Abstract
A 20-year old man was diagnosed with fibrolamellar hepatocellular carcinoma (FLHCC) with multiple lung metastases, and chemotherapy with FOLFOX was administered. Contrast enhanced CT after 3 cycles of FOLFOX showed no disease progression. We therefore performed surgical resection and radiofrequency ablation of the liver lesions and lung metastases, after obtaining the patient's informed consent. The liver lesions and lung metastases tested positive for DNAJB1-PRKACA. The treatment for FLHCC with extrahepatic metastasis has not been established; however, in a few cases, good long-term prognoses were obtained with multidisciplinary therapy. We herein report a case of FLHCC with multiple lung metastases that was treated with multidisciplinary therapies.
Keywords: fibrolamellar hepatocellular carcinoma, multidisciplinary therapy, DNAJB1-PRKACA
Introduction
Fibrolamellar hepatocellular carcinoma (FLHCC) is a rare histological subtype of hepatocellular carcinoma (HCC) with an age-adjusted prevalence of 0.02 per 100,000 persons in the United States (1) and accounts for 0.4% of primary liver cancer cases (2).
There have only a few of case reports from Asian countries, including Japan, where the incidence of FLHCC is even lower than in Western countries. Most cases occur in young individuals without liver disease. Some studies reported that FLHCC progressed gradually. However, whether the prognosis of FLHCC is better than that of conventional HCC is controversial (1-7). Surgical resection is the only curative treatment for FLHCC, and the prognosis after operation has been reported to be better than that of patients with conventional HCC (7). However, there is no consensus on the treatment for locally advanced unresectable cases or cases with distant metastasis, and metastatic disease is reported to be a poor prognostic factor (2,6,8). We herein report a case of FLHCC with multiple lung metastases that was treated with multidisciplinary therapies.
Case Report
A 20-year old man visited a nearby hospital because of left upper abdominal distension. He was found to have hepatic mass lesions and was then referred to our hospital for further examination. He had no relevant medical history including chronic liver diseases and no history of drinking or smoking. His paternal uncle died of progression of a fibrovascular tumor. No yellowing of the bulbar conjunctiva was observed. An enlarged liver was palpated over the epigastric and the left hypochondriac regions. The laboratory data are shown in Table. Protein induced by the absence of vitamin K or antagonist-II(PIVKA-II) and α-fetoprotein (AFP) were elevated, and the indocyanine green retention rate at 15 minutes (ICG R15) and ICG-K value were normal. Abdominal ultrasonography revealed a multi-nodular heterogeneous hypoechoic mass in the left lateral segment of the liver, with a cord-like hyperechoic area in the center of the lesion (Fig. 1). A hypoechoic mass with an internal hyperechoic area was also observed in segment 4. Contrast enhanced computed tomography (CE-CT) revealed a multi-nodular well-defined mass 120×67 mm in size in the left lateral segment of the liver (Fig. 2a). The lesion showed heterogeneous hyperattenuation in the arterial phase, and although it showed a gradual washout of contrast medium, prolonged enhancement was observed in the equilibrium phase. The center of the lesion showed an area of hypoattenuation. There was another hepatic mass lesion in segment 4, measuring 33×20 mm in size. With the exception of the central area, the lesion showed homogeneous hyperattenuation in the arterial phase, and it showed hypoattenuation relative to the background liver parenchyma in the equilibrium phase (Fig. 2b). Moreover, multiple well-defined round metastatic lesions with a maximum size of 15 mm were noted in the lungs (Fig. 2c). On abdominal magnetic resonance imaging (MRI), the tumor was hypointense on T1-weighted images and slightly hyperintense on T2-weighted images (Fig. 3a). No reduction in intensity was noted on in-phase or out-of-phase T1-weighted images. In addition, the hypoattenuated area in the center of the lesion on CE-CT was hypointense on T1-weighted images but hyperintense on T2-weighted images, diffusion-weighted images, and apparent diffusion coefficient (ADC) mapping. On gadolinium-ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) MRI with the exception of the center of the lesion, the tumor showed heterogenous enhancement in the arterial phase and prolonged enhancement in the equilibrium phase. In addition, it was hypointense in the hepatocellular phase (Fig. 3b). These imaging findings especially the contrast enhancement findings were atypical of conventional HCC. Furthermore, this was an early-onset case, and