Abstract
Iron is one of the most abundant transition elements and is indispensable for almost all organisms. While the ability of iron to participate in redox chemistry is an essential requirement for participation in a range of vital enzymatic reactions, this same feature of iron also makes it dangerous in the generation of hydroxyl radicals and superoxide anions. Given the high local oxygen tensions in the lung, the regulation of iron acquisition, utilization, and storage therefore becomes vitally important, perhaps more so than in any other biological system. Iron plays a critical role in the biology of essentially every cell type in the lung, and in particular, changes in iron levels have important ramifications on immune function and the local lung microenvironment. There is substantial evidence that cigarette smoke causes iron dysregulation, with the implication that iron may be the link between smoking and smoking-related lung diseases. A better understanding of the connection between cigarette smoke, iron, and respiratory diseases will help to elucidate pathogenic mechanisms and aid in the identification of novel therapeutic targets.
Keywords: Iron, Cigarette Smoke, Lung Disease
Graphical abstract
Introduction
Iron is an essential nutrient utilized in almost every aspect of normal cell function. Cellular systems require iron for proliferation, DNA biosynthesis, protein function and cell cycle progression. In the lung, the correct regulation of cellular iron metabolism is vital, as highlighted by accumulating evidence that the dysregulation of cellular iron metabolism is associated with a plethora of lung diseases, including but not limited to, chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis and lung cancer [1, 2]. In addition, regulation of iron acquisition by the lung is also critical to the metabolism and growth of most microbes and so the sequestration of iron is of great importance in lung defense. As a result, some of the most abundant proteins found in the alveolar extracellular space are those related to iron metabolism; including transferrin (one of the most abundant bronchoalveolar lavage (BAL) fluid proteins at 44.5%), ferritin, lactoferrin (a glycoprotein belonging to the transferrin family that sequesters iron), ceruloplasmin (a ferroxidase that oxidizes iron to the Fe3+ state), and lipocalin-2 (also known as neutrophil gelatinase-associated lipocalin or NGAL, a siderophore that sequesters iron), all of which are thought to be produced by secretory leukocytes, epithelial and/or endothelial cells of the lung [3–7]. Evidently, a complex extracellular and intracellular iron regulatory system operates within the lungs, which is subject to disruption [8].
The airways are constantly exposed to atmospheric iron sources, with additional occupational exposure to iron-containing environmental particulates and pollutants. Cigarette smoking is a particle related exposure, which consists of more than 5000 chemical constituents [9]. The particulate matter in cigarette smoke is thought to complex host iron and there is substantial evidence that cigarette smoking results in abnormal iron regulation in the lung [10–15]. Specifically, there are abnormally high levels of iron in both current and former smokers with increases in non-heme iron and ferritin levels in the BAL fluid and in alveolar macrophages of smokers when compared to non-smokers and redox-active iron levels in exhaled breath condensate (EBC) from smokers are increased when compared to non-smoking controls [16–25]. Such changes in intracellular and extracellular iron metabolism in the lung may contribute to an array of cellular perturbations including disrupted mitochondrial and lysosomal function, intracellular oxidative stress and cell death, as well as promoting the growth of bacteria, all of which are associated with smoking related diseases in the lung. This review aims to expand and elaborate on the role of cigarette smoking on lung iron metabolism and how this relates to the pathogenesis, susceptibility and progression of a number of chronic lung diseases.
Introduction to Iron Metabolism
The unique chemical properties of iron allow for flexible coordination chemistry involving the accepting or donating of electrons, which facilitates a plethora of diverse biological reactions. As a result of its electropositivity, oxidized forms of iron (Fe3+) display high affinity for oxygen-donor ligands [26]. However, such reactive properties can also promote harmful redox chemistry promoting the generation of reactive oxygen species (ROS), which in large doses can become detrimental in biological systems. As a result, cells have evolved sophisticated mechanisms to control the acquisition, usage, and detoxification of iron to ensure that iron metabolic needs are met, and the risk of iron toxicity is minimized [27].
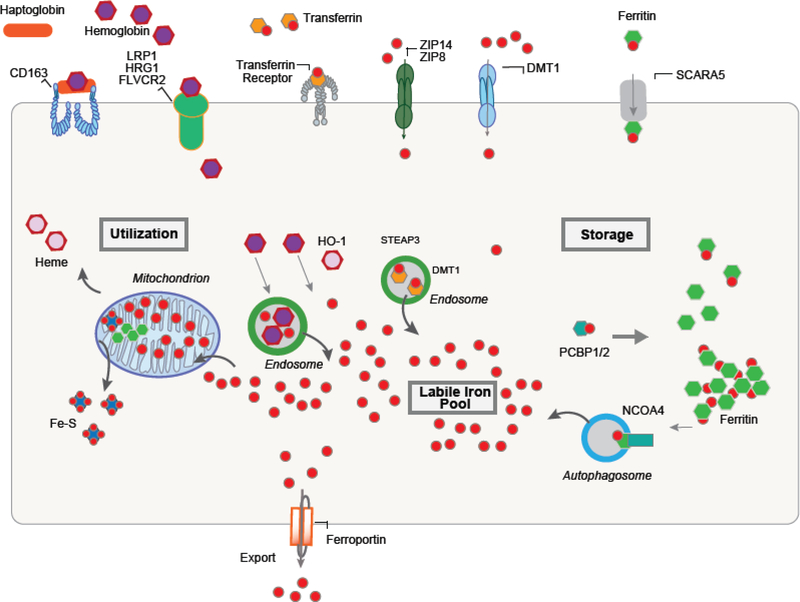
Iron acquisition is mediated through metal or metal-siderophore transporters that transport iron across the plasma membrane at the cell surface or across membranes of intracellular organelles. Iron can be acquired from a variety of forms: transferrin (TF)-bound iron (TBI), non-transferrin-bound iron (NTBI), hemoglobin, and heme. Depending on the cell type, plasma membrane transporters include the transferrin receptor 1 (TFRC1), DMT1 (SLC11A2, solute carrier family 11 member 2), heme-responsive gene-1 (HRG1), CD163 (scavenger receptor that transports hemoglobin-haptoglobin complex), ZRT/IRT-like protein-14 (ZIP14/SLC39A14), ZIP8 (SLC39A8) and natural resistance-associated macrophage protein 1 (NRAMP1, also known as SLC11A1) [28]. These proteins predominantly transport divalent iron and thus require enzymes (reductases) to reduce the abundant ferric iron (Fe3+) to the more soluble ferrous iron (Fe2+). Once iron is acquired it must be transported to sites of utilization such as the mitochondria (heme and Fe-S cluster synthesis), secretory pathways in the endoplasmic reticulum (in yeast), storage into the iron storage protein ferritin or into the vacuole/lysosome to protect cells against iron toxicity (Figure 1) [29]. In mammalian systems, while there are many systems to regulate iron acquisition, there is no regulated mechanism of iron loss and hence iron must be transferred from one tissue to another by cellular iron export into biological fluids (plasma). This process is mediated by the only identified plasma membrane iron exporter ferroportin1 (FPN1) [30].
Figure 1.
In eukaryotes, iron acquisition is mediated through metal or metal-siderophore transporters transferrin receptor, Solute Carrier Family 39 Member 8 (SLC39A8 or ZIP8), Solute Carrier Family 39 Member 14 (SLC39A14 or ZIP14), Solute Carrier Family 11 Member 2 (SLC11A2 or DMT1), the heme receptors/transporters LDL receptor-related protein 1 (LRP1), Solute Carrier Family 48 Member 1 (SLC48A1 or HRG1) and Feline Leukemia Virus Subgroup C Cellular Receptor Family (FLVCR2). Once inside the cell, iron must be transported to sites of utilization such as the mitochondria (heme and Fe-S cluster synthesis), storage into the iron storage protein ferritin or into the lysosome to protect cells against iron toxicity. Poly C binding proteins (PCBP) act as iron chaperones that assists in the mineralization of ferritin. Nuclear receptor coactivator 4 (NCOA4) protein assists in the release of iron from ferritin through an autophagy-mediated mechanism. Iron is exported from the cell by ferroportin1 (FPN1).
Central to the regulation of cellular iron homeostasis are the Iron Regulatory Proteins (IRP1 and IRP2). These cytosolic proteins bind to iron-responsive elements (IREs) on untranslated regions of mRNAs that code for proteins that are involved with iron uptake (TFRC1 and DMT1); storage (ferritin); and export (FPN1), thereby regulating cellular iron availability [31]. They are key regulators of iron sensing (hypoxia inducible factor 2a), uptake (transferrin receptor1, DMT1, FPN1), heme synthesis (5-aminolevulinic acid synthase 2) and iron storage (ferritin) in all cells [32]. Another regulatory mechanism of cellular iron homeostasis is the existence of metallochaperone proteins including the poly C binding proteins (PCBP). PCBP1 is specific to mammals and was originally identified as an iron chaperone for ferritin that assists in the mineralization of ferritin [33]. PCBPs have also been shown to deliver iron to other client proteins regulating iron storage, iron incorporation into proteins and overall iron homeostasis, but do not appear to be required for iron delivery to mitochondria. The nuclear receptor coactivator 4 (NCOA4) protein has been described to assist in the release of iron from ferritin through an autophagy-mediated mechanism [34]. These mechanisms, both delivery of iron for storage and breakdown of ferritin during iron limitation allow for the tight regulation of cellular iron homeostasis (Figure 1).
In healthy organisms, iron is maintained at a stable concentration (0.1%) in the plasma (bound to the transport protein transferrin), to deliver iron largely to the erythropoietic bone marrow, but also to every cell in the body [12]. Erythropoietic tissue in the bone marrow requires up to 20mg of iron to maintain heme biosynthesis [35]. The majority of total iron in the body is therefore bound by hemoglobin in erythrocytes (~50%) or is present in storage compartments of hepatocytes and macrophages (~25%). Most of the iron that enters the extracellular fluid is recycled from senescent erythrocytes and is found in a chelated-state (both Fe2+ and Fe3+ forms of iron are highly reactive) to proteins or organic chelators [12]. Globally, iron regulatory responses are controlled via the action of the small peptide hormone hepcidin [2].
