Abstract
The neural crest is a migratory cell population that contributes to multiple tissues and organs during vertebrate embryonic development. It is remarkable in its ability to differentiate into an array of different cell types, including melanocytes, cartilage, bone, smooth muscle, and peripheral nerves. Although neural crest cells are formed along the entire anterior-posterior axis of the developing embryo, they can be divided into distinct subpopulations based on their axial level of origin. These groups of cells, which include the cranial, vagal, trunk, and sacral neural crest, display varied migratory patterns and contribute to multiple derivatives. While these subpopulations have been shown to be mostly plastic and to differentiate according to environmental cues, differences in their intrinsic potentials have also been identified. For instance, the cranial neural crest is unique in its ability to give rise to cartilage and bone. Here, we examine the molecular features that underlie such developmental restrictions and discuss the hypothesis that distinct gene regulatory networks operate in these subpopulations. We also consider how reconstructing the phylogeny of the trunk and cranial neural crest cells impacts our understanding of vertebrate evolution.
Introduction
The neural crest is a transient, multipotent progenitor population that plays a central role in vertebrate embryonic development. Formation of neural crest cells begins in the early gastrula with the establishment of the neural plate border territory amid the neural plate and the non-neural ectoderm. Subsequently, during neurulation, a subset of neural plate border is specified as the neural crest. This group of cells, which are initially organized in bilateral fields on both sides the neural plate, come to reside on the dorsal aspect of the neural tube as the roof plate closes (Gammill and Bronner-Fraser, 2003; Sauka-Spengler and Bronner-Fraser, 2008; Simoes-Costa and Bronner, 2015). Neural crest cells then undergo an epithelial-tomesenchymal transition and migrate through well-defined pathways to colonize specific locations within the developing embryo. Once they reach their destinations, these cells differentiate into diverse cell types (Martik and Bronner, 2017).
Classical embryology studies, including ablations, cell transplantation, and dye-labeling experiments, have identified the vast array of neural crest derivatives Hall and Hörstadius, 1988; Le Douarin, 1982). These include the neurons and glia of the peripheral and enteric nervous system, craniofacial connective tissue, cartilage and bone, and pigment cells. Other derivatives include part of the smooth muscle of the great vessels, pericytes and secretory cells like the chromaffin cells of the adrenal gland (Etchevers et al., 2001; Le Douarin and Kalcheim, 1999). Indeed, neural crest progenitor cells have the remarkable ability to give rise to both ectodermal and mesodermal-like derivatives and are often referred to as the fourth germ layer (Hall, 2000). Furthermore, since most distinguishing features of vertebrates are neural crest-derived, these cells have important implications in deuterostome evolution and hence are considered a vertebrate synapomorphy (Green et al., 2015).
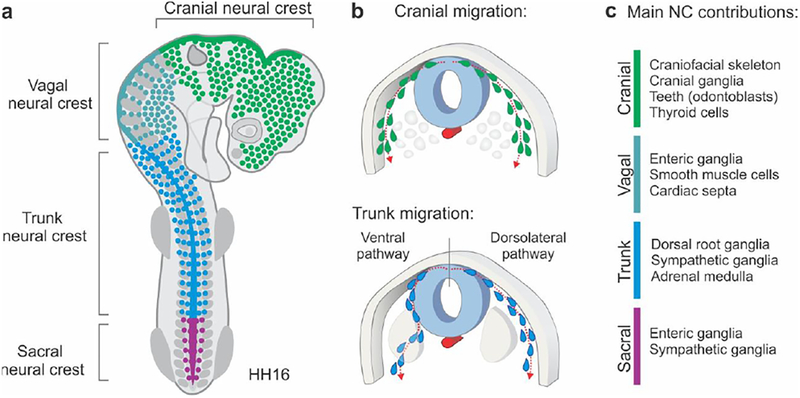
Neural crest cells are formed along most of the anteroposterior axis of the embryo, from the posterior diencephalon (Etchevers et al., 1999) to the lumbosacral region of the embryo (Osorio et al., 2009) (Fig. 1). They can be grouped into four distinct subpopulations according to the position where they are specified: cranial, vagal, trunk or sacral (Fig. 1). The cranial neural crest includes cells that are formed in the cephalic region, from the forebrain to the 6th rhombomere (R6) of the hindbrain. The vagal crest comprises progenitor cells that are specified next to the first seven somites (Le Douarin and Teillet, 1974) in fish, avian and murine embryos. The trunk crest includes the neural crest streams that migrate from the 8th somite until the 27th somite in avian embryos or the 24th somite in the mouse. The sacral neural crest, which has only been identified in amniotes, lies posterior to this boundary (Le Douarin and Kalcheim, 1999). This classification is not merely anatomical, as neural crest cells arising from different axial locations are not equivalent (See Box 1). These subpopulations differ in their migratory patterns, differentiation potentials, and gene expression profiles. For instance, cranial neural crest cells travel as a group, in a wave-like pattern. Alternatively, trunk cells form well-defined streams, and adopt either the ventral or the dorsolateral migratory pathways (Fig. 1b). Such differences in the migratory behaviors between axial levels have been recently reviewed (Kuo and Erickson, 2010; Theveneau and Mayor, 2012). Here, we will focus on the differences in developmental potential between neural crest cells of different axial levels, examining the molecular features that make each population unique. We will also discuss how the phylogeny of the neural crest subpopulations may have affected vertebrate evolution.
Figure 1. Overview of neural crest subpopulations along the anteroposterior axis.
(A) Diagram of neural crest subpopulations in an HH16 chick embryo. The cranial neural crest subpopulation includes neural crest cells that are formed between the forebrain and the 6th rhombomere (R6) of the hindbrain. The vagal crest is adjacent to the first seven somites. The trunk neural crest spans from the 8th to the 27th somite, while the sacral neural crest is positioned posterior to this boundary. (B) Differences in migratory patterns among neural crest subpopulations. While the cranial neural crest migrates dorsolaterally, the trunk neural crest, which is present adjacent to the developing somites, may take either the dorsolateral or ventral pathway of migration. (C) Major derivatives of neural crest subpopulations. The cranial neural crest gives rise to the craniofacial skeleton and is the only subpopulation possessing ectomesenchymal potential in amniotes. HH: Hamburger and Hamilton developmental stages.
Box 1. Overview of Neural Crest Subpopulations
Extensive fate map studies performed in the 1960s and 1970s allowed for the identification of the four distinct neural crest subpopulations, distributed along the anteroposterior axis of the vertebrate embryo. Subsequently, a fifth population, the cardiac neural crest, was identified; it is now considered a subset of the vagal neural crest. Here we present a summary distinctive features of each subpopulation, focusing on their migratory patterns and their respective cellular derivatives.
Cranial Neural Crest
The cranial neural crest is the first subpopulation to undergo an epithelial to mesenchymal transition and delaminate from the neural tube. It gives rise to the majority of the skeletal elements of the face, melanocytes and the neurons and glia of the cranial ganglia (Graham et al., 2004). In amniotes, cranial neural crest cells migrate dorsolaterally and adopt different routes, depending on their axial location along the neural tube. The cells from the forebrain and midbrain regions travel as a continuous mesenchymal sheet of cells (Couly et al., 1995), moving anteriorly over the optic primordia to form fronto-nasal structures. In contrast, neural crest cells from the hindbrain travel laterally and ventrally in defined segmented streams to populate the pharyngeal arches (PA). The developing hindbrain can be divided into 7 segments or rhombomeres (r1–7), and the neural crest streams are formed adjacent to even-numbered rhombomeres, resulting in this segmented pattern. The first stream, also dubbed the trigeminal neural crest, emanates from the midbrain and r1–2. The second stream, or hyoid crest, originates from r4, while the third stream or post-otic crest migrates from r6–7(Kontges and Lumsden, 1996; Kuo and Erickson, 2010; Lumsden et al., 1991). These streams sequentially fill the corresponding pharyngeal arches; trigeminal crest fill PA1 to give rise to jawbones and malleus and incus bones of the middle ear, hyoid crest migrates to PA2 to form hyoid bone and stapes bone of the ear, and post-otic crest travels to PA3 and contributes to thyroid and parathyroid glands. Each of these streams also gives rise to neurons of cranial and jugular ganglion(Noden, 1983).
Vagal neural crest
This subpopulation arises from the axial level of somites 1–7 in the developing embryo and is positioned between the cranial and trunk neural crest. A subset of the anterior vagal crest (somites 1–3) comprises the cardiac neural crest, which is unique in its ability to contribute to the septation of the outflow tract of the heart. This population follows a stereotypic dorsolateral pathway to fill the heart, great vessels and pharyngeal arches 3–6, wherein it differentiates into smooth muscle cells(Kirby et al., 1983; Kirby and Stewart, 1983). In contrast, the late-migrating cells from the same axial level preferentially give rise to melanocytes(Yntema and Hammond, 1954). Two additional populations of vagal neural crest cells arising from somites 1–3 and somites 4–6 travel ventrally to form the enteric nervous system of the foregut and stomach, respectively.(Epstein et al., 1994; Le Douarin and Teillet, 1973) The cells formed next to somite seven only contribute to the dorsal root ganglia of the peripheral nervous system and melanocytes(Reedy et al., 1998).
