict utility of the ELISA for detecting drugs in UC lysates, and to calculate for how long since the last dose was ingested, this approach would still be valid – i.e. sensitive and specific enough. Unless otherwise referenced, all PK parameters were extracted from product monographs.
The Cmax of a single oral dose was calculated using Equation 1. Where Foral is the oral bioavailability of the drug in the body, and Vd is the apparent volume of distribution of the drug.
| (1) |
Once the CmaxOral was calculated, it was multiplied by either 0.03125 or 0.000977, representing the amount of drug left in the body after 5 or 10 half-lives respectively, following a single oral dose of an opioid.
The Cmax for a single IV bolus dose was calculated using Equation 2. Where Cpt is the concentration of drug in the plasma at time t, Cp° is the concentration of drug in plasma at time 0, Kel is the elimination constant of the drug, and t½ is the half-life of the drug, which was multiplied by either 5 or 10 to represent the amount of time passed since injection. When a drug had a range for t½, an average was taken and used in equations. The average t½s were used to calculate Kel for all drugs using Equation 3. All medications that were given IV were assumed to have a one compartment model with instantaneous mixing, and follow first order kinetics for elimination. Drugs typically given medically by infusion were calculated as a bolus to better reflect what illicit drug users may be doing.
| (2) |
| (3) |
The steady state plasma concentration of multiple oral doses was calculated using Equation 4. Where is the mean plasma concentration after multiple oral doses at steady state (at least 5 half-lives have passed), and τ is the dosing frequency of the drug. Once was calculated, it was multiplied as above to determine the amount of drug left in the body after 5 or 10 half-lives respectively. Uniform dosing was assumed. When more than one dose interval (τ) was present, the larger τ was used for lower doses and the smaller τ was used for larger doses to give the widest possible range of concentrations in which to determine opioid use in pregnancy.
| (4) |
For multiple IV doses, the Cmax concentration at steady state was calculated using Equation 5. Where Cpmax is the maximum concentration in the body at steady state following at least 5 half-lives. Once the Cpmax was calculated for a drug, it was multiplied as above to determine the terminal dose in the body.
| (5) |
Demographic and Statistical Analyses
When opioids were detected, opioid positive UC were compared to opioid negative UC using 50 ICD9/ICD10 chart fields for obstetric and neonatal outcomes. The analysis was then repeated with removal of all Caesarean section deliveries to allow for the differentiation between effects observed acutely, and chronic use, since opioids are commonly administered during this procedure and in the Caesarean section deliveries this is almost certainly the source of opioids.
Normality of the data was determined by D’agostino-Pearson Omnibus test for normality or Komolgorov-Smirnoff test for small sample sets. For clinical conditions and demographics with binary outcomes (e.g. sex, delivery method), student’s t-tests were performed between positive and negative groups. For continuous variables (e.g. BMI, age) correlations were performed using Pearson’s or Spearman’s tests as appropriate. All statistical analyses were performed using Graphpad Prism 6.0 for Mac OSX (Graph Pad Prism, San Diego, CA).
Results
Predictive PK analysis
Based on validation parameters and predictive PK analysis, there will be no difficulty in quantitating (above the lower limit of quantitation (LLOQ) of 5.7 ng/ml) or detecting usage (above the limit of sensitivity (LOS) of 1.5 ng/ml) of opioids and/or their metabolites in mothers who ingested high levels of opioids in late pregnancy (Tables S1 – S4). These tables also present the sub-clinical, clinical, and supra-clinical doses that could be detected within five and ten half-lives of either single dose, or multiple dosage administration.
For opioids taken for pain management on a standard oral dosing schedule, PK analysis indicated codeine, its metabolite dihydrocodeine, the morphine metabolite ethylmorphine, and oxycodone could be detected even after 5 half-lives – well beyond therapeutic levels and more sensitive than clinical laboratories calibrated to therapeutic ranges (Tables S1 and S2). For the remainder of the analytes in the oral schedule, and for all IV opioids ingested on a standard medical dosing schedule, only drugs ingested within 5 half-lives (effective elimination) are predicted to be detected (Tables S3 and S4). Predictive PK for some of the water soluble and longer resident metabolites (e.g. morphine-3-glucuronide and morphine-6-glucuronide) could not be performed as data for these compounds do not exist in the literature, but we expect the metabolites to persist beyond 5 half-lives of the parent drug.
