Abstract
The aim of this work was to develop, optimize and characterize a silymarin-laden polyvinylpyrrolidone (PVP)-polyethylene glycol (PEG) polymeric composite to resolve low aqueous solubility and dissolution rate problem of the drug. A number of silymarin-laden polymeric formulations were fabricated with different quantities of PVP K-30 and PEG 6000 by the solvent-evaporation method. The effect of PVP K-30 and PEG 6000 on the aqueous solubility and dissolution rate was investigated. The optimized formulation and its constituents were characterized using powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) techniques. Both the PEG 6000 and PVP K-30 positively affected the aqueous solubility and dissolution rate of the drug. In particular, a formulation consisting of silymarin, PVP K-30 and PEG 6000 (0.25/1.5/1.5, w/w/w) furnished the highest solubility (24.39±2.95 mg/mL) and an excellent dissolution profile (~100% in 40 min). The solubility enhancement with this formulation was ~1150-fold as compared to plain silymarin powder. Moreover, all the constituents existed in the amorphous state in this silymarin-laden PVP-PEG polymeric composite. Accordingly, this formulation might be a promising tool to administer silymarin with an enhanced effect via the oral route.
Keywords: Silymarin, Hydrophilic polymers, Inclusion, Solid dispersion, Aqueous solubility, Dissolution rate
Graphical abstract
1. Introduction
Silybum marianum (milk thistle plant) is a member of family Asteraceae. It is an herbal drug used to rectify hepatic ailments. The extract obtained from fruits and seeds of Silybum marianum is known as silymarin. Silymarin contains four isomeric flavonoids called flavonolignans, namely, silybinin, isosilybinin, silydianin and silychristin. Silybinin is of prime pharmaceutical interest [1], [2], [3]. Silymarin is a well-known hepatoprotective, anticarcinogenic, and anti-inflammatory agent [4]. It is a potent antioxidant which stabilizes cell membranes, detoxifies harmful entities [4], [5] such as thioacetamide [6], diethylnitrosamine [7], carbon tetrachloride, acetaminophen and bromobenzene [8], and regenerates damaged hepatocytes [9]; thus, it has a significant role in the supportive treatment of hepatic illnesses and adversities [10]. Silymarin enhances the survival percentage of cirrhotic patients [11], [12]. It is considered as a powerful hepatic and renal decongestant [10], [13]. Silymarin was used as an antidote for death cap mushroom (Amanita phalloide) [14].
Oral route is considered as the most convenient and safest route for administration of drugs. The absorption of orally administered substances depends upon their adequate solubility in the aqueous gastrointestinal fluid. A substance can only be absorbed when it is in the solution state at the site of administration. On the basis of the aqueous solubility and permeation across cell membranes, Biopharmaceutics Classification System (BCS) has categorized the compounds into four classes. Silymarin is placed under BCS Class II. BCS Class II compounds are either insoluble in aqueous media or possess very low solubility. The compounds presenting aqueous solubility < 100 µg/mL are not adequately absorbed from the gastrointestinal tract (GIT) [15]. Absorption of silymarin from GIT is very poor due to its insufficient solubility and dissolution rate in the aqueous media [16], [17].
There are numerous aqueous solubility and dissolution rate enhancing techniques, for example, particle size reduction or surface area enhancement, nanoparticles, salt forms, use of surfactants, complexation with cyclodextrins, solid dispersions (hydrophilic polymeric drug delivery systems), and encapsulations [18]. These techniques have been effectively used in improving the aqueous solubility and dissolution rate of several natural and synthetic compounds [19], [20], [21]. Numerous solubility enhancing approaches, such as self-emulsifying drug delivery systems (SEDDS) [22], formulation by phosphatidylcholine [23], formulation by means of cyclodextrins [24], preparation with lecithin [25] and solid dispersions [26], [27], have been employed for resolving low solubility and dissolution rate problems of silymarin.
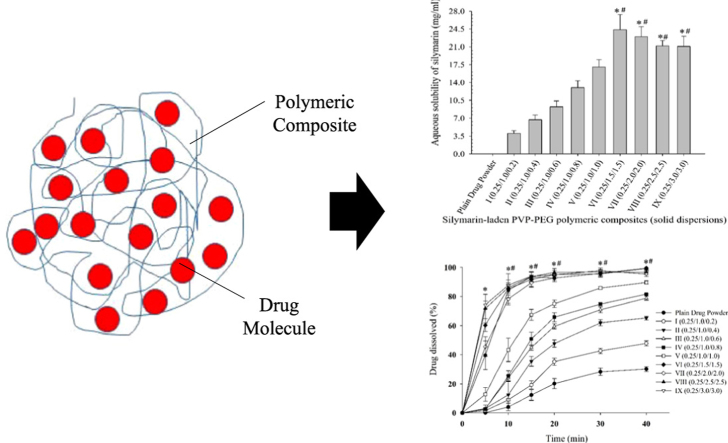
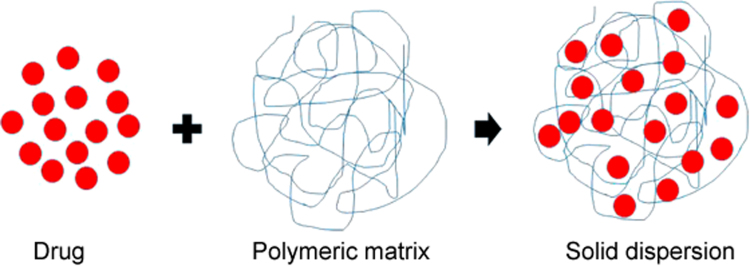
Solid dispersion is a solid drug delivery system in which a poorly water-soluble drug is dispersed into an inert hydrophilic polymeric matrix (Fig. 1). Solid dispersion is a well-known approach to improve the aqueous solubility and dissolution rate of BCS class II drugs [28]. Incorporation of a poorly water-soluble entity in a hydrophilic polymeric matrix or composite might result in its enhanced solubility and dissolution rate in the aqueous media. As the hydrophilic polymer dissolves, the loaded drug presents itself as very fine particles for rapid dissolution [29]. Hydrophilic polymeric matrices, like polyvinylpyrrolidone, hydroxypropyl cellulose, carboxymethylcellulose, poloxamer 188, poloxamer 407, and polyethylene glycol 6000, have been utilized in the preparation of solid dispersions. Solid dispersions can be fabricated via several methods such as melting [30], kneading [31], solvent-evaporation [32] and lyophilisation [33]. The solvent-evaporated solid dispersion is one of the best tools for enhancement of the aqueous solubility and dissolution rate of BCS class II drugs [34], [35]. In this type of solid dispersion, the drug and polymeric matrices are completely dissolved in a solvent system to get an absolutely transparent solution. Subsequently, this clear solution is evaporated to obtain the dried product. In a solvent-evaporated solid dispersion, the drug molecules are uniformly scattered or entrapped into the polymeric composite (Fig. 1).
