Summary
Sleep diary and actigraphy assessments of insomnia symptoms in patients with fibromyalgia (FM) are often discrepant. We examined whether opioid dose and age interact in predicting magnitude or direction of discrepancies. Participants (N=199, M=51.5 years, SD=11.7) with FM and insomnia completed 14 days of diaries and actigraphy. Multiple regressions determined whether average opioid dose and its interaction with age predicted magnitude or direction of diary/actigraphy discrepancies in sleep onset latency (SOL), wake after sleep onset (WASO), and sleep efficiency (SE), controlling for sex, use of sleep medication, evening pain, and total sleep time. Higher opioid dose predicted greater magnitude of discrepancy in SOL and SE. Opioid dose interacted with age to predict direction but not magnitude of discrepancy in SOL and SE. Specifically, higher opioid use was associated with better subjective (shorter SOL, higher SE) than objective reports of sleep among younger adults, and longer subjective than objectively measured SOL among older adults. Opioid dose did not predict magnitude or direction of WASO discrepancies. In FM, higher opioid dose increases diary/actigraphy SOL and SE discrepancies; and direction of discrepancies may depend on age. We speculate that increased opioid use combined with age-related factors, such as slow wave sleep disruption, increased awakenings, and/or cognitive decline, may impact perceived sleep.
Keywords: chronic pain, sleep discrepancy, opioids, aging
Introduction
Patients with fibromyalgia (FM) suffer from chronic widespread pain, with up to 80% experiencing insomnia (Baker et al., 2017). Insomnia symptoms are assessed by sleep diaries (subjective measures) and physiological (objective) measures such as actigraphy. Older adults with insomnia typically report shorter diary than actigraphy estimates (Van Den Berg et al., 2008, Kay et al., 2015), whereas FM patients with poor sleep quality typically report longer subjective than objective estimates (Okifuji and Hare, 2011). Thus, diary and actigraphic methods may measure different constructs. Research shows that increasing age (Van Den Berg et al., 2008), use of sleep medications (Van Den Berg et al., 2008), and pain ratings (Wilson et al., 1998) increase discrepancies. Another potential source of variability between diary/actigraphy measures in FM patients is use of pain medication. Opioid use has been associated with changes in sleep architecture in adults (Dimsdale et al., 2007). Therefore, we aimed to determine whether opioid dose and its interaction with patient age impact the level or direction of diary/actigraphy sleep parameter discrepancies in FM. Knowledge regarding factors that influence sleep ratings in patients with FM and insomnia could improve interpretation of clinical symptoms and understanding of intervention outcomes.
Methods
Participants
Data were derived from a clinical trial investigating the effectiveness of cognitive behavioral therapy for insomnia in patients with FM (NCT02001077; PI, McCrae). Participants (N=235) completed 14 days of baseline sleep diaries (Lichstein et al., 1999) and actigraphy. Criteria for inclusion: at least mild evening pain (10+ on a 0–100 scale), 30+ minutes average diary reported total wake time [sleep onset latency (SOL) + time spent awake after sleep onset (WASO) + early morning awakening (time between last awakening and arising)], and normal global cognition (i.e., scores of ≥24 on the Mini Mental State Examination). There were 199 patients with comorbid FM and insomnia included (see Table 1). The University of Florida Institutional Review Board approved procedures.
Table 1.
Demographics and Sleep Outcomes of Study Sample (N = 199)
| Variable | Mean (SD) | Range |
|---|---|---|
| Age | 51.46 (11.57) | 18–77 |
| Sex (M:F) | 11:188 | -- |
| Average LRD opiates per day | .58 (.93) | .00–4.00 |
| Use of sleep meds (n, %) | 81 (41%) | -- |
| Benzodiazepines (n, %) | 26 (13%) | -- |
| Benzodiazepine-like hypnotics (n, %) | 19 (10%) | -- |
| Antidepressants (n, %) | 41 (21%) | -- |
| Evening pain severity | 51.3 (19.00) | 10.71–95.71 |
| Sleep Diary Measures | ||
| SOL (minutes) | 50.30 (38.98) | 3.07–229.93 |
| WASO (minutes) | 44.05 (36.15) | .93–231.00 |
| TST (minutes) | 398.33 (80.18) | 192.00–631.93 |
| TWT (minutes) | 122.45 (66.44) | 31.92–410.30 |
| TIB (minutes) | 520.87 (77.62) | 343.21–786.43 |
| SE (%) | 76.42 (11.53) | 32.59–93.26 |
| Actigraphy Measures | ||
| SOL (minutes) | 43.11 (35.99) | 1.93–200.65 |
| WASO (minutes) | 51.52 (23.37) | 5.40–147.58 |
| TWT (minutes) | 118.44 (60.36) | 12.67–351.17 |
| TST (minutes) | 394.71 (73.33) | 159.80–822.00 |
| TIB (minutes) | 513.16 (79.61) | 346.43–834.67 |
| SE (%) | 77.29 (9.95) | 31.82–98.71 |
Note. LRD = lowest recommended dosage; SOL = sleep onset latency; WASO = wake after sleep onset; TST = total sleep time; TWT = total wake time; TIB = total time in bed; SE = sleep efficiency
Measures
Demographics
Age, sex, and daily use of sleep/pain medication were collected. Participants who used pain medication provided drug names and daily dosage. Using values in the Physician’s Desk Reference (Staff, 2011), milligrams of opiates were converted into lowest recommended dosage units per day (e.g., codeine: 15mg=1; 30mg=2)
Sleep and Pain Diaries
Participants were instructed to complete a morning diary for 14 days, reporting evening pain severity, total sleep time (TST), SOL, WASO, and time spent in bed (TIB). Sleep efficiency (SE) was computed as: SE=(TST/TIB)*100.
