Abstract
Linezolid is a useful drug for treating drug-resistant tuberculosis. However, the associated toxicities, especially optic neuritis, are a major obstacle for its long-term use. We recently experienced a case of severe optic and peripheral neuropathy during the treatment of multidrug-resistant tuberculosis. The treatment continued for 12 months despite severe optic and peripheral neuropathy. At eight months after the discontinuation of the drug, the optic neuropathy recovered, but the peripheral neuropathy did not. Considering the grave prognosis of drug-resistant tuberculosis, the continuation of linezolid despite neurotoxicity under close observation may be a suitable option.
Keywords: MDR-TB, linezolid, neuropathy
Introduction
Tuberculosis (TB) is fatal disease found worldwide. The prognosis is particularly poor in cases of multidrug-resistant (MDR)-TB, defined as cases resistant to isoniazid and rifampicin, wherein only half of patients who start treatment are cured.
Linezolid is a core second-line agent. However, various adverse effects have proven obstacles to its widespread use. Optic neuropathy should be treated as a medical emergency due to its seriousness (1). We recently experienced a case of serious optic neuropathy induced by linezolid in an MDR-TB patient that was resolved after the discontinuation of the drug.
Case Report
A 38-year-old woman with a history of being treated for pulmonary TB twice was diagnosed again with recurrent pulmonary TB. Her sputum acid fast bacilli (AFB) smear was positive. She started treatment with isoniazid (INH), rifampin (RFP), ethambutol (EMB), and pyrazinamide (PZA). Before taking this TB medication, her corrected visual acuity was 20/20 on the right and 20/30 on the left.
After four months, we obtained the results of a drug susceptibility test showing a Mycobacterium tuberculosis strain resistant to INH, RFP, RBT, EMB, RZA, levofloxacin (LFX), ofloxacin (OFX), periodic acid-Schiff (PAS), and prothionamide (PTH). At that time, the AFB smear and culture became negative, and chest radiography showed improvements in the TB lesion. After a discussion, the patient understood there were only three drugs available for her treatment and that their combination carried a risk of her developing acquired resistance to these remaining drugs as well. After continuing the same regimen for another month, two consecutive sputum AFB smears became positive again. The anti-TB medication was therefore changed to moxifloxacin (MFX), cycloserine (CS), and kanamycin (KM). Although linezolid has been approved for drug-resistant TB in Korea, its administration (600 mg daily) was delayed by 1 month because of the high cost.
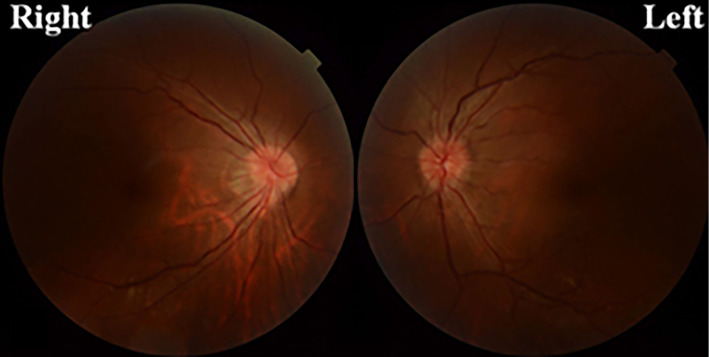
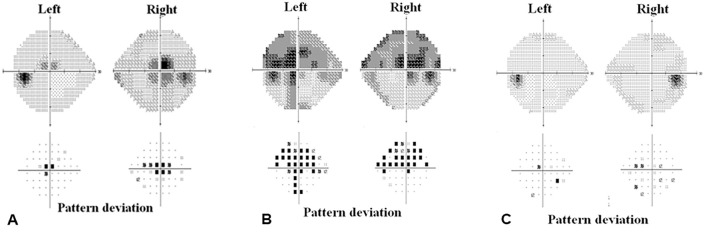
After two months of treatment with linezoid, the patient complained of parasthesia on the distal extremities, suggestive of peripheral neuropathy. She had no disease that might have caused peripheral neuropathy, such as diabetes, chronic kidney disease, alcoholics, or autoimmune diseases. She was also not taking any medications except for anti-TB drugs. We therefore concluded that this was toxic peripheral polyneuropathy, a common side effect of linezolid. The dose of linezolid was reduced to 300 mg daily. At 4 months after starting linezolid medication, she complained of decreased visual acuity. An ophthalmologic examination was highly suggestive of toxic optic neuropathy due to linezolid. The corrected visual acuity was 20/50 on the right and 20/30 on the left. There was no afferent pupillary defect, and her color vision was intact. Visual field testing showed central scotoma, and a fundus examination revealed mild disc swelling in both eyes (Fig. 1, Fig. 2A). At that time, her sputum AFB smears were intermittently positive, although the AFB culture had converted to negative. We recommended that she continue to take linezolid under close ophthalmologic observation.
Figure 1.
A fundus examination revealed mild optic disc swelling in both eyes.
Figure 2.
The serial examination of the visual field showed central scotoma (A) after taking linezolid for four months. It worsened after taking linezolid for 6 months (B) and recovered after discontinuing linezolid for 3 months (C), although treatment was maintained for 12 months despite optic and peripheral neuropathy.