Fig. 1.
Illustration depicting inclusion of drug into the polymeric matrix.
In the present study, several silymarin-laden polyvinylpyrrolidone (PVP)-polyethylene glycol (PEG) polymeric composites (solid dispersions) were prepared with different ratios of PVP K-30 and PEG6000 by the solvent-evaporation method with the aim to enhance the aqueous solubility and dissolution rate of poorly water-soluble silymarin. In vitro solubility and dissolution tests were performed in the aqueous media. The solid state characterization was carried out by powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR).
2. Materials and methods
2.1. Materials
Silymarin was procured from Xi'an Xin Sheng Bio-Chem Co. (Xi'an, Shaanxi, China). PEG 6000 and PVP K-30 were from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals were of reagent grade.
2.2. Preparation of silymarin-laden PVP-PEG polymeric composites
All silymarin-laden polymeric formulations were prepared through the solvent-evaporation method. In this method, drug and excipients were completely dissolved in a solvent to form a clear solution in order to achieve a molecular-level homogeneous mixing. Subsequently, this solution was dried to obtain a solid product in which drug molecules are evenly scattered in the polymeric matrix or composite [36]. For each formulation, accurately weighed quantities of silymarin, PVP K-30 and PEG 6000 were comprehensively dissolved in ethanol to get a transparent solution. Then, the solution was spread in a tray and placed in a tray-dryer at 40 °C. After complete drying, the dried mass was triturated for 15 min using a pestle and mortar. The powdered substance was passed through a sieve No. 60 and preserved in a 45 mL conical tube. The compositions of various formulations are shown in Table 1.
Table 1.
Compositions of silymarin-laden PVP-PEG polymeric composites (solid dispersions).
| Components (g) | I | II | III | IV | V | VI | VII | VIII | IX |
|---|---|---|---|---|---|---|---|---|---|
| Silymarin | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| PVP K− 30 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| PEG 6000 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
2.3. Aqueous solubility of silymarin in PVP-PEG polymeric composites
For each formulation, excess of powder was added to 1 mL distilled water in a 2 mL microtube and vortex-mixed for a minute. After vortex-mixing, all the microtubes were secured on a mechanical shaker in a water-bath and shaken (25 °C, 100 rpm) for 5 days [37]. Then, after centrifugation (5000 g), 500 µL supernatant was carefully taken with the help of a micropipette and appropriately diluted with ethanol. The diluent was analyzed using HALO DB-20 UV–visible spectrophotometer (Dynamica, Victoria, Australia) at 287 nm wavelength for determining concentration of silymarin [38].
2.4. Silymarin content in PVP-PEG polymeric composites
For a formulation, carefully weighed sample equivalent to 50 mg silymarin was dissolved in 100 mL ethanol in a 100 mL measuring flask. The theoretical concentration of this solution was 500 µg/mL. This solution was filtered (0.45 µm) and the filtrate was appropriately diluted with ethanol. The diluent was analyzed using a HALO DB-20 UV–visible spectrophotometer (Dynamica, Victoria, Australia) at 287 nm wavelength for determining concentration of silymarin [38]. The test was performed in triplicate for each formulation. The drug content was determined by the following formula: Xs = Xa/Xt × 100. Where, Xs is the silymarin content, Xa is actual titer quantified by the UV–visible spectrophotometer, and Xt is the theoretical concentration.
2.5. Dissolution of silymarin in PVP-PEG polymeric composites
Dissolution test was performed using the Paddle apparatus (Vision® Classic 6™, Hanson Research Co.; Los Angeles, CA, USA). Each sample equivalent to 50 mg silymarin was sealed in a dialysis membrane (Spectra/Por®, MWCO 3500, Spectrum Laboratories, Rancho Dominguez, CA, USA). Then, the enclosed sample was secured in the sinker and dropped into 900 mL of 2% (w/v) aqueous Tween 80 solution (the dissolution medium) [39], [40] maintained at 37±0.5 °C by a surrounding water-bath. For enhancing dissolution efficiency of poorly soluble drugs, surfactants may be added to the dissolution media at a recommended limit set by The Food and Drug Administration (FDA) [41]; accordingly, the 2% (w/v) aqueous Tween 80 solution was used as dissolution medium in the present study [37], [42]. The paddle rotation was set at 100 rpm [43], [44]. The sink conditions were maintained while conducting the study. At pre-decided time intervals, 1 mL of the medium was sampled, filtered (0.45 µm) and diluted appropriately. The diluent was assayed by HALO DB-20 UV–visible spectrophotometer (Dynamica, Victoria, Australia) at 287 nm wavelength for determining concentration of silymarin [38].