Actigraphy
Participants wore an actigraph (Actiwatch2, Phillips Respironics) on their non-dominant wrist for all 14 baseline days. Actiwatch2 records the intensity and frequency of wrist motion at 32 cycles/second. Activity counts were analyzed in Actiware Sleep Analysis Software v.5.3.2 using validated algorithms (Oakley, 1997) and 30s epochs. Scoring period was set to diary reported initial bedtime and final waketime. SOL, TST, WASO, and SE were computed.
Data Analysis
Two measures of disagreement were calculated for each sleep variable. Magnitude of discrepancy was computed as the absolute difference between average actigraphy and diary estimates. Direction of discrepancy was computed by subtracting actigraphy from diary estimates (i.e., positive values indicate greater diary than actigraphy estimates). Multiple linear regressions were carried out in SPSS (Version 24). Criterion variables included diary and actigraphy estimates of SOL, TST, WASO, and SE. Predictor variables included age, daily opioid dose, and the age by opioid dose interaction term. Patient sex, use of sleep medication, average evening pain ratings, and average TST were entered as covariates. Significant age by opioid dose interactions were clarified by calculating simple slopes of the association between opioid dose and sleep discrepancies for different sample estimated age values characterized as follows: younger (1 SD below mean age, ~40 years), middle-aged (mean age, ~51 years) and older (1 SD above, ~63 years) adults.
Results
Participant characteristics are presented in Table 1.
Results of models predicting magnitude of diary/actigraphy discrepancy are displayed in Table 2. As opioid dose increased, SOL and SE discrepancy increased. Opioid dose did not interact with age in the prediction of SOL, WASO, or SE discrepancy.
Table 2.
Determinants of magnitude of disagreement of sleep parameters
| Variable |
Absolute difference
of sleep parameter (diary – actigraphy)
|
|||
|---|---|---|---|---|
| B | Std error (B) | t | P-value | |
| SOL | ||||
| Opioid dose | 6.10 | 2.29 | 2.66 | .008** |
| Age | .08 | .18 | .46 | .65 |
| Opioid dose x age | .26 | .218 | 1.19 | .24 |
| TST | −.04 | .03 | −1.61 | .11 |
| Use of sleep medicationa | 1.97 | 4.17 | .47 | .64 |
| Evening pain rating | −.07 | .11 | −.63 | .53 |
| Sex | 5.36 | 9.53 | .56 | .57 |
| WASO | ||||
| Opioid dose | 1.59 | 2.05 | .77 | .44 |
| Age | −.22 | .16 | −1.37 | .17 |
| Opioid dose x age | −.12 | .20 | −.60 | .23 |
| TST | .02 | .02 | .75 | .45 |
| Use of sleep medicationa | −1.13 | 3.74 | −.30 | .76 |
| Evening pain rating | .12 | .10 | 1.19 | .23 |
| Sex | 16.81 | 8.5 | 1.97 | .051 |
| SE | ||||
| Opioid dose | 1.92 | .70 | 2.75 | .007** |
| Age | −.02 | .05 | −.29 | .77 |
| Opioid dose x age | −.05 | .07 | −.72 | .77 |
| TST | −.02 | .07 | −.72 | .47 |
| Use of sleep medicationa | −2.95 | 1.17 | −2.32 | .02* |
| Evening pain rating | .03 | .03 | .96 | .34 |
| Sex | 3.15 | 2.89 | 1.09 | .28 |
Note. Multiple regression analyses controlling for patient sex, total sleep time (TST), use of sleep medication, and evening pain ratings. SOL = sleep onset latency; WASO = wake after sleep onset; SE = sleep efficiency
Use of sleep medications entered as a dichotomous covariate (y/n) in regression model. When this variable was entered as a continuous covariate (total average lowest recommended dosage of sleep medications per day), regression results were unchanged.
