Abstract
Introduction
In the present study, we reviewed the efficacy of stereotactic radiosurgery (SRS) alone or in combination with WBRT, for the treatment of patients with BM secondary to SCLC. We further identified patient and treatment specific factors that correlated with improved survival.
Methods
Forty-one patients treated with GKRS for BM secondary to SCLC from 2004 to 2017 at the University of Virginia were identified with histopathologically proven SCLC and included in the study.
Results
Following the first GKRS treatment, the median survival was 6 months (1-41 months). There was no statistical difference in overall survival and tumor control between the patients who had PCI, WBRT or upfront GKRS. The only factor associated with decreased OS after the diagnosis of BM from SCLC was active extracranial disease (P=0.045, HR=2.354).
Conclusion
Stereotactic radiosurgery is a reasonable treatment option for patients with brain metastases of SCLC who had PCI or WBRT failure.
Keywords: Brain metastases, Gamma Knife radiosurgery, small-cell lung cancer, stereotactic radiosurgery, SCLC, SRS
Introduction
Worldwide, lung cancer is the second most common malignancy and is the leading cause of cancer-related mortality in the USA [1]. Small cell lung cancer (SCLC), an epithelial derived neuroendocrine tumor, comprises approximately 15% of all lung cancer diagnoses and is frequently complicated by the development of brain metastasis (BM) [2]. While, 15% of patients with SCLC have evidence of BM at the time of diagnosis [3]. An additional 40% of patients will develop BM within one year of the initial diagnosis [4].
Because of the high incidence of BM, prophylactic cranial irradiation (PCI) or whole brain radiotherapy (WBRT) with upfront systemic chemotherapy have been used as the standard regimen for the majority of the patients with SCLC. While these treatment strategies have been shown to prolong overall survival (OS), the long-term control of metastatic disease within the brain is poor with 12-month control rates ranging between 0% to 14% [4]. The high degree of central nervous system (CNS) disease burden, coupled with risks of WBRT further puts SCLC patients at risk neurocognitive deficits. Thus, targeted treatment strategies that spare radiation doses to normal brain parenchyma are needed for patients with SCLC.
Gamma Knife radiosurgery (GKRS) is emerging as an important treatment option for patients with BM in the setting of SCLC. Several studies have shown favorable local control rate varying from 84% to 95% [5-8]. Additionally, as GKRS is able to deliver targeted radiation, it has been used as an indispensable salvage treatment modality for patients with new BM following WBRT [5-7]. In the present study, we reviewed the efficacy of stereotactic radiosurgery (SRS) alone or in combination with WBRT, for the treatment of patients with BM secondary to SCLC. We further identified patient and treatment specific factors that correlated with improved survival.
Material and Methods
Patient Selection
All patients treated with GKRS for BM secondary to SCLC from 2004 to 2017 at the University of Virginia were identified from an Institutional Review Board (IRB) approved database. Patients were excluded if they did not have histopathologically proven SCLC. We included all patients in the analyses.
Gamma Knife Radiosurgery
GKRS was performed as previously described in a single session [5]. In brief, patients were placed into a Leksell Model G stereotactic frame (Elekta AB, Stockholm, Sweden). All treatment plans were then generated off of thin slice magnetic resonance images (MRI) that were merged with a stereotactic computed tomography (CT) scan. If a MRI was contraindicated, a thin sliced head CT with and without contrast was used for stereotactic planning. The prescription dose was based on tumor volume, tumor location, and the status of prior radiation therapy. Treatment plans were created by a multidisciplinary approach including a neurosurgeon, radiation oncologist, and medical physicist.
Follow-up
Following GKRS, patients underwent clinical assessments and repeat MRIs at 2-3-month intervals. Tumors were considered stable if their volume at last follow-up was within 20% of the original treatment volume. Tumors were considered progressive if they had a volumetric increase of 20% or more while tumor regression was defined as a tumor having decreased by 20% or more by its original volume [9].
Statistical Analysis
The mean, median, range and standard deviation was determined for continuous variables, while frequency and percentages were determined for categorical data. Overall survival (OS) rate was calculated using the Kaplan-Meier product limit method. Univariate analyses were performed using Cox proportional hazards regression model to elucidate prognostic factors for OS based upon previously published SRS series [5-7,10,11]. SPSS software (IBM SPSS version 24) was used for all statistical analyses. A p-value<0.05 was considered statically significant.