2.6. Powder X-ray diffraction analysis
Rigaku X-ray diffractometer diffractometer (D/MAX-2500 PC, Rigaku Corporation; Tokyo, Japan) was used for determining the crystalline property of the samples. X-ray diffraction analysis was accomplished by using Cu Kα1 monochromatic radiation source at 50 kV voltage and 100 mA current. The PXRD patterns were attained in the array of 10–70 °C with 2θ scanning mode, scan speed of 5°/min and a step size of 0.02°/s.
2.7. Differential scanning calorimetry
The thermal characteristics of PVP K-30, PEG 6000, silymarin powder, physical mixture and silymarin-laden PVP-PEG polymeric composites were detected using a differential scanning calorimeter (DSC Q20, TA Instruments; New Castle, Delaware, USA). For this purpose, about 5–10 mg of sample was enclosed in an aluminum crucible and scanned in the calorimeter over a temperature range of 30–120 °C at a heating rate of 15 °C/min. The scanning was carried out in the presence of a nitrogen flow of 30 mL/min. The physical mixture was obtained by triturating silymarin, PEG 6000 and PVP K-30 together at the optimized ratio of 0.25/1.5/1.5 (w/w/w).
2.8. Scanning electron microscopy
The scanning electron microscope (S-4800, Hitachi, Japan) was utilized for the observation of morphological physiognomies of silymarin plain powder and the optimized silymarin-laden PVP-PEG polymeric formulation. The samples were mounted on a brass disc using a double-adhesive carbon tape. Then, to make the samples electronically conductive for imaging, they were subjected to platinum coating under vacuum (8 × 10−3 mbar) at 20 mA current and 90% turbo speed for 3 min using an EMI Teck Ion Sputter (K575K).
2.9. Fourier transform infrared spectroscopy
The Nicolet-6700 spectophotometer (Pittsburgh, PA, USA) was employed for the FTIR analyses of the optimized formulation, physical mixture and individual components. Each sample was properly placed in the crucible below the scanning lens and scanned over a range of 500–4000 cm−1 with 2 cm−1 resolution.
3. Results and discussion
3.1. Constituents and method of preparation
The absorption of silymarin is very poor due to its insufficient solubility in the aqueous gastrointestinal fluid [18]. Solid dispersion improves the aqueous solubility, dissolution rate and absorption of a poorly water-soluble drug [28]. Polymers, like carboxymethylcellulose, polyvinylpyrrolidone, and hydroxypropyl cellulose, and surfactants, such as tweens, spans, poloxamers, polyethylene glycol 6000, and sodium dodecyl sulphate, are utilized in the preparation of solid dispersions. Both the PVP K-30 and PEG 6000 have been successfully used in several formulations to boost the aqueous solubility of silymarin [45], [46], [47]; however, their simultaneous influence on the aqueous solubility of silymarin has not been reported yet. Accordingly, in the present work, both the PVP K-30 and PEG 6000 were used as a hydrophilic polymer and a polymeric surfactant, respectively, to fabricate a hydrophilic polymeric composite. The compositions of various silymarin-laden PVP-PEG polymeric formulations are shown in Table 1. Moreover, the solvent-evaporation method is considered as the best method for the preparation of solid dispersions [32]; therefore, it was adopted in our study. In this method, all the constituents are dissolved in a solvent completely prior to subsequent drying; therefore, a homogeneous mixing is obtained at the molecular-level. Moreover, the drug molecules are distributed or entrapped in the polymeric materials uniformly in the dried product which enhances content uniformity and contact of the drug molecules with the hydrophilic polymeric matrices. Thus, aqueous solubility and dissolution rate of the drug are improved owing to better wetting of the drug by the hydrophilic polymers in the formulation [32]. In order to ensure the maximal removal of solvent from the formulation, the drying was continued at 40 °C until weight of the sample became constant.