Trunk Neural Crest
Trunk neural crest cells originate from the posterior part of the embryo and primarily differentiate into neural and glial cells of the peripheral nervous system, adrenomedullary cells, endoneurial fibroblasts and melanocytes (Kuo and Erickson, 2010; Vega-Lopez et al., 2017). The migration of the trunk neural crest initiates in a crowded environment around the posterior neural tube, in which the cells are in close contact with the prospective epidermis and paraxial mesoderm. This restricts the migration of trunk neural crest cells to specific predetermined routes and makes them especially susceptible to the physical and molecular cues afforded by the surrounding environment. In particular, the somites play an essential role in determining the path and timing of migration by functioning as a source of signaling molecules, as a physical obstacle or as a migration substrate for different streams of trunk neural crest cells(Loring and Erickson, 1987). The trunk neural crest can travel through three different pathways. In mammalian and avian embryos, these are the ventrolateral pathway between the neural tube and somites, the ventromedial pathway between neural tube and sclerotome, and the dorsolateral pathway between the ectoderm and anterior sclerotome. Among those that take the ventral routes, the early migrating cells take the ventromedial/ventrolateral pathway and give rise to dorsal root ganglia of the peripheral nervous system, while the late-migrating cells from the posterior sacral region travel ventrally to differentiate into non-neuronal derivatives (Artinger and Bronner-Fraser, 1992). The cells of the melanocytic lineage migrate through the dorsolateral pathway, being the only trunk crest cells to do so (Henion and Weston, 1997; Reedy et al., 1998).
Sacral Neural Crest
The sacral neural crest has a crucial contribution to the enteric nervous system, giving rise to the ganglia that innervate the hindgut. The sacral crest migrates ventrally and colonizes the gut after the vagal crest. Interestingly, although the sacral neural crest can populate the gut in the absence of vagal crest, it cannot compensate for the lack of vagal derivatives, suggesting molecular differences between the two populations(Burns et al., 2000). The ability to migrate to the hindgut is distinctive of the sacral neural crest, as cranial and trunk neural crest stop migrating at a location much dorsal to the gut. Transplantation experiments have shown that when located at the sacral level, even thoracic crest can invade the gut, while sacral crest grafted in the thorax fails to enter gut (Erickson and Goins, 2000). Thus, the migratory route adopted by neural crest cells located in the sacral region determines their final fate. Indeed, the dorsal location of the gut in the posterior end of the embryo allows the sacral crest to take a more direct route to the gut, populating the enteric mesenchyme at the right developmental window
The developmental potential of neural crest subpopulations
The identification of the full array of neural crest derivatives was a considerable undertaking that spanned more than a century of experiments performed in multiple model organisms. Tissue ablation, cell labeling with vital dyes, cell transplantation, and genetic fate-mapping are among the techniques that were used to map the fate of neural crest cells (Chibon, 1964, 1967; Hörstadius, 1969; Johnston, 1966; Le Douarin, 1982; Le Lievre and Le Douarin, 1975; Noden, 1978; Weston, 1970). Such experiments revealed the extensive contribution of the neural crest to the vertebrate body plan. Furthermore, mapping the derivatives of neural crest cells from different axial levels revealed the specific contribution of each subpopulation (See Box 1). In this context, the chicken-quail chimera experiments pioneered by Nicole Le Douarin were particularly informative. While quail and chick embryos are very similar in size and timing of development, cells from the quail have a unique nuclear morphology that is not observed in other birds. By transplanting quail tissue to chick embryo hosts, Le Douarin and colleagues were able to examine neural crest fate along the anteroposterior axis (Le Douarin and Kalcheim, 1999; Le Douarin et al., 2004). These studies revealed that the contributions of the cranial, vagal and trunk neural crest cells are remarkably diverse (Box 1) (Fig. 1c). However, it was not immediately clear whether those differences were due to cell-intrinsic differences in potential, or a consequence of the distinct environmental signals that are present at each axial level.
To address possible differences between neural crest cell fate and their differentiation potential, researchers employed heterotopic transplantations. In these studies, neural tube explants, containing the premigratory neural crest, were isolated from quail embryos and grafted into a distinct axial level of chick host embryo (Fig. 2) (Chibon, 1967; Le Douarin and Teillet, 1974). Embryos were cultured until late developmental stages and the contributions from the grafted tissue were assessed with histological analysis. The results from these experiments revealed a large degree of developmental plasticity, with neural crest cells often adopting the migratory routes and generating the derivatives of their new axial level (Le Douarin et al., 2004). For instance, when quail cranial or vagal neural crest cells were transplanted to the trunk of a host chick embryo they gave rise to trunk derivatives, differentiating into neurons, glia and pigment cells (Le Douarin and Teillet, 1974). Similarly, when trunk neural crest cells were transplanted to the vagal region, they colonized the gut and differentiated into the enteric nervous system. Thus, early neural crest cells exhibit a large degree of developmental plasticity, and their fate choice is controlled by the environmental signals they encounter during and after migration. While the potential of neural crest cells decrease as these cells migrate (see discussion below), these results are consistent with the idea that early-migrating neural crest cells of distinct axial levels are multipotent and can give rise to an array of cell types (Bronner-Fraser and Fraser, 1988, 1989; Fraser and Bronner-Fraser, 1991).
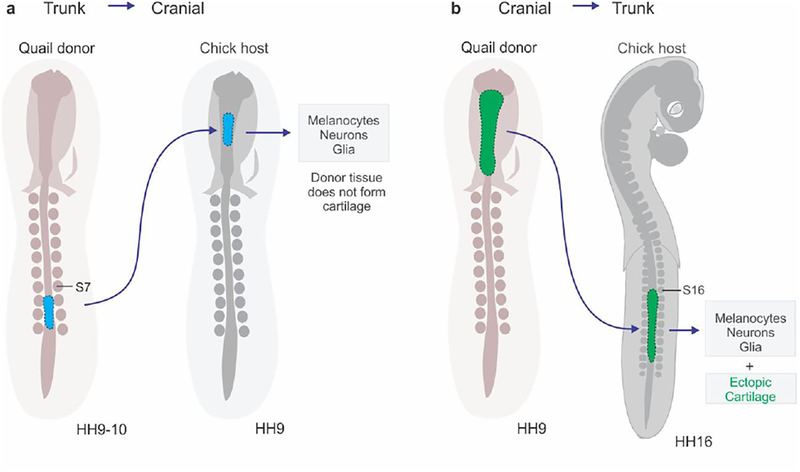
Figure 2: The use of quail-chick chimeras in establishing the differentiation potential of neural crest subpopulations.
(A) Heterotopic grafting of HH9/10 quail trunk neural crest tissue into the midbrain of an HH9 chick host revealed the constrained potential of the trunk, which is not capable of forming cartilage. (B) In the converse experiment, HH9 quail cranial neural crest tissue transplanted into the trunk region of an HH16 chick host gave rise to ectopic cartilage nodules (adapted from Lwigale et. al, 2004 and Le Douarin and Teillet, 1974).
Restrictions of neural crest cell potential along the anteroposterior axis
While all neural crest cells display a high degree of developmental plasticity, cell transplantation experiments also uncovered important differences in potential between axial levels. These developmental restrictions are particularly evident for the mesenchymal derivatives that arise from the cranial and vagal neural crest. Experiments in multiple model organisms indicate that the cranial and vagal neural crest are unique in their potential to contribute to the craniofacial skeleton and the cardiovascular system, respectively (Kirby, 1989; Le Douarin et al., 2004). Several fate mapping studies have suggested that the trunk neural crest of basal vertebrates forms mesenchymal cells that reside in the fin (Collazo et al., 1993; Smith et al., 1994b), but this potential seems to have been lost in amniotes, with the possible exception of chelonians (see discussion below) (Cebra-Thomas et al., 2007).