Iron Handling in the Lung
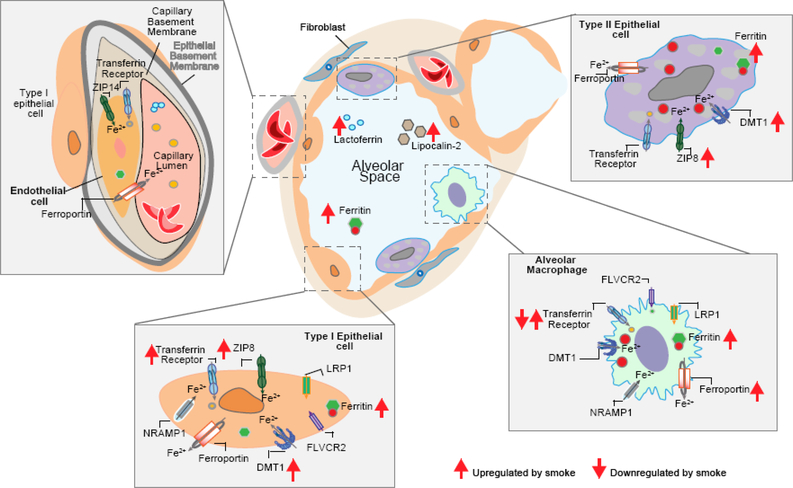
The human lung contains a similar amount of iron per dry weight as the liver (~0.4–0.9mg Fe/g in lung versus 0.2–2mg Fe/g in the liver) and is continuously exposed to exogenous sources of inhaled iron [36–39]. Iron is the most abundant metal present in the atmosphere, and in remote areas, iron levels in air range from 50–90ng/m3, to about 1.3μg/m3 at urban sites. Concentrations of up to 12μg/m3 have been reported in the vicinity of iron- and steel-producing plants and iron is one of the most abundant metals in urban and rural particulate matter [8, 40]. The upper respiratory tract is exposed to ~10–25μg iron daily, approximately 1/1000th of that seen by the gastrointestinal tract accounting for approximately 2–6% of the total amount of iron present in the lung per dry weight [40]. Therefore, in a similar manner to other organs in the body, the lung must obtain its iron from the pulmonary vasculature in the form of transferrin-bound iron, lactoferrin-bound iron, cell free hemoglobin/heme or non-transferrin bound iron (citrate or acetate bound iron). Both alveolar macrophages (AMs) and the bronchial and alveolar epithelia are able to sequester iron by various mechanisms including receptor-mediated uptake of ferric-iron, followed by safe storage within ferritin (ferritin is effective in limiting extra-cellular iron-catalyzed oxidative stress) [2]. In the lung intracellular iron import is chiefly undertaken by TFRC1, DMT1, and in macrophages and neutrophils by NRAMP [41, 42]. Lung cells also express other iron uptake molecules including ZIP-14 and the lactoferrin receptor, low-density lipoprotein receptor-related protein 1 (LRP1) [41, 43–45] (Figure 2). Iron is mostly localized inside lung tissues (55%) with remaining metal associated with bronchoalveolar lavage (BAL) protein as bound (22%) and unbound (5%) forms [46]. One of the most abundant genes in lung tissue is ferritin light chain (FTL) [47], which in addition to ferritin heavy chain (FTH), is localized to lung-resident leukocytes such as AMs and alveolar epithelial cells (AECs) and endothelial cells [43–45]. Lung epithelial, and endothelial cells, along with AMs transport iron out of the cell via FPN1, which also plays an important role in iron detoxification [48]. In macrophages and epithelial cells, FPN1 function is regulated by hepcidin (encoded by HAMP), a hormone chiefly produced in the liver, which regulates, and is in turn regulated by systemic iron levels [49]. Hepcidin expression and release is induced by increased serum iron, by bone morphogenic protein (BMP) signaling [50] and by a number of pro-inflammatory cytokines including IL-6 and IL-22 [51]. Up-regulation of hepcidin expression results in FPN1 endocytosis and proteolysis, preventing cellular iron export and reducing the influx of iron into the plasma from stores, as well as blocking further absorption of dietary iron [12]. Production of hepcidin by AMs has also been described, indicating a specific local role in the lung for this hormone [2, 52].
Figure 2. Iron import and export in the lung and disruption upon smoke exposure.
The lung obtains its iron from the microenvironment along with the pulmonary vasculature. Both alveolar macrophages and the bronchial and alveolar epithelia are able to sequester iron through transferrin receptor 1 (TFRC1), divalent metal transporter 1 (DMT1, also known as SLC11A2), and in macrophages and neutrophils by natural resistance-associated macrophage protein 1 (NRAMP1, also known as SLC11A1). Lung cells also express other iron uptake molecules including ZIP-14 (also known as SLC39A14), and the lactoferrin receptor, low-density lipoprotein receptor-related protein 1 (LRP1). Lung epithelial cells, alveolar macrophages and endothelial cells transport iron out of the cell via the transmembrane iron transporter ferroportin (also known as SLC40A1). Cigarette smoking alters many molecules involved in iron metabolism on lung epithelial cells, alveolar macrophages and possibly endothelial cells.
Cigarette Smoke and Iron
Just How Much Iron is in Cigarette Smoke?
An average cigarette contains 0.5mg of iron per gram of tobacco, but only 0.06% of this is transferred into mainstream cigarette smoke [53]. Therefore, one pack per day smoking (20 cigarettes) results in a daily inhalational iron exposure of about 6μg, which seems at first glance trivial but depending on the excretory capabilities of the lung can accumulate over years and decades. This increased iron load was again demonstrated in a recent study using modern cigarette brands such as Marlboro and Dunhill, analyzing both mainstream and side stream (“second-hand”) smoke [54]. The amount of iron in aerosols derived from electronic cigarettes is significantly less than that of a conventional cigarette, but whether this route of delivery brings additional health hazards over time, especially from other aerosolized metals, remains to be evaluated [55]. Interestingly, the particulate matter in cigarette smoke (and wood smoke) is thought to complex host iron to generate a more severe oxidative and inflammatory response and aqueous extracts of cigarette smoke may react directly with amino acid residues in ferritin and induce the release of iron from ferritin, which can be enhanced under anaerobic conditions [10, 11, 56]. These findings suggest that while the amount of iron found in cigarette smoke is minimal, the ability of cigarette smoke to complex iron inside the lung has important ramifications for cellular iron metabolism and the oxidative stress response system.
Cigarette smoke, Iron and the Lung Epithelium
The epithelial barrier of the lung consists of airway epithelial cells (bronchial epithelial cell) and alveolar epithelial cells. Airway epithelial cells (AECs) line the respiratory tract and secrete numerous substances including mucins, lysozymes, defensins, siderophores and nitric oxide [57, 58]. Ciliated AECs drive the mucociliary escalator, a primary innate defense mechanism, in which motile ciliated epithelial cells eliminate particles and pathogens trapped in mucus from the airways [59]. Pulmonary alveoli, the basic units for gaseous exchange in the lung, are composed of alveolar type 1 (AT1) and alveolar type II (AT2) cells and regulate gaseous exchange and surfactant production, a surface-active lipoprotein complex which reduces the surface tension in the lungs respectively [60]. Iron uptake by AT1 and AT2 cells can occur via TFRC1, DMT1, or the ZIP8/14 transporters (Figure 2) [61]. Once inside, iron must be transported to sites of utilization such as the mitochondria (heme and Fe-S cluster synthesis), secretory pathways in the endoplasmic reticulum (in yeast) [29], storage into the iron storage protein ferritin or into the vacuole/lysosome to protect cells against iron toxicity. AEC with the high level of ferritin present in AECs indicative of the direct interaction between the lung and the external environment [8]. FPN1 is localized to the basolateral membrane of enterocytes, but can be found apically in AECs, where they not only take up but also release iron [48]. Iron can also present to AECs in the form of cell-free heme and hemoglobin, which has been shown to induce mitochondrial dysfunction and cell injury [62].
Cigarette smoke has a deleterious effect on the health and function of epithelial cells of the lung [63]. In general, exposure of rodent models to smoke increases the deposition of iron in lung epithelial cells [18, 31, 64]. Cigarette smoke extract (CSE), an in vitro model for smoke exposure largely increases the concentration of iron inside lung epithelial cells, along with increased ferritin and DMT1 expression [18]. Similarly, exposure of lung epithelial cells to wood smoke results in increased total cell iron levels along with increased ferritin and DMT1 [11, 65]. In addition, ZIP8 mRNA and protein expression is increased in the lung of chronic smokers compared with nonsmokers [66, 67] (Table 1). Inappropriate levels of iron inside an epithelial cell can result in alterations in cellular homeostasis, which may have a profound effect on lung epithelial cell function and survival. Iron accumulation enhances the production of ROS and induces mitochondrial dysfunction [68, 69], both of which are extensively documented in smoke exposed model systems [18, 19, 23, 70–74]. Interestingly, the location of where this iron accumulates inside the cell may also be important. For example, cigarette smoking appears to increase the levels of mitochondrial iron in the lungs of a murine CS-exposure model [31]. Functionally, exposure of pulmonary epithelial cells to iron-containing particulates or CS results in apoptosis, proliferation and fibrosis [75, 76], increased release of cytokines [77] and impaired antimicrobial activity [78].
Table 1.
Alterations of Iron metabolism Proteins upon Cigarette smoke Exposure
| Iron Parameter | Cell type(s) | Regulated | Disease Relevance | Reference |
|---|---|---|---|---|
| Intracellular Iron Regulation | ||||
| Ferritin | Alveolar epithelial cells (AECs) | Increased by cigarette smoke (CS), wood smoke | COPD | [11, 18] |
| Ferritin | Whole lung, AMs | Increased mRNA in COPD, increased sequestration in AMs increased staining in AMs in IPF | COPD (increased disease severity), IPF (increased pulmonary artery pressure) | [16, 17, 19-23] |
| IREB2/IRP2 | Whole lung, AMs | Increased mRNA in whole lung, decreased in AMs in COPD patients | COPD | [21] |
| Mitochondrial Iron | Whole lung | Increased by CS | COPD | [30] |
| Iron Transporters | ||||
| Divalent metal transporter 1 (DMT1) | AECs | Increased by cigarette smoke (CS), wood smoke | COPD | [18] |
| Transferrin Receptor (TfR) | Whole | Increased mRNA in whole lung | COPD | [21] |
| Ferroportin | Whole lung, AMs | Increased mRNA in whole lung, altered in AMs in COPD patients | COPD | [21] |
| ZIP8 | Whole lung | Increased in smokers | Not clear | [64, 65] |
| Extracellular Iron Transporters/Siderophores/Regulators | ||||
| Transferrin (Tf) | Whole lung, AMs | Increased mRNA in whole lung, decreased in AMs in COPD patients | COPD | [21] |
| Lipocalin-2 | AMs, AECs | Increased secretion | COPD, lung cancer, cystic fibrosis | [7, 109–115] |
| Hepcidin | Cellular source? | Pro- form increased in serum of COPD patients; mutation resulting in restrictive disease | COPD, restrictive lung disease | [25, 150] |
Cigarette Smoke, Iron and Immune Cell Regulation
Cigarette smoke exposure has been shown to cause immune cell dysfunction, affecting both innate (AMs, dendritic cells, NK cells, and neutrophils) and adaptive immunity (antibody production and T lymphocytes) [79–82]. While there is little research that connects cigarette smoke, iron, and immune cell regulation all together, smoking is associated with iron loading in AMs, and there is growing evidence that cellular iron status affects macrophage activation and function (Table 1) [19, 22, 23]. AMs exposed to cigarette smoke have been shown to ineffectively clear respiratory pathogens such as S. pneumoniae and P. aeruginosa, as well as damaged epithelial cells [83–85]. Furthermore, macrophages exposed to smoke have defective response to activating stimuli, and in turn ineffectively produce the necessary cytokines for immune activation, especially during infection [86, 87].
