Abstract
Background
Managing virologic failure (VF) in HIV-infected children is especially difficult in resource-limited settings, given limited availability of alternative drugs, concerns around adherence and the development of HIV resistance mutations. We aimed to evaluate four management strategies for children following their first episode of VF by comparing their immunologic and virologic outcomes.
Methods
We included children (aged <16 years) with VF from 8 IeDEA-SA cohorts, initiating cART between 2004–2010, who followed one of four management strategies: continuing on their failing regimen; switching to a second-line regimen; switching to a holding regimen (either lamivudine monotherapy or other non-cART regimen); discontinuing all ART. We compared the effect of management strategy on the 52-week change in CD4% and log10VL from VF, using inverse probability weighting of marginal structural linear models.
Results
982 patients were followed over 54168 weeks. Relative to remaining on a failing regimen, switching to second-line showed improved immunologic and virologic responses 52 weeks after VF with gains in CD4% of 1.5% (95% CI 0.2–2.8) and declines in log10VL of −1.4 copies/mL (95% CI −2.0, −0.8), whilst switching to holding regimens or discontinuing treatment had worse immunologic (−5.4% (95% CI −12.1, 1.3) and −5.6% (95% CI −15.4, 4.1)) and virologic outcomes (0.2 (95% CI −3.6, 4.1) and 0.8 (95% CI −0.6, 2.1) respectively.
Conclusions
The results provide useful guidance for managing children with VF. Consideration should be given to switching children failing first-line cART to second-line, given the improved virologic and immune responses when compared with other strategies.
Keywords: HIV, virologic failure, second-line, resource-limited settings, adherence
Introduction
By 2015 nearly 900 000 HIV-positive children were receiving combination antiretroviral therapy (cART),(1) most of whom are in sub-Saharan Africa. The shift to early cART initiation, results in HIV-infected children being on treatment for long durations, and virologic failure (VF) is a concern; an estimated 20% of children developing VF after 3 years of cART.(2) With limited alternative treatment options, and the difficulties of sustained adherence in this vulnerable group, managing VF in children in resource-limited settings (RLS) is particularly challenging.
Adherence is especially difficult for children due to their dependence on caregivers for cART administration, difficulties facing families in disclosing HIV status to their child, the effects of HIV-associated stigma, and pharmaceutical challenges with pediatric drug formulations, dosing and drug-drug interactions.(3) Adolescents face their own adherence barriers, including the emotional challenges of puberty, and disclosing their HIV-status to others.(4)
Access to pediatric second- or third-line regimens is frequently restricted in RLS, where some newer drugs such as dolutegravir still require dosing studies and registration. Hence the optimal management of children with VF and suspected or proven poor adherence is challenging; switching to a new regimen risks increasing resistance mutations if adherence is not assured. Delays in switching patients from failing regimens are common, but have negative consequences.(2, 5) The South African national guidelines recommend careful assessment of adherence among children with VF before switching to second-line, and suggest “holding strategies” for those with ongoing adherence challenges.(6) “Holding regimens”, such as lamivudine monotherapy (LM) may retain HIV variants with reduced viral fitness, particularly the M184V mutation, slowing immune decline whilst ensuring no new drug resistance develops.(7–9) Complete treatment interruption may also allow adherence barriers to be addressed without new drug resistance emerging.(10) There is little evidence weighing the relative benefits and risks of these different strategies following VF in children. Fairlie et al. compared four management strategies following the most recent episode of VF, in an observational study from US-based pediatric cohorts,(11) but no comparative study has yet been done in RLS in sub-Saharan Africa, or following the first episode of VF.
Using causal inference methods and observational data from over 14,000 children in the International epidemiologic Database to Evaluate AIDS (IeDEA) Southern Africa, we compared immunologic and virologic outcomes after VF for those managed with the following strategies: switching to second-line cART, continuing on failing first-line cART, switching to non-cART holding regimens, and discontinuing all antiretrovirals.
Methods
We included data collected prospectively from eight IeDEA cohorts in South Africa. IeDEA is a multi-regional collaboration of HIV cohort studies.(12) Each study site had institutional ethical approval to contribute data to IeDEA analyses. Children (<16 years) initiating cART from 2004 to 2010, with documented VF after at least six months on cART were included. Final database closure was June 2012.