The neural crest origin of the craniofacial skeleton was identified more than a century ago (Platt, 1893), in experiments conducted in amphibians. This finding was quite controversial at the time (Noden, 1978) due to the popularity of the germ layer theory, which stated that bone and cartilage were of mesodermal origin (Oppenheimer, 1940). Nevertheless, the contribution of cranial neural crest cells to the craniofacial skeleton was confirmed by subsequent experiments (Landacre, 1910; Stone, 1929). Studies in avian embryos allowed for the fate-mapping of the anteroposterior arrangement of the progenitors that form the distinct parts of the skull (Couly et al., 1993; Le Lievre and Le Douarin, 1975; Noden, 1978). Further experiments in birds and amphibians revealed that this potential is unique to the cephalic population. Neural crest cells from tissue grafts dissected from the trunk were unable to give rise to cartilage and bone when grafted to the cranial region (Chibon, 1967; Lwigale et al., 2004; Nakamura and Ayer-le Lievre, 1982) (Fig. 2). Even cardiac neural crest, which can otherwise differentiate into smooth muscle and cardiac tissue, fails to contribute to craniofacial structures when transplanted in the midbrain (Lwigale et al., 2004). Remarkably, quail neural crest cells from cranial neural tube grafts transplanted into the trunk of a chicken embryo can form ectopic cartilage nodules (Le Douarin and Teillet, 1974) (Fig. 2). This suggests that these cells are able to differentiate into cartilage even outside of the cephalic microenvironment.
A similar restriction in potential is observed at the vagal levels. The anterior-most vagal crest (adjacent to somites 1–3) contributes to the heart and the great vessels, hence being termed the cardiac neural crest (Kirby et al., 1983). Intra-vagal transplant experiments show a high degree of plasticity. Posterior vagal crest can occupy the cardiac outflow tract when transplanted to an anterior axial level, while the cardiac crest can populate the gut when grafted posteriorly. In contrast, cells from other axial levels are unable to give rise to cardiac tissue even when grafted in the vagal region. Cranial neural crest cells, when transplanted in the region of the cardiac crest, cannot give rise to a functional heart septum, and instead form ectopic cartilage similar to that found in the face (Kirby, 1989). Trunk derivatives also fail to contribute to the ectomesenchyme for septation of the outflow tract. These results suggest that the cardiac neural crest has diverged from the other subpopulations, acquiring the unique ability to contribute to the cardiovascular system.
Taken together, these studies suggest that the neural crest subpopulations are (i) plastic in respect to most derivatives, being able to differentiate into neural cell types and melanocytes regardless of their axial level of origin, and (ii) restricted in regards to mesenchymal potential. It is important to emphasize that the mesenchymal potentials observed in the cranial and cardiac subpopulations are not interchangeable, as these cells cannot substitute one another. Overall, there seems to be the trend where the neural crest becomes progressively restricted along the anterior-posterior axis (Lwigale et al., 2004), with cranial cells being able to give rise to most cell types and trunk/sacral populations having more restricted developmental potential.
Differences in timing of cell fate restriction
Neural crest subpopulations have essential differences in their patterns of migration, which, in turn influence their timing of cell fate restriction. In the cranial regions, the neural crest engages in collective cell migration, forming a migratory wave that occupies the space between the head mesoderm and the future epidermis (Kuo and Erickson, 2010; Le Douarin, 1980). The early migrating neural crest travels longer distances to populate the distal branchial arches and forms most of the facial structures, including cartilage and connective tissue. The late-migrating crest remains close to the neural tube and contributes mainly to the cranial ganglia (Baker et al., 1997). Compared to its vagal and trunk counterparts, the cranial neural crest population maintains its plasticity the longest during migration. Grafting of early migrating branches of the trigeminal crest into the late-migrating branch and vice versa have shown that late neural crest cells can give rise to all derivatives formed by the early cells (Baker et al., 1997). This suggests that pre-migratory cranial crest is considerably plastic, and that its fate determination depends on the signals that the cell encounters along its migratory routes.
Unlike the cranial neural crest, ventrally migrating trunk crest cells travel within well-defined streams, which are delimited by signals and physical barriers provided by the cells of the somites and the neural tube. This constrains the possible routes that can be adopted by the neural crest cells (Debby-Brafman et al., 1999; Kalcheim and Teillet, 1989; Lallier and Bronner-Fraser, 1988). Furthermore, the timing of migration initiation and the pathways adopted synchronized with the development of the adjacent somites, which imposes strong developmental regulation on the temporal pattern of trunk neural crest migration (Sela-Donenfeld and Kalcheim, 2000). In contrast to cranial cells, the late-migrating trunk neural crest cannot form all the derivatives when transplanted in younger embryos (Artinger and Bronner-Fraser, 1992). These findings indicate that trunk cells become fate-restricted closer to the neural tube than the cranial neural crest. Given the evidence that trunk neural crest cells are still multipotent during migration (Baggiolini et al., 2015; Bronner-Fraser and Fraser, 1989), it is likely that the cells with a more limited repertoire of derivatives (trunk/sacral neural crest cells) commit to their final fates earlier than those with broader potential (cranial neural crest).
Molecular differences underlying neural crest axial identity
Studies from multiple laboratories have identified regulatory factors that control the induction, specification, and differentiation of the neural crest (Nelms and Labosky, 2010; Saint-Jeannet, 2006). Subsequent functional and biochemical analyses have shown that these molecules are connected by regulatory interactions, and thus are part of a gene regulatory network (GRN) that controls neural crest development (Betancur et al., 2010a; Meulemans and Bronner-Fraser, 2004; Simoes-Costa and Bronner, 2015). The neural crest GRN consists of multiple sub-circuits that are sequentially activated to control the major steps in neural crest formation. The earliest module of the network controls neural crest induction and describes the process by which early signalling pathways (Wnts, FGFs and BMPs) establish neural plate border identity by activating specifier genes like Msx1, Pax7 and Zic1 (Hong and Saint-Jeannet, 2007; Prasad et al., 2012) . In the next module of the GRN, the neural plate border genes cooperate to drive expression of a group of genes termed the neural crest specifiers, which include bona fide markers such as FoxD3, Sox10 and Ets1 (Cheung et al., 2005; Honore et al., 2003; Simoes-Costa and Bronner, 2015) Subsequently, these genes will activate transcriptional cascades that drive EMT and migration, cooperating with signaling systems to jumpstart the regulatory circuits that induce cell differentiation (Martik and Bronner, 2017; Simoes-Costa and Bronner, 2015).
The neural crest GRN is comprised of many of molecular players, including signalling proteins, transcription factors, and epigenetic modifiers. It has been compiled from regulatory studies performed in multiple model organisms, and comparative analysis indicates that is highly conserved amongst vertebrates (Sauka-Spengler et al., 2007). Although it is likely one the most comprehensive regulatory network described for any vertebrate cell type, it is far from complete. Transcriptomic analysis has uncovered dozens of uncharacterized neural crest genes (Lumb et al., 2017; Simoes-Costa and Bronner, 2016; Simoes-Costa et al., 2014), and new layers of regulation by epigenetic and post-transcriptional regulators have just begun to be incorporated in the network (Rada-Iglesias et al., 2012). Furthermore, differences in axial level-specific regulation have not been accounted for in the existing versions of the network (Martik and Bronner, 2017; Simoes-Costa and Bronner, 2015). Instead, the neural crest GRN has been typically presented as a composite of the regulatory interactions of different subpopulations, or has favoured the regulatory interactions that take place in the cranial region. This is a consequence of the sheer complexity of this regulatory program and a lack of thorough, comparative molecular studies contrasting neural crest subpopulations. Indeed, while we have just started to explore these molecular differences at a genomic level, there is mounting evidence that supports the hypothesis that the neural crest molecular toolkit differs between axial levels.
Axial-specific factors and enhancers
Studies in distinct model organisms have revealed a small number of neural crest factors that are specifically expressed or enriched at different axial levels. Some examples include transcription factors ID2 and ETS1, and the proto-oncogene RET. Id2, which is present in the cranial neural crest and contributes to neural crest induction, was first characterized in avian embryos and is the first transcription factor reported to be axial-specific (Martinsen and Bronner-Fraser, 1998). Likewise, ETS1 is a cranial-specific transcription factor, which contributes to neural crest delamination and serves as a regulator of several cranial-specific enhancers (Simoes-Costa and Bronner, 2015; Tahtakran and Selleck, 2003; Theveneau et al., 2007). The receptor tyrosine kinase RET, which had been previously linked to the formation of the enteric nervous system (Taraviras et al., 1999), was also shown to be enriched in the vagal neural crest (Simkin et al., 2013). In recent years, transcriptomic analysis has dramatically expanded the number of factors that are exclusively expressed, or enriched, at different axial levels (Lumb et al., 2017; Simoes-Costa and Bronner, 2016; Simoes-Costa et al., 2014). These studies suggest a higher degree of regulatory compartmentalization between the subpopulations than previously thought (see discussion below).