Cigarette smoke also seems to affect macrophage polarization, with transcriptional profiling studies showing suppression of M1-related inflammatory genes and induction of M2-related genes in AMs obtained via lavage fluid from smokers [88]. Classically-activated (M1) macrophages are typically considered pro-inflammatory while alternatively-activated (M2) macrophages are considered anti-inflammatory, but even more relevant is the fact that these different macrophages have opposing expression profiles in the genes involved in iron homeostasis. Genes involved in iron uptake and retention, such as TF, HAMP, and FTH1 are more highly expressed in M1 macrophages, and genes involved in iron release such as FPN1 are more highly expressed in M2 macrophages [89, 90]. This divergence in iron handling likely evolved as a defense stratagem: because of the critical dependence on iron by essentially all microbes, in the event of an infection the host must rigorously sequester iron from its invaders, a key task for its pro-inflammatory immune cells (such as M1 macrophages) [12, 91]. Conversely, M2 macrophages are scavengers that clean up the inflammatory response, and subsequently release iron to surrounding cells for the repair process. Indeed, in vitro studies have demonstrated that supplementing mice with excessive iron biases macrophage polarization toward an M2 phenotype, and dampens the M1 inflammatory response from activating stimuli such as LPS [92]. Whether iron loading is the mechanism behind smoke-dependent macrophage reprogramming remains to be seen.
These immunologic defects have important correlations, as AMs are recognized to be dysfunctional in smoking-related lung diseases such as COPD [93]. These resident cells are the main sentinels of the airway, and even small decreases in function can have profound effects on the microbiologic makeup of their environment, as the lung is constantly exposed to external threats. Infections are by far the most common cause of exacerbations in COPD, and even without overt infection, alveolar macrophage dysfunction, possibly involving iron is likely a factor in causing the changes in lung microbiome seen in COPD [94–96].
Cigarette Smoke, Iron and the Microbial Environment of the Lung
Smoking is a strong risk factor for respiratory infections such as community-acquired pneumonia and tuberculosis, and also modulates diseases of immunity as diverse as from HIV to inflammatory bowel disease [97–101]. Nevertheless, while it is unsurprising that smoke-induced immune cell dysfunction results in increased infection risk, it is clear that lung iron bioavailability also plays a significant role in changing microbial environment in the lung. Virtually all successful pathogens have evolved to acquire iron locally from their host, most by releasing iron scavenging molecules known as siderophores. During infection, the host chelates serum iron to transferrin, lactoferrin, and ceruloplasmin, and relocates iron to reticuloendothelial macrophages while also decreasing the release of iron into the circulation by increasing HAMP and ferritin expression [102–104]. Lung infection similarly results in increased iron storage pathways inside cells and host production of lipocalin-2, which is secreted by neutrophils, AMs and epithelial cells to capture bacterial siderophores and sequester iron away from the pathogen [105–107].
There is substantial evidence behind the importance of iron control in infection. Lipocalin-2 has been shown to be critical in host defense against both extracellular and intracellular pulmonary pathogens [108, 109]. Similarly, exogenous lactoferrin is able reduce the severity of Pseudomonas and M. pneumoniae infections in infectious disease models [110, 111]. Iron itself changes pathogen biology, and was found in one study to be essential to bacterial biofilm formation but not planktonic growth [112]. Many bacteria species have limited capabilities to obtain iron, but can cause fulminant and often lethal infections in iron overloaded hosts [113]. Interestingly, lipocalin-2 is also upregulated in many non-infectious pulmonary diseases, such as COPD, lung cancer, and cystic fibrosis, suggesting that it may have additional roles in the pathogenesis of respiratory diseases [7, 114–120]. Taken together, it seems that by altering iron homeostasis in the lung, cigarette smoke impacts the lung microbial environment directly, in addition to the effect it has on immune cells.
Smoking-Related Respiratory Diseases and Iron
Chronic Obstructive Pulmonary Disease (COPD)
COPD is a progressive debilitating respiratory illness and is among the top four leading causes for mortality worldwide [121]. It can present as chronic cough, sputum production, or dyspnea in an at-risk patient, but can only be diagnosed with a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of less than 0.7, which is indicative of persistent airflow obstruction [122]. COPD pathobiology likely begins in the small airways of the lung, and with gradual airway loss and remodeling, ends in irreversible parenchymal destruction, i.e. emphysema [123, 124]. The pathogenesis of COPD remains poorly understood but involves aberrant inflammatory responses of the lung to noxious particles or gas, most commonly cigarette smoke. While smoking is the leading risk factor for developing COPD, its underlying pathobiology is complex, with likely multiple overlapping genetic and environment factors contributing to disease phenotype.
Multiple genetic-association studies have indicated a role for iron in the genetic susceptibility to COPD, with the most compelling evidence for iron regulatory protein 2 (IREB2) [125–127]. In particular, several single nucleotide polymorphisms (SNPs) such as rs2568494 and rs13180 have appeared in geographically and ethnically diverse populations and from independent analyses [125, 127–129]. IREB2 is co-localized with several components of the nicotinic acetylcholine receptor on chromosome 15q25, and while several additional loci on 15q25 are associated with heavy smoking behavior, the association IREB2 and COPD has also been shown to be smoking independent [130–132]. In experimental COPD models, iron accumulates in the lungs of rodents exposed to smoke and IRP2 deficient mice are protected from the effects of cigarette smoke, likely via mitochondrial iron regulation [18, 31].
Clinically, iron content and iron-binding molecules such as ferritin and lipocalin-2 (NGAL) are increased in the lung tissue, sputum, BAL fluid and alveolar macrophages of smokers, and increased further in those of COPD patients [7, 17, 18, 21]. These iron parameters correlate with clinical measures of disease severity such as the degree of airflow obstruction on pulmonary function tests and the extent of radiographic emphysema, but it is still unknown whether iron overload can be predictive of disease progression or mortality [18, 21]. AMs are known have defective activation and phagocytic ability in COPD, persisting even after smoking cessation, but this has not been linked directly to iron loading [85, 133]. Notably, while some studies indicate a role for systemic iron markers such as serum ferritin in COPD, this has not been demonstrated in other biomarker studies using large, well-characterized cohorts [134–136].
Interstitial Lung Diseases
Interstitial lung diseases (ILDs) consist of a wide spectrum of heterogeneous disorders of known or unknown cause, generally characterized by dyspnea, diffuse parenchymal lung infiltrates, restrictive pulmonary dysfunction, and impaired gaseous exchange [137]. Suggested risk factors for the initiation and progression of ILDs include genetic and epigenetic abnormalities as well as infection or environmental exposures (such as cigarette smoking or silica exposure). Cigarette smoking is a risk factor for the development of several ILDs including desquamative interstitial pneumonia (DIP), respiratory bronchiolitis-associated interstitial lung disease (RBILD), pulmonary Langerhans’ cell histiocytosis (PLCH), and idiopathic pulmonary fibrosis (IPF) [137]. IPF is the most frequent, severe and most studied form of ILDs, where lung function decline is gradual and the overall prognosis of patients with IPF is unpredictable and poor with a median survival after diagnosis of approximately 3.8 years [138]. IPF is characterized by the aberrant deposition of extracellular matrix, as a result of repetitive injury to the alveolar epithelium. Such repetitive injury may be associated with smoking and other environmental factors, such as exposure to dust, silica and infection. As a result, epithelial cells release mediators that activate fibroblast proliferation leading to the presence of abundant foci of highly activated fibroblasts and myofibroblasts that are resistant to apoptosis and lead to extensive lung remodeling. IPF also displays elements of a pathologic adaptive immune response, including dysregulated T-and B-cell responses [139].
Recent studies have shown that total iron levels, the generation of iron-associated oxygen radicals and iron-laden macrophages are elevated in the lungs of IPF patients compared to controls [140–142]. In particular, alveolar macrophage iron accumulation has been described in association with increased capillary density, pulmonary vascular lesions and pulmonary arterial hypertension in IPF [140, 143–145]. Exposure to synthetic fibers, an elevated risk factor for the development of ILD has also been associated with increased deposition of ferruginous bodies in the lung and iron increases the pro-fibrogenic effect of dust suggesting an in vivo accumulation of iron [146, 147]. Rodent models of fibrosis including the bleomycin-induced lung fibrosis model and the silica-induced lung injury model are associated with an accumulation of iron [148–151]. Interestingly, bleomycin directly complexes redox-active iron resulting in DNA degradation and iron deficiency or chelation is associated with a reduction in the severity of bleomycin-induced pulmonary fibrosis. Similarly, an iron-depleted diet diminishes fibrotic injury after dust instillation, whereas loss of DMT1 increases bleomycin-induced lung injury [150–154]. Another recent study showed that a FPN1 mutation resulted in iron accumulation in AMs and lung epithelial cells with subsequent development of restrictive lung disease [155]. Changes in iron metabolism therefore clearly play a role in both human IPF and murine models of this disease where such increases in iron may drive macrophage generation of ROS and promote a heightened pro-inflammatory state [92, 142]. Whether or not smoking plays an important role in these observations remains to be determined. Iron accumulation in the lungs of patients with IPF appears to be smoking-independent, however systemically, iron levels are increased in patients with active IPF and falls in patients with inactive disease, which appeared to be dependent on smoking status, with more serum iron detected in smokers [140–142, 156]. Further studies are required to deduce the role of iron dysregulation in IPF and smoking status.