P < .05;
P < .01
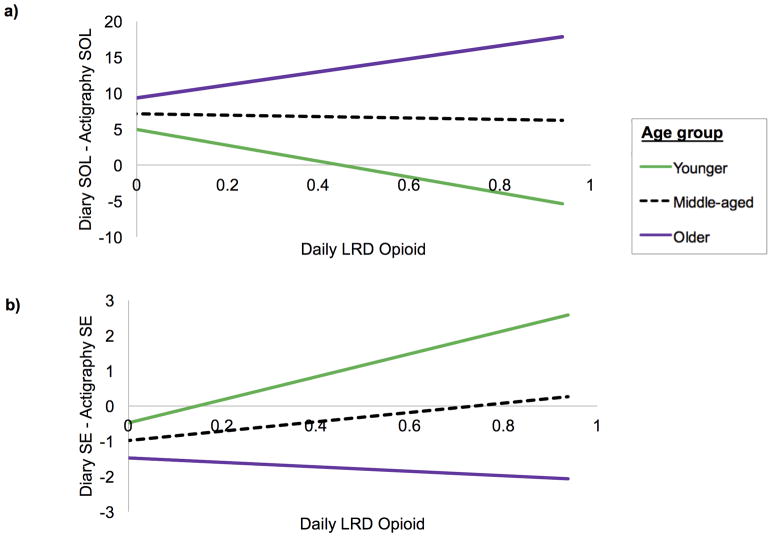
Results of models predicting direction of diary/actigraphy discrepancies are depicted in Table 3. Opioid dose interacted with age to predict SOL and SE discrepancies. As Figure 1a illustrates, higher dosage was associated with shorter diary than actigraphy SOL in younger adults (B=−11.02, SE=4.65, p=.02), and longer diary than actigraphy SOL in older adults (B=9.09, SE=3.66, p=.01). Opioid dose was not associated with SOL discrepancy in middle-aged adults (B=−.97, SE=2.83, p=.73). As Figure 1b illustrates, higher opioid dose predicted greater diary than actigraphy SE in younger adults (B=3.27, SE=1.36 p=.02) and was not a predictor in older (B=−.63, SE=1.07 p=.56) or middle-aged (B=1.32, SE=.83 p=.11) adults. Opioid dose did not predict WASO discrepancies.
Table 3.
Determinants of direction of disagreement of sleep parameters
| Variable |
Difference of sleep
parameter (diary – actigraphy)
|
|||
|---|---|---|---|---|
| B | Std error (B) | t | P-value | |
| SOL | ||||
| Opioid dose | −.97 | 2.83 | −.34 | .73 |
| Age | .19 | .22 | .87 | .38 |
| Opioid dose x age | .88 | .27 | 3.26 | .001** |
| TST | −.16 | .03 | −4.88 | .000*** |
| Use of sleep medicationa | −1.24 | 11.77 | −.11 | .92 |
| Evening pain rating | −.46 | .14 | −3.37 | .001** |
| Sex | −1.24 | 11.77 | −.11 | .92 |
| WASO | ||||
| Opioid dose | −3.07 | 2.50 | −1.23 | .22 |
| Age | −.12 | .19 | −.63 | .53 |
| Opioid dose x age | −.12 | .24 | −.50 | .53 |
| TST | −.26 | .03 | −9.16 | .000*** |
| Use of sleep medicationa | −1.16 | 4.56 | −.25 | .80 |
| Evening pain rating | .20 | .12 | 1.69 | .09 |
| Sex | ||||
| SE | ||||
| Opioid dose | 1.32 | .83 | 1.60 | .11 |
| Age | −.04 | .06 | −.67 | .50 |
| Opioid dose x age | −.17 | .08 | −2.17 | .03* |
| TST | .10 | .009 | 11.00 | .000*** |
| Use of sleep medicationa | −3.36 | 1.51 | −2.23 | .03* |
| Evening pain rating | .04 | .04 | .87 | .39 |
| Sex | −6.07 | 3.44 | −1.77 | .08 |
Note. Multiple regression analyses controlling for patient sex, total sleep time (TST), use of sleep medication, and evening pain ratings. SOL = sleep onset latency; WASO = wake after sleep onset; SE = sleep efficiency
Use of sleep medications entered as a dichotomous covariate (y/n) in regression model. When this variable was entered as a continuous covariate (total average lowest recommended dosage of sleep medications per day), regression results were unchanged.