the patient had no history of chronic liver disease. Thus, a percutaneous liver biopsy was performed to obtain a definitive diagnosis. A histopathological analysis showed polygonal tumor cells with an eosinophilic cytoplasm surrounded by abundant fibrous stroma with a lamellar distribution. On immunohistochemical staining, the lesions were positive for hepatocyte paraffin 1 (Hep-par1). Furthermore, the lesions were positive for cytokeratin 7 (CK7), and CD68, for which conventional HCC lesions are negative. Based on these findings, the tumor was diagnosed as FLHCC with multiple lung metastases. Although there is no established treatment for FLHCC with extrahepatic metastasis, chemotherapy with modified FOLFOX6 [fluorouracil bolus (400 mg/m2), l-leucovorin (200 mg/m2), oxaliplatin (85 mg/m2), fluorouracil (2,400 mg/m2)] was administered on the basis of previous reports in which treatment with FOLFOX and gemcitabine and oxaliplatin (GEMOX) was effective (9-11). CE-CT after 3 cycles of modified FOLFOX6 showed no disease progression. Thus, we proceeded with the plan to perform aggressive surgical resection and local treatment after obtaining the patient's informed consent. Initially, left lateral liver resection and partial liver resection of the lesion in segment 4 were performed (46 days after the first hospitalization). The macroscopic findings of the resected specimens showed a well-defined multi-nodular tumor with a central stellate scar (Fig. 4a). On histopathological examination, the pathological findings of the lesion in the left lateral segment of the liver were similar to the liver biopsy findings (Fig. 4b) and a stellate scar was noted in the central area of the lesion. The stellate scar had a prominent fibrous tissue; however, there was a sparse part of the fibrous tissue that was edematous (Fig. 4c and d). Less fibrous tissue was present in the segment 4 lesion than in the lesion in the left lateral section. Pale bodies were not seen in either lesions. Immunohistochemistry showed that the specimens were positive for Hep-par1, CK7, and CD68, which was consistent with the liver biopsy findings (Fig. 5). The analysis of formalin fixed paraffin embedded (FFPE) specimens with a reverse transcription-polymerase chain reaction (RT-PCR) and direct sequencing showed that the primary lesion and the segment 4 lesion were both positive for DNAJB1-PRKACA, which is a fusion gene that is characteristically found in FLHCC (Fig. 6). As mentioned above, we diagnosed the lesions as FLHCC [expansive growth (eg), capsule formation (fc)+, cancerous infiltration of the capsule (fc-inf)+, formation of fibrous septum within the tumor (sf)+, invasion of the serosa (s) 1, portal vein invasion (vp) 1, hepatic vein invasion (vv) 1, hepatic artery invasion (va) 0, bile duct invasion (b) 0, intrahepatic metastasis (im)+, findings in the non-cancerous portion (f), normal liver (nl)] according to General Rules for the Clinical and Pathological Study of Primary Liver Cancer by the Liver Cancer Study Group of Japan, pT3aN0M1, pstage IVB according to UICC 7th edition. It was difficult to assess the effects of chemotherapy, as the tumor had a significant amount of fibrous tissue; however, given the large number of viable tumor cells, it was considered grade 1 [characteristic cytologic changes of malignancy were present, but few (<10%) or no tumor cell destruction was present] according to Evans' classification (12). The levels of PIVKA-II (25 mAU/mL) and AFP (1.9 ng/mL) were within the normal ranges after the resection of the liver lesions. With regard to the lung lesions, thoracoscopic partial resection (72 days from the first hospitalization) of the left lobe was first performed for the lesions in the left lobe (9 areas, 12 lesions), and radiofrequency ablation (RFA) was subsequently performed for the remaining lesions in the left lobe (16 days from left lobe partial resection, 98 days from the first hospitalization). Thoracoscopic lobectomy of the right lower lobe and partial resection of the right upper and middle lobes were performed for the lesions in the right lobe (34 days from partial resection of the left lobe, 106 days from the first hospitalization), and subsequently, RFA was performed for the remaining lesions (47 days from lobectomy of the right lower lobe and partial resection of the upper and middle lobes, 153 days from the first hospitalization). Similar to the lesion in segment 4, the metastatic lung lesions had relatively little fibrous tissue and were positive for DNAJB1-PRKACA, which was detected by the analysis of frozen specimens. The patient's condition was stable after treatment, and there were no obvious lesions at 18 weeks after RFA of the residual lesions in the right lobe (Fig. 7).