Lung Cancer
An estimated 1.8 million new cases of lung cancer were diagnosed in 2012, accounting for 13% of all cancer diagnoses [157]. Despite being one of the most preventable cancers, lung cancer is the leading cause of cancer deaths in both men and women, especially in developed nations [157]. 85% of patients with lung cancer have non-small cell lung cancer (NSCLC), of which the common subtypes are adenocarcinoma and squamous cell cancer, and the remaining 15% mostly have small cell lung cancer (SCLC) [158]. All lung cancers are associated with cigarette smoking, but the strongest relationships are with squamous cell cancer and SCLC, whereas adenocarcinoma is the most common histology amongst nonsmokers, especially in East Asian women [159].
Abnormal iron metabolism appears to have a role in lung cancer biology. IRP2 has been shown to drive the growth of lung cancer cell lines, an effect exacerbated with iron loading and blunted when IRP2 expression is turned down [160, 161]. Likewise, the proliferative phase of a SCLC cell line was found to be coordinated with increased transferrin expression, and blockage of iron uptake in this setting markedly inhibited growth [162]. NSCLC tumors have been shown to stain strongly for transferrin, with the degree of staining associated with survival in one study, especially in larger tumors (T2 or T3 by TNM staging) [163, 164]. Both serum and BALF ferritin have been found to be useful biomarkers in lung cancer, with serum ferritin in one study correlating with sensitivity to chemotherapy [4, 165]. Lipocalin-2 was found to be associated with radiation sensitivity in a lung cancer line, and with a fivefold increase in hazard ratio for overall survival if strongly stained in lung tumor tissue in another analysis [166, 167]. Unsurprisingly given the poor prognosis of lung cancer, this abundance of preclinical data has encouraged investigators to consider iron pathways as potential therapeutic targets, with already several formulations of iron chelators being studied [168–170]. Further understanding in lung iron biology will likely enhance the development of novel antitumor agents.
Therapeutic Interventions
Cigarette smoke increases the exposure to iron by the respiratory system, but also likely changes the underlying biology of lung cells such that there is both increased iron uptake and decreased iron release [53, 171]. Although not directly etiologically linked, both cigarette smoke and iron status affect epithelial and immune cells in the lung, change the underlying microbial environment, and are associated with a variety of respiratory diseases. Iron chelators may therefore be potentially novel therapeutic agents for these diseases, and while further studies are needed to assess for any accompanying side effects, if these agents can be delivered directly into the airway (perhaps via nebulized therapy), unwanted systemic effects can be minimized [31, 172]. There are a number of iron chelators to choose from and selecting the correct type will be crucial in eliciting the correct therapeutic effect. Factors to consider include: size, as large chelators will unlikely be able to passive diffuse into a cell; selectivity and specificity for iron while not removing other biologically important cations such as copper or zinc; and efficacy in enclosing iron ions and minimizing the formation of reactive oxygen species [173]. Intracellular iron chelators such as deferiprone, which can ferry excess iron between organelles and even between cells, may be more efficacious than deferoxamine, which is larger and may not enter cells as readily [174]. Alternatively, targeting hepcidin may be another approach to treating iron overload, as hepcidin agonists will induce ferroportin expression, leading to the export of excess iron and unloading the cell. This can be achieved either by agents that mimic hepcidin activity such as minihepcidins, or by using agents to stimulate endogenous hepcidin production [49].
There is evidence that suggests it is iron deficiency, rather than iron overload, that is characteristic of COPD, with high serum iron levels and high iron diet both shown to be protective against the development of COPD in smokers [175–177]. In one study, an intravenous iron intervention not only improved hematologic markers in COPD patients hospitalized with exacerbations, but alleviated their sensation of dyspnea [178]. However, there is also evidence to suggest that serum ferritin reduction improves peripheral arterial disease in smokers and that phlebotomies may improve exercise tolerance in COPD patients (with high hematocrit) with PAH and severe secondary polycythemia, provided patients do not enter an iron deficient state [179–181]. The reader is referred to a more comprehensive review on the role of systemic iron mishandling and COPD [2]. Systemic iron deficiency is clearly under recognized in COPD, but it is possible that the blood compartment may not be representative of the local lung environment, especially given the airway’s proximal exposure to cigarette smoke. It therefore follows that nebulized or inhaled therapy may serve to correct a key disease pathway in the lung without excessive systemic adverse effects, much as inhaled corticosteroids have done already in COPD and other airway diseases.
Conclusions
Cigarette smoke drives pathogenic changes in the vast majority of cells in the lung, and underlies or at least contributes to a number of important respiratory diseases. Many of these diseases, despite being leading causes of mortality, still lack effective, etiology-driven treatments [121]. That cigarette smoke additionally results in the dysregulation of iron homeostasis in these same cells strongly suggests a common pathogenic mechanism. While much remains to be learned about iron, cigarette smoke and disease pathogenesis in the lung, evidence for dysregulated iron metabolism is marked in a range of lung diseases, confirming the importance of iron regulatory process in human health and disease. Additional research is desperately needed to further explore the relationship between smoke, iron, and lung disease; the fruit of this labor may not be merely a deeper understanding of mechanism, but also the development of novel therapeutic agents, ones that target iron-related pathways and can possibly change standards of care.
Highlights:
Cigarette smoke induces significant dysregulation of iron homeostasis in the lung
Iron dysregulation is observed in a number of smoking-related lung diseases
Iron is an intriguing target for novel therapies in smoking-related lung diseases
Acknowledgments
S.M.C is supported by the US National Institute of Health–National Heart, Lung and Blood Institute (R00-HL125899). W.Z.Z. is supported by a CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ali MK, Kim RY, Karim R, Mayall JR, Martin KL, Shahandeh A, Abbasian F, Starkey MR, Loustaud-Ratti V, Johnstone D, Milward EA, Hansbro PM, Horvat JC, Role of iron in the pathogenesis of respiratory disease, The international journal of biochemistry & cell biology 88 (2017) 181–195. [DOI] [PubMed] [Google Scholar]
- [2].Cloonan SM, Mumby S, Adcock IM, Choi AMK, Chung KF, Quinlan GJ, The “Iron”-y of Iron Overload and Iron Deficiency in Chronic Obstructive Pulmonary Disease, American journal of respiratory and critical care medicine 196(9) (2017) 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Szabo S, Barbu Z, Lakatos L, Laszlo I, Szabo A , Local production of proteins in normal human bronchial secretion, Respiration; international review of thoracic diseases 39(3) (1980) 172–8. [DOI] [PubMed] [Google Scholar]
- [4].Fracchia A, Ubbiali A, El Bitar O, Pacetti M, Sommariva E, Arreghini M, Longhini E, Bonalumi GP, A comparative study on ferritin concentration in serum and bilateral bronchoalveolar lavage fluid of patients with peripheral lung cancer versus control subjects, Oncology 56(3) (1999) 181–8. [DOI] [PubMed] [Google Scholar]
- [5].Ghio AJ, Carter JD, Dailey LA, Devlin RB, Samet JM, Respiratory epithelial cells demonstrate lactoferrin receptors that increase after metal exposure, Am J Physiol 276(6 Pt 1) (1999) L933–40. [DOI] [PubMed] [Google Scholar]
- [6].Kono S, Yoshida K, Tomosugi N, Terada T, Hamaya Y, Kanaoka S, Miyajima H, Biological effects of mutant ceruloplasmin on hepcidin-mediated internalization of ferroportin, Biochim Biophys Acta 1802(11) (2010) 968–975. [DOI] [PubMed] [Google Scholar]
- [7].Iwamoto H, Gao J, Koskela J, Kinnula V, Kobayashi H, Laitinen T, Mazur W, Differences in plasma and sputum biomarkers between COPD and COPD-asthma overlap, The European respiratory journal 43(2) (2014) 421–9. [DOI] [PubMed] [Google Scholar]
- [8].Ghio AJ, Disruption of iron homeostasis and lung disease, Biochim Biophys Acta 1790(7) (2009) 731–9. [DOI] [PubMed] [Google Scholar]
- [9].Sangani RG, Ghio AJ, Lung injury after cigarette smoking is particle related, International journal of chronic obstructive pulmonary disease 6 (2011) 191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ghio AJ, Stonehuerner J, Quigley DR, Humic-like substances in cigarette smoke condensate and lung tissue of smokers, Am J Physiol 266(4 Pt 1) (1994) L382–8. [DOI] [PubMed] [Google Scholar]
- [11].Ghio AJ, Soukup JM, Dailey LA, Tong H, Kesic MJ, Budinger GR, Mutlu GM, Wood Smoke Particle Sequesters Cell Iron to Impact a Biological Effect, Chemical research in toxicology 28(11) (2015) 2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ganz T, Nemeth E, Iron homeostasis in host defence and inflammation, Nature reviews. Immunology 15(8) (2015) 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kurz T, Gustafsson B, Brunk UT, Cell sensitivity to oxidative stress is influenced by ferritin autophagy, Free Radic Biol Med 50(11) (2011) 1647–58. [DOI] [PubMed] [Google Scholar]
- [14].Ballweg K, Mutze K, Konigshoff M, Eickelberg O, Meiners S, Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells, American journal of physiology. Lung cellular and molecular physiology 307(11) (2014) L895–907. [DOI] [PubMed] [Google Scholar]