Method validation and utility
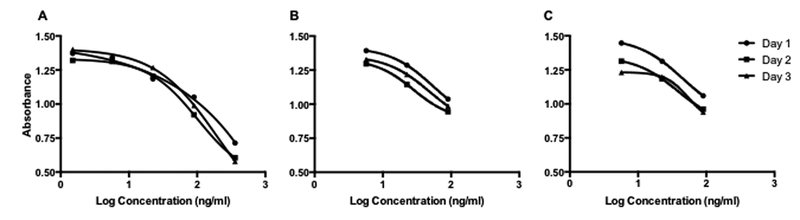
The LOS of commercial standard in buffer, and commercial standards spiked into UC were 0.05 ng/mL and 1.5 ng/mL respectively. The LLOQ in buffer and UC lysate were 0.05 ng/mL and 5.7 ng/ml (Fig 1).
Figure 1: Standard Curves.
1A: Manufacturer’s standard curve in plasma. 1B: Manufacturer’s standard curve spiked into umbilical lysate. 1C: Standard curve of pure codeine at the same concentrations as manufacturer’s standards spiked into UC Lysates. Points are mean ± SD of n = 2 on three days. The lowest concentration on the commercial curve (A, 1.5 ng/mL) was below detection limits and has been removed in (C) and (D). The highest concentration on the commercial curve was not evaluated because pharmacokinetic analysis showed it was far above even what tolerant woman could ingest without acute intoxication.
The method requires 25 μl of UC lysate (equivalent to 25 mg of tissue), allowing for the use of very small pieces of UC. It is possible to process a lysate from fresh UC tissue in 3–5 minutes and, because the ELISA takes ~90 minutes, the technique is very rapid.
Pure codeine spiked into UC lysate approximated the commercial-spiked curve closely, with an LLOQ of 5.7 ng/ml (Fig 1C). However, absolute quantitation of opioids in UC lysate is not possible because several analytes have cross reactivity in excess of 100% in the commercial ELISA. In particular 6-acetylmorphine and 6-acetyl-codeine cross reactivity is 636% and 442% respectively, with thebaine (uncommonly seen in the real world) at 151%. Additionally, several other metabolites are detected at less than 100% cross-reactivity, including morphine 3βD glucuronide (46%), morphine 6βD glucuronide (35%), hydrocodone (22%), hydromorphone (20 %), dihydrocodeine (15%), norcodeine (0.8%) and normorphine (0.6%), according to the manufacturer’s product literature. If testing occurred shortly (minutes to hours) after ingestion, results would initially be several fold higher than true as 6-acetyl morphine is produced quickly, but after this, results would be under predictive of ingestion as the poorly detected glucuronides become more abundant. Despite this, relative levels of quantification can be detected as “high” or “low”, an improvement on the “yes” or “no” previously reported (Montgomery et al. 2006; Wright et al. 2011).
The assay had a 100% screening sensitivity: three UC lysates tested positive by ELISA that had previously tested positive in maternal urine by clinical laboratory screening (confirmed with chart codes). Moreover, 6 samples that had tested negative in clinical screening were also negative in our assay. The assay also had 100% method sensitivity, as a sample spiked with codeine, unknown to the bench analyst, also tested positive.
Population prevalence of opioid use in late 3rd trimester pregnancy
A total of 11 (10.9%) samples tested positive for opioids (average 7.41 μg/ml, range 0.31– 6.98 μg/ml). When births by Caesarean-section were eliminated due to likely use in the procedure, seven samples (7%) tested positive for opioids with an average concentration of 1.45 μg/ml (range 0.31–3.36 μg/ml).
Obstetric and neonatal outcomes associated with opioid detection
All infants exposed to opioids (n = 11) showed trends towards intrauterine growth restriction (IUGR, p = 0.068), admission to the neonatal intensive care unit (NICU, p = 0.079), and length of hospital stay (p = 0.076). Mothers had significantly greater blood loss (p = 0.050), but no other parameters were significant, or trended p < 0.1.
