Hematopoietic stem cells (HSC) are characterized by the ability to self-renew and produce all blood cell lineages throughout life.1 HSC hold enormous value in cell therapy applications aimed at treating hematologic disorders. During ontogeny, HSC are distributed in multiple sites until they subsequently seed the fetal liver (FL) for expansion before migrating to the bone marrow (BM). Each stage is characterized by distinct transcriptional programs and expression of unique surface markers in addition to a subset of generic HSC markers.2 The early stages of HSC development provide important insights into the molecular programs that govern HSC origin and expansion.2,3 Most studies of HSC ontogeny are established in mouse models and the lack of common HSC markers enabling differentiation between mice and human cells makes it difficult to isolate human HSC with optimal purity.4 In general, human HSC are enriched by positively selecting for CD34 and CD90 cells and by negatively selecting for CD38 and CD45RA.5 However, FL HSC are further exclusively enriched with GPI-80 (VNN2) and umbilical cord blood (UCB) HSC with CD49f, showing that human HSC express a subset of unique surface markers during ontogeny.6,7 Using existing surface markers, human HSC are typically enriched with a suboptimal purity of 5 to 10%, which prohibits studies of a pure HSC population, and argues for the need to identify additional markers. Endothelial protein C receptor (EPCR), also known as CD201, is encoded by the PROCR gene. EPCR is the cell surface receptor for activated protein C and essential for anticoagulation and to mediate anti-inflammatory signals.8 A recent study identified EPCR as a marker to purify pre-HSC at the single cell level from mouse AGM region (E11).9 EPCR has also been shown to be a reliable marker for murine HSC from both FL and BM, indicating that it is expressed throughout mouse HSC ontogeny.10,11 However, earlier studies of human cells suggest that EPCR is not associated with HSC activity in adult human BM.11 Recently, it was shown that UM171, a pyrimidoindole compound known to expand human long-term HSC, upregulated EPCR expression in human UCB HSC and that the EPCR+ fraction contained the engraftable HSC.12,13 Gene expression profiling studies have further shown that EPCR is preferentially expressed in HSC compared to multipotent progenitors from both human UCB and FL.7,14 In this study, we therefore carefully evaluated EPCR as a HSC marker during these stages of human ontogeny.
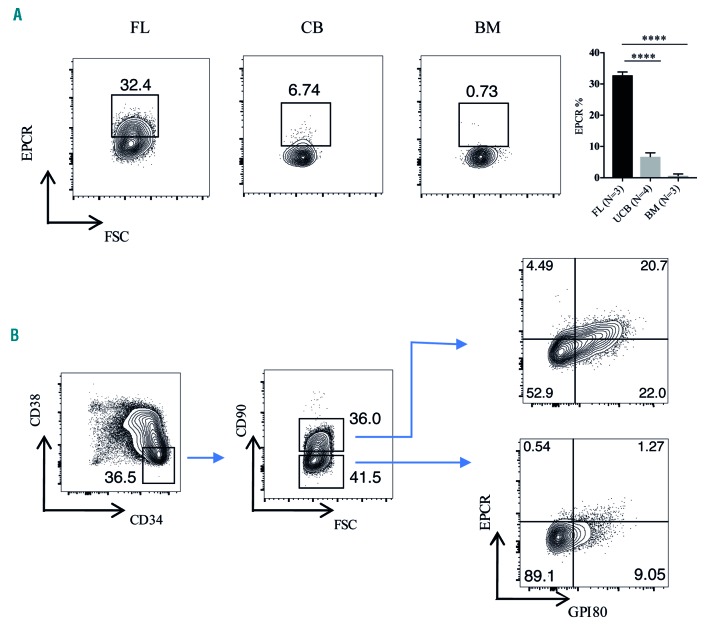
We first examined cell surface EPCR expression using flow cytometry in hematopoietic stem and progenitor cells (HSPC) from human FL, UCB and BM. We observed distinct EPCR expression in the CD34-enriched cells from FL and UCB (Online Supplementary Figure S1). We also observed significant EPCR expression in the CD34+CD38−CD90+ population of FL (Figure 1A), which is known to contain the HSC.7 The frequency of EPCR+ cells within the HSC-enriched population from UCB (CD34+CD38−CD45RA−CD90+) was drastically lower than that in FL, and we did not detect any EPCR+ cells in the BM HSC fraction (CD34+CD38−CD45RA−CD90+) (Figure 1A). Of note, EPCR expression was almost exclusively restricted to the HSC-enriched fractions of both FL and UCB CD34+ cells (Online Supplementary Figure S2) suggesting that it could be a useful marker of HSC in these two sources of cells.
Figure 1.