Table.
Laboratory Data on Admission.
| Peripheral blood | Blood chemistry | Coagulation | ||||||
| WBC | 6,360 | /μL | Alb | 4.1 | g/dL | PT | 12.5 | sec |
| Hb | 15.2 | g/dL | T.Bil | 0.6 | mg/dL | PT-INR | 1.05 | |
| Ht | 47.7 | % | AST | 21 | IU/L | APTT | 31.5 | sec |
| Plt | 34.0 | ×104/μL | ALT | 16 | IU/L | |||
| ALP | 214 | IU/L | HBsAg | (-) | ||||
| Tumor marker | γGTP | 44 | IU/L | HBsAb | (-) | |||
| CEA | 0.8 | ng/mL | BUN | 11 | mg/dL | HBcAb | (-) | |
| CA19-9 | 2.9 | U/mL | CRE | 0.94 | mg/dL | HBV-DNA | (-) | |
| PIVKA-II | 1,033 | mAU/mL | CRP | 2.91 | mg/dL | HCVAb | (-) | |
| AFP | 16.2 | ng/mL | ||||||
| AFP-L3 | 14.6 | % | ICG R15 | 4.6 | % | |||
| ICG K value | 0.21 | |||||||
PIVKA-II: protein induced by vitamin K absence or antagonist-II, AFP: α-fetoprotein, HBsAg: hepatitis B surface antigen, HBsAb: hepatitis B surface antibody, HBcAb: hepatitis B core antibody, HBV: hepatitis B virus, HCV-Ab: hepatitis C virus antibody, ICG: indocyanine green
Figure 1.
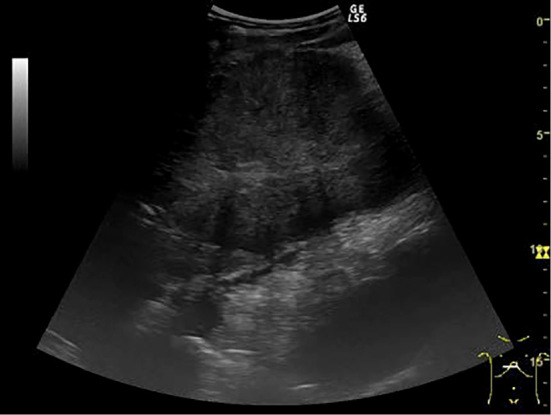
Abdominal ultrasonography (the lesion in the left lateral segment of the liver). A multi-nodular heterogeneous hypoechoic mass with a cord-like hyperechoic area in the center of the lesion.
Figure 2.