Results
Patient Characteristics
We identified 41 patients who underwent GKRS for treatment of BM of SCLC at our institution. Patient demographic information is summarized in Table 1. At the time of GKRS, the median age was 59 years (range 38 to 87 years), and the median Karnofsky performance status (KPS) was 80 (range 70-100). Twenty patients (48%) were male and 21 (52%) female. Thirty-one patients (75.6%) had active extracranial disease. At the first GKRS session, the 41 patients harbored 162 BM (mean 4 BM per patient).
Table 1.
Patient demographics.
| Patient demographics | |
| Age (years), median (range) | 59 (38-87) |
| KPS, median (range) | 80 (70-100) |
| Gender: Female(F), Male (M) | 21F, 20M |
| Active extracranial disease, n (%) | 31 (75.6) |
| BM present at time of diagnosis, n (%) | 18 (43.9) |
| Prophylactic cranial irradiation, n (%) | 13 (31.7) |
| Neurological symptoms at time of BM, n (%) | 31 (75.6) |
| Time from diagnosis to BM (months), median (range) | 6.5 (0-37) |
| WBRT, n (%) | 22 (57.7) |
| WBRT dose (Gy), median (range) | 30 (15-38) |
| Smoking status (current, former, unknown) | 16(39%), 23(56.1%), 2(4.9%) |
| Clinical and radiographic follow-up after GKRS (months), median (range) | 6 (0-43) |
Eighteen patients (43.9%) had metastatic disease to the brain at the time of primary SCLC diagnosis. For all patients, the median duration between the diagnosis of SCLC and BM development was 6.5 months (range 0-37 months). Prior to GKRS, PCI was employed in 13 patients (31.7%) and WBRT in 22 (57.7%). GKRS was used as upfront treatment for BM in 6 patients (14.6%). In this study, 22 patients (53.65%) underwent a single GKRS session while 19 patients (46.35%) required multiple radiosurgeries (range 2-6 times). The study was comprised of 39 patients (95.12%) who were current or former smokers.
At the time of SRS, most patients (n=31, [75.6%]) had neurologic symptoms attributed to their BM and 5 patients (12.2%) had undergone surgical resection of a symptom producing BM. The median follow-up after the diagnosis of SCLC was 21 months (range 3 to 54 months) and the median clinical and radiographic follow-up since GKRS was 6 months (range 0 to 43 months). The median number of BM treated at first GKRS was 3 (range 1 to 15 BM).
Gamma Knife Radiosurgery Parameters
Table 2 summarizes GKRS parameters. At the first GKRS session, the median number of BM treated was 3 (range 1-15). The median prescription dose was 18Gy (range from 10 to 22Gy) to a median isodose of 50%. The median maximum treatment dose was 34 (range 18-50Gy). The median tumor diameter was 1.7cm (range 0.6-6.6cm).
Intracranial disease control after GKRS
Seven patients did not have follow-up imaging secondary to death. In the 34 patients with follow-up imaging, tumor control was evaluated in 119 tumors. In these tumors, by last follow-up, 111 (93.27%) were stable or had regressed whereas 8 (6.7%) had evidence of growth. The median time to tumor progression was 3 months (range 1-24 months). Of the 34 patients with radiographic follow-up, 19 patients (55.9%) developed new BM during follow-up. The mean number of new BM was 2.84, and the median time to new BM was 3 months (range 1-16). The new BM were treated with GKRS in all 19 patients. An additional, 6 required WBRT and chemotherapy following GKRS due to miliary or leptomeningeal disease. In our analysis we did not find any statistically significant factor associated with better or worse local tumor control. Table 3 summarizes these findings.
Table 3.
GKRS results.
| Results | |
| Tumor control after GKRS, n (%) | 32 (94.1) |
| New BM after GKRS, n (%) | 19 (55.9) |
| Time of local control (months), median (range) | 3 (0-24) |
| Number of BM treated at first GKRS, median (range) | 3 (1-15) |
| Severe toxicity after GKRS | 0 |
| Number of GKRS treatments per patient, n, median (range) | 1 (1-6) |
| WBRT after GKRS, n (%) | 6 (14.63%) |
Table 2.
