Abstract
Nivolumab has become the standard second-line chemotherapy for non-small cell lung cancer. A 73-year-old man with stage IV non-small cell lung cancer received 6 cycles of chemotherapy with nab-paclitaxel/carboplatin/bevacizumab followed by 11 cycles of nab-paclitaxel/bevacizumab; however, treatment was stopped due to pneumothorax. One year after therapy started, a nodule appeared in the left upper lung and increased in size. Mycobacterium abscessus subsp. massiliense disease was diagnosed by a sputum analysis. After short antibiotic treatment, nivolumab was administered. Two months after nivolumab treatment, the nodule improved along with a good tumour response. The effectiveness of nivolumab for chronic infectious diseases, such as M. abscessus disease, should be investigated.
Keywords: Mycobacterium abscessus, nivolumab, non-small cell lung cancer
Introduction
Nontuberculous mycobacteria (NTM) inhabit both natural and engineered water systems as well as soil. Although Mycobacterium avium complex (MAC) is generally the most prevalent NTM, M. abscessus accounts for 2-6% of all NTMs in Japan, the US, and Australia (1-3).
NTM infections typically occur in the lungs, and the prevalence of NTM-related pulmonary disease has been increasing globally (4). M. abscessus belongs to a group of rapidly growing mycobacteria and is an emerging cause of NTM lung disease, particularly in patients with pre-existing lung conditions, such as cystic fibrosis, bronchiectasis, and tuberculosis. At present, M. abscessus is the second-most common cause of NTM lung disease in the US and the third-most common after Mycobacterium kansasii in Japan (5, 6). The importance of this species is highlighted by its tendency to be refractory to treatment. At present, lung cancer is the leading cause of cancer-related death worldwide, and the coexistence of lung cancer and NTM lung disease, which share some common predisposing factors (e.g., smoking), is not uncommon.
Recently, antibodies targeting the programmed cell death-1 (PD-1) cell membrane antigen have emerged as a new standard therapy for patients with non-small cell lung cancer (NSCLC). Nivolumab, a fully humanized immunoglobulin G4 antibody, binds to PD-1 on activated immune cells, where it inhibits the immune checkpoint by blocking the interactions of PD-1 with its ligands, PD-L1 and PD-L2. Although nivolumab has shown significant efficacy for the treatment of NSCLC (7), immune checkpoint inhibitors are associated with unique immune-related adverse events.
Although reports from Japan and around the world have described the exacerbation of NTM or M. tuberculosis disease during nivolumab treatment, the effects of this agent on NTM disease remain unknown. We herein report a case of advanced NSCLC, in which NTM disease improved after nivolumab administration, and discuss the potential mechanisms underlying the interactions of infections with immune checkpoint inhibitor therapy.
Case Report
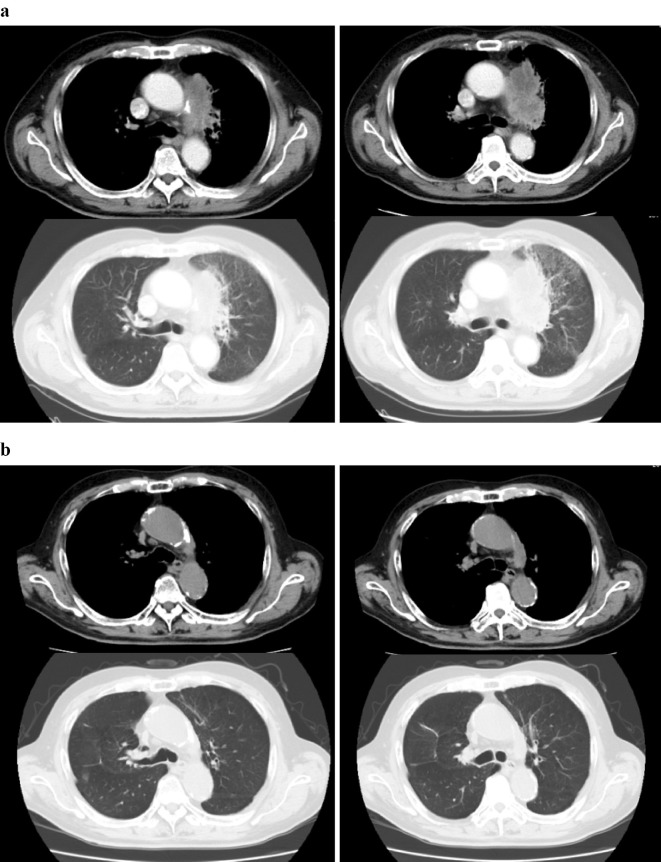
A 73-year-old Japanese man and current smoker (53 pack-years) was diagnosed with NSCLC suggestive of adenocarcinoma, stage IV (T4N2M1a). He presented with massive left pleural effusion and pericardial fluid, as well as mediastinal lymphadenopathy and pleural dissemination with pericardial invasion (Fig. 1a). A mutation analysis of the biopsied tissue revealed that the tumour harboured the wild-type epidermal growth factor receptor (EGFR) gene and the echinoderm microtubule-associated protein-like 4 gene (EML4)-anaplastic lymphoma kinase (ALK) fusion gene. Following pericardial fluid and pleural effusion drainage, the patient received 6 cycles of chemotherapy comprising nanoparticle albumin-bound (nab)-paclitaxel and carboplatin with bevacizumab, followed by 11 cycles of nab-paclitaxel with bevacizumab. This treatment showed a durable response for the NSCLC (Fig. 1b). However, the treatment was later withdrawn because of surgery-adaptive pneumothorax.