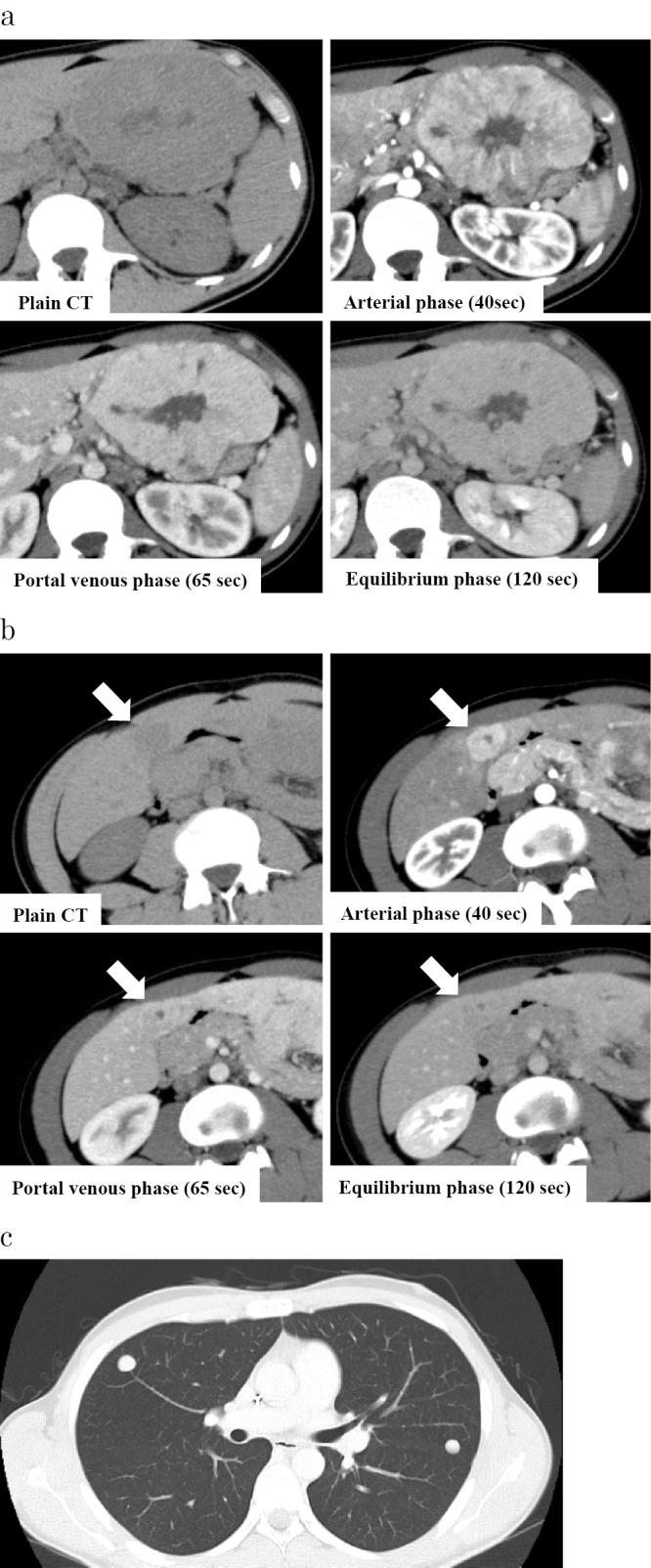
Contrast enhanced computed tomography. a: The lesion in the left lateral segment of the liver. In the arterial phase, the lesion showed heterogeneous hyperattenuation with the hypoattenuation area in the center of the lesion, and although it showed the gradual washout of contrast medium, it was prolonged in the equilibrium phase. b: The lesion in the segment 4 of the liver. The lesion showed homogeneous hyperattenuation in the arterial phase and hypoattenuation in the equilibrium phase (arrow). c: Multiple metastatic lesions in the lungs.
Figure 3.