This idea is consistent with cis-regulatory studies, which have uncovered an unexpected degree of modularity in the enhancers controlling the expression of pan-neural crest genes FoxD3 and Sox10. While both transcription factors are seamlessly expressed in neural crest cells at all axial levels, they are differentially controlled by enhancers that are active in either the cranial or the trunk region. This was first shown in Sox10, which is controlled by enhancers Sox10E1 and Sox10E2. Sox10E1 is a vagal/trunk enhancer present in late migratory neural crest cells, while Sox10E2 is active in migratory cranial neural crest cells. These enhancers seem to be regulated by distinct inputs (Betancur et al., 2010b; Murko and Bronner, 2017). Similarly, the bona fide neural crest marker FoxD3 is regulated by axial level-specific enhancers, NC1 and NC2. These elements are active in the cranial and vagal/trunk neural crest, respectively. Upon analysis of the regulation of each enhancer element, it was reported that Pax7 and Msx1 directly regulate NC1, in concert with cranial-specific transcription factor Ets1. In the trunk, NC2 is also regulated by Pax7 and Ms1, but requires the additional binding of the trunk-enriched transcription factor, Zic1 (Simoes-Costa et al., 2012). These results demonstrate that Ets1 and Zic1 have axial-specific roles in neural crest specification, contributing to the cranial and trunk regulatory programs, respectively.
Taken together, these findings highlight the nuanced regulation of pan-neural crest genes at different locations, and suggest that neural crest specification relies on different inputs according to each axial level. The biological significance of this modular regulation has not yet been fully explored. While it is possible that axial-specific enhancers generate subtle differences in the timing or the levels of expression of pan neural crest genes, these are not evident from transcriptomic analysis (Simoes-Costa and Bronner, 2016) or even in situ hybridization in whole mount embryos. Hence, the existence of cranial vs. trunk enhancers may reflect a requirement for distinct specification programs that are compatible with each axial level. Progenitor cells in the head and the trunk are exposed to distinct signalling inputs (retinoic acid, Wnts, FGFs), and have intrinsic regulatory differences (such as the Hox code, discussed below). Distinct enhancers might have been necessary to reconcile the high level of regulatory heterogeneity between axial levels, and to generate an equivalent transcriptional output in each subpopulation. Furthermore, the modular regulation of neural crest genes may also reflect the evolutionary history of these subpopulations (see discussion in evolution of neural crest subpopulations below). Additional studies that examine the functional consequences of which enhancer is deployed, as well as a survey of axial-specific enhancers in different species, are required to elucidate this intriguing property of the neural crest gene regulatory network.
Role of the Hox Code
The homeobox-containing protein (Hox) cluster plays a central role in patterning the vertebrate embryo along the anteroposterior axis, making it a likely player in the definition of neural crest subpopulations. In particular, Hox genes have been shown to play a central part in the segmentation of the cranial crest. The cranial neural crest can be subdivided into an anterior Hox− population and a posterior Hox+ population (Trainor and Krumlauf, 2000). Importantly, Hox gene expression also impacts the potential of neural crest cells. The absence of nested Hox gene expression in the anterior cranial neural crest (midbrain/r1-r2 of the hindbrain) has been shown to be necessary for the formation of ectomesenchymal derivatives. Ectopic expression of Hoxa2 in the mesencephalic and r1-r2 streams of chick embryos results in complete loss of jaw and frontonasal structures, while overexpression of Hoxa3 and Hoxb4 displays drastic reduction in the formation of the facial skeleton (Creuzet et al., 2002). Conversely, Hox expression in the posterior cranial neural crest (r4-r6), as well as the vagal and trunk neural crest appears to be required for differentiation. Studies in mouse, Xenopus, and zebrafish embryos have shown that inactivation of Hoxa2 in the r4 neural crest streams results in the transformation of pharyngeal arch (PA) 2 elements into structures of PA1, such as Merkel’s cartilage and malleus and incus bones of middle ear(Minoux et al., 2009).
The apparent significance of Hox patterning in the morphogenesis of neural crest-derived tissues has raised the question of how this gene family is regulated and how its expression relates to the neural crest gene regulatory network(Parker et al., 2018). A recent study comparing the transcriptomes of Hox+ and Hox− cranial crest in mice reported an enrichment of genes responsive to the retinoic acid signaling pathway in the Hox− population. Further, several other members of the Hox family proteins including Hoxb1, Hoxc5 and Hoxc11 were also found to be upregulated in the r4 stream (Lumb et al., 2017). It has been previously reported that Hox1 family members are responsive to retinoic acid signaling, and thus it likely that in the Hox+ cranial neural crest, Hoxa2 functions downstream of the retinoic acid pathway to activate transcription of other Hox family proteins. This generates a unique “Hox code”, which fine-tunes the migration and differentiation of r4 and r6 streams of cranial crest, patterning the developing vertebrate head(Parker et al., 2018).
It is possible that Hox genes may act directly on neural crest genes to modulate the neural crest specification program. In mice, microarray analysis of Hoxa1 null embryos revealed a downregulation in neural crest genes, Zic1, Hnf1b and Foxd3(Makki and Capecchi, 2011). Furthermore, knockdown of Hoxa2 results in the ectopic expansion of the expression domain of the ectomesenchymal factor, Sox9. Sox9 transcriptionally activates the cartilage-specific gene, Col2a1, and epistatic experiments have shown that Sox9 is responsible for ectopic cartilage formation in Hoxa2 mutants(Bell et al., 1997).
The vagal and trunk neural crest also comprise the Hox+ neural crest population. However, these neural crest components differ from the cranial Hox+ population. Indeed, while Hoxa2 is the predominant Hox family protein expressed in the cephalic neural crest, Hoxb5 has been shown to be enriched in vagal and trunk neural crest (Kam and Lui, 2015). Hoxb5 promotes the transcription of RET, and inhibition of Hoxb5 specifically in the vagal neural crest results is reduced crest migration and delayed gut development (Lui et al., 2008). In the trunk neural crest, Hoxb5 is hypothesized to induce the expression of classical neural crest factors Sox9 and FoxD3 to promote neurogenic fate in the early migrating trunk neural crest cells(Kam and Lui, 2015). Moreover, posterior Hox genes such as Hox6, Hox7, Hox8, and Hox9 are also exclusively expressed in the vagal and trunk neural crest. These proteins are essential for posterior neural tube closure and migration of neural crest cells originating therein. Interestingly, ubiquitous expression of Hoxa7 results in severe craniofacial defects (Balling et al., 1989), which highlights how the topological restriction of specific Hox genes recapitulates anatomical differences inherent in neural crest cells. Additionally, unlike members of Hox1-Hox5 family, the above proteins respond to FGF signaling and not retinoic acid signaling(Nelms and Labosky, 2010), which is supportive of the hypothesis that environmental cues contribute to differences in development of neural crest subpopulations.
The absence of Hox expression in the anterior cranial neural crest has been implicated in the inherent plasticity of this cell population(Helms et al., 2005). For example, transplants of single neural crest cells from r2 to r6 in the developing zebrafish embryo revealed a striking switch in Hox gene expression of the transplanted cell, such that it adopted the profile of the surrounding tissue(Schilling et al., 2001). These cells also acquired the ability to form the neuronal and skeletal derivatives reminiscent of the donor site, indicating that Hox status may play a role in dictating the differentiation potential among neural crest subpopulations along the anteroposterior axis. The plasticity of the anterior Hox− cranial crest has established this progenitor pool as a potential candidate for regenerative medicine. In the case of bone regeneration, skeletal progenitor cells derived from the Hox− neural crest are able to adopt the Hox status of the surrounding tissue when grafted to an injury site(Leucht et al., 2008). Upon the acquisition of this identity, these cells maintain this gene expression profile throughout their lifetime
Hox gene expression along the anteroposterior axis serves as an exciting avenue for exploring axial level differences among neural crest subpopulations. Additionally, the existence of Hox− and Hox+ cranial neural crest populations showcase the nuanced regulation of axial level-specific gene expression even within the same neural crest subpopulation. Though many parallels have been drawn between the Hox Code and differentiation of neural crest progenitors, particularly in the case of cranial neural crest derivatives, many of the regulatory interactions between these two gene sets remain to be discovered(Parker et al., 2018).
Axial-specific gene regulatory networks
To further examine the regulatory programs that control distinct neural crest subpopulations, we recently employed axial-specific enhancers to perform a comprehensive transcriptome analysis of cranial vs. trunk neural crest cells. Comparative transcriptional profiling of these two neural crest cell populations, which were isolated using the FoxD3 NC1 and NC2 enhancers, revealed hundreds of cranial and trunk-enriched genes (Simoes-Costa and Bronner, 2016). Functional and biochemical analysis of a group of transcription factors found to be cranially enriched allowed for the delineation of a cranial-specific regulatory sub-circuit (Fig. 3a). Remarkably, ectopic expression of three components of this circuit (Ets1, Sox8, and Tfap2b) in the trunk neural crest cells were able to form ectopic cartilage nodules when transplanted to the head, while engraftment of untreated trunk neural crest could only give rise to neurons and melanocytes (Simoes-Costa and Bronner, 2016). This study emphasizes the importance of axial-specific genetic programs in cell fate determination within the neural crest.