GKRS parameters.
| GKRS Parameters | |
| Upfront GKRS, n (%) | 6 (14.6) |
| Repeated GKRS, n (%) | 19 (46.3%) |
| Systemic therapy at time of GKRS, n (%) | 26 (63.4) |
| Prescription dose (Gy), median (range) | 18 (10-22) |
| Isodose line (%) median (range) | 50 (40-95) |
| Maximum dose (Gy), median (range) | 34 (18-50) |
| Number of BM at first GKRS treatment, median (range) | 3 (1 to 15) |
| Maximum diameter (cm) of BM, median, range | 1.7 (0.6-6.6) |
| Follow-up since GKRS (months), median (range) | 6 (0-43) |
Survival
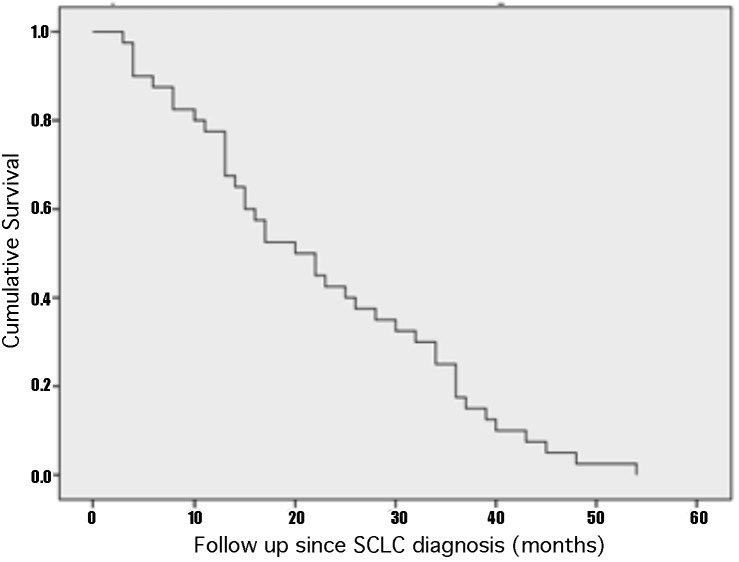
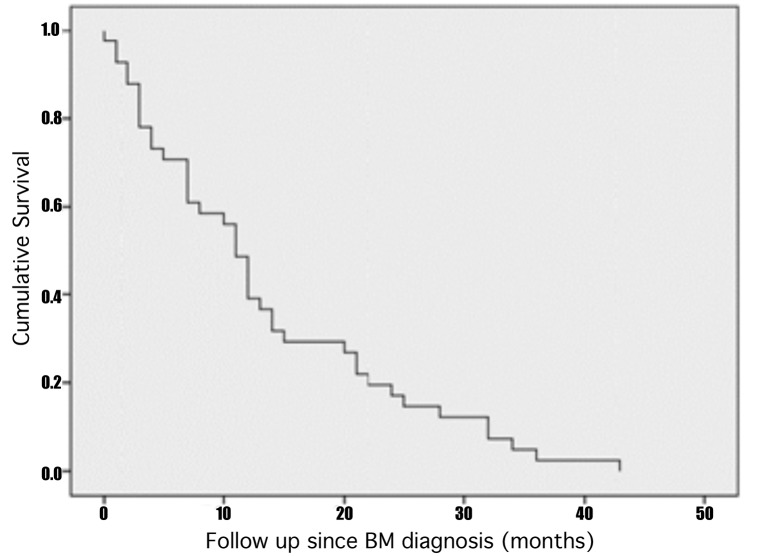
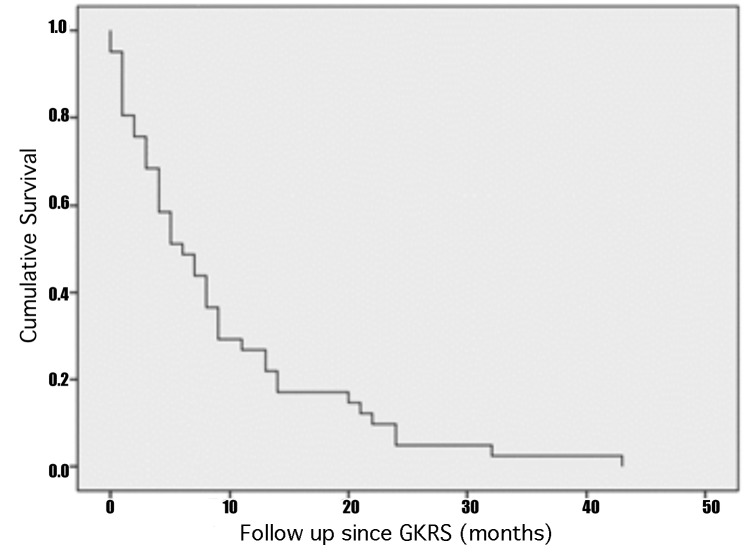
The median overall survival after SCLC diagnosis was 20 months (3-54 months), while the median OS after BM diagnosis was 11 months (1-43 months). Following the first GKRS treatment, the median survival was 6 months (1-41 months). There was no statistical difference in overall survival and tumor control between the patients who had PCI, WBRT or upfront GKRS. The only factor associated with decreased OS after the diagnosis of BM from SCLC was active extracranial disease (P=0.045, HR=2.354). The 5 and 11-month actuarial survival rate following GKRS was 50% and 25%, respectively. At last follow-up, one patient had an OS of 43 months. Figure 1 shows the Kaplan-Meier curves with the OS after SCLC diagnosis (A), after BM diagnosis (B) and after initial GKRS treatment (C).