The expression of endothelial protein C receptor during different stages of human hematopoietic stem cell ontogeny. (A) EPCR expression in human FL (week 16), UCB and BM HSC. Cells were gated on the CD34+CD38−CD90+ population for FL cells and CD34+CD38−CD45RA−CD90+ population for UCB and BM cells. (B) FL CD34-enriched HSPC were stained for CD34, CD38, CD90, EPCR and GPI-80, and analyzed by flow cytometry. The gating strategy to evaluate EPCR and GPI-80 co-expression in CD34+CD38−CD90+ and CD34+CD38−CD90− populations is shown. EPCR: endothelial protein C receptor; FSC: forward scatter; FL: fetal liver; UCB: umbilical cord blood; BM: bone marrow; HSC: hematopoietic stem cells; HSPC: hematopoietic stem and progenitor cells. ****P≤0.0001.
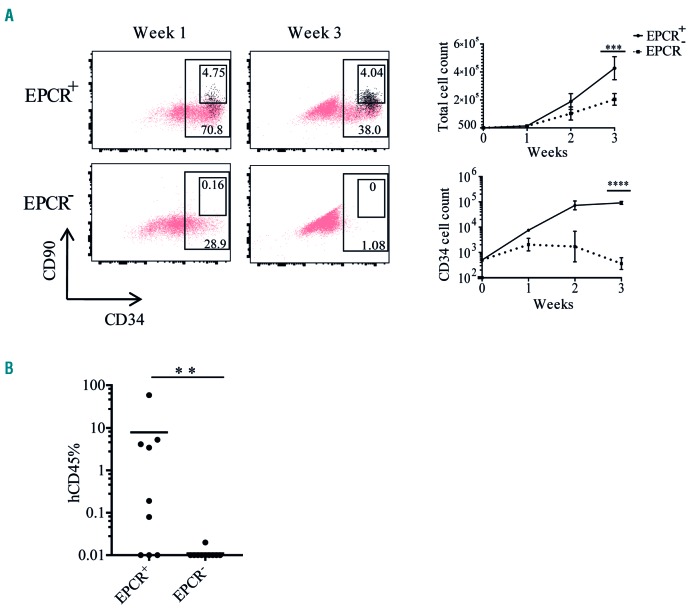
Next, we examined EPCR expression in FL in more detail. To evaluate whether EPCR expression is associated with the recently identified FL HSC marker GPI-80,7 we analyzed EPCR and GPI-80 expression within the CD34+CD38−CD90+ population. The vast majority of EPCR+ cells expressed GPI-80 and, more importantly, the GPI-80+ population was divided in terms of EPCR expression (Figure 1B). To assess whether EPCR is a marker of functional HSPC activity, we evaluated the in vitro expansion activity of EPCR+ and EPCR− cells obtained from the CD34+CD38−CD90+ population. Following 1 week of culture, most of the progeny of the EPCR+ fraction maintained expression of CD34 and some cells also expressed the HSC-associated marker CD90 as well as EPCR, whereas the EPCR− fraction had lost almost all CD34 expression and failed to generate any CD90+ or EPCR+ cells (Figure 2A). Similarly, at 3 weeks of culture, progeny of the EPCR+ fraction still retained expression of CD34, CD90 and EPCR, while the EPCR− fraction had completely lost all CD34 expression, indicating that the most immature portion of functionally active HSPC is highly enriched in the EPCR+ fraction (Figure 2A). Accordingly, the EPCR+ cell fraction also showed a substantially larger total cell expansion over time, compared to the EPCR− fraction (Figure 2A). To determine further whether EPCR enriches for bona fide long-term repopulating HSC, we transplanted EPCR+ and EPCR− cells, obtained from the FL CD34+CD38−CD90+ population, into sub-lethally irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NSG) mice. EPCR+ cells generated robust multilineage engraftment whereas the EPCR− fraction failed to engraft (Figure 2B and Online Supplementary Figure S3A). Among the engrafted mice we also observed CD34+CD38low cells which contain HSPC (Online Supplementary Figure S3B). Altogether the above data demonstrate that EPCR is a defining marker of human FL HSC, which, in combination with current markers, should enable isolation of higher purity HSC.
Figure 2.