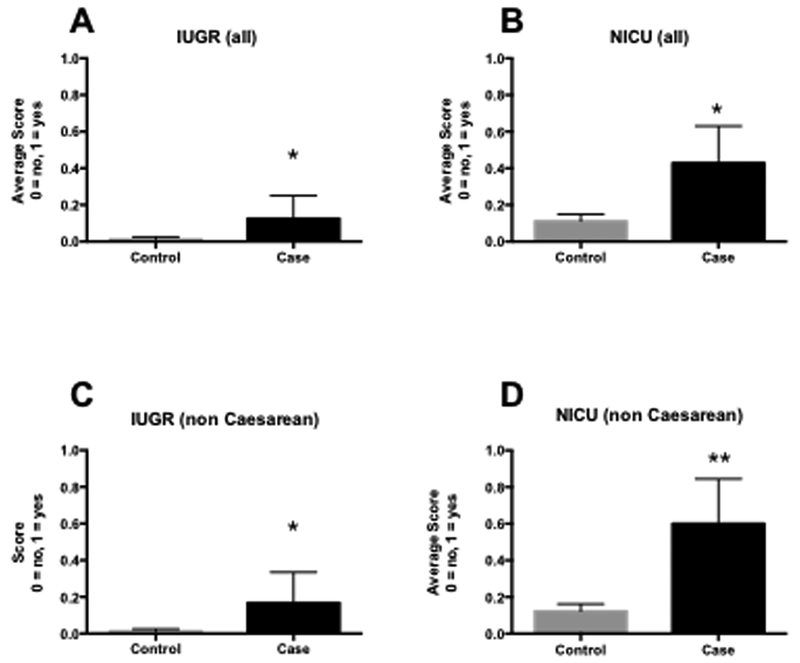
When adjusted for preterm birth (delivery <37 weeks), smoking and advanced maternal age, which are independent risk factors for IUGR and NICU admission (Ananth et al. 2004); all opioid exposed babies were significantly more likely to have IUGR (p = 0.035, Fig 2A), to be sent to NICU (p = 0.02, Fig 2B), and mothers were significantly more likely to have chronic hypertension (p = 0.009), but neither length of stay, nor blood loss was significantly different from controls (p = 0.05, p = 0.17 respectively). The adjusted odds ratios for IUGR and NICU admissions were 10.9 and 6 respectively.
Figure 2: Comparison of likelihood of IUGR (2A, 2C) and admission to NICU (2B, 2D) in opioid exposed neonates, adjusted for pre-term birth and advanced maternal age.
2A: Likelihood of IUGR in all opioid exposed (case) vs. non-exposed (control) neonates. 2B: Likelihood of admission to NICU in all opioid exposed (case) vs. non-exposed (control) neonates. 2C: Likelihood of IUGR in non-caesarean-birth opioid exposed (case) vs. non-exposed (control) neonates. 2D: Likelihood of admission to NICU in non-caesarean-birth opioid exposed (case) vs. non-exposed (control) neonates. Bars are means ± SEM. * p < 0.05, ** p < 0.01.
For non-caesarean section opioid-exposed infants, adjusted for preterm birth, smoking, and AMA, IUGR was significantly associated with opioid positive status (p = 0.007, Fig 2C) and admission to NICU (p = 0.0003, Fig 2D). Other factors associated with these outcomes were not adjusted for because they were ruled out by the inclusion/exclusion criteria or were not available (e.g. socio-economic status). After adjustment for AMA and preterm birth, chronic hypertension in pregnancy (p = 0.25), maternal blood loss (p = 0.460), and membrane rupture (p = 0.83) were not significant variables. Overall, opioid ingestion, independent of cesarean section, AMA, or preterm birth, was associated with an odds ratio for IUGR of 15.6 and admission to the NICU of 21.