Figure 1.
A - Kaplan-Meier curve of cumulative survival after the initial diagnosis of SCLC showing that the median OS after SCLC diagnosis was 20 months (3-54 months). B - Kaplan-Meier curve of the cumulative survival after BM diagnosis demonstrating that the median OS after BM diagnosis was 11 months (1-43 months). C - Kaplan-Meier curve of cumulative survival after the first GKRS treatment indicating that the median survival was 6 months (1-41 months).
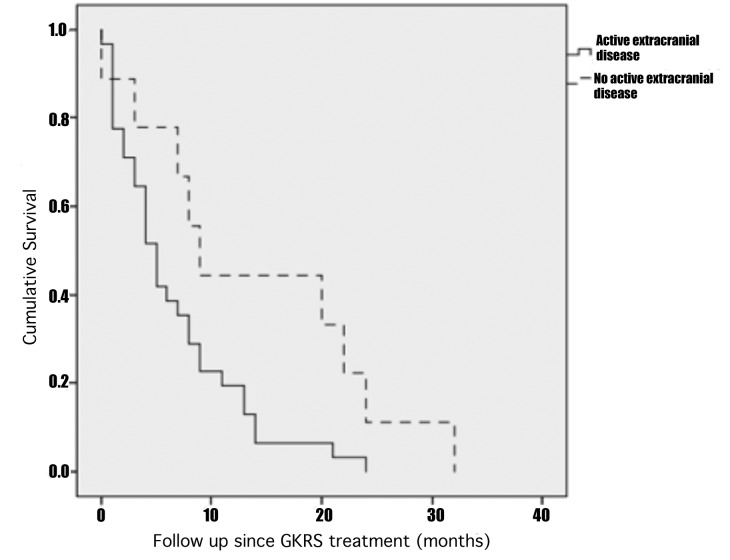
The prognostic factor that was statically significant in univariate analysis was active extracranial disease (p=0.050, HR=2.254) that was a factor related to a lower survival rate than patients without active extracranial disease as is illustrated by Kaplan-Meier curve in Figure 2. Since no other variable had a p value < 0.10 and given the statistical power of the current study, we only performed univariate analyses as shown in Table 4.
Figure 2.
Kaplan-Meier curve of cumulative survival after GKRS illustrating the worse OS of patients with active extracranial disease that had a median OS of 5 months (range 0-24 months) in comparison to patients without active extracranial disease that had a median OS of 9 months (range 0-32 months).
Table 4.
Univariate analysis of factors related to poor overall survival from time of GKRS.
| Univariate Analysis | ||||
| 95% CI | ||||
| Variables | P | HR | Lower | Upper |
| Age at time of initial GKRS | 0.503 | 1.015 | 0.973 | 1.059 |
| KPS | 0.117 | 0.972 | 0.937 | 1.007 |
| Active extracranial disease | 0.050 | 2.254 | 1.001 | 5.075 |
| BM at time of SCLC diagnosis | 0.926 | 0.970 | 0.514 | 1.834 |
| Pre-GKRS WBRT | 0.699 | 1.207 | 0.465 | 3.134 |
| More than 5 BM at time of initial GKRS | 0.370 | 1.369 | 0.689 | 2.720 |
| Concurrent systemic therapy at time of GKRS | 0.197 | 0.634 | 0.317 | 1.267 |
Discussion
Brain metastases are the most common malignant tumor of the brain with over 200,000 cases diagnosed each year in the US alone. Although contemporary 1-year survivorship has increased compared to 1980s, optimizing the management of patients with BM is an ongoing effort [12,13].