After six months of treatment with linezolid, she complained of a worsening visual acuity and visual field. She could no longer read the facial expressions of other people, even at a distance of one meter. She said, “I am living in a world of monochrome film.” Her corrected visual acuity was 20/300 on the right and 20/200 on the left, and the bilateral central scotoma had become more prominent on visual field testing (Fig. 2B). After a long discussion, she insisted on continuing to take linezolid, as her AFB smear and culture had been negative for two months at that time. After six months of severe optic neuropathy, we decided to stop linezolid and added bedaquiline, a newly available drug. KM was discontinued after 7 months of linezolid, and all medication were stopped after 20 months of negative conversion. The total duration of treatment was 30 months, and that of linezolid was 12 months.
Three months after stopping linezolid, her corrected visual acuity improved to 20/50 on the right and 20/25 on the left. Her visual field test recovered to almost normal (Fig. 2C). Sixteen months after stopping linezoid, her color vision recovered to nearly normal, but the paraesthesia of the feet did not fully recover. Her AFB smear and culture remained negative for eight months after the discontinuation of all drugs. During and after taking anti-TB drugs, including linezolid, there were no renal or hepatic abnormalities nor any hematologic side effects, such as thrombocytopenia.
Discussion
Linezolid is a useful drug for treating MDR- and extensive drug-resistant (XDR)-TB. However, its associated toxicities are a major problem, especially those associated with the optic nerve. Whether or not the neurotoxicity is reversible, especially in the optic nerve, how severe the toxicity can be before it is irreversible, and how long treatment can be continued before the toxicity becomes irreversible are all questions in need of answers. We encountered a patient who developed peripheral and optic neuropathy due to linezolid but still continued treatment for another eight months. At 16 months after the discontinuation of the drug, her optic neuropathy was completely recovered, although her peripheral neuropathy was not.
Linezolid, introduced in 2000, is a synthetic oxazolidinone broad-spectrum antibiotic with efficacy against Gram-positive organisms and mycobacteria. Linezolid has also been used off-label for MDR-TB (2). Clinical trials of linezolid have shown culture conversion rates of 73-87% in drug-resistant pulmonary TB (3). The major adverse events of linezolid are reported to be myelosuppression and neuropathy. A recent systematic review and a meta-analysis by Sotgiu et al. found that 13% of linezolid-treated patients developed optic neuropathy, which has also been reported in different case series as well as isolated case reports (4).
The clinical manifestations of linezolid-induced toxic optic neuropathy are very similar to those of ethambutol and include variable progressive vision loss, decreased color vision, and, most commonly, cecocentral visual field defects (5). In cases of ethambutol-induced optic neuropathy, the immediate termination of therapy is the only way to stop the progression and allow for the recovery of vision (6).
The mechanism underlying linezolid-induced optic neuropathy is unclear at present, but parallels may be drawn with other agents toxic to the peripheral and optic nerve. Linezolid inhibits bacterial protein synthesis by binding to the 70S ribosomal initiation complex. Although this complex is not present in mammalian cells, it has been shown to reduce the mitochondrial respiratory chain enzyme activity in experimental animal models and in a patient receiving linezolid therapy who developed optic neuropathy (7). In addition, linezolid has high central nervous system and intraocular penetration, which may potentiate its toxic neuropathic effects (8). The findings of optic toxicity include varying reductions in vision, normal pupils and extraocular muscles, and unremarkable fundoscopy findings, with the possibility of swollen optic discs in the acute stage of optic neuropathy. Other important findings of toxic optic neuropathy include decreased color vision and cecocentral visual field defects (9).
Linezolid also causes axonal peripheral neuropathy that, like optic neuropathy, occurs after prolonged exposure. However, in contrast to optic neuropathy, the peripheral neuropathy has not been reported to improve, even after discontinuation of the drug (10).
Conclusion
Linezolid-induced toxic optic neuropathy is a medical emergency. However, considering the serious prognosis of MDR-/XDR-TB, continuing linezolid despite toxic optic neuropathy should be considered under close observation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis. 2016 Update. 22-32 2016 . [PubMed]
- 2. Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet 42: 1129-1140, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Chang KC, Leung CC, Daley CL. Linezolid for multidrug-resistant tuberculosis - authors' reply. Lancet Infect Dis 13: 16-17, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Sotgiu G, Centis R, D'Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 40: 1430-1442, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Karuppannasamy D, Raghuram A, Sundar D. Linezolid-induced optic neuropathy. Indian J Ophthalmol 62: 497-500, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grzybowski A, Zulsdorff M, Wilhelm H, Tonagel F. Toxic optic neuropathies: an updated review. Acta Ophthalmol 93: 402-410, 2015. [DOI] [PubMed] [Google Scholar]
- 7. De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 42: 1111-1117, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Rucker JC, Hamilton SR, Bardenstein D, Isada CM, Lee MS. Linezolid-associated toxic optic neuropathy. Neurology 66: 595-598, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Libershteyn Y. Ethambutol/Linezolid Toxic Optic Neuropathy. Optom Vis Sci 93: 211-217, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Rho JP, Sia IG, Crum BA, Dekutoski MB, Trousdale RT. Linezolid-associated peripheral neuropathy. Mayo Clin Proc 79: 927-930, 2004. [DOI] [PubMed] [Google Scholar]