Abstract
The importance of effects related to the repair of sublethal radiation damage as treatment duration varies, partly a function of dose-rate, is a current controversy in clinical radiosurgery. Cell survival studies have been performed to verify the importance of this effect in relation to established models.
Mammalian V79-4 cells were irradiated in vitro with γ-rays, either as an acute exposure in a few minutes, where the effects of sublethal irradiation damage repair over the period of exposure can be ignored, or as protracted exposures delivered over 15–120 min. Protraction was achieved either by introducing a variable time gap between two doses of 7 Gy, or as a continuous exposure at lower dose rates so that a range of doses were delivered in fixed times of 30, 60 or 120 min.
For all doses there was a progressive reduction in efficacy with increasing overall treatment time. This was illustrated by the progressive increase in clonogenic cell survival with a resulting right shift of the survival curves. Cell survival curves for irradiations given either as an acute exposure (6.1 Gy/min), over fixed times (30, 60 and 120 min) or for a fixed low dose-rate (0.2 Gy/min) were well fitted by the Linear Quadratic (LQ) model giving an α/β ratio of 4.0 Gy and a single repair half-time of 31.5 min.
The present results are consistent with published data with respect to the response of solid tumors and normal tissues, whose response to both continuous and fractionated irradiation is also well described by the LQ model. This suggests the need for dose compensation in radiosurgical treatments, and other forms of radiotherapy, where dose is delivered over a similar range of protracted overall treatment times, perhaps as a prerequisite to full biological effective dose treatment planning.
Keywords: Biological effective dose, overall treatment time, dose-rate, sublethal repair, radiosurgery
Introduction
The variation in the duration of radio-surgical treatments, using the various platforms, is considerable and there are multiple factors that lead to this variability. Historically, using the Gamma Knife, emphasis has been placed on the decay of cobalt-60, with a half-life of 5.26 years. Thus for similar treatment plans, the beam-on time will become progressively longer with time such that, by one half-life, the beam-on time would double. However, even for sources of fixed activity the introduction of progressive sector blocking would have a similar effect. In addition to the beam-on time there may be scheduled or unscheduled time gaps in treatment. For the Model B and C Gamma Knife, the scheduled time gaps between different isocenters can be long to allow for collimator changes and patient positioning. The introduction of the Perfexion® and Icon® machines have significantly reduced the variability in set up times. However, the ease and timing of collimator and positioning changes with the latest technology have frequently lead to an increase in the complexity of treatments with the use of more iso-centers than would have been practical using the older devices. This, to an extent, mitigates the effect of longer scheduled gaps, a feature of all historical Gamma Knife treatments, except for those that just involved a single isocenter. In this simple case, the source activity, the degree of beam/sector blocking and the individual patient geometry are the only factors that influence the duration of the total overall treatment time, barring the introduction of any unscheduled pauses in treatment that are applicable using all Gamma Knife models.
For Cyberknife treatments, the total overall treatment time is related to the number of beams and nodes, the collimation system used and time taken to monitor/re-establish patient position. For the linac, treatment duration also depends on the treatment delivery technique employed, including the number of beams or arcs, the collimation system used, the time taken to monitor/re-establish patient position and the dose rate used. For all technologies, significant unscheduled interruptions to patient treatment can still occur, which may be patient related or due to technical faults, resulting in an increase in the overall treatment time.
Retrospective analysis of largely clinical Gamma Knife data has frequently resulted in confounding results. In an analysis of cases where morbidity was related to the exposure of cranial nerves [1], morbidity was reported to be higher in those that were treated in shorter times with new sources compared with those treated in a longer time with older sources, an effect that was said to be related to the significantly reduced dose-rate. In contrast, a multi-variate analysis of the complications associated with the treatment of arterio-venous malformations (AVM’s) found no such correlation within the range of overall treatment times examined [2], although there were many more treatment variables in patients treated for the closure of AVM’s. There is also a suggestion, in the same publication, that a smaller number of iso-centers may increase the efficacy of a given prescription dose [2].
Many of these clinical studies look on the dose-rate as being a fixed parameter which is reduced daily to take account of the decay of the cobalt-60 sources. This parameter is measured in the center of a 160 cm diameter spherical phantom. This is defined as the calibration dose-rate (standard output) of the machine. For dose calculations in a patient, this calibration dose-rate, in a given treatment, does not directly relate to the dose-rate in the tissues at a specific position in the treatment volume due to the different isocenters/ collimators/ degree of sector blocking used. In fact in any treatment, for each individual voxel on the prescription dose iso-surface, the dose-rate will vary for each of the different isocenters and the dose-rate will be zero between iso-centers (beam-off time). The summation of the individual doses from each isocenter represents the total dose to that voxel. Each voxel is unique with respect to the dose/dose-rate prescription received [3]. Thus, the concept of a uniform dose-rate in Gamma Knife radiosurgery is not valid. The only constant in any treatment is the overall treatment time and this is applicable to all voxels in the treatment volume, irrespective of cumulative dose to any specific voxel. This will be equally true for the other radiosurgical platforms.