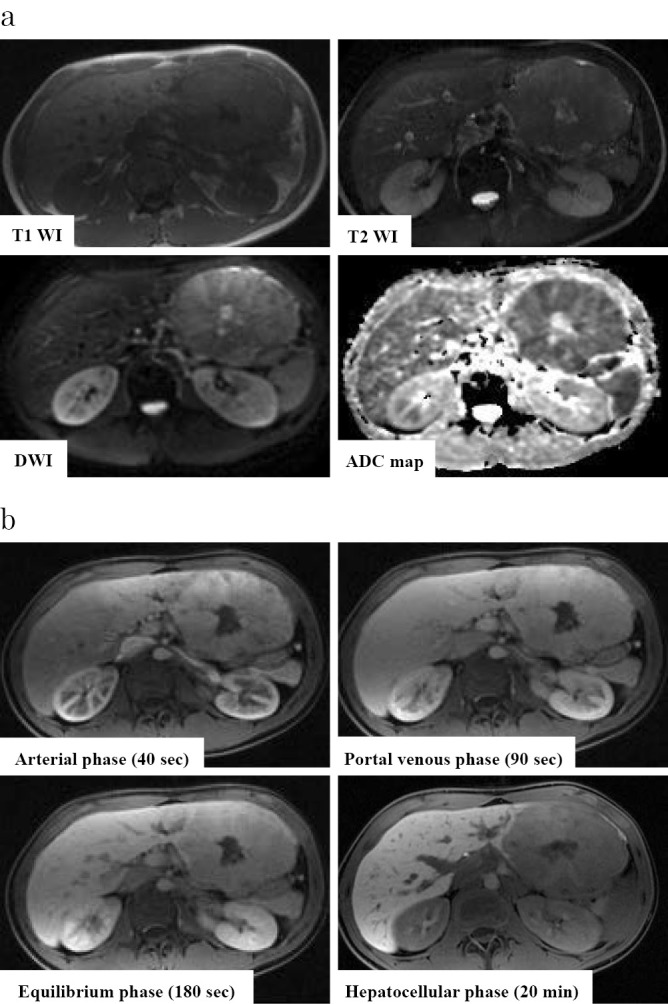
Abdominal MRI (the lesion in the left lateral segment of the liver). a: The tumor was hypointense on T1-weighted images and hyperintense on T2-weighted images. In addition, the center of the lesion was hypointense on T1-weighted images hyperintense on T2-weighted images. b: EOB-MRI. Excluding the center of the lesion, the tumor showed heterogeneous enhancement in the arterial phase and prolonged enhancement in the equilibrium phase. In addition, it was hypointense on the hepatocellular phase.
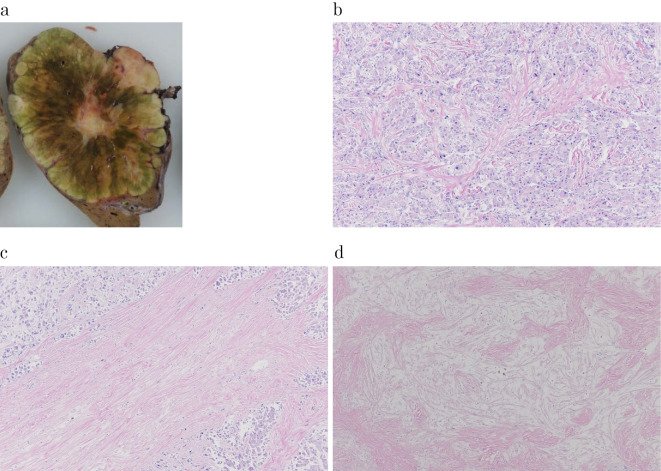
Figure 4.
The histopathological analysis (the lesion in the left lateral segment of the liver). a: A well-defined multi-nodular tumor with a central stellate scar. b: The histopathological analysis showed polygonal tumor cells with an eosinophilic cytoplasm surrounded by abundant fibrous stroma. Hematoxylin and Eosin (H&E) staining, × 100. c, d: The stellate scar had prominent fibrous tissue; however, there was a sparse part of the fibrous tissue that was edematous. H&E staining, × 100.
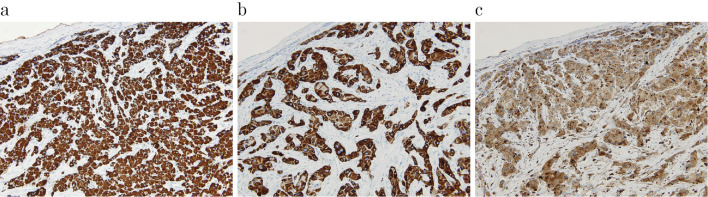
Figure 5.