P < .05;
P < .01;
P < .001
Figure 1.
Direction of sleep diary/actigraphy discrepancies in SOL (a) and SE (b) as a function of average daily opioid dose (lowest recommended dosage, LRD) and age (younger adults, middle-aged adults, older adults)
Discussion
This study examined independent and interactive effects of opioid dose and age on the magnitude and direction of diary/actigraphy sleep parameter discrepancies in patients with FM and insomnia. Findings show that higher opioid dose increases the magnitude of diary/actigraphy discrepancies. However, the direction of discrepancy depends on age, with higher opioid dose in younger adults (~40 years) leading to shorter SOL and higher SE subjective than objective estimates. In contrast, higher dose led to longer subjective than objectively measured SOL among older adults (~63 years).
There are several potential explanations for these variations in diary/actigraphy estimates. First, research shows that, relative to younger adults, older adults experience less slow wave sleep (SWS), and opioid use has been shown to decrease amount of SWS (Wang and Teichtahl, 2007). Thus, in older adults with FM, there may be an additive effect of age and opioid dose on SWS, leading to a greater likelihood of attributing lighter sleep as ‘wake’, resulting in perceptions of longer sleep onset. Second, as older adults experience more brief nighttime awakenings than younger adults (Scullin and Bliwise, 2015), it is possible that opioid use exacerbates this effect and contributes to sleep misperception. Third, given that both opioid use (Schiltenwolf et al., 2014) and FM (Glass, 2008) have been associated with worse cognition, normal age-related cognitive decline may exacerbate cognitive effects, increasing the likelihood of subjective/objective SOL discrepancies. However, it would be important for future research to compare results to a control group without FM in order to provide evidence for a specific opioid/cognition association. For younger adults, in the absence of decreased SWS, higher opioid dose may have a greater sedative effect, resulting in lower self-report versus actigraphic sleep estimates, as well as greater subjective than objective SE.
A potential limitation concerns the validity of actigraphy in distinguishing sleep from wake. While actigraphy versus polysomnography estimates of SE are highly correlated, SOL estimates show only weak correlation (Lichstein et al., 2006). Thus, diary versus polysomnography patterns of discrepancy and associations with opioid dose and age should also be compared. Additionally, given that the average age of FM participants was approximately 51 years, results for younger adults (~40 years) are not necessarily representative of the young adult population. Finally, future work should compare results to those in older adults with insomnia and no FM, in order to determine whether findings are specific to FM – important information for the clinical care of these comorbid conditions.
Results suggest that associations between opioid dose and direction of diary/actigraphy discrepancies depend on patient age, with older adults most vulnerable to perceptions of worse sleep onset. Because self-reported sleep is strongly associated with health outcomes in older adults (Dew et al., 2003), it may be important for clinicians to carefully monitor opioid dosage in older adults with FM. Implications of the associations between higher opioid dose and better subjective than objective reports of sleep in younger adults remain to be determined.
Acknowledgments
Funding: National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR055160, R01AR005160-S1; McCrae, PI). Data collected as part of clinical trial NCT02001077 (University of Florida; McCrae, PI).
Footnotes
Conflicts of Interest: Authors declare no conflicts of interest.
Author Contributions: A.F.C. and M.B.M conducted analyses. A.F.C drafted the manuscript. C.S.M. drafted and carried out protocol. All authors revised draft.
References
- Baker S, Mcbeth J, Chew-Graham CA, Wilkie R. Musculoskeletal pain and co-morbid insomnia in adults; a population study of the prevalence and impact on restricted social participation. BMC Fam Pract. 2017;18:17. doi: 10.1186/s12875-017-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, Dejardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–36. [PubMed] [Google Scholar]
- Glass JM. Fibromyalgia and cognition. The Journal of clinical psychiatry. 2008;69:20–24. [PubMed] [Google Scholar]
- Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective–objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J Sleep Res. 2015;24:32–39. doi: 10.1111/jsr.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, Riedel BW, Means MK. Psychological treatment of late-life insomnia. 1999 [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–39. [PubMed] [Google Scholar]
- Oakley N. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend: Mini Mitter, Cambridge Neurotechnology. 1997 [Google Scholar]
- Okifuji A, Hare BD. Nightly analyses of subjective and objective (actigraphy) measures of sleep in fibromyalgia syndrome: what accounts for the discrepancy? The Clinical journal of pain. 2011;27:289. doi: 10.1097/AJP.0b013e31820485db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltenwolf M, Akbar M, Hug A, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17:9–20. [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10:97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff P. Physicians’ desk reference. PDR Network. 2011 [Google Scholar]
- Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11:35–46. doi: 10.1016/j.smrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75:75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]