Despite these known variations in overall treatment time in radiosurgery and evidence from a number of early radiobiological studies [4, 5], which associated the effects of protracted periods of exposure as a result of variable dose-rates and/or gaps in the dose delivery with the repair of sublethal radiation damage, no systematic method has yet been adopted to correct the total physical dose delivered to take the phenomenon of repair into account in radio-surgical treatments. Indeed, one recent study was undertaken to support a view that no such changes are required [6]. However, this study had serious limitations as discussed previously [7].
In the present study, mammalian V79-4 cells, a cell line frequently used for mechanistic studies because of their high platting efficiency and the formation of discrete colonies, have been irradiated with a range of doses using cobalt-60 sources, delivered as continuous exposures over fixed overall treatment times of 30, 60 or 120 min while maintaining the cells at 37oC. In addition, cells were also irradiated at a high dose-rate (6.1 Gy/min) and therefore short exposure times, in order to mimic the situation where no repair of sublethal damage is likely to occur over the period of exposure. Studies were also carried out using 137Cs γ-rays (dose-rate 2.04 Gy/min). The effects of two high dose-rate sub-fractions of 7 Gy were used to examine the effects of variable duration of time gaps, compared with a single continuous high dose-rate exposure of 14 Gy. These irradiations, inclusive of the time gaps, were carried out with cells incubated at either 37oC, on ice, or at room temperature (20oC).
Materials and Methods
Cells culture and clonogenic survival
V79-4 mammalian cells were used in the study. They were routinely cultured at 37oC, gassed with a 5% CO2/95% air mixture in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 1% l-glutamine (Sigma), 1% penicillin/streptomycin (Sigma) and 10% fetal bovine serum (Gibco). Approximately 3 h prior to irradiation, cells were harvested with trypsin/EDTA (HyClone) and seeded into T25 flasks at a density calculated to yield ~200 surviving colonies following irradiation. For protracted radiation exposures, the caps of the T25 flasks were sealed on removal from the incubator (to maintain the optimum pH of the medium) and only opened following irradiation when returned to the incubator. After 8 days incubation, the cells were fixed, using 80% methanol in water (>10 min), stained with 1% methylene blue in water (>30 min) and the surviving cell colonies (>50 cells) counted using an automated colony counter (ColCount; Oxford Optronix, Abingdon UK). The fraction of surviving cells was calculated with respect to the cloning efficiency (plating efficiency) of sham-irradiated cells.
Protracted continuous radiation exposures.
Continuous protracted exposures were carried out using the cobalt-60 γ-ray sources at the Medical Research Council, Harwell, Oxfordshire, UK. During irradiation, cells were kept at 37oC using ‘in house’ made incubators. These consisted of a resistive electrical heating mat coupled to an Arduino-powered PID controller housed within a Styrofoam box. The T25 flasks along with the temperature probe monitor were placed on a 6 mm Perspex build-up sheet positioned 1 mm above the heated mat. Six of these incubators, each containing three T25 flasks, were irradiated simultaneously on shelves at six different distances from the sources within the irradiation room to vary the dose received by each set of flasks for a given fixed exposure time. An additional incubator was positioned outside the irradiation room to provide cells for sham-irradiated control data.
At these set distances the dose-rates, and hence exposure times, could be further varied by changing the number of cobalt-60 sources used. Cells were irradiated to obtain survival curves for protracted exposures of 30 min (four sources), 60 min (two sources) and 120 min (one source). In addition, acute radiation exposures, delivered in an overall treatment time of <3 min, a time scale over which repair of sublethal damage would be negligible, were also carried out at a fixed dose-rate of 6.1 Gy/min with the T25 flasks at a distance of ~17 cm above all four cobalt-60 sources. This approach contrasts to standard clonogenic survival curves, where cells are exposed at different fixed dose-rates and thus increasing exposure times as the dose is escalated.
Experiments with variable time gaps in the radiation exposure
Irradiations were carried out using a cesium-137 γ-ray irradiator (GSR D1, Gamma-Service Medical GmbH, Leipzig, Germany) at the University of Oxford. Cells were irradiated at an average dose-rate of 2.04 Gy/min to four T25 flasks at a time, positioned on a 6 mm thick Perspex build-up shelf. Flasks were given a total dose of 14 Gy either as a single continuous exposure, or as two exposures of 7 Gy separated by a variable time gap, ranging from 15 min to 120 min. At least 15 min prior to irradiation, the lids of the flasks were sealed and incubated at the required temperature, either 37oC, room temperature (20oC) or placed on ice, to ensure that cells reached the required temperature and that they were maintained at these temperatures between the two doses. Following the final exposure, all flasks were unsealed and returned to the 37oC incubator.