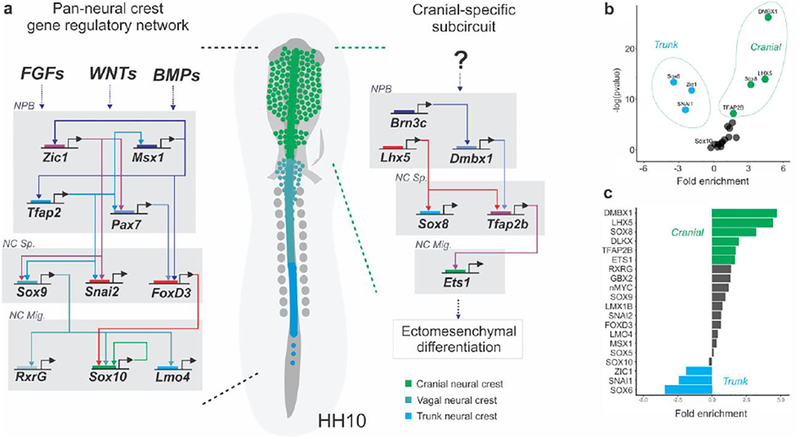
Figure 3. Axial-specific features of the neural crest gene regulatory network (GRN).
(A) Simplified diagram of the pan-neural crest gene regulatory network, which does not account for differences among subpopulations, and the cranial-specific subcircuit contributing to ectomesenchymal differentiation. Neural plate border genes (HH5–6) regulate the expression of neural crest specification and migration genes (HH8–10) (B-C) Enrichment of neural crest genes in the cranial vs. trunk populations suggests the presence of axial-specific circuits. NPB: Neural Plate Border; NC Sp: Neural Crest Specification; NC. Mig: Neural Crest Migration
Analysis of the cranial and trunk neural crest transcriptomes may reveal the axial level-specific identity of existing players within the neural crest GRN (Simoes-Costa and Bronner, 2015). For example, in addition to the cranial-specific factors identified in Simoes-Costa et al, 2016 (Brn3c, LHX5, Dmbx1, ETS1, Sox8, and TFAP2b) other factors such as Zic1, GATA2 and Sox6 were found to be enriched in the trunk neural crest (Fig. 3b). Lastly, neural crest genes found not to be differentially expressed in either the cranial or trunk neural crest (such as Sox10 and FoxD3) presumably coordinate both axial levels (Fig. 3c). By combining information from the existing neural crest GRN and the cranial and trunk transcriptomes, it is possible to predict gene regulatory subcircuits for axial-specific programs (Fig. 3). While the direct interactions of these cranial-specific factors with the promoters of their downstream targets have been shown in cranial neural crest (Simoes-Costa and Bronner, 2015), much less is known in the context of the trunk. Intriguingly, a comparison of the trunk and cranial neural crest transcriptomes showed that there is larger number of transcripts enriched in the posterior population (327 trunk genes vs. 216 cranial genes). This surplus of trunk-enriched genes may indicate the existence of a number of regulatory mechanisms unique to the posterior neural crest. First, the nested expression of Hox genes, which are highly transcribed in the posterior region of the embryo, may modulate the developmental potential of these cells. Second, the complex migratory patterns exhibited by the trunk crest may require a larger molecular toolkit, including membrane-bound receptors that trigger responses to attractant and repellent cues. Finally, it is also possible that a trunk-specific regulatory program acts to suppress skeletogenic potential. Additional genomic and functional analyses (Cheung et al., 2005) and biochemical studies will be necessary to delineate the structure and importance of this putative trunk regulatory program.
The architecture of cranial and trunk regulatory sub-circuits may inform upon the specific migratory patterns, fate decisions, and clarify the phylogeny of neural crest axial levels. It is likely that a number of factors present at several axial levels contribute to multiple programs, but may have different inputs/outputs in each context. The evidence detailed above supports the existence of subpopulation-specific genetic programs governing neural crest specification. However, axial level-specific regulation has not been studied in the context of terminal differentiation. It will be interesting to explore the network architecture governing differentiation of common neural crest derivatives, such as neurons, glia, and melanocytes, which are derived from every subpopulation. Given the differences observed in specification, it is possible that the formation of same cell type requires divergent regulatory programs at distinct axial levels. Thus, network analysis remains the suggested strategy for decoding the regulatory basis of neural crest axial identity.
The evolutionary history of neural crest subpopulations
Neural crest cells give rise to multiple elements that characterize the body plan of vertebrates, and thus are thought to have played a central role in their emergence and diversification. This idea was initially proposed in ‘New Head’ hypothesis, which suggests that the origin and diversification of vertebrates is linked to the transition from a passive to an active mode of predation (Gans and Northcutt, 1983). According to this idea, this shift is characterized by a number of co-opted and newly established structures that are of neural crest and placodal origin, including the formation of the jaw and the acquisition of an elaborate set of sense organs, replacing filter-feeding with selective predation. These new feeding mechanisms came with drastic implications, including increased body size and metabolic output. The New Head hypothesis distinguishes the neural crest as one of the defining factors giving rise to these new structures, ultimately driving the diversification of the vertebrate clade.
Given the indispensable role of the neural crest in vertebrate evolution, researchers have long sought to discover the origins of this intriguing cell population and how it attained its unique properties. While neural crest cells are a vertebrate innovation, work conducted in urochordates and cephalochordates, the closest invertebrate relatives to vertebrates, has suggested that the neural crest has even more ancient roots. Indeed, homologs to a number of bona fide neural crest genes, including BMP2/4, Pax3/7, Msx, and Snail are expressed at the neural plate border in the cephalochordate, amphioxus (Holland and Holland, 2001). Likewise, the urochordate, Ciona intestinalis, expresses Snail at the dorsal neural tube (Nieto, 2002). This perhaps indicates that though these invertebrate chordate species do not have neural crest, at least some of the gene regulatory machinery was present prior to its induction (Manzanares and Nieto, 2003; Yu et al., 2008). The first evidence of migratory neural crest-like cells was identified in the ascidian urochordate, Ecteinascidia turbinate. These cells were found to migrate from the neural tube and populate the body wall, giving rise to pigment cells. They also expressed neural crest markers, HNK-1 and Zic, suggesting that they may share a common ancestor with the neural crest (Jeffery et al., 2004). Since these cells were only able to contribute to pigmentation, it is possible that the other lineages of the neural crest were only acquired within vertebrate chordates. More recently, studies in Ciona have presented evidence of neural crest like-cells, giving rise to both melanocytes and peripheral neurons (Abitua et al., 2012; Stolfi et al., 2015).
Rebuilding the phylogeny of neural crest subpopulations
The evolutionary history of neural crest subpopulations is still an open question, although recent findings have shed light on the order in which these populations appeared. Studies on the agnathan vertebrate, Petromyzon marinus (sea lamprey), indicate that it lacks vagal neural crest cells despite having both cranial and trunk subpopulations (Green et al., 2017). Furthermore, fate mapping experiments with DiI-labelling revealed that in these animals the enteric nervous system is partly derived from Schwann cell precursors that are formed from the trunk neural crest (Green et al., 2017). These results indicate that the split between the cranial and trunk neural crest took place during early neural crest evolution. In contrast, the vagal neural crest, which is the most recent neural crest subpopulation, is a shared gnasthome trait.
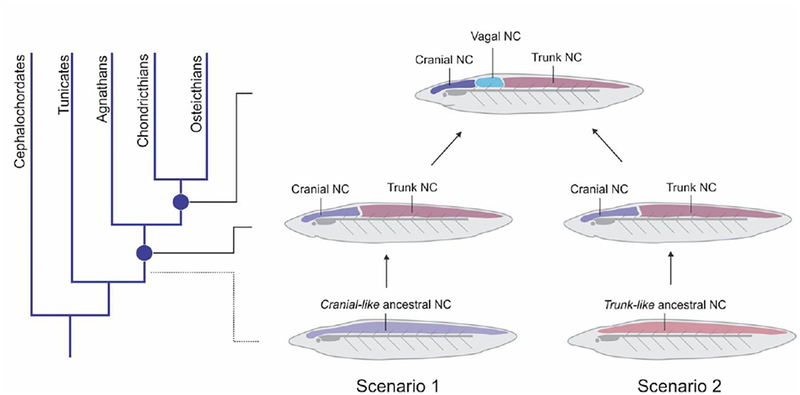
The acquisition of skeletogenic potential by the neural crest is crucial for clarifying the phylogeny of neural crest subpopulations. Elucidating the developmental potential of the ‘ancient neural crest’ will provide insight into which subpopulation arose first during vertebrate evolution. Here, we present two scenarios for the evolution of the neural crest subpopulations (Fig. 4). The first scenario proposes that the ancient neural crest possessed sketogenic potential and thus would be a more plastic cell population akin to the cranial neural crest. Subsequently, the posterior neural crest subpopulations diverged to develop their unique properties and lost most of this ectomesenchymal potential. The second scenario postulates that the ancient neural crest displayed restricted developmental potential reminiscent of the trunk neural crest. This suggests that skeletogenic potential is a derived feature, arising later during evolution. The following sections summarize the evidence in support of each scenario.