All study sites are part of the South African national ART program where virologic monitoring is routinely used. Table 1 provides details of the recommended first-line regimens during the study period.(13–15) The recommended second-line regimen prior to 2010 was to switch from an efavirenz-based to a lopinavir/ritonavir(LPV/r)-based regimen and switch at least one nucleoside reverse transcriptase inhibitor (NRTI). Prior to 2010, children failing protease-inhibitor (PI)-based first-line were switched to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based cART. From 2010 guidelines recommended specialist referral for children failing PI-based regimens as they require resistance testing and potentially integrase inhibitors, second generation PIs or NNRTIS such as darunavir and etravirine.(13)
Table 1.
Standard First-line Antiretroviral Therapy Regimens for HIV-infected Children in South Africa 2004–2012
| Before 2007 | 2007–2010 | After 2010 | |
|---|---|---|---|
| Co-infected with tuberculosis | Stavudine, Lamivudine, Ritonavir | Stavudine, Lamivudine, LPV/r + Ritonavir | Abacavir, Lamivudine, LPV/r + Ritonavir |
| Less than 6 months old | Stavudine, Lamivudine, Ritonavir | Stavudine, Lamivudine LPV/r | Abacavir, Lamivudine, LPV/r |
| Less than 3 years old or less than 10 kg | Stavudine, Lamivudine, LPV/r | Stavudine, Lamivudine, LPV/r | Abacavir, Lamivudine, LPV/r |
| Older than 3 years old and more than 10kg | Stavudine, Lamivudine, Efavirenz | Stavudine, Lamivudine, Efavirenz | Abacavir, Lamivudine, Efavirenz |
LPV/r: Lopinavir/ritonavir
We defined virologic failure (VF) as having at least two consecutive viral loads (VL) >1000 copies/ml, measured at least one month and less than one year apart. The date of VF was the date of the second unsuppressed VL. We considered only the first episode of VF.
We defined cART as comprising at least three antiretroviral drugs from at least two different drug classes. We compared four management strategies:
Remaining on a failing first-line regimen, with at most one single drug substitution not constituting a drug class change.
Switching to second-line: changing at least one drug including a change in drug class.
Switching to a holding regimen: a regimen not meeting the definition of cART, such as one or more drugs from a single class, or only two drugs from two drug classes.
Treatment interruption (TI): discontinuing all antiretroviral drugs.
The outcomes were the CD4% and log10VL slope, which were calculated at each time point after VF with a recorded CD4% or log10VL measure, as the difference between the current CD4%/ log10VL and the CD4%/ log10VL at VF, divided by the time in weeks between these two measurements. Patients were censored for death, transferring care or loss to follow-up (nine months without a recorded clinic visit prior to database closure).
Outcomes were modelled based on follow-up time on each strategy. Initially, we derived unweighted linear regression models for each outcome, adjusting for management strategy (allowing a four week lag to ensure the outcome reflected the appropriate strategy), covariates at VF, and weeks since failure. To estimate the effect of management strategy on the outcomes we adjusted for the time-dependent confounders VL and CD4%, which may have influenced both clinician choice of management strategy and the respective outcome, using inverse probability of treatment weighting (IPTW) of marginal structural linear models.(16) To estimate the IPTW, we fitted a pooled multinomial logistic regression model for management strategy choice until a switch to second-line was made.(17) We used different model specifications, based on in/exclusion of variables, interaction terms, and the functional form of the variables. All models used stabilized weights as previously described.(17) We chose the final model based on Bayesian model averaging and the stability of the weights.(18) The final marginal structural model was a linear model with the slope of CD4% or log10VL (from VF to the current time point) as the outcome, weights to adjust for the confounding, and conditional on time and covariates at VF, including immune suppression, age, VL, being on a PI-based regimen and level of care. The stabilized weights had a mean of 1.008, minimum of 0.000924 and maximum of 12.2686.
Results
Across the eight sites, 1,347 (19%) of 7,053 children with recorded VL measurements on cART experienced VF, and 365 were excluded due to incomplete CD4% and regimen data. The final dataset comprised 982 children (46% female), with median age at VF of 6.1 years (IQR 2.7–10.7), and median duration on cART of 1.4 years (see Table, Supplemental Digital Content 1). Median time between first and second consecutive VL >1000 was 90 days (IQR 66–140). Comparing patients based on their first change in management strategy, 557 (57%) remained on failing regimens throughout follow-up, 335 (34%) switched to second-line, 25 (3%) switched to holding regimens and 65 (7%) interrupted treatment. Most patients (95%) changed strategies only once. Among those switched to holding regimens, 80% were placed on NRTI-only regimens. Children switched to second-line were older, had lower median CD4% at cART start, at failure and lower nadir CD4%. Few patients (32/398, 8%) on PI-based first-line were switched to NNRTI-based second-line.