After adjustment, maternal hypertension, maternal blood loss, and length of membrane rupture were observed to be primarily driven by gestational and maternal age status, and not opioid exposure. However, both acute and chronic opioid use are associated with IUGR and admission to NICU, with outcome associations much stronger for previous opioid exposure than for acute use of opioids during the birthing process.
Discussion
We have successfully validated a commercial opioid ELISA for use in UC lysates, as a sensitive, accurate, and reliable tool for population screening. In addition, because UCs are usually discarded, collection is safe for both patients and medical staff, rapid, and low-risk. Our screen of 101 individuals showed several significant differences in maternal and neonatal outcomes affected by use of opioids in the third trimester, including a prevalence of opioid use (non-cesarean section births) of 7% and significant associations with IUGR and admission to NICU, which is consistent with the literature (Liu et al. 2010; Pritham et al. 2012).
Despite many samples having concentrations that fall within the standard curve, this method is not absolutely quantitative due to the fact that the commercial antibody has cross-reactivity with multiple parent drugs and metabolites. For example, in a patient who had ingested morphine, the parent drug would be detected (with 100% accuracy and quantitation), but the metabolite, 6-acetylmorphine, would also be detected, and due to the variable affinity of the antibody, it may appear as though the concentration was 6-fold greater than the actual metabolite concentration. Additionally, the final and most abundant metabolites, morphine 3βD glucuronide, and morphine 6βD glucuronide, would be detected but, at only 40% and 35% of their true concentrations, respectively. Due to the variable residence times of each metabolite, the concentration of detected opioids will vary widely, with high values being returned shortly after ingestion due to the presence of the early metabolite 6-acetylmorphine, and much lower values returned after a longer period following ingestion due to the prevalence of the more poorly detected glucuronide metabolites. This makes it impossible to estimate with any confidence actual ingestion of parent drug. In spite of this, when the level of opioids in UCs from non-cesarean births was compared to UCs from cesarean births, it was still possible to distinguish between “high” and “low” levels of opioid ingestion. Additionally, these are archival tissues collected 2012 – 2014, and we do not know what the stability of the analytes is. It is possible that we have lost some sample to degradation, although this is not likely to be a large proportion as all cords were kept at −80 °C and had never been thawed until this evaluation. However, again, without a stability study, absolute quantitation is not possible, and our opioid prevalence rates may be lower than the real world.
Although UCs could be a valuable screening tool to assist in diagnosis of NAS/NOWS, it may also provide clinicians with additional information as to the prevalence of medically necessary opioid use in third trimester. Further investigation to define what constitutes a “low” or “high” level is necessary, along with better reporting of opioid use during labor than is available from the ICD9/ICD10 codes. This is particularly important in light of very recent evidence that UC is less sensitive than meconium for most illicit drugs – and hence may only be suitable for detecting very high levels of usage, potentially missing some lower, medically appropriate dosages during the third trimester (Colby 2017). Additionally, because UC are less sensitive than blood or urine, their value primarily lies in the non-invasive sampling and rapid acquisition of samples, as compared to invasive blood sampling, and/or the considerable time takes to acquire blood or urine samples during or after labour.
In 2013, NAS incidence in the United States ranged from 0.7 cases per 1,000 hospital births in Hawaii to 33.4 cases per 1,000 hospital births in West Virginia (Ko et al. 2016). In contrast to the recent focus on opioid misuse, there are very few studies on therapeutic opioid use in pregnancy. Broussard et al. (2011) reported 2.6% prevalence of therapeutic opioid use in birth defect cases, and 2.0% prevalence in controls, concluding that opioid treatment was significantly associated with cardiovascular defects, spina bifida, and gastroschisis, although this study was not adjusted for confounding by indication (Broussard et al. 2011). Similarly, Yazdy et al. (2015) reported medical usage of opioids up to 2.0% in pregnancy with a mean duration of treatment of 90 days, which was associated with neural tube defects (Yazdy et al. 2015). Significantly, both studies relied on self-report, which is well-known to return lower prevalence than clinical screening studies due to recall, and expectation biases (Kilpatrick et al. 2000). Both of these studies had a much lower prevalence of opioid detection than the 7% observed in our study. Due to the fact that all of the tissues used in our study were from a single source (the Hawaii Biorepository), and our selectivity and sensitivity for detection (as compared to medical laboratory screening) was 100%, we believe that some of the opioid usage observed is due to administration during birth, for pain relief, but not identified in the ICD9/10 fields. The remainder being due to either therapeutic use or misuse of opioids during the late third trimester. Although this is an important consideration in determining the true prevalence of opioid use in the third trimester (as opposed to administration during birth), this would not detract from the UC as a screening tool for the attending physician at the point of care as physicians would know whether opioids were administered during birth. Although it is possible that the prevalence of opioid use is slightly over-estimated here, we suspect that usage of opioids in pregnancy is higher than previous reports.