- [15].Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K, PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis, Autophagy 11(3) (2015) 547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mateos F, Brock JH, Perez-Arellano JL, Iron metabolism in the lower respiratory tract, Thorax 53(7) (1998) 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Corhay JL, Weber G, Bury T, Mariz S, Roelandts I, Radermecker MF, Iron content in human alveolar macrophages, The European respiratory journal 5(7) (1992) 804–9. [PubMed] [Google Scholar]
- [18].Ghio AJ, Hilborn ED, Stonehuerner JG, Dailey LA, Carter JD, Richards JH, Crissman KM, Foronjy RF, Uyeminami DL, Pinkerton KE, Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect, American journal of respiratory and critical care medicine 178(11) (2008) 1130–8. [DOI] [PubMed] [Google Scholar]
- [19].Thompson AB, Bohling T, Heires A, Linder J, Rennard SI, Lower respiratory tract iron burden is increased in association with cigarette smoking, The Journal of laboratory and clinical medicine 117(6) (1991) 493–9. [PubMed] [Google Scholar]
- [20].McGowan SE, Henley SA, Iron and ferritin contents and distribution in human alveolar macrophages, The Journal of laboratory and clinical medicine 111(6) (1988) 611–7. [PubMed] [Google Scholar]
- [21].Philippot Q, Deslee G, Adair-Kirk TL, Woods JC, Byers D, Conradi S, Dury S, Perotin JM, Lebargy F, Cassan C, Le Naour R, Holtzman MJ, Pierce RA, Increased iron sequestration in alveolar macrophages in chronic obstructive pulmonary disease, PloS one 9(5) (2014) e96285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wesselius LJ, Flowers CH, Skikne BS, Alveolar macrophage content of isoferritins and transferrin. Comparison of nonsmokers and smokers with and without chronic airflow obstruction, The American review of respiratory disease 145(2 Pt 1) (1992) 311–6. [DOI] [PubMed] [Google Scholar]
- [23].Wesselius LJ, Nelson ME, Skikne BS, Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers, American journal of respiratory and critical care medicine 150(3) (1994) 690–5. [DOI] [PubMed] [Google Scholar]
- [24].Nelson ME, O’Brien-Ladner AR, Wesselius LJ, Regional variation in iron and iron-binding proteins within the lungs of smokers, American journal of respiratory and critical care medicine 153(4 Pt 1) (1996) 1353–8. [DOI] [PubMed] [Google Scholar]
- [25].Mumby S, Saito J, Adcock IM, Chung KF, Quinlan GJ, Decreased breath excretion of redox active iron in COPD: a protective failure?, The European respiratory journal (2015). [DOI] [PubMed] [Google Scholar]
- [26].Haas KL, Franz KJ, Application of metal coordination chemistry to explore and manipulate cell biology, Chemical reviews 109(10) (2009) 4921–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paul BT, Manz DH, Torti FM, Torti SV, Mitochondria and Iron: current questions, Expert review of hematology 10(1) (2017) 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knutson MD, Iron transport proteins: Gateways of cellular and systemic iron homeostasis, The Journal of biological chemistry 292(31) (2017) 12735–12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiao G, Wan Z, Fan Q, Tang X, Zhou B, The metal transporter ZIP13 supplies iron into the secretory pathway in Drosophila melanogaster, eLife 3 (2014) e03191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI, Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter, Nature 403(6771) (2000) 776–81. [DOI] [PubMed] [Google Scholar]
- [31].Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, Pabon MA, Konrad C, Polverino F, Siempos II, Perez E, Mizumura K, Ghosh MC, Parameswaran H, Williams NC, Rooney KT, Chen ZH, Goldklang MP, Yuan GC, Moore SC, Demeo DL, Rouault TA, D’Armiento JM, Schon EA, Manfredi G, Quackenbush J, Mahmood A, Silverman EK, Owen CA, Choi AM, Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice, Nat Med (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wilkinson N, Pantopoulos K, The IRP/IRE system in vivo: insights from mouse models, Frontiers in pharmacology 5 (2014) 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shi H, Bencze KZ, Stemmler TL, Philpott CC, A cytosolic iron chaperone that delivers iron to ferritin, Science (New York, N.Y.) 320(5880) (2008) 1207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC, Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy, Nature 509(7498) (2014) 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kautz L, Nemeth E, Molecular liaisons between erythropoiesis and iron metabolism, Blood 124(4) (2014) 479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takemoto K, Kawai H, Kuwahara T, Nishina M, Adachi S, Metal concentrations in human lung tissue, with special reference to age, sex, cause of death, emphysema and contamination of lung tissue, International archives of occupational and environmental health 62(8) (1991) 579–86. [DOI] [PubMed] [Google Scholar]
- [37].Diez M, Arroyo M, Cerdan FJ, Munoz M, Martin MA, Balibrea JL, Serum and tissue trace metal levels in lung cancer, Oncology 46(4) (1989) 230–4. [DOI] [PubMed] [Google Scholar]
- [38].Van Eijk HG, Wiltink WF, Bos G, Goossens JP, Measurement of the iron content in human liver specimens, Clinica Chimica Acta 50(2) (1974) 275–280. [Google Scholar]
- [39].Sirlin CB, Reeder SB, Magnetic resonance imaging quantification of liver iron, Magnetic resonance imaging clinics of North America 18(3) (2010) 359–81, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].W.H. Organization, Guidelines for drinking-water quality, World Health Organization; Geneva, 1996. [Google Scholar]
- [41].Ghio AJ, Carter JD, Richards JH, Richer LD, Grissom CK, Elstad MR, Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome, Crit Care Med 31(2) (2003) 395–400. [DOI] [PubMed] [Google Scholar]
- [42].Kim J, Molina RM, Donaghey TC, Buckett PD, Brain JD, Wessling-Resnick M, Influence of DMT1 and iron status on inflammatory responses in the lung, American journal of physiology. Lung cellular and molecular physiology 300(4) (2011) L659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Du Y, Guo M, Whitsett JA, Xu Y, ‘LungGENS’: a web-based tool for mapping single-cell gene expression in the developing lung, Thorax 70(11) (2015) 1092–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, Proteomics. Tissue-based map of the human proteome, Science (New York, N.Y.) 347(6220) (2015) 1260419. [DOI] [PubMed] [Google Scholar]
- [45].Lindskog C, Fagerberg L, Hallstrom B, Edlund K, Hellwig B, Rahnenfuhrer J, Kampf C, Uhlen M, Ponten F, Micke P, The lung-specific proteome defined by integration of transcriptomics and antibody-based profiling, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 28(12) (2014) 5184–96. [DOI] [PubMed] [Google Scholar]
- [46].Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M, Manganese and iron transport across pulmonary epithelium, American journal of physiology. Lung cellular and molecular physiology 290(6) (2006) L1247–59. [DOI] [PubMed] [Google Scholar]
- [47].Wu L, Ma L, Nicholson LF, Black PN, Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD, Respiratory medicine 105(3) (2011) 329–36. [DOI] [PubMed] [Google Scholar]
- [48].Yang F, Haile DJ, Wang X, Dailey LA, Stonehuerner JG, Ghio AJ, Apical location of ferroportin 1 in airway epithelia and its role in iron detoxification in the lung, American journal of physiology. Lung cellular and molecular physiology 289(1) (2005) L14–23. [DOI] [PubMed] [Google Scholar]
- [49].Arezes J, Nemeth E, Hepcidin and iron disorders: new biology and clinical approaches, International journal of laboratory hematology 37 Suppl 1 (2015) 92–8. [DOI] [PubMed] [Google Scholar]
- [50].Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY, Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression, Nature genetics 38(5) (2006) 531–9. [DOI] [PubMed] [Google Scholar]
- [51].Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T, IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin, The Journal of clinical investigation 113(9) (2004) 1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nguyen NB, Callaghan KD, Ghio AJ, Haile DJ, Yang F, Hepcidin expression and iron transport in alveolar macrophages, American journal of physiology. Lung cellular and molecular physiology 291(3) (2006) L417–25. [DOI] [PubMed] [Google Scholar]
- [53].Mussalo-Rauhamaa H, Leppänen A, Salmela SS, Pyysalo H, Cigarettes as a source of some trace and heavy metals and pesticides in man, Arch Environ Health 41(1) (1986) 49–55. [DOI] [PubMed] [Google Scholar]
- [54].Behera SN, Xian H, Balasubramanian R, Human health risk associated with exposure to toxic elements in mainstream and sidestream cigarette smoke, Sci Total Environ 472 (2014) 947–956. [DOI] [PubMed] [Google Scholar]
- [55].Palazzolo DL, Crow AP, Nelson JM, Johnson RA, Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel, Front Physiol 7 (2016) 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moreno JJ, Foroozesh M, Church DF, Pryor WA, Release of iron from ferritin by aqueous extracts of cigarette smoke, Chemical research in toxicology 5(1) (1992) 116–23. [DOI] [PubMed] [Google Scholar]
- [57].Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R, The great big alveolar TI cell: evolving concepts and paradigms, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 25(1) (2010) 55–62. [DOI] [PubMed] [Google Scholar]
- [58].Guillot L, Nathan N, Tabary O, Thouvenin G, Le Rouzic P, Corvol H, Amselem S, Clement A, Alveolar epithelial cells: master regulators of lung homeostasis, The international journal of biochemistry & cell biology 45(11) (2013) 2568–73. [DOI] [PubMed] [Google Scholar]
- [59].Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, An CH, Shan B, Franks JM, Haley KJ, Owen CA, Tesfaigzi Y, Washko GR, Quackenbush J, Silverman EK, Rahman I, Kim HP, Mahmood A, Biswal SS, Ryter SW, Choi AM, Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction, The Journal of clinical investigation 123(12) (2013) 5212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Whitsett JA, Wert SE, Weaver TE, Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease, Annual review of medicine 61 (2010) 105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang X, Ghio AJ, Yang F, Dolan KG, Garrick MD, Piantadosi CA, Iron uptake and Nramp2/DMT1/DCT1 in human bronchial epithelial cells, American journal of physiology. Lung cellular and molecular physiology 282(5) (2002) L987–95. [DOI] [PubMed] [Google Scholar]
