Acute myeloid leukemia (AML) represents a heterogeneous group of disorders characterized by the rapid expansion of immature myeloid cells (blasts) in the bone marrow (BM). There is wide disease heterogeneity, and patient risk-stratification principally relies on cytogenetic-molecular data.1 A proportion of patients are refractory to standard first-line chemotherapy, and current salvage therapy rarely yields durable remissions. The failure of current therapies to eradicate leukemic initiating cells (LIC) and chemotherapy refractoriness/toxicity are the major causes underlying AML progression/relapse. The high rate of mortality and morbidity in AML reinforces the need for greater understanding of the leukemic BM microenvironment to support the development of more durable front-line treatments.
Bone-marrow mesenchymal stem/stromal cells (BMSCs) are key components of the hematopoietic niche thought to contribute to leukemia pathogenesis. BMSCs maintain hematopoiesis and coordinately regulate regenerative responses through dynamic cell-cell interaction and paracrine communication with hematopoietic stem and progenitor cells (HSPCs).2 Additionally, healthy BMSCs have proven potential as “live” medicines for cell therapy applications thanks to their anti-inflammatory and/or regenerative properties.2 Importantly, BMSCs have been implicated in the pathogenesis of multiple hematologic malignancies. BMSCs from AML patients have been poorly characterized thus far and conflicting results exist.3,4 Previously, we performed a detailed characterization of BMSCs from a large cohort of AML BMSCs from a large cohort of AML patients risk-stratified as low- (LR), intermediate- (IR) and high-risk (HR), and reported that differences in the in vitro cytokine secretion of AML-derived BMSCs (AML-BMSC) correlate with patient overall survival despite the fact that they were not tumor-related, and demonstrated only minimal functional differences from BMSCs derived from age-matched healthy donors (HD-BMSCs).5 Since HSPC failure, aberrant myeloid differentiation, hematopoietic displacement, therapy resistance, and clonal evolution all underlie the pathogenesis of AML, we herein wanted to further understand the contribution of BMSCs to the pathogenesis of AML. We have thus analyzed the capacity of BMSCs risk-stratified AML patients (i) to support cultured CD34+ HSPC and their in vivo repopulating potential, and (ii) to suppress inflammation in vivo using an experimental model of severe colitis.
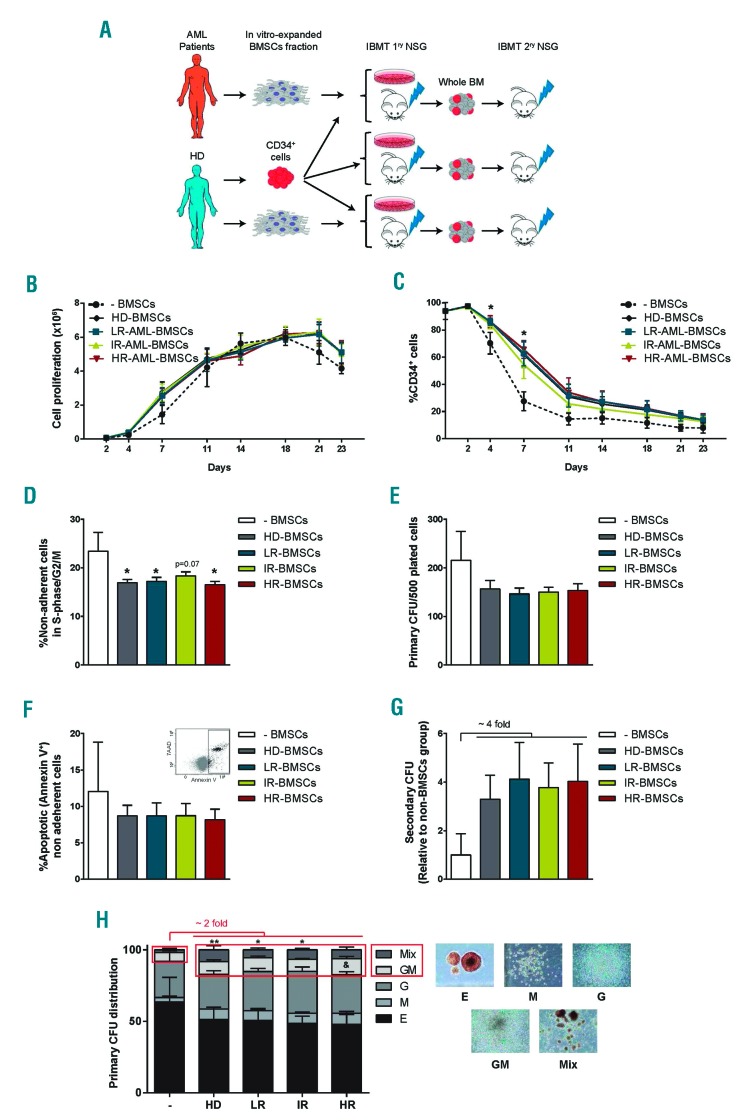
Several groups have provided evidence of a role for BMSCs in AML. The precise role of these cells however, remains unclear. One hypothesis is that AML-BMSCs become intrinsically altered such that they adopt a phenotype that provides reduced support for normal hematopoiesis.6, 7 To address this hypothesis, we co-cultured BMSCs obtained from 15 risk-stratified AML patients (5 from each risk group, Online Supplementary Table S1) and 5 age-matched HD with CD34+ HSPCs purified from umbilical cord blood (CB) (Figure 1A). BMSCs were fully characterized elsewhere and were always used in early passage (p1-p3).5 CB-CD34+ cells cultured in cytokine-supplemented media alone (-BMSCs) were used as a further control to compare the overall capacity of BMSCs from either source to support HSPCs. Over a 