Irradiations involving protracted continuous exposure or two relatively short exposures with a variable gap were carried out, for convenience, with cobalt-60 and cesium-137 γ-ray sources, respectively. A comparison of the survival curve obtained using cobalt-60 and cesium-137 γ-rays showed no statistical significant differences (Appendix A). Thus the results for continuous and doses involving a variable time gap can be compared.
Dosimetry
Prior to performing the cell survival experiments, dose-rate measurements were performed at each of the shelf positions using Gafchromic™ EBT3 film (International Specialty Products, Wayne, NJ). Measurements were made for 5 different exposure times (covering a range of doses up to ~3.5 Gy) with films positioned between the build-up sheet and the flasks containing the same volume of medium used for the experiments. The films were scanned as 48-bit RGB TIFF images at 300 dpi resolution using an Epson® Expression 10000 XL flatbed scanner (Plainfield, IN) 24 h after irradiation. The dose to each film was then calculated using the optical density of the red channel and corrected using the optical density of the blue channel in conjunction with a calibration curve. A calibration curve was obtained over a range of doses (0–5 Gy) using 6 MV x-rays from a calibrated [8] clinical linear accelerator. The gradient of the linear fit to the dose vs. exposure time response fit corresponds to the dose-rate at each of the different irradiation positions.
Results
Effects of overall treatment time on clonogenic cell survival following continuous irradiation
The changes in clonogenic cell survival after irradiation given over fixed periods of time, namely 30, 60 and 120 min (and therefore variable dose-rate) are illustrated in Figure 1 along with the data for acute radiation exposure (6.1 Gy/min). In addition, these data were used to derive a cell survival curve for continuous exposures using a low dose-rate of 0.2 Gy/min.
Figure 1.
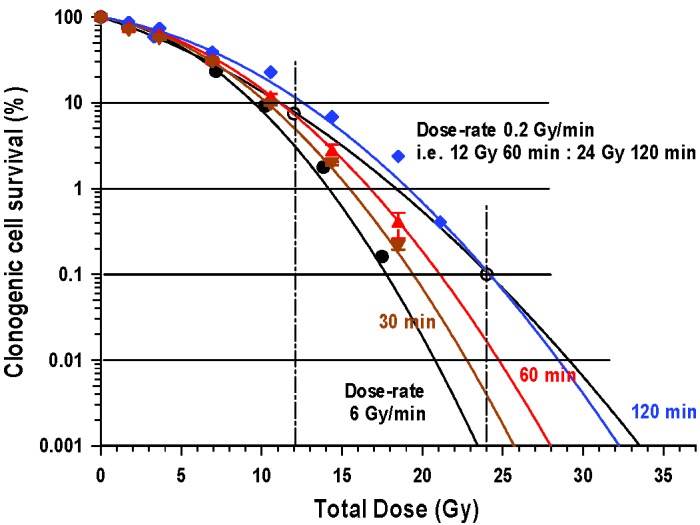
Variations in the percentage clonogenic survival of V79-4 cells after irradiation with various doses of cobalt-60 γ-rays. Doses were delivered over fixed times of 30 (▼), 60 (▲) or 120 (♦) min. By way of comparison, cells were also irradiated either acutely (6 Gy/min), where exposure times were short (<3 min) and where little or no repair took place over the period of exposure, or at 0.2 Gy/min where exposure times were long. The slope of a cell survival curve given at low dose-rate tended to be shallower than those where various doses are delivered in a fixed time period. All irradiations were carried out with the cells at 37oC. Error bars indicate ± SEM.
The five resulting cell survival curves have been collectively fitted using the linear-quadratic model with a single repair component added to take account of the repair of sublethal damage as exposure times are increased, namely:-
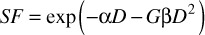
where G is the generalized Lea-Catcheside time factor [9], which accounts for repair of lesions during protracted exposures, such that
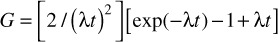
where λ is defined as:
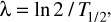
T1/2 being the half-time repair (in minutes). The log transformation of the surviving fraction, SF, was fitted as a function of dose using the indictor model [10] and performed using the IBM SPSS® statistical analysis software package (IBM United Kingdom Limited). The experimental data were well described by the combined curve fitting approach producing a common α/β ratio of 4.0 Gy (α = 0.072 ± 0.011; β = 0.018 ± 0.018) and a single repair half time of 31.5 min (λ = 0.022 ± 0.002).