- [62].Chintagari NR, Jana S, Alayash AI, Oxidized Ferric and Ferryl Forms of Hemoglobin Trigger Mitochondrial Dysfunction and Injury in Alveolar Type I Cells, American journal of respiratory cell and molecular biology 55(2) (2016) 288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Feldman C, Anderson R, Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems, The Journal of infection 67(3) (2013) 169–84. [DOI] [PubMed] [Google Scholar]
- [64].Ghio AJ, Pritchard RJ, Dittrich KL, Samet JM, Non-heme (Fe3+) in the lung increases with age in both humans and rats, The Journal of laboratory and clinical medicine 129(1) (1997) 53–61. [DOI] [PubMed] [Google Scholar]
- [65].Soukup JM, Dailey LA, Ghio AJ, Particle retention by respiratory epithelial cells is associated with persistent biological effect, Inhalation toxicology 27(7) (2015) 335–41. [DOI] [PubMed] [Google Scholar]
- [66].Napolitano JR, Liu MJ, Bao S, Crawford M, Nana-Sinkam P, Cormet-Boyaka E, Knoell DL, Cadmium-mediated toxicity of lung epithelia is enhanced through NF-kappaB-mediated transcriptional activation of the human zinc transporter ZIP8, American journal of physiology. Lung cellular and molecular physiology 302(9) (2012) L909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD, ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading, The Journal of biological chemistry 287(41) (2012) 34032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huang ML, Lane DJ, Richardson DR, Mitochondrial mayhem: the mitochondrion as a modulator of iron metabolism and its role in disease, Antioxidants & redox signaling 15(12) (2011) 3003–19. [DOI] [PubMed] [Google Scholar]
- [69].Chen C, Paw BH, Cellular and mitochondrial iron homeostasis in vertebrates, Biochim Biophys Acta 1823(9) (2012) 1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chung KF, Adcock IM, Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction, The European respiratory journal 31(6) (2008) 1334–56. [DOI] [PubMed] [Google Scholar]
- [71].Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, Bittner A, Rao N, Murphy MP, Kirkham PA, Chung KF, Adcock IM, Copdmap, Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease, J Allergy Clin Immunol 136(3) (2015) 769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rahman I, Adcock IM, Oxidative stress and redox regulation of lung inflammation in COPD, The European respiratory journal 28(1) (2006) 219–42. [DOI] [PubMed] [Google Scholar]
- [73].Kamp DW, Greenberger MJ, Sbalchierro JS, Preusen SE, Weitzman SA, Cigarette smoke augments asbestos-induced alveolar epithelial cell injury: role of free radicals, Free Radic Biol Med 25(6) (1998) 728–39. [DOI] [PubMed] [Google Scholar]
- [74].Wesselius LJ, Williams WL, Bailey K, Vamos S, O’Brien-Ladner AR, Wiegmann T, Iron uptake promotes hyperoxic injury to alveolar macrophages, American journal of respiratory and critical care medicine 159(1) (1999) 100–6. [DOI] [PubMed] [Google Scholar]
- [75].Dai J, Xie C, Churg A, Iron loading makes a nonfibrogenic model air pollutant particle fibrogenic in rat tracheal explants, American journal of respiratory cell and molecular biology 26(6) (2002) 685–93. [DOI] [PubMed] [Google Scholar]
- [76].Churg A, Sun J, Zay K, Cigarette smoke increases amosite asbestos fiber binding to the surface of tracheal epithelial cells, Am J Physiol 275(3 Pt 1) (1998) L502–8. [DOI] [PubMed] [Google Scholar]
- [77].Smith LA, Paszkiewicz GM, Hutson AD, Pauly JL, Inflammatory response of lung macrophages and epithelial cells to tobacco smoke: a literature review of ex vivo investigations, Immunologic research 46(1–3) (2010) 94–126. [DOI] [PubMed] [Google Scholar]
- [78].Gally F, Chu HW, Bowler RP, Cigarette Smoke Decreases Airway Epithelial FABP5 Expression and Promotes Pseudomonas aeruginosa Infection, PloS one 8(1) (2013) e51784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gaschler GJ, Zavitz CCJ, Bauer CMT, Skrtic M, Lindahl M, Robbins CS, Chen B, Stämpfli MR, Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages, American journal of respiratory cell and molecular biology 38(2) (2008) 218–226. [DOI] [PubMed] [Google Scholar]
- [80].Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L, Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming, J Immunol 175(4) (2005) 2684–2691. [DOI] [PubMed] [Google Scholar]
- [81].Horvath KM, Herbst M, Zhou H, Zhang H, Noah TL, Jaspers I, Nasal lavage natural killer cell function is suppressed in smokers after live attenuated influenza virus, Respir Res 12 (2011) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Feng Y, Kong Y, Barnes PF, Huang F-F, Klucar P, Wang X, Samten B, Sengupta M, Machona B, Donis R, Tvinnereim AR, Shams H, Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis, Infect Immun 79(1) (2011) 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Phipps JC, Aronoff DM, Curtis JL, Goel D, O’Brien E, Mancuso P, Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae, Infect Immun 78(3) (2010) 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stämpfli MR, Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection, American journal of respiratory and critical care medicine 170(11) (2004) 1164–1171. [DOI] [PubMed] [Google Scholar]
- [85].Hodge S, Hodge R Fau-Scicchitano G, Scicchitano PN Fau-Reynolds R, Reynolds M Fau-Holmes Pn, Holmes M, Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells, Immunol Cell Biol 81(4) (2003) 289–96. [DOI] [PubMed] [Google Scholar]
- [86].Hristova M, Spiess PC, Kasahara DI, Randall MJ, Deng B, van der Vliet A, The tobacco smoke component, acrolein, suppresses innate macrophage responses by direct alkylation of c-Jun N-terminal kinase, American journal of respiratory cell and molecular biology 46(1) (2012) 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].van Zyl-Smit RN, Binder R Fau-Meldau A, Meldau PL Fau-Semple R, Semple A Fau-Evans Pl, Evans P Fau-Smith A, Smith ED Fau-Bateman P, Bateman K Fau-Dheda Ed, Dheda K, Cigarette smoke impairs cytokine responses and BCG containment in alveolar macrophages, Thorax 69(4) (2014) 363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shaykhiev R, Krause J Fau-Salit A, Salit Y Fau-Strulovici-Barel J, Strulovici-Barel B-G Fau-Harvey Y, Harvey TP Fau-O’Connor Bg, O’Connor RG Fau-Crystal Tp, Crystal RG Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease, J Immunol. 183(4) (2009) 2867–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, Zammataro L, Girelli D, Cairo G, Differential regulation of iron homeostasis during human macrophage polarized activation, Eur J Immunol 40(3) (2010) 824–835. [DOI] [PubMed] [Google Scholar]
- [90].Galvan-Pena S, O’Neill LA, Metabolic reprograming in macrophage polarization, Frontiers in immunology 5 (2014) 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cairo G, Recalcati S, Mantovani A, Locati M, Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype, Trends Immunol 32(6) (2011) 241–247. [DOI] [PubMed] [Google Scholar]
- [92].Agoro R, Taleb M, Quesniaux VFJ, Mura C, Cell iron status influences macrophage polarization, PloS one 13(5) (2018) e0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW, Identification of an autophagy defect in smokers’ alveolar macrophages, J Immunol 185(9) (2010) 5425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sethi S, Sethi R, Eschberger K, Lobbins P, Cai X, Grant BJ, Murphy TF, Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease, American journal of respiratory and critical care medicine 176(4) (2007) 356–61. [DOI] [PubMed] [Google Scholar]
- [95].Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC, The lung tissue microbiome in chronic obstructive pulmonary disease, American journal of respiratory and critical care medicine 185(10) (2012) 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mohan S, Ho T, Kjarsgaard M, Radford K, Borhan AS, Thabane L, Nair P, Hemosiderin in sputum macrophages may predict infective exacerbations of chronic obstructive pulmonary disease: a retrospective observational study, BMC pulmonary medicine 17(1) (2017) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF, Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team, N Engl J Med 342(10) (2000) 681–689. [DOI] [PubMed] [Google Scholar]
- [98].Shang S, Ordway D, Henao-Tamayo M, Bai X, Oberley-Deegan R, Shanley C, Orme IM, Case S, Minor M, Ackart D, Hascall-Dove L, Ovrutsky AR, Kandasamy P, Voelker DR, Lambert C, Freed BM, Iseman MD, Basaraba RJ, Chan ED, Cigarette smoke increases susceptibility to tuberculosis--evidence from in vivo and in vitro models, J Infect Dis 203(9) (2011) 1240–1248. [DOI] [PubMed] [Google Scholar]
- [99].Gajalakshmi V, Peto R, Kanaka TS, Jha P, Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls, Lancet 362(9383) (2003) 507–515. [DOI] [PubMed] [Google Scholar]
- [100].Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, Markowitz N, Rosen MJ, Mangura BT, Hopewell PC, Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group, N Engl J Med 333(13) (1995) 845–851. [DOI] [PubMed] [Google Scholar]
- [101].Birrenbach T, Böcker U, Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications, Inflamm Bowel Dis 10(6) (2004) 848–859. [DOI] [PubMed] [Google Scholar]
- [102].White KN, Conesa C, Sanchez L, Amini M, Farnaud S, Lorvoralak C, Evans RW, The transfer of iron between ceruloplasmin and transferrins, Biochim Biophys Acta 1820(3) (2012) 411–6. [DOI] [PubMed] [Google Scholar]
- [103].Eid C, Hemadi M, Ha-Duong NT, El Hage Chahine JM, Iron uptake and transfer from ceruloplasmin to transferrin, Biochim Biophys Acta 1840(6) (2014) 1771–81. [DOI] [PubMed] [Google Scholar]
- [104].Samygina VR, Sokolov AV, Bourenkov G, Petoukhov MV, Pulina MO, Zakharova ET, Vasilyev VB, Bartunik H, Svergun DI, Ceruloplasmin: macromolecular assemblies with iron-containing acute phase proteins, PloS one 8(7) (2013) e67145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Carraway MS, Ghio AJ, Taylor JL, Piantadosi CA, Induction of ferritin and heme oxygenase-1 by endotoxin in the lung, Am J Physiol 275(3 Pt 1) (1998) L583–92. [DOI] [PubMed] [Google Scholar]
- [106].Damron FH, Oglesby-Sherrouse AG, Wilks A, Barbier M, Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia, Scientific reports 6 (2016) 39172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Seifert M, Nairz M, Schroll A, Schrettl M, Haas H, Weiss G, Effects of the Aspergillus fumigatus siderophore systems on the regulation of macrophage immune effector pathways and iron homeostasis, Immunobiology 213(9–10) (2008) 767–78. [DOI] [PubMed] [Google Scholar]