At study closure, 9 (1%) patients had died (all having remained on their failing regimen), 216 (22%) transferred care, 90 (9%) were lost to follow-up (88 having remained on their failing regimen, 1 after switch to second-line and 1 after TI), and 667 (68%) remained in care at their original treatment site. Figure 1 shows management strategies at five points during the 52-week study period, 73% of follow-up time was spent on failing regimens, 24% on second-line, 1% on holding regimens, and 2% interrupting treatment. Median time to first change in strategy was 168 days (IQR 79–365), with median time from VF to second-line 115 days (IQR 62–203), to holding regimens 90 days (IQR 30–154), and to TI 123 days (IQR 52–259).
Figure 1.
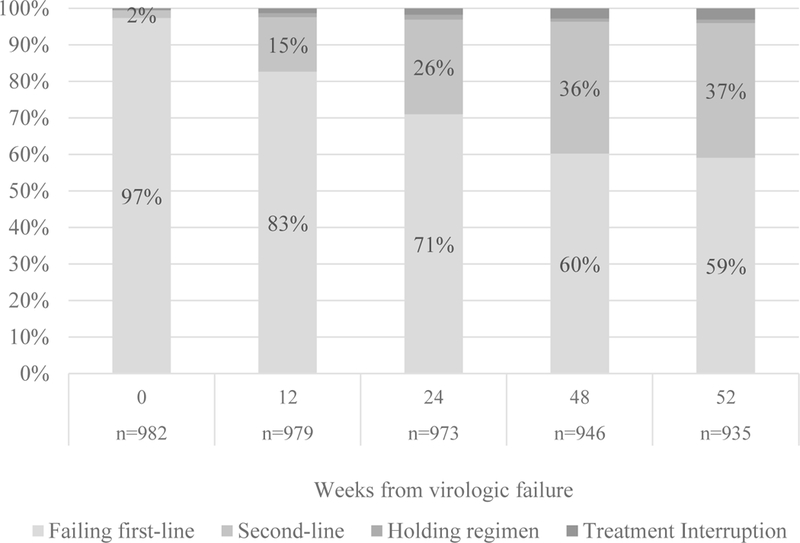
Management Strategies followed from Virologic Failure during follow-up
Choice of management strategy
Choice of management strategy was strongly associated with type of regimen at failure. Compared to those on PI-based first-line, those on NNRTI-based regimens were more likely to switch strategies than remain on failing first-line (Table 2). Those with the highest VL at VF (VL>50,000 copies/ml), were more likely to interrupt treatment (aHR 5.9, 95% CI 2.5, 13.8). Having a mid-range VL at VF (10,000–50,000 vs 1,000–5,000copies/ml) was associated with switching to a holding regimen (aHR 2.7, 95% CI 1.1, 7.0), indicating clinician preference to switch partially adherent patients to this strategy.
Table 2.
Associations with Management Strategy Choice in Children with Virologic Failure, Relative to Remaining on a Failing Regimen
| aHR (95% CI)* |
|||
|---|---|---|---|
| Characteristics at virologic failure | Switch to second- line |
Switch to holding regimen |
Treatment interruption |
| Type of first-line regimen (NNRTI-based vs PI-based) | 8.86 (5.96–13.18) | 3.18 (1.95–5.18) | 1.87 (1.28–2.73) |
| Gender (female vs male) | 0.81 (0.65–1.01) | 0.83 (0.58–1.20) | 1.13 (0.86–1.48) |
| Calendar year of ART start | 1.06 (0.99–1.13) | 1.32 (1.19–1.46) | 1.10 (1.02–1.19) |
| Type of facility (primary vs secondary/tertiary) | 2.97 (1.99–4.44) | 2.73 (1.31–5.69) | 22.78 (5.62–92.31) |
| Age in years at failure | 1.07 (1.01–1.13) | 0.95 (0.85–1.06) | 1.20 (1.12–1.28) |
| Viral load at failure (copies/ml) | |||
| 1000–5000 | 1 | 1 | 1 |
| 5000–10000 | 1.73 (0.80–3.75) | 1.25 (0.43–3.69) | 3.18 (1.06–9.52) |
| 10000–50000 | 1.63 (0.77–3.46) | 2.72 (1.06–6.95) | 5.72 (2.42–13.50) |
| >50000 | 1.45 (0.68–3.10) | 0.44 (0.16–1.16) | 5.93 (2.54–13.84) |
| Viral load at failure x Age in years at failure | |||
| VL >1000 and VL<5000 x Age at failure | 1 | 1 | 1 |
| VL>=5000 and VL <10000 x Age at failure | 0.95 (0.88–1.03) | 0.99 (0.86–1.14) | 0.76 (0.67–0.86) |