Figure 4. Scenarios for the evolution of neural crest subpopulations.
Skeletogenic potential impacts the phylogeny of the neural crest subpopulations. A skeletogenic ancient crest implies that the trunk subpopulation gradually lost the potential to give rise to ectomesenchyme in vivo (scenario 1), during vertebrate evolution. Alternatively, if the ancient crest was trunk-like, it is likely that a skeletogenic cranial neural crest appeared much later (scenario 2), possibly co-opting mesenchymal genetic programs that exist in other cell types.
Scenario 1: A skeletogenic ancient crest
Early theories of an ancient neural crest that displayed chondrocytic potential came from observations of the fossil record. Ostracoderms (armored jawless fish) were amongst the earliest vertebrates and possessed a mineralized exoskeleton composed of dermal bone and dentine. Though this primitive armour has subsequently been lost in extant species, it has been suggested that teleost and cartilaginous fish retain components of this skeleton in the form of scales, fin rays, or dermal denticles. These skeletal elements could have played an important role in vertebrate evolution, functioning as protection or for sensory organ enhancement (Donoghue and Sansom, 2002). Were the exoskeletons of the earliest vertebrates neural crest-derived? This would suggest that the ancient neural crest possessed skeletogenic potential, not only on the cephalic regions, but also in the trunk. Observations of a bona fide cranial neural crest origin of dentine in higher vertebrates suggests that the ostracoderm exoskeleton was derived from neural crest (Smith and Hall, 1990). While controversial (see discussion of alternative scenario below) this idea is supported by fate mapping studies showing the contribution of the trunk neural crest to the caudal fin mesenchyme of zebrafish (Kague et al., 2012) and Xenopus (Collazo et al., 1993). More recently, the trunk neural crest has also been shown to contribute to the dermal denticles of the little skate, Leucoraja erinacea (Gillis et al., 2017; Smith et al., 1994a).
Studies that argue for the existence of ectomesenchymal capacity in the trunk neural crest also exist in amniotes. Chelonians, or turtles, are among the few vertebrate species that possess an extensive trunk exoskeleton. For this reason, the embryonic origins of the turtle shell have been studied extensively to determine whether it may be neural crest-derived. Multiple studies have reported the contribution of trunk neural crest cells to the turtle plastron bones, located on the ventral surface of the shell (Cebra-Thomas et al., 2007; Cebra-Thomas et al., 2013; Clark et al., 2001; Gilbert et al., 2007). These studies suggest that a late-migrating population of trunk neural crest cells, expressing similar markers to those of the cranial neural crest, populate the developing plastron (Cebra-Thomas et al., 2013).
It is possible that if the ancient neural crest had originally possessed ectomesenchymal potential, remnants may still exist in all neural crest subpopulations. Following this premise, much work has been done in vitro in attempts to induce skeletogenesis in trunk neural crest cells. Several studies have revealed that under certain culture conditions, avian trunk neural crest cells may be induced to produce bone and cartilage, often by modifying growth factor signaling (Calloni et al., 2007; Coelho-Aguiar et al., 2013; McGonnell and Graham, 2002). These studies have suggested that trunk neural crest cells possess latent chondrocytic potential that is suppressed in vivo in higher vertebrates (Coelho-Aguiar et al., 2013). These studies raise the possibility that the basal neural crest evolved with the potential to form ectomesenchyme, but that regulatory mechanisms arose during evolution to suppress skeletogenesis. The molecular mechanisms governing this suppression and pathways by which it may resurface remain unresolved. Nevertheless, assignment of skeletogenic potential to the ancient neural crest provides a compelling evolutionary scenario that bridges information obtained the fossil record with results from developmental studies.
Scenario 2: The ectomesenchyme as an evolutionary novelty
Early heterotopic grafting experiments conducted in amphibians and amniotes have established that, in vivo, skeletogenic potential is restricted to the cranial neural crest. Accordingly, upon their presentation of the ‘new head’ hypothesis, Gans Northcutt proposed that many changes in the vertebrates body plan occurred anterior to the existing nerve tube (Gans and Northcutt, 1983). They postulated that the vertebrate head, derived from cranial neural crest, represents an addition rather than a modification of the trunk. This supports the idea that a skeletogenic cranial subpopulation emerged later during evolution, and that the ancient neural crest possessed a more restricted differentiation potential, reminiscent of the trunk. According to this hypothesis, the cranial neural crest subsequently co-opted mesenchymal regulatory circuits from other cell types, which allowed for the emergence of an elaborate craniofacial skeleton.
Consistent with this, a number of studies have refuted the idea that the trunk neural crest displays ectomesenchymal potential. For example, experiments exploring the contribution of the trunk neural crest to the fin/scale mesenchyme in teleost fish have reported an exclusively mesodermal origin of these tissues (Lee et al., 2013a; Lee et al., 2013b; Mongera and Nusslein-Volhard, 2013; Shimada et al., 2013). While much of the data supporting a trunk neural crest origin of skeletal tissues in extant species has relied heavily on fate mapping via administration of lipophilic fluorescent dye, genetic fate-mapping has continuously contested these arguments. In amniotes, some studies suggest a mesodermal origin for the turtle carapace, being derived from endoskeletal ribs rather than from a skeletogenic trunk neural crest (Hirasawa et al., 2013). Furthermore, while some studies have used the HNK-1 antibody to identify neural crest derivatives in chelonians, it is important to consider the limitations of this reagent as a diagnostic marker for the neural crest lineage. Since the HNK-1 epitope is present in multiple cell types (Bronner-Fraser, 1986; Tucker et al., 1984), its use for the mapping of neural crest derivatives has been strongly questioned. Thus, the observation that trunk neural crest cells are able to give rise to ectomesenchyme in vivo is still contentious.
Despite the body of research conducted in efforts to elucidate the status of the ancient neural crest, its nature still remains controversial. It is possible the ancient neural crest was neither cranial nor trunk in nature, but that these subpopulations both diverged from a common ancestral cell type. Thus far, we have discussed the cranial vs trunk identity of the ancient neural crest only in terms of its skeletogenic potential. However, these two subpopulations differ in many aspects, including their migratory patterns, cis-regulatory inputs, and gene expression profiles (Kuo and Erickson, 2010; Simoes-Costa and Bronner, 2016). Though many questions still remain unanswered, it is clear that the analysis of an array of chordate species will continue be indispensable in elucidating the origins of neural crest subpopulations. To date, many studies have drawn conclusions from classical developmental biology techniques such as fate mapping via heterotropic grafting or DiI labeling. Conflicting results from these experiments highlight how technically complex fate-mapping is as a technique, and the need of additional strategies to scrutinize neural crest potential. In this context, understanding the molecular circuits controlling skeletogenic differentiation in the early neural crest (Simoes-Costa and Bronner, 2016) may be particularly informative. New technologies allowing for comprehensive genomic analysis of non-traditional model organisms may provide insight as to the molecular mechanisms governing the divergence of neural crest subpopulations over evolutionary time. Additionally, paleontological studies may continue to uncover morphological features in extinct species that will clarify the developmental potential of neural crest cells, shedding light in the evolutionary history of these cell populations.
Conclusions
The unique properties of the neural crest set vertebrates apart from other chordate species. The broad potential of this cell type and its importance in the establishment of the vertebrate body plan has fascinated scientists for decades. Though much work has been conducted to characterize this cell population in great detail, to date, we still have a limited understanding of the molecular asymmetries that result from axial-specific differences in potential and behaviour. However, with the advent of improved genomic techniques, it is becoming increasingly easier to investigate the molecular mechanisms governing the emergence of the neural crest subpopulations. Here, we have described in detail the demarcation of the neural crest into four distinct anatomical components, the cranial, vagal, trunk, and sacral neural crest. It is important to emphasize that this demarcation only partially recapitulates the heterogeneity characteristic of this stem cell population. Indeed, as we have highlighted above, even cells within the same neural crest population differ considerably in their temporal and spatial patterns of migration, lineage plasticity and differentiation abilities. These differences are a consequence of the unique transcriptional circuits that are active in individual neural crest components. Ultimately, much remains unknown regarding the molecular underpinnings of neural crest subpopulations. Further extrapolation of these mechanisms will have important consequences regarding our understanding of the emergence of the vertebrate clade.
Highlights.