A progressive shift of the curves to the right, indicative of reduced cell killing as the irradiation time is increased from 30 min out to 120 min, relative to an acute exposure (<3 min exposures given at 6.1 Gy/min), are illustrated in Figure 1. Even for a 30 min protraction of the exposure time, there was significant repair when compared with acute exposures. These clonogenic survival curves reflect the clinical situation where the exposure time is fixed, but different cells are exposed at different dose-rates depending on where they are positioned with respect to the prescription isodose surface. This is in contrast to standard clonogenic survival curves where cells are exposed at a fixed dose-rate but for different exposure periods to give a prescribed dose. The relationship between cell survival and a fixed low dose-rate (0.2 Gy/min) is less steep than for exposures given over a fixed time of 120 min.
Based on data from these curves it is possible to illustrate the reduction in biological effectiveness, in terms of clonogenic cell survival, for different radiation doses as the overall treatment time is protracted, as is illustrated for doses in the range 12–20 Gy, in Figure 2. For all radiation exposures at 37oC as the exposure time is increased the efficacy is reduced and gradual dose escalation is required to maintain the same level of biological effect. By way of an example, 12 Gy delivered in 30 min is associated with a surviving fraction of 5%. Equivalent cell kill is achieved with 13 Gy delivered in 60 min and a little over 14 Gy in 120 min.
Figure 2.
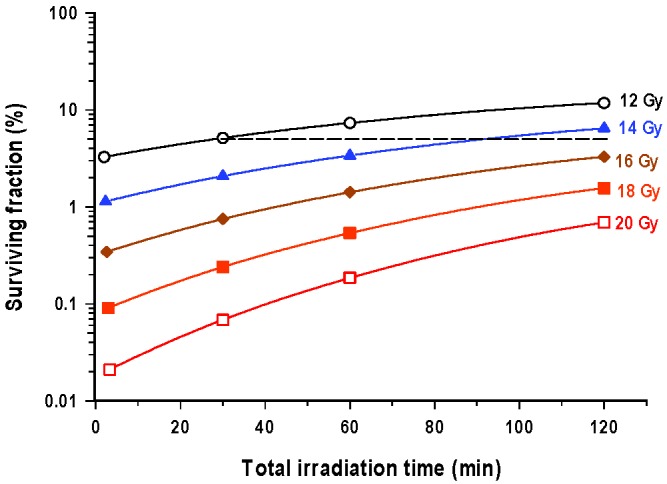
Variation in the percentage clonogenic survival of V79-4 cells with changes in the duration of radiation exposure, for doses in the range 12–20 Gy of cobalt-60 γ-rays. For all doses there is a reduction in efficacy of a given dose as the period of exposure is progressively increased. Thus, for a given effect the dose needs to be progressively escalated in order to maintain a given level of effect. For example, for an exposure of 12 Gy in 30 min the dose would need to be increased to 13 Gy for a 60 min exposure period and to a little over 14 Gy if the exposure period was increased to 120 min. All irradiations were carried out with the cells at 37oC.
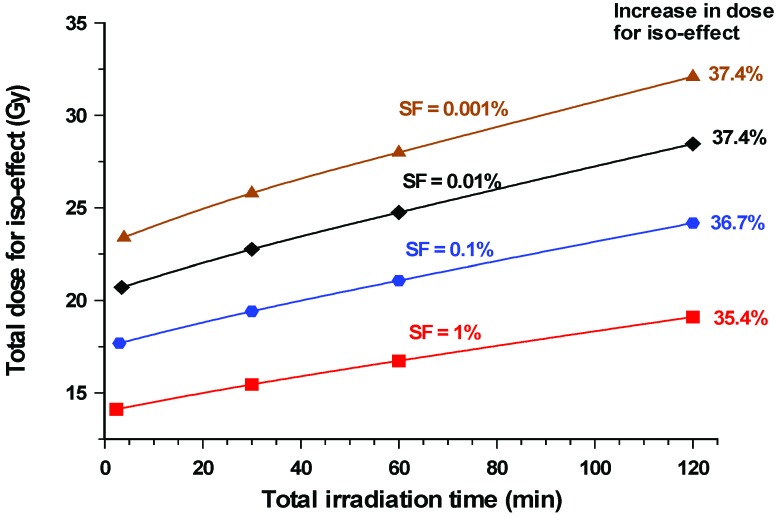
In an alternative approach, curves showing the relationship between dose and exposure time for a given level of iso-effect, in this case the surviving fraction, gives an indication as to how cell killing reduces (due to an increase in clonogenic cell survival) with exposure time. This is illustrated in Figure 3. When exposure times are increased from 30 to 120 min, the total dose would need to be increased by, on average, 22.5% depending on the level of cell survival under consideration. This increased to ~37% when compared to an acute exposure
Figure 3.