In this study, we observed significantly higher IUGR and admissions to NICU in opioid positive pregnancies, after adjustment for confounding variables. IUGR would be expected to be an outcome measure that closely represents adverse effects of opioids on the developing fetus due to either long term therapeutic use, or misuse. In contrast, NICU admissions can be due to acute ingestion of opioids during birth, affecting neonatal heartbeat, and respiration, as well as longer-term ingestion causing NAS or other complications. Although the odds ratios we have derived are correct to the data, they are likely exaggerated due to the small number of cases (n = 11 for all opioid detection, n = 7 after caesarean births were removed) relative to controls (n = 90 for both). Moreover, in adjusting for AMA and pre-term delivery, we have likely amplified the effects of the small sample size. Despite this, our results match with previously reported effects of both acute and chronic opioid usage in pregnancy and childbirth with respect to higher levels of IUGR (Liu et al. 2010) and NICU admissions (Pritham et al. 2012). Ultimately, this lends further support to the idea of using UC lysates as a sensitive and appropriate screening tool.
The commercial ELISA used here is able to recognize the morphine-like opioid and opiate backbone of parent drugs and their metabolites but not the backbones of some synthetic opioids including pethidine (meperidine), fentanyl, carfentanyl or remifentanil, so cannot be used to detect these compounds. This is an important consideration as North America has increasingly seen contamination of the illicit drug supply with these synthetic drugs (O’Donnell et al. 2017). In moving this concept forward, a multi-antibody approach might be more successful in covering the spectrum of opioids that may be both used and misused in pregnancy.
Specifically, we contend that this approach can be clinically useful because UCs are immediately available at birth, the average UC lysate takes 3–5 minutes to make in a prepared laboratory, and the ELISA test itself only takes ~2hrs. However, we suggest that the process may be able to be further simplified by taking the antibodies out of the ELISA plate and developing a test, analogous to modern home pregnancy test, in which UC lysate can be dropped onto a strip or device that immediately returns a yes/no answer. Despite this, the concern remains that umbilical cords cannot be sampled prior to birth, so are only of use for post-natal diagnosis of maternal opioid use and/or NAS. This means that improved pre-natal screening techniques are still needed. Also, to our knowledge there is no specific mechanism for binding of opioids in UC, but due to the highly perfused nature of the tissue (the mother exchanges her blood through the cord/placenta every 5–7 minutes at term) and the very fat soluble nature of opioid drugs, significant diffusion into UC is expected. This opioid test could be enhanced by using multiple antibodies specific for different opioid backbones embedded on the strip with different colors indicating positive results. Similar technology has already been commercially developed for testing Malaria antibodies, and is rapid, reliable, and inexpensive (Markakpo et al. 2016).
Finally, in developing these types of assays for opioid detection, a cautionary note should be included. Drug testing for pregnant women in general, is very controversial. It has a wide range of ethical and legal implications, e.g. women can, and have, been arrested for urine drug tests that they did not consent to, losing custody based on the results (Terplan and Minkoff 2017). Umbilical cord testing has great potential, however, a strong distinction between opioid misuse, medically appropriate opioid use during pregnancy, and opioid administration during labor will need to be developed in order to balance the scientific screening aspects with legal and ethical issues for mothers and their newborn children.