Neural crest cells are divided in distinct subpopulations that differ in migratory behaviour and developmental potential;
We postulate that axial-specific genetic circuits operate in each subpopulation and underlie their unique features;
We discuss how the phylogeny of neural crest subpopulations impacts our understanding of vertebrate evolution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abitua PB, Wagner E, Navarrete IA, Levine M, 2012. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature 492, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artinger KB, Bronner-Fraser M, 1992. Partial restriction in the developmental potential of late emigrating avian neural crest cells. Dev Biol 149, 149–157. [DOI] [PubMed] [Google Scholar]
- Baggiolini A, Varum S, Mateos JM, Bettosini D, John N, Bonalli M, Ziegler U, Dimou L, Clevers H, Furrer R, Sommer L, 2015. Premigratory and migratory neural crest cells are multipotent in vivo. Cell stem cell 16, 314–322. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA, 1997. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development 124, 3077–3087. [DOI] [PubMed] [Google Scholar]
- Balling R, Mutter G, Gruss P, Kessel M, 1989. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox−1.1 in transgenic mice. Cell 58, 337–347. [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KKH, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PPL, Cheah KSE, 1997. SOX9 directly regulates the type-II collagen gene. Nat Genet 16, 174–178. [DOI] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T, 2010a. Assembling neural crest regulatory circuits into a gene regulatory network. Annual review of cell and developmental biology 26, 581–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T, 2010b. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc Natl Acad Sci U S A 107, 3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M, 1986. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Developmental biology 115, 44–55. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser S, 1988. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature 335, 161–164. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser S, 1989. Developmental potential of avian trunk neural crest cells in situ. Neuron 3, 755–766. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Champeval D, Le Douarin NM, 2000. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Developmental Biology 219, 30–43. [DOI] [PubMed] [Google Scholar]
- Calloni GW, Glavieux-Pardanaud C, Le Douarin NM, Dupin E, 2007. Sonic Hedgehog promotes the development of multipotent neural crest progenitors endowed with both mesenchymal and neural potentials. Proc Natl Acad Sci U S A 104, 19879–19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Betters E, Yin M, Plafkin C, McDow K, Gilbert SF, 2007. Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evol Dev 9, 267–277. [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Terrell A, Branyan K, Shah S, Rice R, Gyi L, Yin M, Hu Y, Mangat G, Simonet J, Betters E, Gilbert SF, 2013. Late-emigrating trunk neural crest cells in turtle embryos generate an osteogenic ectomesenchyme in the plastron. Dev Dyn 242, 1223–1235. [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J, 2005. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell 8, 179–192. [DOI] [PubMed] [Google Scholar]
- Chibon P, 1964. [Analysis by the Method of Nuclear Labelling with Tritiated Thymidine of Derivatives of the Cephalic Neural Crest in the Urodele Pleurodeles Waltlii Michah]. C R Hebd Seances Acad Sci 259, 3624–3627. [PubMed] [Google Scholar]
- Chibon P, 1967. [Nuclear labelling by tritiated thymidine of neural crest derivatives in the amphibian Urodele Pleurodeles waltlii Michah]. Journal of embryology and experimental morphology 18, 343–358. [PubMed] [Google Scholar]
- Clark K, Bender G, Murray BP, Panfilio K, Cook S, Davis R, Murnen K, Tuan RS, Gilbert SF, 2001. Evidence for the neural crest origin of turtle plastron bones. Genesis 31, 111–117. [DOI] [PubMed] [Google Scholar]
- Coelho-Aguiar JM, Le Douarin NM, Dupin E, 2013. Environmental factors unveil dormant developmental capacities in multipotent progenitors of the trunk neural crest. Dev Biol 384, 13–25. [DOI] [PubMed] [Google Scholar]
- Collazo A, Bronner-Fraser M, Fraser SE, 1993. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development 118, 363–376. [DOI] [PubMed] [Google Scholar]
- Couly G, Coltey P, Eichmann A, Le Douarin NM, 1995. The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mech Dev 53, 97–112. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM, 1993. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117, 409–429. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Vincent C, Le Douarin NM, 2002. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development 129, 4301–4313. [DOI] [PubMed] [Google Scholar]
- Debby-Brafman A, Burstyn-Cohen T, Klar A, Kalcheim C, 1999. F-Spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron 22, 475–488. [DOI] [PubMed] [Google Scholar]
- Donoghue PC, Sansom IJ, 2002. Origin and early evolution of vertebrate skeletonization. Microsc Res Tech 59, 352–372. [DOI] [PubMed] [Google Scholar]
- Epstein ML, Mikawa T, Brown AM, McFarlin DR, 1994. Mapping the origin of the avian enteric nervous system with a retroviral marker. Dev Dyn 201, 236–244. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Goins TL, 2000. Sacral neural crest cell migration to the gut is dependent upon the migratory environment and not cell-autonomous migratory properties. Dev Biol 219, 79–97. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, Le Douarin NM, 1999. Anterior cephalic neural crestis required for forebrain viability. Development 126, 3533–3543. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF, 2001. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128, 1059–1068. [DOI] [PubMed] [Google Scholar]
- Fraser SE, Bronner-Fraser M, 1991. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development 112, 913–920. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Bronner-Fraser M, 2003. Neural crest specification: migrating into genomics. Nature reviews. Neuroscience 4, 795–805. [DOI] [PubMed] [Google Scholar]
- Gans C, Northcutt RG, 1983. Neural crest and the origin of vertebrates: a new head. Science 220, 268–273. [DOI] [PubMed] [Google Scholar]
- Gilbert SF, Bender G, Betters E, Yin M, Cebra-Thomas JA, 2007. The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. Integr Comp Biol 47, 401–408. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Alsema EC, Criswell KE, 2017. Trunk neural crest origin of dermal denticles in a cartilaginous fish. Proc Natl Acad Sci U S A 114, 13200–13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Begbie J, McGonnell I, 2004. Significance of the cranial neural crest. Dev Dyn 229, 5–13. [DOI] [PubMed] [Google Scholar]
- Green SA, Simoes-Costa M, Bronner ME, 2015. Evolution of vertebrates as viewed from the crest. Nature 520, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Uy BR, Bronner ME, 2017. Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature 544, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK, 2000. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evolution & development 2, 3–5. [DOI] [PubMed] [Google Scholar]
- Hall BK, Hörstadius S,1988. The neural crest: including a facsimile reprint of The neural crest by Sven Hörstadius. Oxford University Press, London ; New York. [Google Scholar]
- Helms JA, Cordero D, Tapadia MD, 2005. New insights into craniofacial morphogenesis. Development 132, 851–861. [DOI] [PubMed] [Google Scholar]
- Henion PD, Weston JA, 1997. Timing and pattern of cell fate restrictions in the neural crest lineage. Development 124, 4351–4359. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Nagashima H, Kuratani S, 2013. The endoskeletal origin of the turtle carapace. Nat Commun 4, 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland LZ, Holland ND, 2001. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate? J Anat 199, 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Saint-Jeannet JP, 2007. The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol Biol Cell 18, 2192–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore SM, Aybar MJ, Mayor R, 2003. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev Biol 260, 79–96. [DOI] [PubMed] [Google Scholar]
- Hörstadius S, 1969. The neural crest; its properties and derivatives in the light of experimental research, Facsim. reprint of the 1950 edition ed. Hafner Pub. Co., New York,. [Google Scholar]
- Jeffery WR,Strickler AG, Yamamoto Y, 2004. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature 431, 696–699. [DOI] [PubMed] [Google Scholar]
- Johnston MC, 1966. A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat Rec 156, 143–155. [DOI] [PubMed] [Google Scholar]
- Kague E, Gallagher M, Burke S, Parsons M, Franz-Odendaal T, Fisher S, 2012. Skeletogenic fate of zebrafish cranial and trunk neural crest. PloS one 7, e47394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C, Teillet MA, 1989. Consequences of somite manipulation on the pattern of dorsal root ganglion development. Development 106, 85–93. [DOI] [PubMed] [Google Scholar]
- Kam MK, Lui VC, 2015. Roles of Hoxb5 in the development of vagal and trunk neural crest cells. Dev Growth Differ 57, 158–168. [DOI] [PubMed] [Google Scholar]
- Kirby ML, 1989. Plasticity and predetermination of mesencephalic and trunk neural crest transplanted into the region of the cardiac neural crest. Developmental biology 134, 402–412. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE, 1983. Neural crest cells contribute to normal aorticopulmonary septation. Science 220, 1059–1061. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Stewart DE, 1983. Neural crest origin of cardiac ganglion cells in the chick embryo: identification and extirpation. Dev Biol 97, 433–443. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A, 1996. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229–3242. [DOI] [PubMed] [Google Scholar]
- Kuo BR, Erickson CA, 2010. Regional differences in neural crest morphogenesis. Cell Adh Migr 4, 567–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier TE, Bronner-Fraser M, 1988. A spatial and temporal analysis of dorsal root and sympathetic ganglion formation in the avian embryo. Dev Biol 127, 99–112. [DOI] [PubMed] [Google Scholar]
- Landacre FL, 1910. The origin of the cranial ganglia in Ameiurus. J Comp Neurol Psycho 20, 309–411. [Google Scholar]