| VL>=10000 and VL <50000 x Age at failure | 0.95 (0.88–1.02) | 0.86 (0.75–0.99) | 0.82 (0.75–0.89) |
| VL>=50000 x Age at failure | 0.94 (0.87–1.01) | 1.08 (0.95–1.22) | 0.74 (0.68–0.82) |
| Time-varying covariates | |||
| Current Log10VL (per 1 Log10 increase) | 1.58 (1.38–1.79) | 2.33 (1.89–2.88) | 2.18 (1.89–2.52) |
Hazard ratios derived from pooled unweighted multinomial logistic regression fit on subsample of person-weeks of follow-up during which a switch to new regimen had not yet occurred
aHR: adjusted hazard ratio
Outcomes: Change in CD4% and VL
Figure 2A and2B shows the model estimates for the difference in mean 52-week change in CD4% and log10VL after VF, relative to remaining on a failing regimen and switching to second-line respectively. The final weighted model indicates that switching to second-line, even after operational delay, results in increases in CD4% and declines in VL compared to remaining on a failing regimen. Switching to a holding regimen or interrupting treatment results in declines in CD4% and increases in VL, albeit with wide confidence intervals.
Figure 2.
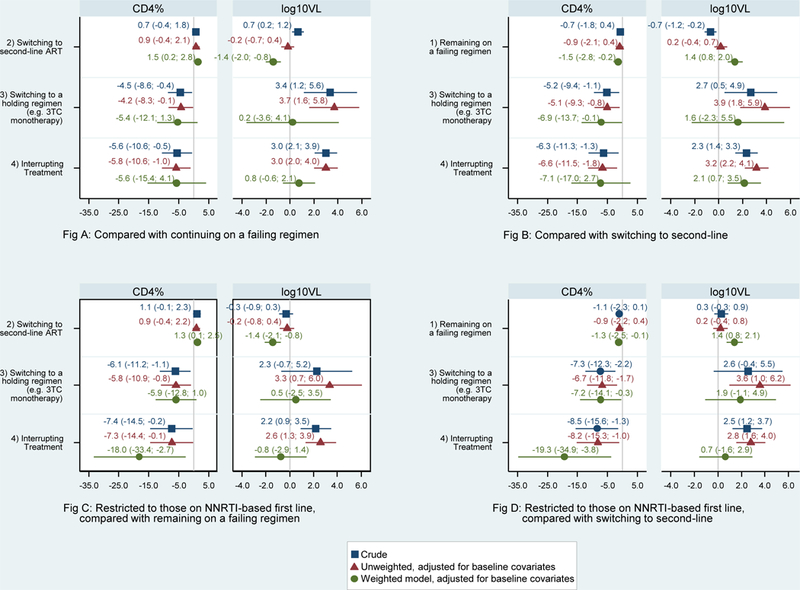
Estimated change in CD4% and log viral load at 12 months after virologic failure from crude, unweighted and weighted generalized linear models
We conducted a separate analysis restricted to children on NNRTI-based first-line, for whom alternative regimens at the time were more robust than for those failing PI-based first-line (Figures 2C and2D). Overall, the results were similar except the estimated declines in CD4% for those interrupting treatment compared to switching to second-line were significant and much larger than for all patients. When we compared switching to a holding regimen relative to TI we found that those interrupting treatment experienced bigger declines in CD4% (−12.1, 95% CI −29.1; 4.9). Due to small numbers switching to second-line NNRTI from a PI-based first-line, we were unable to conduct a restricted analysis for this group.
Estimates were stable in sensitivity analyses exploring alternative model specifications, with estimates for holding regimens or TI being more sensitive to model specifications given their smaller sample sizes (not published).
Discussion
This collaborative analysis of almost 1000 children with VF from eight South African cohorts showed that switching to second-line cART results in the best immunologic and virologic outcomes. The relative immunologic benefits and risks of using holding regimens was quantified; for patients with VF on NNRTI-based first-line, switching to a holding regimen may be better than TI, but has worse outcomes than remaining on failing first-line, while switching to LPV/r-based second-line cART, if available and where adherence is addressed, has the best outcomes.