3.2. Solubility test
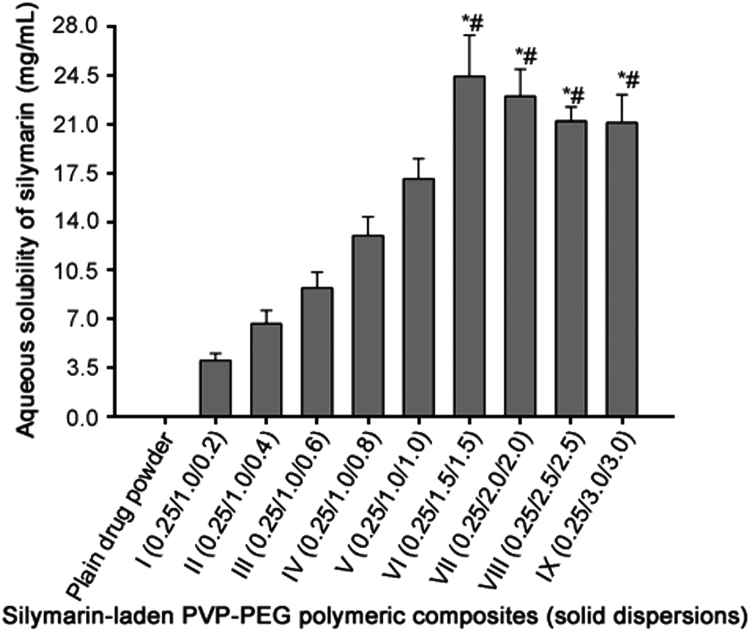
All of the PVP-PEG polymeric composites positively influenced the aqueous solubility (Fig. 2). The solubility of silymarin plain powder in water was (0.023±0.008) mg/mL. Moreover, the aqueous solubility of silymarin with formulation I, II, III, IV, V, VI, VII, VIII and IX (Table 1) was (4.06±0.50) mg/mL, (6.69±0.93) mg/mL, (9.25±1.11) mg/mL, (13.01±1.34) mg/mL, (17.07±1.42) mg/mL, (24.39±2.95) mg/mL, (22.98±1.98) mg/mL, (21.19±1.01) mg/mL and (21.08±1.99) mg/mL, respectively (Fig. 2). The aqueous solubility of silymarin with formulations VI-IX was significantly higher (P < 0.05) than those with formulations I-V; however, solubility results of silymarin with formulations VI-IX were not significantly different (P > 0.05) from one another. In particular, the aqueous solubility enhancement with silymarin-laden PVP-PEG polymeric formulation VI was apparently the highest. The increased aqueous solubility of silymarin by PVP-PEG polymeric composites might be attributed to the improved wetting owing to the presence of hydrophilic polymers and solubilizing effect of PEG 6000.
Fig. 2.
Effect of PVP K-30 and PEG 6000 on the aqueous solubility of silymarin in the PVP-PEG polymeric composites. Each value represents the mean ± S.D. (n = 3) * p < 0.05 compared with the plain drug powder and formulations I-V. #p > 0.05 compared with the formulations VI-IX.
3.3. Drug content determination
Prior to dissolution test, drug content was determined in each formulation. All the formulations exhibited drug content within 99%–101% range. It suggested that silymarin remained stable during the preparation and drying process. Moreover, drug molecules are uniformly distributed in the polymeric composite prepared by the solvent evaporation method [32].
3.4. Dissolution test
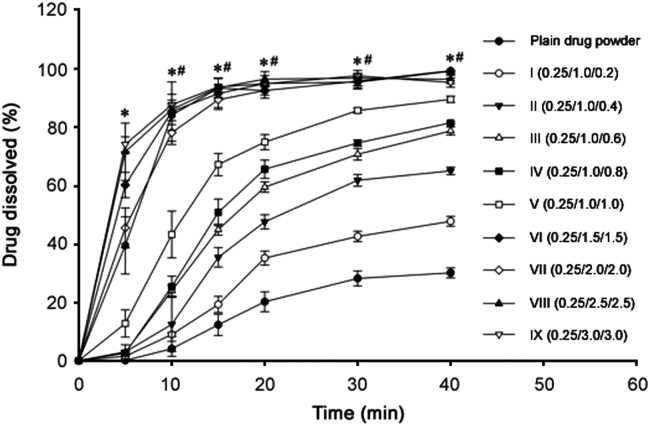
The dissolution profiles of the samples are shown in Fig. 3. Like solubility results, all the formulations significantly improved dissolution rate of silymarin. At 20 min, the dissolution of silymarin with plain powder, formulation I, II, III, IV, V, VI, VII, VIII and IX were (20.18±3.40)%, (35.19±2.41)%, (47.63±2.42)%, (59.57±1.79)%, (65.69±3.11)%, (74.98±2.59)%, (95.14±2.69)%, (92.69±1.52)%, (96.60±2.63)% and (95.01±0.46)%, respectively. Like the aqueous solubility results, dissolution rate results of silymarin with formulations VI-IX were significantly higher (P < 0.05) than those with formulations I-V; nevertheless, dissolution results with formulations VI-IX were not significantly different (P > 0.05) from one another. Thus, on the basis of the highest apparent aqueous solubility and excellent dissolution, silymarin-laden PVP-PEG polymeric formulation VI consisting of silymarin, PVP K-30 and PEG 6000 (0.25/1.5/1.5, w/w/w) was selected for further study. As the hydrophilic polymeric matrix dissolves, the loaded drug presents itself as very fine particles for rapid dissolution [29].
Fig. 3.
Effect of PVP K-30 and PEG 6000 on the dissolution of silymarin in the PVP-PEG polymeric composites. Each value denotes the mean ± S.D. (n = 6) * p < 0.05 compared with the plain drug powder and formulations I-V. #p > 0.05 compared with the formulations VI-IX.