SCLC comprises of 15 to17% of all new lung cancer diagnoses each year. The incidence of SCLC has been decreasing in the last two decades, which has been attributed to decreasing use of tobacco related products [3]. At the time of initial cancer diagnosis, approximately 15% of SCLC patients have BM. This rate increases to 50 to 80% of within 24 months [14]. Although there have been improvements in the diagnosis and management of patients with SCLC, the median survival of patients with disseminated disease is 10-12 months [15]. Thus, there is a need for improved management of patients with metastatic SCLC.
In addition to systemic chemotherapy and given the high incidence of BM in patients with SCLC, PCI has long been advocated for patients with limited or extensive disease [16,17]. In a randomized, multicenter clinical trial, Ben et al [16] showed that PCI reduced the incidence of BM and prolonged progression free survival in patients with limited-stage disease that responded to initial therapy. PCI is used as standard of care and has Level 1 evidence to support its use [18]. PCI in patients with extensive systemic disease appears to decrease the risk of new symptomatic brain metastases [11,16] although this is controversial. The EORTC trial showed that patients with extracranial metastases had improved OS with PCI, but these results were limited in generalizability as patients were only screened for BM if they had neurological symptoms. Another phase III trial reported that patients with extensive systemic disease without brain metastases did not benefit from PCI [11].
Despite these conflicting results, PCI and WBRT are used as standard treatment for brain metastases secondary to SCLC [19,20]. Nevertheless, between17% to 33% of patients treated with PCI will develop new BM and WBRT is invariably complicated by neurocognitive deficits [21-23]. Furthermore, despite the radiosensitivity of SCLC, both local and distant failures are not uncommon. In the current study, frequent of field recurrences occurred, and 55.9% of patients underwent repeat GKRS after prior PCI, WBRT, or prior GKRS. Given favorable local tumor control and ease of repeatability, SRS has recently been used in patients with a limited number of BM. Since SRS delivers localized radiation and is able to spare normal brain appreciable ionizing radiation, the risk of neurotoxicity is reduced [5,24]. Indeed, the lower GKRS doses required for tumor control confer increased safety and have led to a low rate of radiotoxicity as was observed in patients reported herein.
Unlike with more radioresistant histologies like melanoma and renal cell carcinoma, SCLC is highly radiosensitive, plus many of the patients had had prior WBRT. Thus, we tended to utilize a lower prescription dose for this cohort that other BM patients who had more radioresistant histologies and less frequently WBRT prior to SRS. Further study of the optimal SRS dose for single and hypofractioned techniques in SCLC patients is warranted. Also, the likely coupling of SRS and target systemic therapies in BM with SCLC may favorably impact the rate of local and distant tumor progression. Immune checkpoint inhibitors [39]. Further study is required to elucidate the SRS optimal dose and fractionation scheme and adjuvant therapy regimen for SCLC patients with BM so as to achieve favorable intracranial tumor control an avoid the detrimental effects of historically employed wide field cranial radiation therapy techniques.
Some trials for BM have shown that the use of SRS alone in patients with 1 to 3 metastases resulted in less cognitive deterioration with no difference in overall survival, suggesting that GKRS is viable treatment option for patients with limited central nervous system (CNS) disease [25]. Other studies have suggested that in patients with 1-3 BM, GKRS following WBRT improved local control and OS than WBRT alone and that the radiosurgery boost was not associated with any increased adverse radiation effect [26]. The JLGK0901 clinical trial from Japan analyzed BM from different types of primary malignancies, including SCLC patients. This study demonstrated that that SRS without WBRT in patients with five to ten brain metastases was non-inferior to that in patients with two to four brain metastases [27]. The current study given its retrospective design lacks robust neurocognitive endpoints, but this information should be a focus for future studies.