To our knowledge, this is the first study comparing management strategies for children with VF from sub-Saharan Africa, where the burden of pediatric HIV is highest, and treatment options most limited. This study also represents the best comparative data on the use of holding regimens. Our estimates are likely conservative and potentially underestimate the true effect size for two reasons: (i) we considered immunologic and virologic trajectories from “baseline” measurements taken at VF, however most patients first spent time on their failing regimen before switching strategies; (ii) we assumed patients were adherent to their assigned strategies, but misclassification of strategy may have occurred, with unrecorded TI being most likely. Since VL monitoring for patients on non-cART regimens is not routinely performed, our virologic results for holding regimens and TI have very wide confidence intervals. Apart from VL as a proxy measure, no adherence data was available. Adherence is likely a key consideration of management strategy choice and hence there may be unmeasured residual confounding. We had no data on drug resistance mutations, and could not assess its effect on immunologic and virologic trajectories. Remaining on failing NNRTI-based cART, runs a higher risk of accumulating drug resistance(19) compared to remaining on failing PI-based cART, where resistance is less likely unless there has been exposure to unboosted PI treatment.(20) Outcomes of remaining on failing regimens may therefore differ depending on regimen.
Our results are largely in agreement with Fairlie et al’s study which was of similar design. They found switching to new cART had better immunologic outcomes compared to remaining on a failing regimen, with TI having the worst outcomes. Conclusions about their drug-sparing group could not be reached due to small numbers and large variety in the drug-sparing regimens used.(11) Clinician’s decision-making of management strategy may not be comparable to our study, since treatment options, availability of resistance testing and resistance patterns were likely different for the mostly heavily treatment–experienced US-based study population.
Compared to TI, switching to a holding regimen resulted in smaller increases in VL, and for those on NNRTI-based first-line, much smaller immune decline. Whilst there are few comparative studies addressing holding regimens in children and adolescents, there is evidence in adults that LM has better immunologic and virologic outcomes than TI.(21) The IMPAACT P1094 study enrolled children failing non-NNRTI-based cART, and aimed to compare outcomes of those randomized to continue with failing non-NNRTI-based cART versus changing to either lamivudine or emtricitabine monotherapy. The study was discontinued due to lack of enrolment, but the available data indicates greater immune decline for those on holding regimens.(22) Descriptive studies of LM use found that most children experience substantial immune decline and some experience clinical deterioration, and recommend avoiding this strategy in those with low CD4 counts, and considering it only whilst awaiting second- or third-line drugs.(7, 23)
Understanding the virologic and immunologic consequences of TI provides a useful benchmark for comparison. Planned TI is recommended in some countries for children to preserve future cART options, avoid long-term ART toxicity and because of difficulties in maintaining adherence.(24) Whilst this approach is severely detrimental in adults, outcomes are better among younger children or those with higher nadir CD4%.(25, 26) Unplanned TI, a consequence of poor or non-adherence to treatment, often occurs at a poorer clinical and immunological state, and increases the risk of AIDS-defining illness and hospitalization.(25) We could not determine the reason for TI in our study, but those interrupting treatment had the highest VL at VF, suggesting unplanned TI in those non-adherent. Several studies among children with planned TI, who were virally suppressed with high CD4, as well as those with unplanned TI, have documented rapid immune decline and virologic rebound in a similar range to our findings,(10, 25–28) although estimates have great inter-subject variability.
In conclusion, switching to second-line results in the best immunologic and virologic outcomes for children with VF. Where children are failing NNRTI-based cART and PI-based regimens are accessible, strong consideration should be given to early switch to second-line. Switching to holding regimens should be carefully considered, and reserved for use whilst awaiting access to suitable regimens. The most appropriate management strategy choice requires consideration of the available future treatment options and current adherence. Our findings inform that choice by quantifying the relative immunologic and virologic costs of delayed switching, holding regimens and TI in comparison to switching to second-line.
Supplementary Material
Acknowledgements
Lucy Profitt for her initial work on this concept.
Sources of Funding: This study was supported by funding from the United States National Institutes of Health Grant 5U01AI069924. ME is supported by the Swiss National Science Foundation, grant 32FP30–174281.
Footnotes
Conflicts of Interest: The authors declare that there are no conflicts of interest.
References
- 1.Number of children (<15 years) receiving ART globally, and by WHO region, 2000–2015 Available at: http://www.who.int/hiv/data/pedartregions2016.png?ua=1. Accessed 18 September 2017.