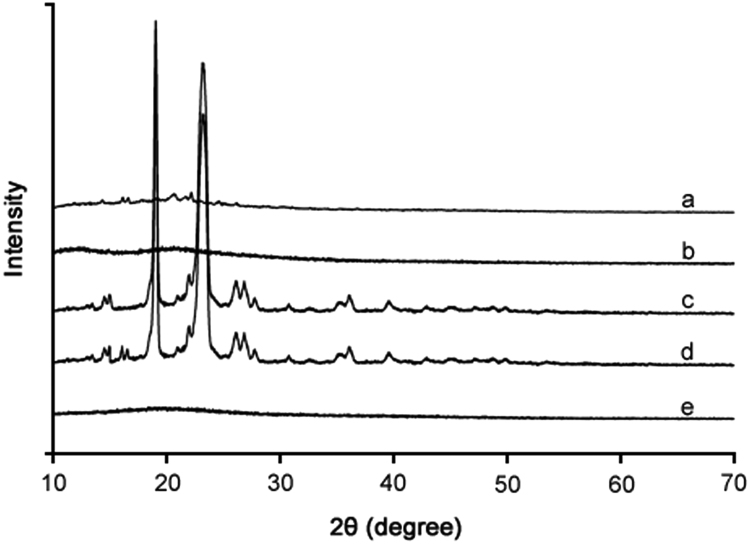
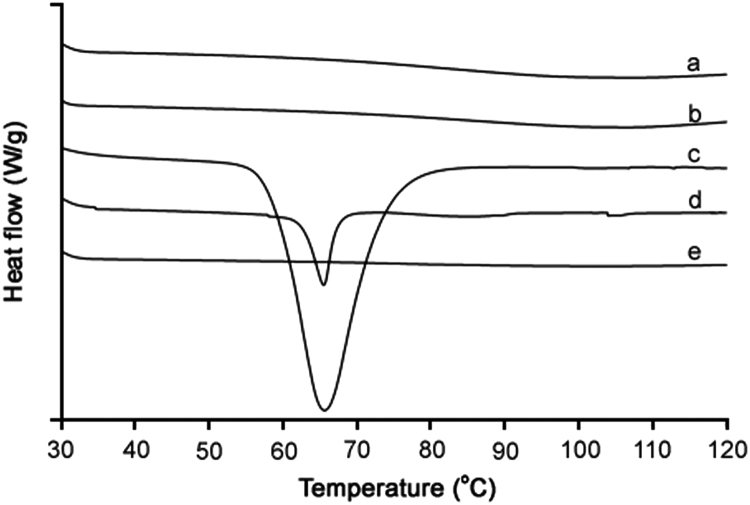
3.5. Crystalline property
The crystalline property of the samples was perused using XRD and DSC analyses. The XRD patterns and DSC thermograms are shown in Fig. 4 and Fig. 5, respectively. In the XRD analysis, silymarin plain powder showed some very minute peaks (Fig. 4a), suggesting the presence of some crystalline component in the extract. PVP K-30 did not generate any peak (Fig. 4b); thus, it was amorphous in nature. PEG 6000 produced some very sharp peaks in about 15–30° range (Fig. 4c), suggesting its semi-crystalline or crystalline nature. The physical mixture also exhibited sharp peaks (Fig. 4d). However, the crystalline components were converted to the amorphous state in the silymarin-laden PVP-PEG polymeric formulation VI (Fig. 4e) as no peak was observed in the XRD pattern. The DSC endotherms of silymarin plain powder (Fig. 5a) and PVP K-30 (Fig. 5b) did not show any sharp endotherm in 30–120 °C range; however, a broad slight curve in 70–120 °C range was observed in case of both the samples. This broad endothermic depression might be due to the escape of moisture from the sample [36]. This suggested that silymarin extract and PVP K-30 might be hygroscopic in nature. PEG 6000 showed a deep endotherm at about 65 °C, suggesting that it was crystalline in nature (Fig. 5c). This endotherm was also observed in the thermogram of physical mixture (Fig. 5d). On the other hand, thermogram of silymarin-laden PVP-PEG polymeric formulation VI did not show such an endotherm or broad depression (Fig. 5e). This suggested that all the components were converted into the amorphous state and the formulation was completely dry.
Fig. 4.
PXRD patterns: (a) plain drug powder, (b) PVP K-30, (c) PEG 6000, (d) physical mixture, and (e) silymarin-laden PVP-PEG polymeric composite VI.
Fig. 5.
DSC thermograms: (a) plain drug powder, (b) PVP K-30, (c) PEG 6000, (d) physical mixture, and (e) silymarin-laden PVP-PEG polymeric composite VI.
In the solvent-evaporation method of solid dispersion preparation, the drug and polymeric components are completely dissolved in a solvent to get a clear solution prior to subsequent drying. During drying process, the polymeric components exert inhibitory effect on recrystallization. Thus, in the final dry product, the drug molecules are uniformly scattered in the polymeric matrix and all the components usually exist in the amorphous state. The improvement in solubility and dissolution rate was due to better wetting of silymarin by the hydrophilic polymers, solubilizing effect of PEG 6000, complete transformation of the crystalline constituents to the amorphous form and molecular-level uniform intermingling of silymarin, PVP K-30 and PEG 6000 in the PVP-PEG polymeric composite. In amorphous forms, more surface area is exposed to the surrounding dissolution medium; therefore, dissolution is more.
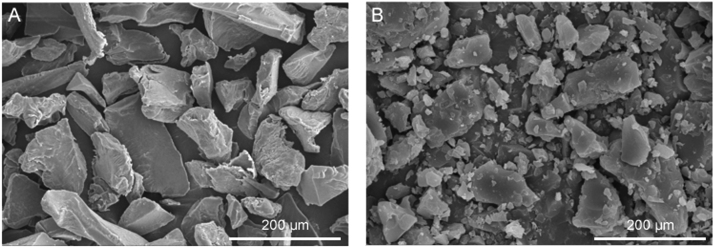
3.6. Shape and surface morphology
The morphological features of silymarin plain powder and silymarin-laden PVP-PEG polymeric formulation VI are shown in Fig. 6. The scanning electron micrograph of silymarin plain powder revealed irregular shape and surface of the particles (Fig. 6A). Likewise, the particles of silymarin-laden PVP-PEG polymeric formulation VI were of irregular shape and surface, and from the scanning electron micrograph it can be observed they were in the micro-size range (Fig. 6B).
Fig. 6.
SEM: (A) plain drug powder (× 200), and (B) silymarin-laden PVP-PEG polymeric composite VI (× 2000).