Endothelial protein C receptor is a marker of fetal liver hematopoietic stem cells. (A) To measure the in vitro expansion activity of EPCR+ and EPCR− populations obtained from the FL CD34+CD38−CD90+ population, 500 cells from each population were sorted and expanded in serum-free expansion medium (SFEM) supplemented with STF (stem cell factor, FMS-like tyrosine kinase 3 ligand and thrombopoietin). The first panel shows the flow cytometric analysis of the HSPC markers CD34 and CD90 in the progeny of EPCR+ and EPCR− populations. EPCR+ cells are highlighted in black. The second panel shows the expansion rate of total cells and CD34+ cells derived from EPCR+ and EPCR− cells over 3 weeks in culture. The experiment was performed in three independent samples in duplicate. Error bars represent the mean ± standard deviation. (B) To evaluate the in vivo repopulating activity of EPCR+ and EPCR− populations, FACS-sorted cells from both populations were transplanted into sublethally (300 cGy) irradiated NSG mice (1000 cells/mouse) and the levels of human cell engraftment in BM were analyzed after 20 weeks. The experiments were performed for two samples. EPCR: endothelial protein C receptor; HSPC: hematopoietic stem and progenitor cells; BM: bone marrow. ***P≤0.001; ****P≤0.0001.
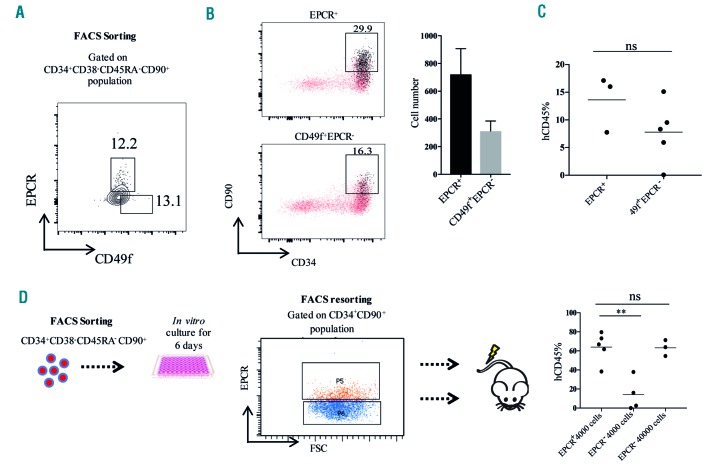
To further assess the relevance of EPCR expression in human UCB-derived cells we evaluated it in combination with CD49f, which together with CD34+CD38−CD45RA−CD90+ can be used to enrich HSC to a purity of 5 to 10%.6 To explore whether EPCR expression specifically marks UCB HSC, we analyzed the in vitro expansion activity of CD34+CD38− CD45RA−CD90+EPCR+ and CD34+CD38−CD45RA− CD90+CD49f+EPCR− populations (Figure 3A). Following 1 week of in vitro culture, we observed that the progeny from both populations were able to generate CD34+CD90+ HSPC. Surprisingly progeny from the EPCR− population generated EPCR+ cells that were enriched within the CD34+CD90+ HSPC fraction (Figure 3B), indicating fluctuations of EPCR expression and that there may be a population of EPCR−cells at the apex of the HSPC hierarchy in UCB. A similar phenomenon has been observed for the HSC-associated CD90 marker, with CD90− HSPC consistently generating CD90+ HSC both during in vitro culture and in vivo in transplanted NSG mice.6 To evaluate the in vivo repopulating activity of the EPCR+ and CD49f+EPCR− HSC populations derived from the CD34+CD38−CD45RA−CD90+ cells, we transplanted both populations into sublethally irradiated NSG mice and found that both populations were capable of establishing robust multilineage engraftment (Figure 3C and Online Supplementary Figure S4A,B). Further staining with CD34 and CD38 revealed that both EPCR+ and CD49f+EPCR− HSC populations generated CD34+CD38low HSPC in the NSG BM (Online Supplementary Figure S4C). The above data support the existence of EPCR+ HSC in human UCB; however, unlike what we found for FL, EPCR does not exclusively separate the HSC pool in UCB. Although we did not detect any significant difference in the engraftment levels between EPCR+ and EPCR− cells more experiments would have to be carried out to assess whether there are any qualitative differences between these HSC populations. Recently Fares et al. identified EPCR as a reliable surface marker of in vitro-expanded UCB HSC.12 In accordance with that study, we observed that the NSG repopulating activity of in vitro-expanded UCB cells was highly enriched in the CD34+CD90+EPCR+ fraction, and that EPCR marked the vast majority of repopulating HSC in the cultured UCB cells (Figure 3D and Online Supplementary Figure S5A) without any skewing in the lineages (Online Supplementary Figure S5B). Both HSC isolated from FL and UCB HSC in culture are known to be actively cycling, while the majority of freshly isolated UCB CD34+CD38-cells are quiescent.7,15 Since the FL HSC activity was exclusively present in the EPCR+ fraction, and in vitro culture with cytokines increased the fraction of EPCR+ UCB HSC, we hypothesized that EPCR expression in human HSC may be linked to the cell cycle. Cell cycle analysis of UCB CD34+ cells showed that the vast majority of both CD34+CD38−EPCR+ and CD34+CD38−EPCR− cells reside in the G0 phase (Online Supplementary Figure S6), suggesting that EPCR expression is not specifically associated with an active cell cycle state of human HSC.