23-day period, CB-CD34+ cells expanded to give rise to a similar number of total cells (Figure 1B) concomitant with cellular differentiation as measured by loss of CD34+ cells (Figure 1C), regardless of whether they were cultured without or with BMSCs, either from HD or AML patients. Compared to cytokine-alone controls, however, co-culture of CD34+ cells with either HD-or AML-BMSCs, resulted in a significant delay in differentiation within the initial 7-8 days of culture, as measured by retention of CD34 expression (Figure 1C). This correlated with a consistent decrease in the frequency of cycling CD34+ HSPCs (Figure 1D). On the other hand, CD34+ cells underwent apoptosis at the same frequency in all BMSC cultures, suggesting a major role for BMSCs in reducing proliferation and differentiation (Figure 1E). To determine whether AML-BMSCs have specific impairments in their capacity to maintain functional hematopoietic progenitor cells, non-adherent cells were harvested for 4 days in AML- and HD-BMSCs co-cultures and plated in serial CFC. While neither HD- nor AML-BMSCs affected the frequency of the myeloid committed progenitors detected (Figure 1F), cells co-cultured with BMSCs showed a 4-fold increase (n=9 per risk group, P≥0,05) in the overall number of CFC detected after serial re-plating in secondary assays (Figure 1G). The enhanced clonogenic capacity detected in secondary, but not primary CFC was similar in HD, LR, IR and HR-BMSCs co-cultures, suggesting that, as detected with healthy BMSCs, AML-BMSCs support the maintenance of HSPCs with a more immature phenotype linked to a higher long-term differentiation potential. This notion is supported by the phenotype/scoring of the primary CFCs, which consistently showed a significantly higher frequency of immature CFC-Mix from CD34+ cells exposed to either HD- or AML-BMSCs (8.7±3.2% -BMSCs vs. 13.3±2.4% HD-, 11.1±1.4% LR-, 11.3±1.1% IR- and 13.3±2.1% HR-AML-BMSCs, Figure 1H). A similar significant trend was found among primitive CFC-GM colonies.
Figure 1.
BMSCs from risk-stratified AML patients support in vitro homeostasis of CB-CD34+ HSPCs cells. (A) Schematic depicting the experimental design. (B) In vitro expansion kinetics of CB-CD34+ HSPCs alone (n=9) or co-cultured on BMSCs from HD (n=9) and AML patients (n=27; LR, n=9; IR, n=9; HR, n=9). This “n” is applicable to all subsequent graphs of this figure. (C) Kinetics of loss of CD34 antigen over time in CD34:BMSCs co-cultures. (D) Proportion of cycling CD34+ cells measured at day 11 of CD34:BMSCs co-cultures. (E) Proportion of apoptotic CB-CD34+ cells (Annexin V+) measured at day 11 of CD34:BMSCs co-cultures. (F) The primary clonogenic potential of CB-CD34+ HSPCs previously co-cultured 4 days with HD- or AML-BMSCs. (G) Clonogenic potential of CD34+ progenitors replated in secondary CFC assays normalized against -BMSCs. CFU-Mix (also known as CFU-GEMM) is a Colony-Forming Unit-Granulocyte, Erythrocyte, Monocyte/macrophage, Megakaryocyte. (H) Scoring of primary CFCs. CD34+ cells not previously exposed to BMSCs, were used as base-line control for CD34: BMSCs co-cultures. Because HSPCs gradually lose CD34 expression, we refer to them, we refer to them as “non-adherent cells” rather than “CD34+ cells”. *P< 0.05; ** P<0.01 for CFU-Mix and & P<0.05 for CFU-GM between BMSC groups and -BMSCs.