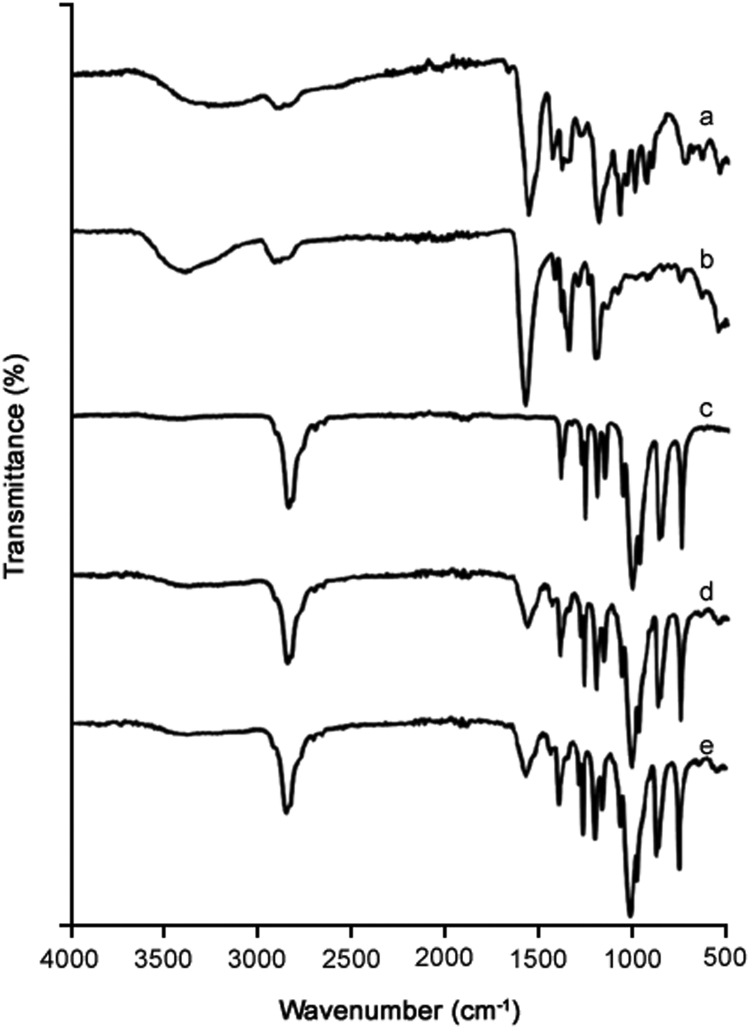
3.7. FTIR spectroscopic investigation
The FTIR spectra of silymarin plain powder (Fig. 7a), PVP K-30 (Fig. 7b), PEG 6000 (Fig. 7c), physical mixture (Fig. 7d) and silymarin-laden PVP-PEG polymeric formulation VI (Fig. 7e) are shown. The FTIR spectrum of silymarin plain powder revealed several peaks corresponding to flavonolignans. Peak appearing at 1083.7 cm−1 confirmed the benzopyran ring with concomitant presence of out of plane —C—H deformations at 820.2 cm−1. Peak appearing at 1633.4 cm−1 confirmed the presence of reactive flavonolignan ketone, whilst the aromatic ring stretching vibrations were evident at 1511.7 cm−1 [48], [49]. FTIR spectra of physical mixture and the formulation also revealed reactive flavonolignan ketone peak without shifting, thus endorsing the absence of interaction between silymarin and polymers. The remaining characteristic silymarin peaks were overlapped with polymers peaks; therefore, it was not possible to suggest any possible interactions between silymarin and polymers.
Fig. 7.
FTIR spectra: (a) plain drug powder, (b) PVP K-30, (c) PEG 6000, (d) physical mixture, and (e) silymarin-laden PVP-PEG polymeric composite VI.
3.8. Subsequent studies
The present method of preparation is very convenient, cost-efficient and simpler than the previously employed methods. Moreover, amelioration in the aqueous solubility and dissolution rate with silymarin-laden PVP-PEG polymeric composite VI is better than those obtained via the other costly methods [27], [50]. Accordingly, these rapidly dissolving silymarin-laden PVP-PEG polymeric composites might be a promising drug delivery system to administer the drug with improved solubility in the aqueous gastrointestinal fluid. However, in vivo evaluation in animal subjects will be carried out in our forthcoming studies to further investigate the effect of this formulation on the oral bioavailability of silymarin and their capability to protect hepatocytes.
4. Conclusion
Silymarin-laden PVP-PEG polymeric composite VI, consisting of silymarin, PVP K-30 and PEG 6000 at the weight ratio of 0.25/1.5/1.5 (w/w/w), showed the highest apparent aqueous solubility of the drug (24.39±2.95 mg/mL) and an excellent dissolution (~100% at 40 min). Moreover, all the components were in the amorphous state and had no drug-excipient or excipient-excipient interactions. The enhancement in solubility and dissolution is attributed to better wetting of silymarin by the hydrophilic polymers, solubilizing effect of PEG 6000, complete conversion of the crystalline components to the amorphous state and molecular level homogeneousness of silymarin, PVP K-30 and PEG 6000 in PVP-PEG polymeric composite. Accordingly, this formulation can be used as an effective drug delivery system to administer silymarin orally with enhanced solubility and dissolution rate.