Immunohistochemistry (the lesion in the left lateral segment of the liver). a: Hep-par 1 staining, × 200. b: CK7 staining, × 200. c: CD68 staining, × 200.
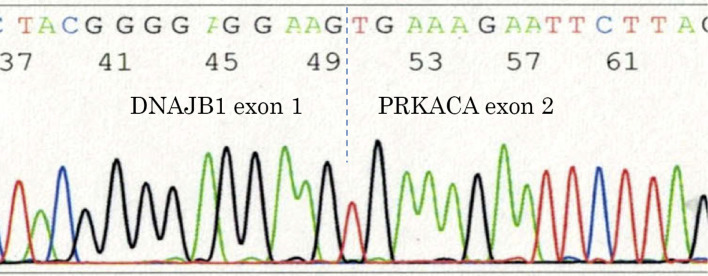
Figure 6.
DNAJB1-PRKACA fusion. RT-PCR and direct sequencing showed the fusion of exon 1 from DNAJB1 with exon 2 from PRKACA.
Figure 7.
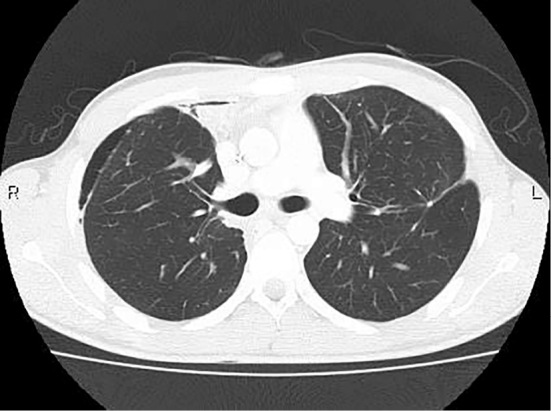
Computed tomography after the operation and radiofrequency ablation for lung metastases. There were no obvious lesions in the lungs.
Discussion
FLHCC is a rare tumor with an age-adjusted incidence of 0.02 per 100,000 persons in the United States (1). In Asian countries including Japan, the incidence is even lower; however, the actual incidence is unknown and there are only a few case reports. The characteristics of FLHCC include an early onset at around age 10-29 years of age, no sex difference (or a somewhat higher prevalence in women), normal background liver status, and a large tumor size (4,5,8,13-16). FLHCC is categorized as pure-type FLHCC or mixed-type FLHCC, which contains a conventional HCC component. However, mixed-type FLHCC has the characteristics of conventional HCC, such as low methylation of LINE-1 and abnormal methylation of RASSF1A (17), and is thought to be a different disease entity from true FLHCC. Some of the previously reported cases included mixed-type FLHCC (13,14), and caution is required when examining treatment policies and prognostic predictions for FLHCC. The imaging characteristics include heterogeneous hyperattenuation in the arterial phase; diverse contrast enhancement findings in the portal venous and equilibrium phases, which reflect the various degrees of fibrosis; and areas of hypoattenuation at the center of the lesion due to a stellate scar with a prominent fibrous tissue (18,19). In the present case, a stellate scar was also noted in the central area of the lesion, and prominent fibrous tissue was observed. However, there was a sparse part in the fibrous tissue that was edematous. Although the reason for these findings is unknown, we considered that the cause might have been associated with the center of the primary lesion, which was hyperintense on T2-weighted images, diffusion-weighted images, and ADC- mapping. In comparison to the primary lesion, the lesion in segment 4 of the liver and the metastatic lesions in the lungs, which were small in size, had little fibrous tissue. Unlike the primary lesion, we thought that this was the reason for the hypoattenuation (relative to the background liver parenchyma) observed in the portal venous and equilibrium phases on CE-CT. Most studies reported that the median diameter of FLHCC lesions is ≥10 cm (5,6,13,15,16), and the fibrous stroma may increase in size as the lesion grows. Hence, caution is required when diagnosing small lesions based on imaging inspections, as the contrast enhancement effect may vary in small lesions.