- [108].Chan YR, Liu DA Fau-Pociask Js, Pociask M Fau-Zheng Da, Zheng TA Fau-Mietzner M, Mietzner T Fau-Berger Ta, Berger TW Fau-Mak T, Mak MC Fau-Clifton Tw, Clifton RK Fau-Strong Mc, Strong P Fau-Ray Rk, Ray JK Fau-Kolls P, Kolls JK, Lipocalin 2 is required for pulmonary host defense against Klebsiella infection, J Immunol 182(8) (2009) 4947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Saiga H, Nishimura H Fau-Kuwata J, Kuwata M Fau-Okuyama H, Okuyama S Fau-Matsumoto M, Matsumoto S Fau-Sato S, Sato M Fau-Matsumoto S, Matsumoto S Fau-Akira M, Akira Y Fau-Yoshikai S, Yoshikai K Fau-Honda Y, Honda M Fau-Yamamoto K, Yamamoto K Fau-Takeda M, Takeda K, Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium, J Immunol. 181(12) (2008) 8521–7. [DOI] [PubMed] [Google Scholar]
- [110].Wu Q, Jiang MN Fau-Minor D, Minor RJ Fau-Martin Mn, Martin HW Fau-Chu Rj, Chu HW, In vivo function of airway epithelial TLR2 in host defense against bacterial infection, American journal of physiology. Lung cellular and molecular physiology 300(4) (2011) 579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Valenti P, Frioni A, Rossi A, Ranucci S, De Fino I, Cutone A, Rosa L, Bragonzi A, Berlutti F, Aerosolized bovine lactoferrin reduces neutrophils and pro-inflammatory cytokines in mouse models of Pseudomonas aeruginosa lung infections, Biochem Cell Biol. 95(1) (2017) 41–47. [DOI] [PubMed] [Google Scholar]
- [112].Ojha A, Hatfull GF, The role of iron in Mycobacterium smegmatis biofilm formation: the exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth, Mol Microbiol 66(2) (2007) 468–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wright LM Fau-Simpson Ac, Simpson JD Fau-Oliver Lm, Oliver JD , Role of iron in the pathogenesis of Vibrio vulnificus infections, Infect Immun 34(2) (1981) 503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Keatings VM, Barnes PJ, Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects, American journal of respiratory and critical care medicine 155(2) (1997) 449–53. [DOI] [PubMed] [Google Scholar]
- 115].Wang XR, Li YP, Gao S, Xia W, Gao K, Kong QH, Qi H, Wu L, Zhang J, Qu JM, Bai CX, Increased serum levels of lipocalin-1 and −2 in patients with stable chronic obstructive pulmonary disease, International journal of chronic obstructive pulmonary disease 9 (2014) 543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cockayne DA, Cheng DT, Waschki B, Sridhar S, Ravindran P, Hilton H, Kourteva G, Bitter H, Pillai SG, Visvanathan S, Muller KC, Holz O, Magnussen H, Watz H, Fine JS, Systemic biomarkers of neutrophilic inflammation, tissue injury and repair in COPD patients with differing levels of disease severity, PloS one 7(6) (2012) e38629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Eagan TM, Damas JK, Ueland T, Voll-Aanerud M, Mollnes TE, Hardie JA, Bakke PS, Aukrust P, Neutrophil gelatinase-associated lipocalin: a biomarker in COPD, Chest 138(4) (2010) 888–95. [DOI] [PubMed] [Google Scholar]
- [118].Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS, McCubrey JA, Libra M, Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer, Oncotarget 5(6) (2014) 1576–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Song B, Zhang H, Jiang L, Chi Y, Tian J, Du W, Yu B, Han Z, Down-regulation of lipocalin 2 suppresses the growth of human lung adenocarcinoma through oxidative stress involving Nrf2/HO-1 signaling, Acta biochimica et biophysica Sinica 47(10) (2015) 805–14. [DOI] [PubMed] [Google Scholar]
- [120].Eichler I, Nilsson M, Rath R, Enander I, Venge P, Koller DY, Human neutrophil lipocalin, a highly specific marker for acute exacerbation in cystic fibrosis, The European respiratory journal 14(5) (1999) 1145–9. [DOI] [PubMed] [Google Scholar]
- [121].Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet 390(10100) (2017) 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A, Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary, American journal of respiratory and critical care medicine 195(5) (2017) 557–582. [DOI] [PubMed] [Google Scholar]
- [123].McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Pare PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC, Small-airway obstruction and emphysema in chronic obstructive pulmonary disease, N Engl J Med 365(17) (2011) 1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, Wouters EF, Chronic obstructive pulmonary disease, Nature reviews. Disease primers 1 (2015) 15076. [DOI] [PubMed] [Google Scholar]
- [125].DeMeo DL, Mariani T, Bhattacharya S, Srisuma S, Lange C, Litonjua A, Bueno R, Pillai SG, Lomas DA, Sparrow D, Shapiro SD, Criner GJ, Kim HP, Chen Z, Choi AMK, Reilly J, Silverman EK, Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene, Am J Hum Genet 85(4) (2009) 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ding Y, Yang D, Xun X, Wang Z, Sun P, Xu D, He P, Niu H, Jin T, Association of genetic polymorphisms with chronic obstructive pulmonary disease in the Hainan population: a case-control study, International journal of chronic obstructive pulmonary disease 10 (2015) 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hardin M, Zielinski J, Wan ES, Hersh CP, Castaldi PJ, Schwinder E, Hawrylkiewicz I, Sliwinski P, Cho MH, Silverman EK, CHRNA3/5, IREB2, and ADCY2 are associated with severe chronic obstructive pulmonary disease in Poland, American journal of respiratory cell and molecular biology 47(2) (2012) 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Du Y, Xue Y, Xiao W, Association of IREB2 Gene rs2568494 Polymorphism with Risk of Chronic Obstructive Pulmonary Disease: A Meta-Analysis, Med Sci Monit 22 (2016) 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ziółkowska-Suchanek I, Mosor M, Gabryel P, Grabicki M, Żurawek M, Fichna M, Strauss E, Batura-Gabryel H, Dyszkiewicz W, Nowak J, Susceptibility loci in lung cancer and COPD: association of IREB2 and FAM13A with pulmonary diseases, Scientific reports 5 (2015) 13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Saccone NL, Culverhouse RC, Schwantes-An T-H, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliövaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang B-Z, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PAF, Nöthen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ, Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD, PLoS Genet 6(8) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhou H, Yang J, Li D, Xiao J, Wang B, Wang L, Ma C, Xu S, Ou X, Feng Y, Association of IREB2 and CHRNA3/5 polymorphisms with COPD and COPD-related phenotypes in a Chinese Han population, J Hum Genet 57(11) (2012) 738–746. [DOI] [PubMed] [Google Scholar]
- [132].Siedlinski M, Tingley D, Lipman PJ, Cho MH, Litonjua AA, Sparrow D, Bakke P, Gulsvik A, Lomas DA, Anderson W, Kong X, Rennard SI, Beaty TH, Hokanson JE, Crapo JD, Lange C, Silverman EK, C.O.a.E. Investigators, Dissecting direct and indirect genetic effects on chronic obstructive pulmonary disease (COPD) susceptibility, Hum Genet 132(4) (2013) 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hodge S, Hodge J Fau-Ahern G, Ahern H Fau-Jersmann J, Jersmann M Fau-Holmes H, Holmes PN Fau-Reynolds M, Reynolds PN, Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease, American journal of respiratory cell and molecular biology 37(6) (2007) 748–55. [DOI] [PubMed] [Google Scholar]
- [134].Ghio AJ, Hilborn ED, Indices of iron homeostasis correlate with airway obstruction in an NHANES III cohort, International journal of chronic obstructive pulmonary disease 12 (2017) 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Lee CH, Goag EK, Lee SH, Chung KS, Jung JY, Park MS, Kim YS, Kim SK, Chang J, Song JH, Association of serum ferritin levels with smoking and lung function in the Korean adult population: analysis of the fourth and fifth Korean National Health and Nutrition Examination Survey, International journal of chronic obstructive pulmonary disease 11 (2016) 3001–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Keene JD, Jacobson S, Kechris K, Kinney GL, Foreman MG, Doerschuk CM, Make BJ, Curtis JL, Rennard SI, Barr RG, Bleecker ER, Kanner RE, Kleerup EC, Hansel NN, Woodruff PG, Han MK, Paine R, Martinez FJ, Bowler RP, O’Neal WK, C.O.a.S.I. ‡, Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts, American journal of respiratory and critical care medicine 195(4) (2017) 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ryu JH, Colby TV, Hartman TE, Vassallo R, Smoking-related interstitial lung diseases: a concise review, European Respiratory Journal 17(1) (2001) 122. [DOI] [PubMed] [Google Scholar]
- [138].Tashiro J, Rubio GA, Limper AH, Williams K, Elliot SJ, Ninou I, Aidinis V, Tzouvelekis A, Glassberg MK, Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis, Frontiers in Medicine 4 (2017) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].O’Dwyer DN, Ashley SL, Moore BB, Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis, American journal of physiology. Lung cellular and molecular physiology 311(3) (2016) L590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Puxeddu E, Comandini A, Cavalli F, Pezzuto G, D’Ambrosio C, Senis L, Paci M, Curradi G, Sergiacomi GL, Saltini C, Iron laden macrophages in idiopathic pulmonary fibrosis: the telltale of occult alveolar hemorrhage?, Pulmonary pharmacology & therapeutics 28(1) (2014) 35–40. [DOI] [PubMed] [Google Scholar]
- [141].Sangiuolo F, Puxeddu E, Pezzuto G, Cavalli F, Longo G, Comandini A, Di Pierro D, Pallante M, Sergiacomi G, Simonetti G, Zompatori M, Orlandi A, Magrini A, Amicosante M, Mariani F, Losi M, Fraboni D, Bisetti A, Saltini C, HFE gene variants and iron-induced oxygen radical generation in idiopathic pulmonary fibrosis, The European respiratory journal 45(2) (2015) 483–90. [DOI] [PubMed] [Google Scholar]