Acknowledgments
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are thankful to University of Central Punjab and COMSATS University Islamabad (Lahore Campus) for providing research materials and lab facilities.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Kvasnička F., Bıba B., Ševčík R. Analysis of the active components of silymarin. J. Chromatogr. A. 2003;990:239–245. doi: 10.1016/s0021-9673(02)01971-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee J.I., Hsu B.H., Wu D. Separation and characterization of silybin, isosilybin, silydianin and silychristin in milk thistle extract by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A. 2006;1116:57–68. doi: 10.1016/j.chroma.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 3.Lu C., Lu Y., Chen J. Synchronized and sustained release of multiple components in silymarin from erodible glyceryl monostearate matrix system. Eur. J. Pharm. Biopharm. 2007;66:210–219. doi: 10.1016/j.ejpb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Post-White J., Ladas E.J., Kelly K.M. Advances in the use of milk thistle (Silybum marianum) Integr. Cancer Ther. 2007;6:104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 5.Gopalakrishnan R., Sundaram J., Sattu K. Dietary supplementation of silymarin is associated with decreased cell proliferation, increased apoptosis, and activation of detoxification system in hepatocellular carcinoma. Mol. Cell Biochem. 2013;377:163–176. doi: 10.1007/s11010-013-1582-1. [DOI] [PubMed] [Google Scholar]
- 6.Madani H., Talebolhosseini M., Asgary S. Hepatoprotective activity of Silybum marianum and Cichorium intybus against thioacetamide in rat. Pak. J. Nutr. 2008;7:172–176. [Google Scholar]
- 7.Pradeep K., Mohan C.V.R., Gobianand K. Silymarin modulates the oxidant–antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007;560:110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Parkki M. Blackwell Science Ltd Po Box 88; Osney Mead, Oxford, Oxon, England OX2 ONE: 1976. Effects of Silymarin on Carbontetrachloride, Paracetamol and Bromobenzene Caused Deterioration on Detoxifying Enzymes, Acta Physiologica Scandinavica. pp. 139. [Google Scholar]
- 9.Fintelmann V. Modern phytotherapy and its uses in gastrointestinal conditions. Planta Med. 1991;57:S48–S52. [PubMed] [Google Scholar]
- 10.Flora K., Hahn M., Rosen H. Milk thistle (Silybum marianum) for the therapy of liver disease. Am. J. Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P., Dragosics B., Dittrich H. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J. Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 12.Singh R.P., Agarwal R. A cancer chemopreventive agent silibinin, targets mitogenic and survival signaling in prostate cancer. Mutat. Res. 2004;555:21–32. doi: 10.1016/j.mrfmmm.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Foster S. Milk thistle: silybum marianum. Bot. Ser. 1991 [Google Scholar]
- 14.Floersheim G., Eberhard M., Tschumi P. Effects of penicillin and silymarin on liver enzymes and blood clotting factors in dogs given a boiled preparation of Amanita phalloides. Toxicol. Appl. Pharmacol. 1978;46:455–462. doi: 10.1016/0041-008x(78)90091-1. [DOI] [PubMed] [Google Scholar]
- 15.Dressman J., Butler J., Hempenstall J. The BCS: where do we go from here? Pharm. Technol. 2001;25:68–77. [Google Scholar]
- 16.Provinciali M., Papalini F., Orlando F. Effect of the silybin-phosphatidylcholine complex (IdB 1016) on the development of mammary tumors in HER-2/neu transgenic mice. Cancer Res. 2007;67:2022–2029. doi: 10.1158/0008-5472.CAN-06-2601. [DOI] [PubMed] [Google Scholar]
- 17.Škottová N., Krečman V. Dietary silymarin improves removal of low density lipoproteins by the perfused rat liver. Acta Univ. Palacki. Olomuc. Fac. Med. 1998;141:39–40. [PubMed] [Google Scholar]
- 18.Yang K.Y., Yousaf A.M., Hwang D.H. Silymarin-loaded solid nanoparticles provide excellent hepatic protection: physicochemical characterization and in vivo evaluation. Int. J. Nanomed. 2013;8:3333–3343. doi: 10.2147/IJN.S50683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goddeeris C., Coacci J., Van G. den Mooter, Correlation between digestion of the lipid phase of smedds and release of the anti-HIV drug UC 781 and the anti-mycotic drug enilconazole from smedds. Eur. J. Pharm. Biopharm. 2007;66:173–181. doi: 10.1016/j.ejpb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Leuner C., Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000;50:47–60. doi: 10.1016/s0939-6411(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 21.Perrut M., Jung J., Leboeuf F. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes: part I: micronization of neat particles. Int. J. Pharm. 2005;288:3–10. doi: 10.1016/j.ijpharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Woo J.S., Kim T.-S., Park J.-H. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharm. Res. 2007;30:82–89. doi: 10.1007/BF02977782. [DOI] [PubMed] [Google Scholar]
- 23.Barzaghi N., Crema F., Gatti G. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990;15:333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 24.Arcari M., Brambilla A., Brandt A. A new inclusion complex of silibinin and beta-cyclodextrins: in vitro dissolution kinetics and in vivo absorption in comparison with traditional formulations. Boll. Chim. Farm. 1992;131:205–209. [PubMed] [Google Scholar]