- 2.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr 2011;56:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele RG, Nelson TD, Cole BP. Psychosocial functioning of children with AIDS and HIV infection: review of the literature from a socioecological framework. J Dev Behav Pediatr 2007;28:58–69. [DOI] [PubMed] [Google Scholar]
- 4.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc 2013;16:18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen ML, van der Laan MJ, Napravnik S, et al. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS 2008;22:2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Consolidatd Guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults National Department of Health, South Africa; 2015. [Google Scholar]
- 7.Linder V, Goldswain C, Adler H, et al. Lamivudine Monotherapy: Experience of Medium-term Outcomes in HIV-infected Children Unable to Adhere to Triple Therapy. Pediatr Infect Dis J 2016;35:e199–205. [DOI] [PubMed] [Google Scholar]
- 8.Opravil M, Klimkait T, Louvel S, et al. Prior therapy influences the efficacy of lamivudine monotherapy in patients with lamivudine-resistant HIV-1 infection. J Acquir Immune Defic Syndr 2010;54:51–58. [DOI] [PubMed] [Google Scholar]
- 9.Gianotti N, Tiberi S, Menzo S, et al. HIV-1 replication capacity and genotype changes in patients undergoing treatment interruption or lamivudine monotherapy. J Med Virol 2008;80:201–208. [DOI] [PubMed] [Google Scholar]
- 10.Siberry GK, Patel K, Van Dyke RB, et al. CD4+ lymphocyte-based immunologic outcomes of perinatally HIV-infected children during antiretroviral therapy interruption. J Acquir Immune Defic Syndr 2011;57:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairlie L, Karalius B, Patel K, et al. CD4+ and viral load outcomes of antiretroviral therapy switch strategies after virologic failure of combination antiretroviral therapy in perinatally HIV-infected youth in the United States. AIDS 2015;29:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012;41:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Antiretroviral Treatment Guidelines In: National Department of Health SA, : Jacana; 2004. [Google Scholar]
- 14.Ren Y, Nuttall JJ, Egbers C, et al. Effect of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 2008;47:566–569. [DOI] [PubMed] [Google Scholar]
- 15.Chadwick EG, Capparelli EV, Yogev R, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS 2008;22:249–255. [DOI] [PubMed] [Google Scholar]
- 16.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 17.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: A tutorial. Stat Sci 1999;14:382–401. [Google Scholar]
- 19.Team P-S, Babiker A, Castro H, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis 2011;11:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zyl GU, Rabie H, Nuttall JJ, Cotton MF. It is time to consider third-line options in antiretroviral-experienced paediatric patients? J Int AIDS Soc 2011;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castagna A, Danise A, Menzo S, et al. Lamivudine monotherapy in HIV-1-infected patients harbouring a lamivudine-resistant virus: a randomized pilot study (E-184V study). AIDS 2006;20:795–803. [DOI] [PubMed] [Google Scholar]
- 22.Agwu A, Warshaw M, Siberry GK, et al. 3TC/FTC monotherapy vs. continuing failing cART as a bridging ART strategy in persistently nonadherent HIV-infected youth with M184V resistance: results of IMPAACT P1094. In: 6th International Workshop on HIV Pediatrics. Melbourne, Australia2014. [Google Scholar]
- 23.Linder V, Goldswain C, Boon G, et al. Lamivudine monotherapy as a safe option for HIV-infected paediatric clients with adherence challenges: new evidence from a large South African cohort. J Int AIDS Soc 2014;17:19763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Management of HIV in Children and Adults. Ministry of Health, Malawi 2011.
- 25.Saitoh A, Foca M, Viani RM, et al. Clinical outcomes after an unstructured treatment interruption in children and adolescents with perinatally acquired HIV infection. Pediatrics 2008;121:e513–521. [DOI] [PubMed] [Google Scholar]
- 26.Paediatric European Network for Treatment of A. Response to planned treatment interruptions in HIV infection varies across childhood. AIDS 2010;24:231–241. [DOI] [PubMed] [Google Scholar]
- 27.Gibb DM, Duong T, Leclezio VA, et al. Immunologic changes during unplanned treatment interruptions of highly active antiretroviral therapy in children with human immunodeficiency virus type 1 infection. Pediatr Infect Dis J 2004;23:446–450. [DOI] [PubMed] [Google Scholar]
- 28.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013;382:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