- [142].Lee J, Arisi I, Puxeddu E, Mramba LK, Amicosante M, Swaisgood CM, Pallante M, Brantly ML, Skold CM, Saltini C, Bronchoalveolar lavage (BAL) cells in idiopathic pulmonary fibrosis express a complex pro-inflammatory, pro-repair, angiogenic activation pattern, likely associated with macrophage iron accumulation, PloS one 13(4) (2018) e0194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kim KH, Maldonado F, Ryu JH, Eiken PW, Hartman TE, Bartholmai BJ, Decker PA, Yi ES, Iron deposition and increased alveolar septal capillary density in nonfibrotic lung tissue are associated with pulmonary hypertension in idiopathic pulmonary fibrosis, Respir Res 11 (2010) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fukihara J, Taniguchi H, Ando M, Kondoh Y, Kimura T, Kataoka K, Furukawa T, Johkoh T, Fukuoka J, Sakamoto K, Hasegawa Y, Hemosiderin-laden macrophages are an independent factor correlated with pulmonary vascular resistance in idiopathic pulmonary fibrosis: a case control study, BMC pulmonary medicine 17(1) (2017) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Colombat M, Mal H, Groussard O, Capron F, Thabut G, Jebrak G, Brugiere O, Dauriat G, Castier Y, Leseche G, Fournier M, Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: Histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics, Human pathology 38(1) (2007) 60–5. [DOI] [PubMed] [Google Scholar]
- [146].Ghio AJ, Funkhouser W, Pugh CB, Winters S, Stonehuerner JG, Mahar AM, Roggli VL, Pulmonary fibrosis and ferruginous bodies associated with exposure to synthetic fibers, Toxicologic pathology 34(6) (2006) 723–9. [DOI] [PubMed] [Google Scholar]
- [147].Churg A, Wright JL, Airway wall remodeling induced by occupational mineral dusts and air pollutant particles, Chest 122(6 Suppl) (2002) 306s–309s. [DOI] [PubMed] [Google Scholar]
- [148].Hay JG, Haslam M Fau-Turner-Warwick Pl, Turner-Warwick GJ Fau-Laurent M, Laurent GJ, The effects of iron and desferrioxamine on the lung injury induced by intravenous bleomycin and hyperoxia, Free Radic Res Commun 4(2) (1987) 109–14. [DOI] [PubMed] [Google Scholar]
- [149].Khazri O, Charradi K, Limam F, El May MV, Aouani E, Grape seed and skin extract protects against bleomycin-induced oxidative stress in rat lung, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 81 (2016) 242–249. [DOI] [PubMed] [Google Scholar]
- [150].Chandler DB, Barton DD Fau-Briggs Jc 3rd, Briggs TW 3rd Dd Fau-Butler, Butler JI Fau-Kennedy Tw, Kennedy WE Fau-Grizzle Ji, Grizzle JD Fau-Fulmer We, Fulmer JD, Effect of iron deficiency on bleomycin-induced lung fibrosis in the hamster, Am Rev Respir Dis. 137(1) (1988) 85–9. [DOI] [PubMed] [Google Scholar]
- [151].Ghio AJ, Jaskot RH, Hatch GE, Lung injury after silica instillation is associated with an accumulation of iron in rats, Am J Physiol 267(6 Pt 1) (1994) L686–92. [DOI] [PubMed] [Google Scholar]
- [152].von Bonsdorff L, Lindeberg E, Sahlstedt L, Lehto J, Parkkinen J, Bleomycin-detectable iron assay for non-transferrin-bound iron in hematologic malignancies, Clinical Chemistry 48 (2002) 307+. [PubMed] [Google Scholar]
- [153].Chandler DB, Fulmer JD, The effect of deferoxamine on bleomycin-induced lung fibrosis in the hamster, The American review of respiratory disease 131(4) (1985) 596–8. [DOI] [PubMed] [Google Scholar]
- [154].Yang F, Stonehuerner JG, Richards JH, Nguyen NB, Callaghan KD, Haile DJ, Ghio AJ, Deficiency in the divalent metal transporter 1 increases bleomycin-induced lung injury, Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 23(4) (2010) 657–67. [DOI] [PubMed] [Google Scholar]
- [155].Neves J, Leitz D, Kraut S, Brandenberger C, Agrawal R, Weissmann N, Muhlfeld C, Mall MA, Altamura S, Muckenthaler MU, Disruption of the Hepcidin/Ferroportin Regulatory System Causes Pulmonary Iron Overload and Restrictive Lung Disease, EBioMedicine 20 (2017) 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Jack CI, Jackson MJ, Johnston ID, Hind CR, Serum indicators of free radical activity in idiopathic pulmonary fibrosis, American journal of respiratory and critical care medicine 153(6 Pt 1) (1996) 1918–23. [DOI] [PubMed] [Google Scholar]
- [157].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, Global cancer statistics, 2012, CA: a cancer journal for clinicians 65(2) (2015) 87–108. [DOI] [PubMed] [Google Scholar]
- [158].Herbst RS, Morgensztern D, Boshoff C, The biology and management of non-small cell lung cancer, Nature 553(7689) (2018) 446–454. [DOI] [PubMed] [Google Scholar]
- [159].Sun S, Schiller JH, Gazdar AF, Lung cancer in never smokers--a different disease, Nature reviews. Cancer 7(10) (2007) 778–90. [DOI] [PubMed] [Google Scholar]
- [160].Maffettone C, Chen G, Drozdov I, Ouzounis C, Pantopoulos K, Tumorigenic properties of iron regulatory protein 2 (IRP2) mediated by its specific 73-amino acids insert, PloS one 5(4) (2010) e10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Khiroya H, Moore JS, Ahmad N, Kay J, Woolnough K, Langman G, Ismail I, Naidu B, Tselepis C, Turner AM, IRP2 as a potential modulator of cell proliferation, apoptosis and prognosis in nonsmall cell lung cancer, The European respiratory journal 49(4) (2017). [DOI] [PubMed] [Google Scholar]
- [162].Vostrejs M, Moran PL, Seligman PA, Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation, The Journal of clinical investigation 82(1) (1988) 331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Whitney JF, Clark JM, Griffin TW, Gautam S, Leslie KO, Transferrin receptor expression in nonsmall cell lung cancer. Histopathologic and clinical correlates, Cancer 76(1) (1995) 20–25. [DOI] [PubMed] [Google Scholar]
- [164].Kukulj S, Jaganjac M, Boranic M, Krizanac S, Santic Z, Poljak-Blazi M, Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer, Medical oncology (Northwood, London, England) 27(2) (2010) 268–77. [DOI] [PubMed] [Google Scholar]
- [165].Shi HB, Li XD, Jiang JT, Zhao WQ, Ji M, Wu CP, Serum ferritin is elevated in advanced non-small cell lung cancer patients and is associated with efficacy of platinum-based chemotherapy, Journal of cancer research and therapeutics 10(3) (2014) 681–5. [DOI] [PubMed] [Google Scholar]
- [166].Shiiba M, Saito K, Fushimi K, Ishigami T, Shinozuka K, Nakashima D, Kouzu Y, Koike H, Kasamatsu A, Sakamoto Y, Ogawara K, Uzawa K, Takiguchi Y, Tanzawa H, Lipocalin-2 is associated with radioresistance in oral cancer and lung cancer cells, International journal of oncology 42(4) (2013) 1197–204. [DOI] [PubMed] [Google Scholar]
- [167].Ruiz-Morales JM, Dorantes-Heredia R, Arrieta O, Chavez-Tapia NC, Motola-Kuba D, Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) prognostic value in lung adenocarcinoma, Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 36(5) (2015) 3601–10. [DOI] [PubMed] [Google Scholar]
- [168].Lui GY, Obeidy P, Ford SJ, Tselepis C, Sharp DM, Jansson PJ, Kalinowski DS, Kovacevic Z, Lovejoy DB, Richardson DR, The iron chelator, deferasirox, as a novel strategy for cancer treatment: oral activity against human lung tumor xenografts and molecular mechanism of action, Molecular pharmacology 83(1) (2013) 179–90. [DOI] [PubMed] [Google Scholar]
- [169].Yu Y, Suryo Rahmanto Y, Richardson DR, Bp44mT: an orally active iron chelator of the thiosemicarbazone class with potent anti-tumour efficacy, British journal of pharmacology 165(1) (2012) 148–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ohara T, Noma K, Urano S, Watanabe S, Nishitani S, Tomono Y, Kimura F, Kagawa S, Shirakawa Y, Fujiwara T, A novel synergistic effect of iron depletion on antiangiogenic cancer therapy, International journal of cancer 132(11) (2013) 2705–13. [DOI] [PubMed] [Google Scholar]
- [171].Ghio AJ, Hilborn ED, Stonehuerner JG, Dailey LA, Carter JD, Richards JH, Crissman KM, Foronjy RF, Uyeminami DL, Pinkerton KE, Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect, American Journal of Respiratory and Critical Care Medicine 178(11) (2008) 1130–1138. [DOI] [PubMed] [Google Scholar]
- [172].Yatmark P, Morales NP, Chaisri U, Wichaiyo S, Hemstapat W, Srichairatanakool S, Svasti S, Fucharoen S, Effects of Iron Chelators on Pulmonary Iron Overload and Oxidative Stress in beta-Thalassemic Mice, Pharmacology 96(3–4) (2015) 192–9. [DOI] [PubMed] [Google Scholar]
- [173].Zhou T, Ma Y, Kong X, Hider RC, Design of iron chelators with therapeutic application, Dalton transactions (Cambridge, England : 2003) 41(21) (2012) 6371–89. [DOI] [PubMed] [Google Scholar]
- [174].Sohn YS, Breuer W, Munnich A, Cabantchik ZI, Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications, Blood 111(3) (2008) 1690–9. [DOI] [PubMed] [Google Scholar]
- [175].Nickol AH, Frise MC, Cheng HY, McGahey A, McFadyen BM, Harris-Wright T, Bart NK, Curtis MK, Khandwala S, O’Neill DP, Pollard KA, Hardinge FM, Rahman NM, Armitage AE, Dorrington KL, Drakesmith H, Ratcliffe PJ, Robbins PA, A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease, BMJ open 5(7) (2015) e007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Shibata Y, Inoue S, Igarashi A, Yamauchi K, Abe S, Aida Y, Nunomiya K, Sato M, Nakano H, Sato K, Watanabe T, Konta T, Ueno Y, Kato T, Kayama T, Kubota I, Elevated serum iron is a potent biomarker for spirometric resistance to cigarette smoke among Japanese males: the Takahata study, PloS one 8(9) (2013) e74020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hirayama F, Lee AH, Oura A, Mori M, Hiramatsu N, Taniguchi H, Dietary intake of six minerals in relation to the risk of chronic obstructive pulmonary disease, Asia Pac J Clin Nutr 19(4) (2010) 572–7. [PubMed] [Google Scholar]
- [178].Silverberg DS, Mor R, Weu MT, Schwartz D, Schwartz IF, Chernin G, Anemia and iron deficiency in COPD patients: prevalence and the effects of correction of the anemia with erythropoiesis stimulating agents and intravenous iron, BMC pulmonary medicine 14 (2014) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].DePalma RG, Zacharski LR, Chow BK, Shamayeva G, Hayes VW, Reduction of iron stores and clinical outcomes in peripheral arterial disease: outcome comparisons in smokers and non-smokers, Vascular 21(4) (2013) 233–41. [DOI] [PubMed] [Google Scholar]
- [180].Martinez JA, Guerra CC, Nery LE, Jardim JR, Iron stores and coagulation parameters in patients with hypoxemic polycythemia secondary to chronic obstructive pulmonary disease: the effect of phlebotomies, Sao Paulo medical journal = Revista paulista de medicina 115(2) (1997) 1395–402. [DOI] [PubMed] [Google Scholar]
- [181].Chetty KG, Light RW, Stansbury DW, Milne N, Exercise performance of polycythemic chronic obstructive pulmonary disease patients. Effect of phlebotomies, Chest 98(5) (1990) 1073–7. [DOI] [PubMed] [Google Scholar]