Variation in radiation dose from cobalt-60 γ-rays required to produce a given iso-effect in V79-4 cells. The iso-effect assessed was a given level of clonogenic cell survival. This was in the range 1.0–0.001%. The iso-effective dose increased progressively as the irradiation time was gradually increased and represented an approximate 36% increase in dose when acute exposures, where little or no repair will occur over the period of exposure, are compared to 120 min exposures. All irradiations were carried out with the cells at 37oC.
In the studies involving variable time gaps the total exposure times (beam-on time) were relatively short: 3.4 min for the initial 7 Gy. However, with time gaps of 15, 30 and 45 min, the level of cell killing, as compared with a single acute exposure (6.9 min) of 14 Gy, was progressively reduced (clonogenic cell survival increases) when cells were maintained at 37oC for the duration of the study. Indeed, at 30 min the effect was comparable with that seen after continuous protracted exposure over this time period. It should be noted that the actual dose used for protracted exposure was found, retrospectively, to be slightly higher at 14.37 Gy. However, the surviving fraction after 14 Gy can be determined from the actual cell survival curves. This similarity between exposures involving time gaps and those delivered continuously was even seen at 60 min (time gap vs continuous exposure). However, for time gaps of 90 and 120 min, the reduction in efficacy, judged by the level of clonogenic cell survival, slowed considerably compared with that seen after comparable continuous exposures involving no time gaps (Figure 3). The effects found to be associated with increasing time gap size seen for irradiation when cells were maintained at 37oC were lost when the cells were maintained on ice or at room temperature for the required period.
Discussion
The most striking feature of the present study was that repair of sublethal irradiation damage, equated with the repair of sublethal DNA damage, but as assessed by the reduction in cell killing or an increase in clonogenic cell survival, was associated with a protraction of the irradiation time. This was irrespective of whether the cells received a continuous exposure or if the dose was given as a two equal higher doses separated by a variable time gap in the total overall treatment time. This was provided the cells were irradiated at 37oC, or normal body temperature, over the total period of exposure, including any time gaps. When comparable irradiations were delivered either with the cells on ice or at room temperature, no such repair was seen. This effect has been reported in a number of cell lines [11]. The results thus further question and warn against reliance on results obtained where the temperature was not controlled, for example the study of Niranjan et al [6] where irradiations started with cells that had been stored on ice, and with the phantom used for irradiation being at an unrecorded room temperature. Rat 9L-gliosarcoma cells in this study [6] are known to be capable of the repair of sublethal damage at 37oC. It was the loss of this effect when cells are irradiated at 41oC that accounted for the radiation enhancing effect of mild hyperthermia [12]. In addition, in this questionable study [6], fixed calibration doses rates of 0.77–2.937 Gy/min were used, with cells placed in the center of the standard phantom. Cells were irradiated to total doses of 4, 8 and 16 Gy as continuous exposures. Thus, the exposure times, as pointed out previously [7], were in the range 5–20 min for the lowest dose-rate and shorter for the two higher dose-rates. This is not the time range associated with the majority of radiosurgery treatments and even it irradiations had been carried out at 37oC the finding would not have been directly clinically relevant as the majority of overall treatment times would have been short relative to the duration of the fast component of repair.
Figure 4.
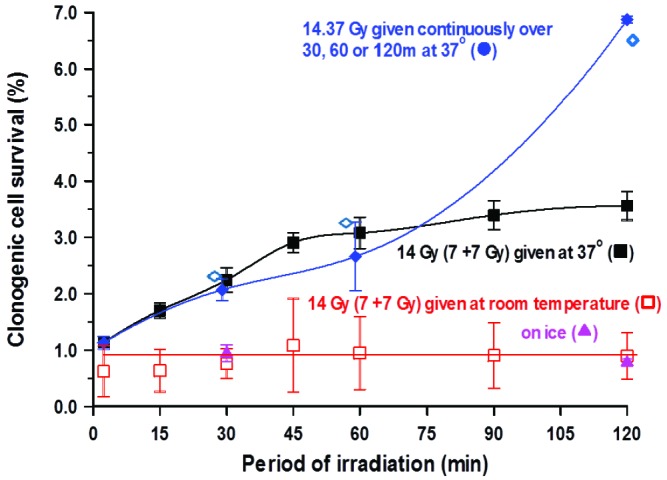
Variation in the percentage clonogenic cell survival of V79-4 cells with two doses of 7 Gy of cesium-137 γ-rays, each dose being delivered in approximately 3 min with variable time gaps of 15–120 min between the two doses. The effects of irradiation at 37oC (■) with those where, over the period of irradiation, cells were either held at room temperature (□) or on ice (▲). The percentage clonogenic survival of cells at room temperature and on ice does not change significantly with changes in the time gap between the two doses of 7 Gy. For irradiation at 37oC, the loss of efficacy was approximately 2% with a 60 min time gap after the initial dose of 7 Gy, however, in the second hour the additional loss of efficacy was only 0.5%. These results have been compared with those irradiated continuously over 30, 60 and 120 min (♦). In these studies, the actual data point was for a dose of 14.37 Gy, although a value for 14 Gy (◊) was also obtained by extrapolation from the relative cells survival curve. The two data sets were very comparable for exposures up to 60 min. but for exposures spread over 120 min greater recovery was seen after continuous exposure. Error bars indicate ±SEM.