There are few appreciable series analyzing SRS for BM from SCLC as an upfront treatment or after fractionated cranial radiation therapy and these are summarized in Table 5. A recently published study by Robin et al [28] comparing 5752 patients who had WBRT and 200 patients who had SRS alone showed favorable survival outcomes with SRS alone compared with WBRT ± SRS. The improved outcomes with SRS remained consistent in multivariable and propensity score-matched analyses controlling for important confounders. There is one ongoing clinical trial called Whole Brain Radiation Therapy Alone vs. Radiosurgery for SCLC Patients With 1-10 Brain Metastases (ENCEPHALON) [29] which may elucidate some questions regarding neurocognition, tumor control, OS, quality of life (QoL) with the use of WBRT and SRS to the treatment of BM from SCLC.
Table 5.
SRS series for SCLC BM
| SRS series for SCLC BM | ||||||
| Studies | Year | Number of patients | Radiation therapy combination | Tumor control rate at 1 year | Median Survival (months) | Statistically difference in survival between those treated with WBRT and SRS |
| Serizawa et al.9 | 2002 | 34 | SRS | 94.5% | 9.1 | no |
| Sheehan et al.8 | 2005 | 27 | SRS+WBRT | 81% | 4.5 after SRS | not reported |
| Wook et al.41 | 2011 | 50 | PCI+SRS, WBRT+SRS and SRS alone | 76.4% | 4.8 after SRS | no |
| Wegner et al.10 | 2011 | 44 | PCI+SRS, PCI+WBRT+SRS, WBRT+SRS, SRS | 86% | 9 after SRS | Yes (WBRT+SRS) |
| Olson et al. 42 | 2012 | 27 | PCI+WBRT, WBRT+SRS | 75% | 3 after SRS | not reported |
| Harris et al.43 | 2012 | 51 | SRS+WBRT | 57% | 5.9 after SRS | not reported |
| Nagazaki et al.17 | 2013 | 44 | SRS+WBRT | 95.8% (4months FU) | 5.8 after SRS | not reported |
| Yomo et al.44 | 2014 | 41 | SRS alone | 86% | 8.1 after SRS | not reported |
| Yomo et al.45 | 2015 | 70 | SRS alone, WBRT+SRS | 77% | 7.8 after SRS | no |
| Baernhardt et al.31 | 2016 | 76 | PCI+SRS, PCI+Re-WBRT | Not reported | 3 (WBRT) and 5 (SRS) | no |
Most of studies examining SRS for BM secondary to SCLC have investigated SRS following WBRT [5-7,10,30-33]. Yomo et al [32] treated patients with upfront GKRS while Sheehan et al [5] examined the use of GKRS following WBRT. Tumor control in these studies has ranged between 81 to 95%. In our study, the tumor control rate was 93.27%, which is consistent with previous studies. As extracranial disease progression often leads to shortened OS in this patient population, avoiding the neurocognitive effects of WBRT may be especially appealing to these patients. Moreover, contemporary SRS devices now allow for treatment of patients with 10 or more brain metastases thereby making SRS feasible for SCLC patients with low to moderate intracranial tumor burden. Of course, the current study and the prior ones noted in Table 4 support the use of SRS in SCLC patients with reasonable performance status and intracranial progression after prior fractionated radiotherapy [6,34,35]. The OS and the rate of neurological death were not significant different comparing groups treated with WBRT or WBRT plus GKRS or even with repeated WBRT [5-7,25,26,34-38].
Study Limitations
There are several limitations to this study. It is a retrospective analysis from a single center and it is subjected to biases and systemic error. The relatively small number of patients, and the heterogeneity of the treatment algorithm (e.g. some had previous PCI, others WBRT after diagnosis of BM, and still others had upfront GKRS), and also the relatively short follow-up limits the statistical analyses.
Conclusion
Stereotactic radiosurgery is a reasonable treatment option for patients with brain metastases of SCLC who had PCI or WBRT failure. GKRS is a safe and effective treatment for tumor control in these patients and confers a low risk of complications. Despite treatment with WBRT, GKRS, and systemic therapies, patients with BM of SCLC still have a poorer OS and are at a high risk of brain failure with frequent new metastatic lesions as compared to patients with other cancer histologies. Further investigation into the optimal role of SRS for SCLC is required.