Figure 3.
Endothelial protein C receptor expression in umbilical cord blood hematopoietic stem cells. (A) The gating strategy used to obtain the UCB-derived EPCR+ and 49f+EPCR− populations. (B) To measure the in vitro expansion activity of EPCR+ and CD49f+EPCR− populations, 500 cells from each population were sorted and expanded in serum-free expansion medium (SFEM) supplemented with STF (stem cell factor, FMS-like tyrosine kinase 3 ligand and thrombopoietin). Flow cytometric analysis of CD34, CD90 and EPCR expression in the progeny of UCB-derived EPCR+ and CD49f+EPCR- populations is shown. EPCR+ cells are highlighted in black. The experiment was performed in three independent samples in duplicate. (C) To evaluate the in vivo repopulating activity of EPCR+ and CD49f+EPCR− populations, 200 cells from each population were sorted and transplanted into sublethally (300 cGy) irradiated NSG mice and the levels of human cell engraftment in the BM were analyzed after 16 weeks. (D) To evaluate whether EPCR expression enriches for long-term HSC in cultured UCB HSPC, EPCR+ cells (4000/mouse) and EPCR− cells (4000/mouse in one group and 40000/mouse in the second group) derived from CD34+CD90+ population were transplanted into sublethally irradiated NSG mice. At week 16, the frequency of hCD45 cells in BM was evaluated. EPCR: endothelial protein C receptor; UCB: umbilical cord blood; BM: bone marrow; HSPC: hematopoietic stem and progenitor cells. **P≤0.01.
Overall the above data suggest that EPCR is expressed during earlier stages of human HSC ontogeny and is gradually lost as the cells migrate to the BM, in contrast to the situation in mice, in which it marks HSC throughout ontogeny. Although human and murine HSC are characterized by distinct markers, our study supports that EPCR expression is conserved during early ontogeny. EPCR may mark a more fetal-associated subset of human HSC and it is possible that UCB contains a mixture of such fetal-associated HSC and more adult-like HSC. This could have implications for efforts to expand UCB HSC for clinical benefit, as different culture conditions may influence these two subsets distinctly. Further studies are required to assess this in greater detail. Identifying suitable HSC markers during early stages of human ontogeny paves the way to study molecular regulators of HSC emergence and expansion which eventually could lead to the development of in vitro HSC production and expansion for clinical applications. The addition of EPCR to existing markers could enhance the enrichment of FL HSC.
Supplementary Material
Acknowledgements
We thank Roman Galeev and Kees-Jan Pronk for providing normal bone marrow samples. We would also like to thank the staff at BMC FACS core and the animal house facilities for their excellent support.
Footnotes
Funding: this work was funded by grants from the Swedish Research Council, the Swedish Cancer Foundation, the Swedish Pediatric Cancer Foundation, Knut och Alice Wallenbergs Stiftelse and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement n. 648894) to JL. The work was also supported by the HematoLinné and StemTherapy programs at Lund University.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. [DOI] [PubMed] [Google Scholar]
- 2.McKinney-Freeman S, Cahan P, Li H, et al. The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell. 2012;11(5):701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe RG, Mandelbaum J, Zon LI, Daley GQ. Engineering hematopoietic stem cells: lessons from development. Cell Stem Cell. 2016;18(6):707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larochelle A, Savona M, Wiggins M, et al. Human and Rhesus macaque hematopoietic stem cells cannot be purified based only on SLAM family markers. Blood. 2011;117(5):1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notta F, Doulatov S, Laurenti E, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. [DOI] [PubMed] [Google Scholar]
- 7.Prashad SL, Calvanese V, Yao CY, et al. GPI-80 defines self-renewal ability in hematopoietic stem cells during human development. Cell Stem Cell. 2015;16(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood. 2014;124(10):1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Li XL, Wang WL, et al. Tracing haematopoietic stem cell formation at single-cell resolution. Nature. 2016;533(7604):487–492. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki H, Arai F, Kubota Y, Dahl M, Suda T. Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood. 2010;116(4):544–553. [DOI] [PubMed] [Google Scholar]
- 11.Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107(6):2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fares I, Chagraoui J, Lehnertz B, et al. EPCR expression marks UM171-expanded CD34+ cord blood stem cells. Blood. 2017;129(25):3344–3351. [DOI] [PubMed] [Google Scholar]
- 13.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doulatov S, Vo LT, Chou SS, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13(4):459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chute JP, Saini AA, Chute DJ, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100(13):4433–4439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.