The observation that there was a significant reduction in cell killing, as indicated by increased clonogenic cell survival, when continuation radiation exposure over 30 min, or as two separate equal exposure separated by 15 or 30 min, were compared with those delivered acutely (<3 min exposure times) suggested that the initially repair of sublethal radiation damage is rapid. However, the study using two doses with a separation of 60–120 min suggest that over longer times, the net rate of repair, as indicated by increased clonogenic cell survival, is less. Repair between doses can only apply to the first half of the total dose delivered. When delivered as a continuous exposure over 120 min as opposed to 60 min, a greater amount of repair occurs due to reduced miss-repair of sublethal damage due to the increased temporal separation of these lesions. When suitable data are available, repair of sublethal damage is usually assessed using a bi-exponential model to give a slow and fast component of repair [13, 14]. The fast half-time for repair in these studies was of the order of 10 min. The slow component of repair based on these studies was slightly longer than the maximum 2 h irradiation times associated with the present studies. Considering this and the limited number of data points, it would be difficult to accurately determine the half-times for both fast and slow-repair using a two component repair model. The use of a 1-component repair model for the present data set resulted in a repair half time of ~30 min, clearly the result of the impact of the slow component of repair on the fast repair parameter.
A single half time for repair was also found to be adequate to explain the dose sparing associated with the protraction of a 2 Gy dose fraction in an IRMT schedule lasting for 30 or 60 min compared with the short exposure times associated with conventional radiotherapy [15]. The in vivo model used the evaluation of regrowth delay in a transplanted Balb/C breast adenocarcinoma. The degree of regrowth delay in this model is a function of the level of clonogenic cell survival: the lower the level of cell survival, the greater the regrowth delay. Protraction of an acute dose exposure to 30 or 60 min significantly reduced the regrowth delay because of increased repair (increased cell survival) over these more protracted exposures. Use of the LQ model with the single repair component suggested total dose increases of 16 and 24%, respectively, to compensate. The validity of these predictions was demonstrated experimentally. Reductions in tumor regrowth delay are also found after the same treatment protraction after large individual doses of 18 Gy [16]. This loss of efficacy even for IMRT type of exposures has also been reported for in vitro studies [17].
The underlying mechanism responsible for these effects observed both in vitro and in vivo is the repair of radiation damage to DNA. In studies of the repair of DNA strand breaks, models of the kinetics have reported up to 3 repair parameters [18] with repair half times of ~2.5 h, 15 min and a very short one with a half time of 2.28 min. It would not be practical to resolve half times of a few minutes either in vitro or in vivo. Thus, fast half times of the order of 10 min may represent an averaging process. Repair rate are independent of the total physical dose (i.e., they follow first order kinetics).
These findings are equally applicable to other radiosurgical technologies. Treatment duration can vary significantly with the complexity of CyberKnife treatments (number of robotic arm positions or nodes used, number of beams and monitor units), and the collimator system used, i.e. fixed, iris or micro multi-leaf collimator (mMLC) [20]. In a recent survey of intracranial treatments delivered in England using linac based technology, different centers used static conformal fields, static conformal arcs or volumetric arc therapy [21]. Overall treatment times varied widely with each of these techniques.
Model predictions could also be used to allow for changes in the prescribed radiosurgery dose with either the activity of the sources used in the case of Gamma Knife or the complexity of any form of radiosurgical treatment. In this respect, the concept of Biologically Effective Dose (BED) is directly related to the LQ model and thence a given BED value is in effect iso-effective for a given level of clonogenic cell survival. This principle has been discussed elsewhere in relation to its application to radiosurgery [7, 19] and recently a simplified approach to the modification of dose to take account of changes in overall treatment time was proposed [22]. The underlying basis of which is directly related to the effects at a cellular level reported in the present publication.