Surgical treatment is the first-line treatment for FLHCC. The median overall survival (OS) for FLHCC after surgical resection is 78 months for localized lesions and 46 months for regional lesions, and the prognosis is better than that of conventional HCC (49 and 23 months for localized and regional lesions, respectively) (7). However, differences in background factors, such as the age at onset and history of chronic liver disease, may be involved in these differences. One recent report suggested that the median OS and 5-year OS of patients with FLHCC and conventional HCC did not differ to a statistically significant extent when the patients were ≤40 years of age or had no underlying cirrhosis (2). In any case, the general condition and liver function are maintained in most patients, and we believe that aggressive surgical resection should be performed in cases for which R0 resection is possible. As described above, the prognosis of surgical resection is relatively good; however, the recurrence rate is reported to be 66.7-83.7% and the median recurrence-free survival (RFS) is 13.9-33 months (4,14,16). Thus, the possibility of recurrence must be considered during the follow-up of the patient. There are also reports suggesting that surgical resection was effective for recurrent lesions (14,20), and it is desirable to select the surgical procedure in view of the remnant liver volume. On the other hand, the prognosis of unresectable cases, such as those with extrahepatic metastasis, is similar to that of conventional HCC, and the median OS and 5-year OS are reported to be 12-20 months and 0-7.4%, respectively (2,14,15). One reason for the poor prognosis is that no effective treatments have been established thus far. Although there are reports on the effectiveness of chemotherapy with GEMOX or FOLFOX for unresectable cases (9-11), there is no established regimen. The only clinical trial on chemotherapy for FLHCC is a phase II trial that examined the efficacy of fluorouracil (200 mg/m2, days 1-21, every 28 days) + IFNα2b (4 million units/m2, 3 times a week, subcutaneously) in 2003. The results showed that the objective response rate (ORR) and median OS were 62.5% (5/8) and 23.1 months, respectively, in FLHCC (n=8), which were better than those in conventional HCC (n=28, ORR: 14.3%, median OS: 15.5 months). However, there was no significant difference due to the small number of cases (21). Based on previous reports, chemotherapy with FOLFOX was administered to the patient in the present case. Although it was difficult to assess the effects of chemotherapy because the treatment period was short and the tumor contained abundant fibrous stroma, the examination of the liver resection specimens could not confirm a sufficient treatment effect. Recently, the expression of epidermal growth factor receptor (EGFR) and the activation of the mammalian target of rapamycin (mTOR) pathway in FLHCC have been reported (20,22), and molecularly targeted drugs such as everolimus and erlotinib may be effective. The efficacy of local therapy, such as transarterial chemoembolization (TACE) and RFA, on extrahepatic metastasis cases has not been elucidated; however, there have been cases in which multidisciplinary therapy combining surgical resection, local therapy, and chemotherapy were performed with relatively positive outcomes (20). Thus, although further investigation is required, we believe that aggressive multidisciplinary therapy may be a viable option for distant metastatic or recurrent lesions. No obvious residual or recurrent lesions were noted in this patient. Nevertheless, careful follow-up of the patient will be conducted and surgical resection or local therapy will be considered, depending on the reserve capacity of the remaining organs if a recurrent lesion appears.