Figure 1.
Before (a) and after (b) four cycles of treatment with nab-paclitaxel plus carboplatin with bevacizumab.
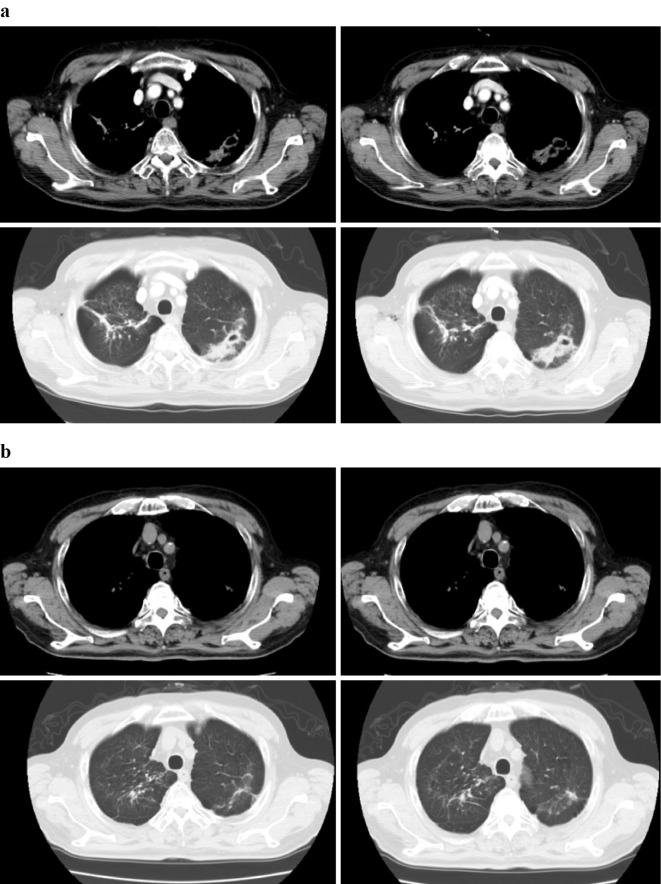
One year after therapy initiation (five months before pneumothorax), nodules with cavitation and a disseminated focus appeared in the left upper lung, increasing in size. At the onset of the pneumothorax, these left upper lobe nodules continued to increase in size, and imaging findings suggested mycobacterial disease (Fig. 2a). Following repeated sputum examinations, the patient received a diagnosis of M. abscessus subsp. massiliense lung disease. Two weeks’ treatment with a combination of imipenem (1,000 mg/day) and amikacin (200 mg/day) was performed for the M. abscessus lung disease. However, the antibiotic treatment was ineffective, and we wanted to treat the patient with nivolumab as recurrence of the lung cancer had been incidentally confirmed on a surgery specimen obtained during pneumothorax surgery. The interaction of antibiotics and nivolumab was unknown, and so we discontinued the medication. Subsequently, nivolumab was administered intravenously at a dose of 3 mg/kg every 2 weeks as a second-line treatment for lung cancer. After two months, the M. abscessus nodule with cavitation and disseminated focus improved, and continuous improvements were visible on computed tomography (Fig. 2b). A regular follow-up sputum examination was not performed because the patient was unable to expectorate sputum due to improvement. However, were able to obtain a single sputum sample after nivolumab therapy, which was negative on culture. The patient remains on nivolumab therapy, which has not only maintained tumour shrinkage but has also effectively treated the M. abscessus infection.
Figure 2.
Mycobacterium abscessus nodule with cavity before (a) and two months after (b) treatment with nivolumab.