Conclusion
The results of the present study indicate that when V79-4 mammalian cells are irradiated with a range of doses, either as an acute exposure (<3 min) or as a protracted exposure over 15 to 120 min, efficacy is progressively reduced as the overall treatment time is increased. This applies irrespective of whether irradiation was delivered as a continuous exposure, or as two doses with a variable time gap. This loss of efficacy was illustrated by the progressive increase in the surviving clonogenic cell fraction of over this period. Similar effects have also been reported in solid tumors, the results of which are assessed by regrowth delay, and in irradiated normal tissues. Thus, repair of sublethal damage is a significant factor in radiosurgical treatments and should be accounted for with varying overall treatment times.
Acknowledgements
The majority of the studies reported in this paper were carried out by one of us (SH) as part of the requirements for the degree of Master of Science in Radiation Biology, University of Oxford, for which individual support was provided by the USNI Fitzgerald Fund. The authors also gratefully acknowledge funding from the Medical Research Council Strategic Partnership (MC-PC-12004) for the CRUK/MRC Institute for Radiation Oncology. Finally, the authors would like to thank MRC Harwell for the use of their irradiation facility.
Appendix A
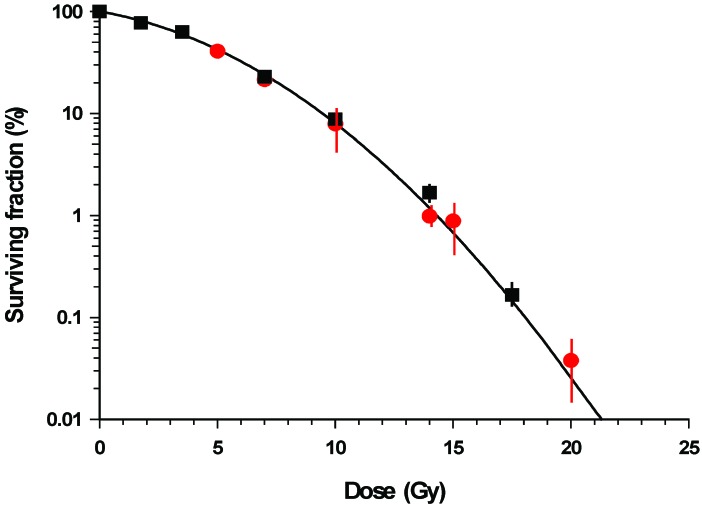
For convenience in the present series of experiments, that involved protracted continuous exposure or two relatively short exposures with a variable gap, γ-ray from cobalt-60 and cesium-137 sources were used, respectively. A comparison of the survival curve obtained using cobalt-60 and cesium-137 γ-rays showed no statistical significant differences (Figure A1) and thus results from the two studies can be compared.
Figure A1.
Variations in the percentage clonogenic survival of V79-4 cells following acute radiation exposure with either cobalt-60 (■) or (λ) cesium-137 γ-rays. Doses were delivered at 6 Gy/min or 2.04 Gy/min for cobalt-60 and cesium-137 γ-rays, respectively. Error bars indicate ±SEM
Footnotes
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception, design: John W Hopewell, Ian Paddick, Mark A Hill
Data collection: Steven Hallgren, James M Thompson, Amy Elliott
Data analysis, interpretation: Steven Hallgren, John W Hopewell, Mark A Hill, Bleddyn Jones, Ian Paddick
Manuscript writing: Steven Hallgren, Mark Hill, John W Hopewell, Ian Paddick
Final approval of manuscript: Steven Hallgren, Mark A Hill, James M Thompson, Amy Elliott, Ian Paddick, Bleddyn Jones, John W Hopewell
References
- 1. Kondziolka D, Lunsford LD, Maitz A, Flickinger JC. Radiobiologic considerations in gamma knife radiosurgery. Prog Neurol Surg 1998;14:21-38. [Google Scholar]
- 2. Flickinger JC, Kondziolka D, Pollock BE, Maitz AH, Lunsford LD. Complications from arteriovenous malformation radiosurgery: Multivariate analysis and risk modelling. Int J Radiat Oncol Biol Phys 1997;38:485-490. [DOI] [PubMed] [Google Scholar]
- 3. Hopewell JW, Millar WT, Lindquist C, Nordström H, Lidberg P, Gårding J. Application of the concept of biologically effective dose (BED) to patients with Vestibular Schwannomas treated by radiosurgery. J Radiosurg SBRT 2013;2:259-271. [PMC free article] [PubMed] [Google Scholar]
- 4. Bedford JS, Mitchell JB. Dose-rate effects in synchronous mammalian cells in culture. Radiat Res 1973;54:316-327 [PubMed] [Google Scholar]
- 5. KK Fu, Philips TL, Kane LK, Smith V. Tumor and normal tissue response to irradiation in vivo: Variation with decreasing dose rates. Radiology 1975;114:709-716. [DOI] [PubMed] [Google Scholar]
- 6. Niranjan A, Gobbel G, Novotny J, Jr., Bhatnagar J, Fellows W, Lunsford LD. Impact of decaying dose rate in gamma knife radiosurgery: In vitro study on 9L rat gliosarcoma cells. J Radiosurg SBRT 2012;1:257-264. [PMC free article] [PubMed] [Google Scholar]