DNAJB1-PRKACA, a fusion gene that is characteristically found in FLHCC, was detected in both the primary lesion and the lung metastases of this patient. DNAJB1-PRKACA is formed by the fusion of DNAJB1, which codes for heat shock protein 40 (hsp 40), and PRKACA, which codes for the cAMP-dependent protein kinase catalytic subunit, as a result of the partial deletion of chromosome 19. It was first reported in 2014 by Honeyman et al. as a fusion gene specific to FLHCC (23). While almost all cases of FLHCC tested positive for DNAJB1-PRKACA in subsequent reports, the background liver and other types of primary liver cancer are negative (24-26). Negative results are also observed for scirrhous HCC, the histological findings of which are sometimes difficult to distinguish from those of FLHCC. Although the role of DNAJB1-PRKACA in the carcinogenic process of FLHCC is currently unclear, it may be used as a biomarker for diagnostic purposes or for measuring the treatment effect if it is confirmed that the proteins produced by DNAJB1-PRKACA are secreted into the blood. Additionally, if it is proven to be a key driver of carcinogenesis, it may become a therapeutic target for developing molecularly targeted drugs, and this warrants further investigation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 39: 798-803, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Eggert T, McGlynn KA, Duffy A, et al. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J 1: 351-357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Njei B, Konjeti VR, Ditah I. Prognosis of patients with fibrolamellar hepatocellular carcinoma versus conventional hepatocellular carcinoma: a systematic review and meta-analysis. Gastrointest Cancer Res 7: 49-54, 2014. [PMC free article] [PubMed] [Google Scholar]
- 4. Kaseb AO, Shama M, Sahin IH, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology 85: 197-203, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wahab MA, El Hanafy E, El Nakeeb A, et al. Clinicopathological features and surgical outcome of patients with fibrolamellar hepatocellular carcinoma (experience with 22 patients over a 15-year period). World J Gastrointest Surg 9: 61-67, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darcy DG, Malek MM, Kobos R, et al. Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J Pediatr Surg 50: 153-156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mayo SC, Mavros MN, Nathan H, et al. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg 218: 196-205, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol 18: 1417-1423, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Fonseca GM, Varella AD, Coelho FF, et al. Downstaging and resection after neoadjuvant therapy for fibrolamellar hepatocellular carcinoma. World J Gastrointest Surg 6: 107-111, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gras P, Truant S, Boige V, et al. Prolonged complete response after GEMOX chemotherapy in a patient with advanced fibrolamellar hepatocellular carcinoma. Case Rep Oncol 5: 169-172, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fakih M. A case of fibrolamellar cancer with a palliative response and minor radiographic regression with erlotinib and bevacizumab combination therapy. Am J Ther 21: e207-e210, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 127: 1335-1339, 1992. [DOI] [PubMed] [Google Scholar]
- 13. Chagas AL, Kikuchi L, Herman P, et al. Clinical and pathological evaluation of fibrolamellar hepatocellular carcinoma: a single center study of 21 cases. Clinics 70: 207-213, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 106: 1331-1338, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Mavros MN, Mayo SC, Hyder O, et al. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg 215: 820-830, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Herman P, Chagas AL, Perini MV, et al. Surgical treatment of fibrolamellar hepatocellular carcinoma: an underestimated malignant tumor? Hepatobiliary Pancreat Dis Int 13: 618-621, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Malouf GG, Brugieres L, Le Deley MC, et al. Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. Cancer 118: 4981-4990, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma 2: 151-157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ganeshan D, Szklaruk J, Kundra V, et al. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol 202: 544-552, 2014. [DOI] [PubMed] [Google Scholar]
- 20. Riehle KJ, Yeh MM, Yu JJ, et al. mTORC1 and FGFR1 signaling in fibrolamellar hepatocellular carcinoma. Mod Pathol 28: 103-110, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol 21: 421-427, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Buckley AF, Burgart LJ, Kakar S. Epidermal growth factor receptor expression and gene copy number in fibrolamellar hepatocellular carcinoma. Hum Pathol 37: 410-414, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 343: 1010-1014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham RP, Jin L, Knutson DL, et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol 28: 822-829, 2015. [DOI] [PubMed] [Google Scholar]
- 25. Cornella H, Alsinet C, Sayols S, et al. Unique genomic profile of fibrolamellar hepatocellular carcinoma. Gastroenterology 148: 806-818.e10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reid LM, Sethupathy P. The DNAJB1-PRKACA chimera: candidate biomarker and therapeutic target for fibrolamellar carcinomas. Hepatology 63: 662-664, 2016. [DOI] [PubMed] [Google Scholar]