Abbreviations
| BM | brain metastases |
| CNS | central nervous system |
| CT | computed tomography |
| GKRS | Gamma Knife radiosurgery |
| IRB | Institutional Review Board |
| KPS | Karnofsky performance scale |
| MRI | magnetic resonance imaging |
| OS | overall survival |
| PCI | prophylactic cranial irradiation |
| SCLC | small-cell lung cancer |
| SRS | stereotactic radiosurgery |
| WBRT | whole brain radiation therapy |
Acknowledgments
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Diogo Cordeiro, Zhiyuan Xu, Matthew Shepard, Chelsea Li, Jason Sheehan
Data collection: Diogo Cordeiro, Zhiyuan Xu, Matthew Shepard, Chelsea Li, Jason Sheehan
Data analysis and interpretation: Diogo Cordeiro, Zhiyuan Xu, Matthew Shepard, Chelsea Li, Jason Sheehan
Manuscript writing: Diogo Cordeiro, Zhiyuan Xu, Matthew Shepard, Darrah Sheehan, Chelsea Li, Jason Sheehan
Final approval of manuscript: Diogo Cordeiro, Zhiyuan Xu, Matthew Shepard, Darrah Sheehan, Chelsea Li, Jason Sheehan
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):1130. [DOI] [PubMed] [Google Scholar]
- 2. Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78(8):1781-1788. [PubMed] [Google Scholar]
- 3. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24(28):4539-4544. [DOI] [PubMed] [Google Scholar]
- 4. Slotman BJ, Mauer ME, Bottomley A, Faivre-Finn C, Kramer GW, Rankin EM, Snee M, Hatton M, Postmus PE, Collette L, Senan S. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: Short-term health-related quality of life and patient reported symptoms—Results of an international phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;278:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheehan J, Kondzioka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: outcomes and prognostic factors. Journal of neurosurgery. 2005;102(Suppl):247-254. [DOI] [PubMed] [Google Scholar]
- 6. Serizawa T, Ono J, Iichi T, Matsuda S, Sato M, Odaki M, Hirai S, Osato K, Saeki N, Yamaura A. Gamma knife radiosurgery for metastatic brain tumors from lung cancer: a comparison between small cell and non-small cell carcinoma. Journal of neurosurgery. 2002;97(5 Suppl):484-488. [DOI] [PubMed] [Google Scholar]
- 7. Wegner RE, Olson AC, Kondziolka D, Niranjan A, Lundsford LD, Flickinger JC. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. International journal of radiation oncology, biology, physics. 2011;81(3):e21-27. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto M, Barfod BE, Urakawa Y. Gamma knife radiosurgery for brain metastases of non-lung cancer origin: focusing on multiple brain lesions. Prog Neurol Surg. 2009;22:154-169. [DOI] [PubMed] [Google Scholar]
- 9. Snell JW, Sheehan J, Stroila M, Steiner L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note. Journal of neurosurgery. 2006;104(1):157-162. [DOI] [PubMed] [Google Scholar]
- 10. Nakazaki K, Higuchi Y, Nagano O, Serizawa T. Efficacy and limitations of salvage gamma knife radiosurgery for brain metastases of small-cell lung cancer after whole-brain radiotherapy. Acta neurochirurgica. 2013;155(1):107-113; discussion 113-104. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663-671. [DOI] [PubMed] [Google Scholar]
- 12. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65(1):5-29. [DOI] [PubMed] [Google Scholar]
- 13. Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117(11):2505-2512. [DOI] [PubMed] [Google Scholar]
- 14. Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100(4):801-806. [DOI] [PubMed] [Google Scholar]
- 15. Byers LA, Rudin CM. Small cell lung cancer: Where do we go from here? Cancer. 2015;121(5):664-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S. EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med, 2007;357:664-672. [DOI] [PubMed] [Google Scholar]
- 17. Auperin A, Arriagada R, Pignon J-P, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission: Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-484. [DOI] [PubMed] [Google Scholar]
- 18. PDQ Adult Treatment Editorial Board. Small cell lung cancer treatment (PDQ): Health professional version. National Cancer Institute: Bethesda, MD; 2002. [Google Scholar]
- 19. Castrucci WA, Knisely JP. An update on the treatment CNS of metastases in small cell lung cancer. Cancer J. 2008;14(3):138-146. [DOI] [PubMed] [Google Scholar]