- 7. Hopewell JW, Millar WT, Paddick Lindquist C. Impact of decaying dose-rate in Gamma Knife radiosurgery – Letter to the Editor. J Radiosurgery and SBRT, 2013;2:251-253. [PMC free article] [PubMed] [Google Scholar]
- 8. Lillicrap SC, Owen B, Williams JR, Williams PC. Code of practice for high-energy photon therapy dosimetry based on the NPL absorbed dose calibration service. Phys Med Biol 1990;35:1355–1360. [Google Scholar]
- 9. Lea DE, Catcheside DG. The mechanism of the induction by radiation of chromosome aberrations in Tradescantia. J Genet 1942, 44:216-245. [Google Scholar]
- 10. Chalmers AJ, Bentzen SM, Buffa FM. A general framework for quantifying the effects DNA of repair inhibitors on radiation sensitivity as a function of dose. Theoretical Biol Med Modelling 2007;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steel GG, Down JD, Peacock JH, Stephens TC. Dose-rate effects and the repair of radiation damage. Review article. Radioth Oncol 1986;5:321-331. [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Armour EP, Corry PM, Martinez A. Elimination of dose-rate effects by mild hyperthermia. Int J Radiat Oncol Biol Phys 1992;24:965-73. [DOI] [PubMed] [Google Scholar]
- 13. Pop LA, Millar WT, van der Plas M, van der Kogel AJ. Radiation tolerance of rat spinal cord to pulsed dose rate (PDR-) brachytherapy: The impact of differences in temporal dose distribution. Radiother Oncol 2000;55:301-315. [DOI] [PubMed] [Google Scholar]
- 14. Millar WT, Hopewell JW. Effects of very low dose-rate 90Sr/90Y exposure on the acute moist desquamation response of pigskin: Comparison based on predictions from dose fractionation studies at high dose-rate with incomplete repair. Radioth Oncol. 2007;83:187-195. [DOI] [PubMed] [Google Scholar]
- 15. Nikzada S, Hashemia B, Hasan ZS, Mozdarani H, Baradar-Ghahfsarakhi M, Amini P. The application of the linear quadratic model to compensate the effects of prolonged fraction delivery time on a Balb/C breast adenocarcinoma tumor: An in vivo study. Int J Radiat Biol 2015; 10.3109/09553002.2016.1117677 [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Xiong X-P, Zhu G-P, Guo-Pei Z, Shao-Qin H, Chao-Su H, Hong-Mei Y. The in vivo study of the radiobiological effects of prolonged delivery time to tumour control in C57Bl mice implanted with Lewis lung cancer. Radiat Oncol 2011;6:4 http://www.ro-journal.com/content/6/1/4 (6 pp.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joiner MC, Mogili N, Marples B, Burmeister J. Significant dose can be lost by extended delivery times in IMRT with x-rays but not high-LET radiations. Med Phys 2010;2457-2465. [DOI] [PubMed] [Google Scholar]
- 18. Dikomey E, Franzke J. Three classes DNA of strand breaks induced by X-irradiation and internal β-rays. Int J Radiat Biol 1986;50:893-908. [DOI] [PubMed] [Google Scholar]
- 19. Millar WT, Hopewell JW, Paddick I, Lindquist C, Nordströn H, Lidberg P, Gårding J. The role of the concept of biologically effective dose (BED) in treatment planning in radiosurgery. Physica Medica 2015;31:627-633. [DOI] [PubMed] [Google Scholar]
- 20. Kathriarachchi V, Shang C, Evans G, Leventouri1 T, Kalantzis1 G. Dosimetric and radiobiological comparison of CyberKnife M6TM InCise multileaf collimator over IRISTM variable collimator in prostate stereotactic body radiation therapy. J Med Phys 2016;41:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eaton DJ, Lee J, Patel R, Millin AE, Paddick I, Walker C. Stereotactic radiosurgery for benign brain tumors: Results of multi-center bench mark planning studies. Practical Radiation Oncology 2018;8:e295–e304. 10.1016/j.prro.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 22. Jones B, Hopewell JW. Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: Derivation of ‘protective’ dose modification factors. Bri J Radiol 2018;92:20180111. 10.1259/bjr.20180111 (pp 1-11) [DOI] [PMC free article] [PubMed] [Google Scholar]