Discussion
M. abscessus disease is resistant to many antibiotics and is therefore difficult to treat. However, this species is usually susceptible to some parenteral agents (amikacin, cefoxitin, and imipenem) and macrolides (clarithromycin and azithromycin) (8, 9). At present, the American Thoracic Society/Infectious Diseases Society of America recommends a combination therapy of intravenous amikacin with cefoxitin or imipenem and an oral macrolide (10). However, unsatisfactory responses to the recommended treatment doses have been observed, so the ideal therapeutic regimens and treatment durations have not yet been established.
Patients with M. abscessus infection were found to have lower initial sputum conversion rates than M. massiliense (11). In our case, the M. abscessus shadow improved after nivolumab administration, and a subsequent sputum examination was negative. Although we should have performed prolonged combination therapy including a macrolide and parental drugs for M. abscessus disease, we gave priority to the anticancer therapy. We note that, in addition to the characteristic antibiotic resistance of M. abscessus disease, the patient had only received antibiotic therapy for two weeks. We do not believe that we would have seen an effective response to antibiotics within such a short period. Therefore, we attribute the remission of the M. abscessus shadow in this case to nivolumab treatment.
Regarding the mechanism by which nivolumab may have resolved the M. abscessus infection, we hypothesise that this drug activates T lymphocytes, which may have primed the patient's immune system to effectively target a chronic infection. Specifically, nivolumab blocks the PD-1/PD-L1 pathway and can thus restore the function to exhausted cytotoxic T cells (CTLs); activated CTLs may cure a chronic M. abscessus infection. This finding is promising, as immunosuppressive molecules, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), PD-1, and PD-L1, are markedly overexpressed in the tumour microenvironment, and the consequent CTL exhaustion critically hinders viral clearance and presents a major hurdle to treating chronic infections (12). According to Reungwetwattana et al., immune checkpoint inhibitors do not suppress T cells but instead reactivate CTLs and enhance the immune system (13). Therefore, blocking the PD-1 pathway may improve the lymphocyte function and reduce apoptosis.
Although concerns have been raised regarding the potentially harmful effects of cytotoxic chemotherapy for lung cancer and consequent immune suppression on the course of NTM lung disease (14), in the present case, nivolumab appears to have resolved the infection. Still, many reports have described the development of acute pulmonary tuberculosis during nivolumab treatment, and therefore, this risk must be considered separately from chronic infectious disease (15). Further research is needed to elucidate the precise mechanisms of action.
This study is associated with one limitation that should be taken into consideration. We should have performed bronchoscopy for the new shadow, as the possibility of cancer could not be denied. The radiologic appearance of cavitated satellite nodules combined with the simultaneous detection of M. abscessus from sputum led to the diagnosis of nontuberculous lung disease.
While the results achieved in the present case are indeed promising, we feel that a systematic survey of the effectiveness of nivolumab for chronic infectious disease, such as M. abscessus infection, is still warranted.
The patient provided his written informed consent for the publication of this case report and accompanying images.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank the patient as well as his family and caregivers.
References
- 1. Sakatani M. The non-tuberculous mycobacteriosis. Kekkaku 80: 25-30, 2005(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 2. Winthrop KL, McNelley E, Kendall B, et al. . Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 182: 977-982, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis 16: 1576-1583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griffith DE, Aksamit T, Brown-Elliott BA, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Prevots DR, Shaw PA, Strickland D, et al. . Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182: 970-976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Namkoong H, Kurashima A, Morimoto K, et al. . Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan (1). Emerg Infect Dis 22: 1116-1117, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakahama K, Tamiya A, Taniguchi Y, et al. . Severe acute interstitial lung disease after nivolumab in three non-small cell lung cancer patients with imaging findings of airway obstruction adjacent to lung tumors. J Infect Chemother 23: 826-829, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med 156: S1-25, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Griffith DE, Aksamit T, Brown-Elliott BA, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 21: 1638-1646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koh WJ, Jeon K, Lee NY, et al. . Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183: 405-410, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Shu CC, Wang JY, Wu MF, et al. . Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci Rep 7: 42004, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reungwetwattana T, Adjei AA. Anti-PD-1 antibody treatment and the development of acute pulmonary tuberculosis. J Thorac Oncol 11: 2048-2050, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Tsuji T, Tsuyuguchi K, Tachibana K, et al. . Analysis of the impact of lung cancer treatment on nontuberculous mycobacterial lung diseases. Respir Investig 55: 45-50, 2017. [DOI] [PubMed] [Google Scholar]
- 15. Fujita K, Terashima T, Mio T. Anti-PD1 antibody treatment and the development of acute pulmonary tuberculosis. J Thorac Oncol 11: 2238-2240, 2016. [DOI] [PubMed] [Google Scholar]