- 20. Postmus PE, Haaxma-Reiche H, Gregor A, Groen HJ, Lewinski T, Scolard T, Kirkpatrick A, Curran D, Sahmoud T, Giaccone G. Brain-only metastases of small cell lung cancer; efficacy of whole brain radiotherapy. An EORTC phase II study. Radiotherapy and oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 1998;46(1):29-32. [DOI] [PubMed] [Google Scholar]
- 21. D’Ambrosio DJ, Cohen RB, Glass J, Konski A, Buyyounouski MK, Feigenberg SJ. Unexpected dementia following prophylactic cranial irradiation for small cell lung cancer: Case report. Journal of neuro-oncology. 2007;85(1):77-79. [DOI] [PubMed] [Google Scholar]
- 22. McDuff SG, Taich ZJ, Lawson JD, Sanghvi P, Wong ET, Barker FG, 2nd, Hochberg FH, Loeffler JS, Warnke PC, Murphy KT, Mundt AJ, Carter BS, McDonald CR, Chen CC. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry. 2013;84(12):1384-1391. [DOI] [PubMed] [Google Scholar]
- 23. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789-796. [DOI] [PubMed] [Google Scholar]
- 24. Adler JR, Cox RS, Kaplan I, Martin DP. Stereotactic radiosurgical treatment of brain metastases. Journal of neurosurgery. 1992;76(3):444-449. [DOI] [PubMed] [Google Scholar]
- 25. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, 2nd, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016;316:401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672. [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395. [DOI] [PubMed] [Google Scholar]
- 28. Robin TP, Bernard L, Jones BL, Amini A, Koshy M, Gaspar LE, Liu AK, Nath SK, Kavanagh BD, Camidge DR, Rusthoven CG. Radiosurgery alone is associated with favorable outcomes for brain metastases from small-cell lung cancer. Lung Cancer, 2018;120:88. [DOI] [PubMed] [Google Scholar]
- 29. Whole brain radiation therapy alone vs. radiosurgery for SCLC patients with 1-10 brain metastases. ClinicalTrials.gov Identifier: NCT03297788. Sponsor: Juergen Debus. Collaborator: Heidelberg University [Google Scholar]
- 30. Olson AC, Wegner RE, Rwigema JC, Heron DE, Burton SA, Mintz AH. Clinical outcomes of reirradiation of brain metastases from small cell lung cancer with Cyberknife stereotactic radiosurgery. J Cancer Res Ther. 2012;8(3):411-416. [DOI] [PubMed] [Google Scholar]
- 31. Harris S, Chan MD, Lovato JF, Ellis TL, Tatter SB, Bourland JD, Munley MT, deGuzman AF, Shaw EG, Urbanic JJ, McMullen KP. Gamma knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. International Journal of Radiation Oncology, Biology, Physics. 2012;83(1):e53-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yomo S, Hayashi M. Upfront stereotactic radiosurgery in patients with brain metastases from small cell lung cancer: retrospective analysis of 41 patients. Radiat Oncol 2014;9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yomo S, Hayashi M. Is stereotactic radiosurgery a rational treatment option for brain metastases from small cell lung cancer? A retrospective analysis of 70 consecutive patients. BMC Cancer 2015;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooper JS, Steinfeld AD, Lerch IA. Cerebral metastases: Value of reirradiation in selected patients. Radiology. 1990;174(3 Pt 1):883-885. [DOI] [PubMed] [Google Scholar]
- 35. Wong WW, Schild SE, Sawyer TE, Shaw EG. Analysis of outcome in patients reirradiated for brain metastases. International Journal of Radiation Oncology, Biology, Physics. 1996;34(3):585-590. [DOI] [PubMed] [Google Scholar]
- 36. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. International Journal of Radiation Oncology, Biology, Physics. 1999;45(2):427-434. [DOI] [PubMed] [Google Scholar]
- 37. Jo KW, Kong DS, Lim DH, Ahn YC, Nam DH, Lee JI. The role of radiosurgery in patients with brain metastasis from small cell lung carcinoma. J Korean Neurosurg Soc. 2011;50(2):99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bernhardt D, Bozorgmehr F, Adeberg S, Opfermann N, von Eiff D, Rieber J, Kappes J, Foerster R, König L, Thomas M, Debus J, Steins M, Rieken S. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer. 2016;101:76-81. [DOI] [PubMed] [Google Scholar]
- 39. Gadgeel SM. Targeted therapy and immune therapy for small cell lung cancer. Curr Treat Options Oncol. 2018. Sep 10;19(11):53. [DOI] [PubMed] [Google Scholar]