- 25.Morazzoni P., Magistretti M., Giachetti C. Comparative bioavailability of silipide, a new flavanolignan complex, in rats. Eur. J. Drug Metab. Pharmacokinet. 1992;17:39–44. doi: 10.1007/BF03189986. [DOI] [PubMed] [Google Scholar]
- 26.Chen W., Xia H., Wu W. Optimized preparation of silymarin dripping pills by a central composite design response surface method. Chin. Trad. Herb. Drug. 2005;36:679–683. [Google Scholar]
- 27.Hwang D.H., Kim Y.-I., Cho K.H. A novel solid dispersion system for natural product-loaded medicine: silymarin-loaded solid dispersion with enhanced oral bioavailability and hepatoprotective activity. J. Microencapsul. 2014;31:619–626. doi: 10.3109/02652048.2014.911375. [DOI] [PubMed] [Google Scholar]
- 28.Chiou W.L., Riegelman S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971;60:1281–1302. doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- 29.Vasconcelos T., Sarmento B., Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today. 2007;12:1068–1075. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Ming-Thau S., Ching-Min Y., Sokoloski T.D. Characterization and dissolution of fenofibrate solid dispersion systems. Int. J. Pharm. 1994;103:137–146. [Google Scholar]
- 31.Modi A., Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS PharmSciTech. 2006;7:E87–E92. doi: 10.1208/pt070368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joe J.H., Lee W.M., Park Y.-J. Effect of the solid-dispersion method on the solubility and crystalline property of tacrolimus. Int. J. Pharm. 2010;395:161–166. doi: 10.1016/j.ijpharm.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Patel T., Patel L., Patel T. Enhancement of dissolution of fenofibrate by solid dispersion technique. Int. J. Res. Pharm. Sci. 2010;1:127–132. [Google Scholar]
- 34.Craig D.Q.M. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 2002;231:131–144. doi: 10.1016/s0378-5173(01)00891-2. [DOI] [PubMed] [Google Scholar]
- 35.Taylor L., Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 1997;14:1691–1698. doi: 10.1023/a:1012167410376. [DOI] [PubMed] [Google Scholar]
- 36.Yousaf A.M., Kim D.W., Oh Y.-K. Enhanced oral bioavailability of fenofibrate using polymeric nanoparticulated systems: physicochemical characterization and in vivo investigation. Int. J. Nanomed. 2015;10:1819–1830. doi: 10.2147/IJN.S78895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yousaf A.M., Kim D.W., Kim D.S. Influence of polyvinylpyrrolidone quantity on the solubility, crystallinity and oral bioavailability of fenofibrate in solvent-evaporated microspheres. J. Microencapsul. 2016;33:365–371. doi: 10.1080/02652048.2016.1194906. [DOI] [PubMed] [Google Scholar]
- 38.Moin A.M., Patel C., Dave J. Validated method for silymarin by spectrophotometry in bulk drug and pharmaceutical formulations. J. Chem. Pharm. Res. 2010;2:396–400. [Google Scholar]
- 39.Shah V., Konecny J., Everett R. In vitro dissolution profile of water-insoluble drug dosage forms in the presence of surfactants. Pharm. Res. 1989;6:612–618. doi: 10.1023/a:1015909716312. [DOI] [PubMed] [Google Scholar]
- 40.Shah V.P., Noory A., Noory C. In vitro dissolution of sparingly water-soluble drug dosage forms. Int. J. Pharm. 1995;125:99–106. [Google Scholar]
- 41.Kim K.S., Jin S.G., Mustapha O. Novel fenofibric acid-loaded controlled release pellet bioequivalent to choline fenofibrate-loaded commercial product in beagle dogs. Int. J. Pharm. 2015;490:273–280. doi: 10.1016/j.ijpharm.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 42.Lee S.N., Poudel B.K., Tran T.H. A novel surface-attached carvedilol solid dispersion with enhanced solubility and dissolution. Arch. Pharm. Res. 2013;36:79–85. doi: 10.1007/s12272-013-0008-7. [DOI] [PubMed] [Google Scholar]
- 43.B.C. Sherman, Choline fenofibrate delayed release compositions, United States Patent Application 20120225946, 2012.
- 44.Srinarong P., Faber J., Visser M. Strongly enhanced dissolution rate of fenofibrate solid dispersion tablets by incorporation of superdisintegrants. Eur. J. Pharm. Biopharm. 2009;73:154–161. doi: 10.1016/j.ejpb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Li F.Q., Hu J.H. Improvement of the dissolution rate of silymarin by means of solid dispersions. Chem. Pharm. Bull. 2004;52:972–973. doi: 10.1248/cpb.52.972. [DOI] [PubMed] [Google Scholar]
- 46.Qiu M.F., Jia W., Li S.S. A new silymarin preparation based on solid dispersion technique. Adv. Ther. 2005;22:595–600. doi: 10.1007/BF02849953. [DOI] [PubMed] [Google Scholar]
- 47.Sun N., Wei X., Wu B. Enhanced dissolution of silymarin/polyvinylpyrrolidone solid dispersion pellets prepared by a one-step fluid-bed coating technique. Powder Technol. 2008;182:72–80. [Google Scholar]
- 48.Das S., Roy P., Auddy R.G. Silymarin nanoparticle prevents paracetamol-induced hepatotoxicity. Int. J. Nanomed. 2011;6:1291. doi: 10.2147/IJN.S15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Nahas A.E., Allam A.N., Abdelmonsif D.A. Silymarin-loaded eudragit nanoparticles: formulation, characterization, and hepatoprotective and toxicity evaluation. AAPS PharmSciTech. 2017;18:3076–3086. doi: 10.1208/s12249-017-0799-9. [DOI] [PubMed] [Google Scholar]
- 50.Seo Y.G., Kim D.W., Yousaf A.M. Solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced oral bioavailability of poorly water-soluble tacrolimus: physicochemical characterisation and pharmacokinetics. J. Microencapsul. 2015;32:503–510. doi: 10.3109/02652048.2015.1057252. [DOI] [PubMed] [Google Scholar]