Supplementary Material
Acknowledgments
Funding: The Hawaii Biorepository is funded in part by NIH funding grant MD007601
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- Ananth C, Balasubramanian B, Demissie K, Kinzler W. 2004. Small-for-gestational-age births in the United States: an age-period-cohort analysis. Epidemiology 15(1):28–35. . [DOI] [PubMed] [Google Scholar]
- Babb M, Koren G, Einarson A. 2010. Treating pain during pregnancy. Canadian Family Physician. 56(1):25, 27. [PMC free article] [PubMed] [Google Scholar]
- Broussard C, Rasmussen S, Reefhuis J, Friedman J, Jann M, Riehle-Colarusso T, Honein M, National B, Defects, Prevention, Study. 2011. Maternal treatment with opioid analgesics and risk for birth defects. American Journal of Obstetrics and Gynecology. 204(4):314.e311–311. [DOI] [PubMed] [Google Scholar]
- Carter P 1978. Preparation of ligand-free human serum for radioimmunoassay by adsorption on activated charcoal. Clinical Chemistry. 24(2):362–364. [PubMed] [Google Scholar]
- Casagrande D, Gugala Z, Clark SM, Lindsey RW. 2015. Low Back Pain and Pelvic Girdle Pain in Pregnancy. Journal of the American Academy Orthopedic Surgery. 23(9):539–549. [DOI] [PubMed] [Google Scholar]
- Colby J 2017. Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure. Clinical Biochemistry. 50(13–14):784–790. [DOI] [PubMed] [Google Scholar]
- Collier A, Sato B, Milam K, Wright T. 2015. Methamphetamine, smoking, and gestational hypertension affect norepinephrine levels in umbilical cord tissues. Clinical and Experimental Obstetrics and Gynecology 42(5):580–585 [PubMed] [Google Scholar]
- Jones J, Jones M, Jones B, Sulaiman K, Plate C, Lewis D. 2015. Detection of Codeine, Morphine, 6-Monoacetylmorphine, and Meconin in Human Umbilical Cord Tissue: Method Validation and Evidence of In Utero Heroin Exposure. Therapeutic Drug Monitoring. 37(1):45–52. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Reis M. 2016. Ongoing Pharmacological Management of Chronic Pain in Pregnancy. Drugs. 76(9):915–924. [DOI] [PubMed] [Google Scholar]
- Kilpatrick B, Howlett M, Sedgwick P, Ghodse AH. 2000. Drug use, self report and urinalysis. Drug and Alcohol Dependence. 58(1–2):111–116. [DOI] [PubMed] [Google Scholar]
- Kim J, de Castro A, Lendoiro E, Cruz-Landeira A, López-Rivadulla M, and Concheiro M 2018. Detection of in utero cannabis exposure by umbilical cord analysis. Drug Testing and Analalysis. 10(4), 636–643. [DOI] [PubMed] [Google Scholar]
- Ko J, Patrick S, Tong V, Patel R, Lind J, Barfield W. 2016. Incidence of Neonatal Abstinence Syndrome - 28 States, 1999–2013. Morbidity and Mortality Weekly. 65(31):799–802. [DOI] [PubMed] [Google Scholar]
- Kocherlakota P 2014. Neonatal abstinence syndrome. Pediatrics. 134(2):e547–561. [DOI] [PubMed] [Google Scholar]
- Lendoiro E, Quintela O, de Castro A, Cruz A, López-Rivadulla M, Concheiro M. 2012. Target screening and confirmation of 35 licit and illicit drugs and metabolites in hair by LC-MSMS. Forensic Science International 217(1–3):207–215. . [DOI] [PubMed] [Google Scholar]
- Liu A, Sithamparanathan S, Jones M, Cook C, Nanan R. 2010. Growth restriction in pregnancies of opioid-dependent mothers. Archives of Diseases in Childhood Fetal and Neonatal Edition 95(4):F258–262. [DOI] [PubMed] [Google Scholar]
- Markakpo U, Bosompem K, Dzodzomenyo M, Danso-Appiah A, Essuman E, Anyan W, Suzuki M, Stephens J, Anim-Baidoo I, Asmah R et al. 2016. Minimising invasiveness in diagnostics: developing a rapid urine-based monoclonal antibody dipstick test for malaria. Tropical Medicine and Interntional Health. 21(10):1263–1271. [DOI] [PubMed] [Google Scholar]
- Montgomery D, Plate C, Alder S, Jones M, Jones J, Christensen R. 2006. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. Journal of Perinatology. 26 (1):11–14. [DOI] [PubMed] [Google Scholar]