- Le Douarin N, 1980. Migration and differentiation of neural crest cells. Curr Top Dev Biol 16, 31–85. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, 1982. The neural crest. Cambridge University Press, Cambridge Cambridgeshire ; New York. [Google Scholar]
- Le Douarin N, Kalcheim C, 1999. The neural crest, 2nd ed. Cambridge University Press, Cambridge. [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E, 2004. Neural crest cell plasticity and its limits. Development 131, 4637–4650. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA, 1973. The migration of neural crest cells to the wall of the digestive tract in avian embryo. Journal of embryology and experimental morphology 30, 31–48. [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA, 1974. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Developmental biology 41, 162–184. [DOI] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM, 1975. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. Journal of embryology and experimental morphology 34, 125–154. [PubMed] [Google Scholar]
- Lee RT, Knapik EW, Thiery JP, Carney TJ, 2013a. An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 140, 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Thiery JP, Carney TJ, 2013b. Dermal fin rays and scales derive from mesoderm, not neural crest. Curr Biol 23, R336–337. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA, 2008. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 135, 2845–2854. [DOI] [PubMed] [Google Scholar]
- Loring JF, Erickson CA, 1987. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol 121, 220–236. [DOI] [PubMed] [Google Scholar]
- Lui VC, Cheng WW, Leon TY, Lau DK, Garcia-Barcelo MM, Miao XP, Kam MK, So MT, Chen Y, Wall NA, Sham MH, Tam PK, 2008. Perturbation of hoxb5 signaling in vagal neural crests down-regulates ret leading to intestinal hypoganglionosis in mice. Gastroenterology 134, 1104–1115. [DOI] [PubMed] [Google Scholar]
- Lumb R, Buckberry S, Secker G, Lawrence D, Schwarz Q, 2017. Transcriptome profiling reveals expression signatures of cranial neural crest cells arising from different axial levels. BMC Dev Biol 17, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A, Sprawson N, Graham A, 1991. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 113, 1281–1291. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Conrad GW, Bronner-Fraser M, 2004. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development 131, 1979–1991. [DOI] [PubMed] [Google Scholar]
- Makki N, Capecchi MR, 2011. Identification of novel Hoxa1 downstream targets regulating hindbrain, neural crest and inner ear development. Dev Biol 357, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares M, Nieto MA, 2003. A celebration of the new head and an evaluation of the new mouth. Neuron 37, 895–898. [DOI] [PubMed] [Google Scholar]
- Martik ML, Bronner ME, 2017. Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet 33, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsen BJ, Bronner-Fraser M, 1998. Neural crest specification regulated by the helix-loop-helix repressor Id2. Science 281, 988–991. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Graham A, 2002. Trunk Neural Crest Has Skeletogenic Potential. Current Biology 12, 767–771. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M, 2004. Gene-regulatory interactions in neural crest evolution and development. Developmental cell 7, 291–299. [DOI] [PubMed] [Google Scholar]
- Minoux M, Antonarakis GS, Kmita M, Duboule D, Rijli FM, 2009. Rostral and caudal pharyngeal arches share a common neural crest ground pattern. Development 136, 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongera A, Nusslein-Volhard C, 2013. Scales of fish arise from mesoderm. Curr Biol 23, R338–339. [DOI] [PubMed] [Google Scholar]
- Murko C, Bronner ME, 2017. Tissue specific regulation of the chick Sox10E1 enhancer by different Sox family members. Dev Biol 422, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Ayer-le Lievre CS, 1982. Mesectodermal capabilities of the trunk neural crest of birds. Journal of embryology and experimental morphology 70, 1–18. [PubMed] [Google Scholar]
- Nelms BL, Labosky PA, 2010. Transcriptional control of neural crest development Morgan & Claypool Life Sciences, San Rafael, Calif. [PubMed] [Google Scholar]
- Nieto MA, 2002. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3, 155–166. [DOI] [PubMed] [Google Scholar]
- Noden DM, 1978. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Developmental biology 67, 296–312. [DOI] [PubMed] [Google Scholar]
- Noden DM, 1983. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 96, 144–165. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JM, 1940. The non-specificity of the germ-layers. Quarterly Review of Biology 15, 1–27. [Google Scholar]
- Osorio L, Teillet MA, Palmeirim I, Catala M, 2009. Neural crest ontogeny during secondary neurulation: a gene expression pattern study in the chick embryo. The International journal of developmental biology 53, 641–648. [DOI] [PubMed] [Google Scholar]
- Parker HJ, Pushel I, Krumlauf R, 2018. Coupling the roles of Hox genes to regulatory networks patterning cranial neural crest. Dev Biol. [DOI] [PubMed] [Google Scholar]
- Platt JB, Ectodermic origin of the cartilages of the head. Anat. Anz, 506–509. [Google Scholar]
- Platt JB, 1893. Ectodermic origin of the cartilages of the head. . Anat. Anz, 506–509. [Google Scholar]
- Prasad MS, Sauka-Spengler T, LaBonne C, 2012. Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Developmental biology 366, 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T, Wysocka J, 2012. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11, 633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy MV, Faraco CD, Erickson CA, 1998. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev Biol 200, 234–246. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J-P, 2006. Neural crest induction and differentiation Springer Science+Business Media; ; Landes Bioscience/Eurekah.com, New York, N.Y. Georgetown, Tex. [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M, 2008. A gene regulatory network orchestrates neural crest formation. Nature reviews. Molecular cell biology 9, 557–568. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, Bronner-Fraser M, 2007. Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell 13, 405–420. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Prince V, Ingham PW, 2001. Plasticity in zebrafish hox expression in the hindbrain and cranial neural crest. Dev Biol 231, 201–216. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C, 2000. Inhibition of noggin expression in the dorsal neural tube by somitogenesis: a mechanism for coordinating the timing of neural crest emigration. Development 127, 4845–4854. [DOI] [PubMed] [Google Scholar]
- Shimada A, Kawanishi T, Kaneko T, Yoshihara H, Yano T, Inohaya K, Kinoshita M, Kamei Y, Tamura K, Takeda H, 2013. Trunk exoskeleton in teleosts is mesodermal in origin. Nature Communications 4, 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin JE, Zhang D, Rollo BN, Newgreen DF, 2013. Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS One 8, e64077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Bronner ME, 2015. Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Bronner ME, 2016. Reprogramming of avian neural crest axial identity and cell fate. Science 352, 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Tan-Cabugao J, Antoshechkin I, Sauka-Spengler T, Bronner ME, 2014. Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Res 24, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa MS, McKeown SJ, Tan-Cabugao J, Sauka-Spengler T, Bronner ME, 2012. Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest is Encrypted in the genome. PLoS Genet 8, e1003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Hickman A, Amanze D, Lumsden A, Thorogood P, 1994a. Trunk Neural Crest Origin of Caudal Fin Mesenchyme in the Zebrafish Brachydanio rerio. Proceedings of the Royal Society B: Biological Sciences 256, 137–145. [Google Scholar]
- Smith M, Hickman A, Amanze D, Lumsden A, Thorogood P, 1994b. Trunk Neural Crest Origin of Caudal Fin Mesenchyme in the Zebrafish Brachydanio-Rerio. P Roy Soc B-Biol Sci 256, 137–145. [Google Scholar]
- Smith MM, Hall BK, 1990. Development and Evolutionary Origins of Vertebrate Skeletogenic and Odontogenic Tissues. Biological Reviews 65, 277–373. [DOI] [PubMed] [Google Scholar]
- Stolfi A, Ryan K, Meinertzhagen IA, Christiaen L, 2015. Migratory neuronal progenitors arise from the neural plate borders in tunicates. Nature 527, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, 1929. Experiments showing the role of migrating neural crest (mesectoderm) in the formation of head skeleton and loose connective tissue in Rana palustris. Wilhelm Roux Arch Entwickl Mech Org 118, 40–77. [DOI] [PubMed] [Google Scholar]
- Tahtakran SA, Selleck MAJ, 2003. Ets-1 expression is associated with cranial neural crest migration and vasculogenesis in the chick embryo. Gene Expression Patterns 3, 455–458. [DOI] [PubMed] [Google Scholar]
- Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V, 1999. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 126, 2785–2797. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Duband JL, Altabef M, 2007. Ets-1 confers cranial features on neural crest delamination. PLoS One 2, e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R, 2012. Neural crest delamination and migration: from epithelium-tomesenchyme transition to collective cell migration. Developmental biology 366, 34–54. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R, 2000. Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity. Nat Rev Neurosci 1, 116–124. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP, 1984. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell differentiation 14, 223–230. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez GA, Cerrizuela S, Aybar MJ, 2017. Trunk neural crest cells: formation, migration and beyond. Int J Dev Biol 61, 5–15. [DOI] [PubMed] [Google Scholar]
- Weston JA, 1970. The migration and differentiation of neural crest cells. Adv Morphog 8, 41–114. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS, 1954. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol 101, 515–541. [DOI] [PubMed] [Google Scholar]
- Yu JK, Meulemans D, McKeown SJ, Bronner-Fraser M, 2008. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res 18, 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]