- Montgomery D, Plate C, Jones M, Jones J, Rios R, Lambert D, Schumtz N, Wiedmeier S, Burnett J, Ail S et al. 2008. Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey. Journal of Perinatology 28(11):750–753. [DOI] [PubMed] [Google Scholar]
- Noormohammadi A, Forinash A, Yancey A, Crannage E, Campbell K, Shyken J. 2016. Buprenorphine Versus Methadone for Opioid Dependence in Pregnancy. Annals of Pharmacotherapy. 50:666–672. . [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Gladden R, Seth P. 2017. Trends in Deaths Involving Heroin and Synthetic Opioids Excluding Methadone, and Law Enforcement Drug Product Reports, by Census Region - United States, 2006–2015. Morbidity and Mortality Weekly. 66(34):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick S, Davis M, Lehmann C, Cooper W. 2015. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. Journal of Perinatology. 650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price H, Collier A. 2017. Analgesics in Pregnancy: An Update on Use, Safety and Pharmacokinetic Changes in Drug Disposition. Current Pharmaceutical Design. August 25. doi: 10.2174/1381612823666170825123754. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Pritham U, Paul J, Hayes M. 2012. Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome. Journal of Obstetrics, Gynecology and Neonatal Nursing. 41(2):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sances G, Granella F, Nappi RE, Fignon A, Ghiotto N, Polatti F, Nappi G. 2003. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 23(3):197–205. [DOI] [PubMed] [Google Scholar]
- Smith D 2017. Medicalizing the Opioid Epidemic in the U.S. in the Era of Health Care Reform. Journal of Psychoactive Drugs. 49(2):95–101. [DOI] [PubMed] [Google Scholar]
- Terplan M, Minkoff H. 2017. Neonatal Abstinence Syndrome and Ethical Approaches to the Identification of Pregnant Women Who Use Drugs. Obstetrics and Gynecology. 129(1):164–167. [DOI] [PubMed] [Google Scholar]
- Tocaciu S, Robinson BW, Sambrook PJ. 2016. Severe odontogenic infection in pregnancy - A timely reminder. Australian Dental Journal. [DOI] [PubMed] [Google Scholar]
- Tolia V, Patrick S, Bennett M, Murthy K, Sousa J, Smith P, Clark R, Spitzer A. 2015. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. New England Journal of Medicine. 372(22):2118–2126. [DOI] [PubMed] [Google Scholar]
- Tunc V, Camurdan A, Ilhan M, Sahin F, Beyazova U. 2008. Factors associated with defecation patterns in 0–24-month-old children. European Journal of Pediatrics. 167(12):1357–1362. [DOI] [PubMed] [Google Scholar]
- Van Marter LJ, Leviton A, Allred EN, Pagano M, Sullivan KF, Cohen A, Epstein MF. 1996. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal antiinflammatory drug consumption during pregnancy. Pediatrics. 97(5):658–663. [PubMed] [Google Scholar]
- Venuto RC, O’Dorisio T, Stein JH, Ferris TF. 1975. Uterine prostaglandin E secretion and uterine blood flow in the pregnant rabbit. Journal of Clinical Investigation. 55(1):193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder C, Winhusen T. 2015. Pharmacological Management of Opioid Use Disorder in Pregnant Women. CNS Drugs. 29:625–636. . [DOI] [PubMed] [Google Scholar]
- Wright TE, Milam KA, Rougee L, Tanaka MD, Collier AC. 2011. Agreement of umbilical cord drug and cotinine levels with maternal self-report of drug use and smoking during pregnancy. Journal of Perinatology. 31(5):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdy MM, Desai RJ, Brogly SB. 2015. Prescription Opioids in Pregnancy and Birth Outcomes: A Review of the Literature. Journal of Pediatric Genetetics. 4(2):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.