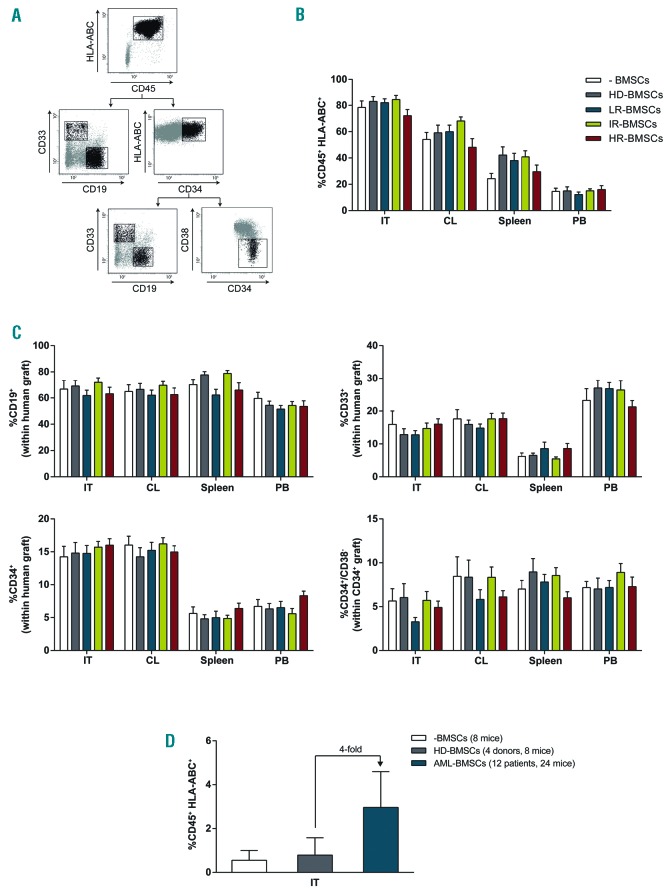
We next compared the ability of CB-CD34+ cells cultured alone, or co-cultured with HD- and AML-BMSC to reconstitute the hematopoietic system of NSG mice. Hence, 5×104 CB-CD34+ cells cultured for 4 days with 3×105 HD- or risk-stratified AML-BMSCs were both co-transplanted intratibia in irradiated NSG mice, and were then sacrificed 7 weeks later for chimerism analysis in the injected tibiae, contralateral (CL) bone, peripheral blood (PB) and spleen (Figure 1A). Human multilineage reconstitution was determined by flow cytometry using anti-CD45, anti-HLA-ABC, anti-CD19, anti-CD33 and anti-CD34 (Figure 2A). CD34+ cells co-transplanted with either HD- or risk-stratified AML-BMSCs conferred similar levels of engraftment, ranging between 72% and 85% (Figure 2B). As a defining property of repopulating cells is the migration to and multilineage engraftment in multiple hematopoietic tissues, we next assessed the ability of transplanted CD34+ cells to migrate to the CL tibiae and extramedullary tissues and differentiate into myeloid and lymphoid lineages. Human cell engraftment in the hematopoietic tissues of all transplanted animals was always multilineage and consisted of CD19+ B-lymphoid cells (~60-70%), CD33+ myeloid cells (~15-20%) as well as primitive CD34+ (~5-15%) and CD34+CD38− progenitor cells (~5-15% of the CD34+ graft). Mice injected with CD34+ cells cultured and transplanted alone, or co-cultured and co-transplanted with either HD- or AML-BMSCs co-cultures showed very similar levels of hematopoietic reconstitution in CL, PB and spleen (Figure 2C). When equal numbers of primograft human engrafted cells were transplanted into secondary recipients, we found that although the differences are not significant, HSPCs initially exposed to AML-BMSCs displayed a higher (P=0.1) long-term BM hematopoietic reconstitution than those HSPCs exposed to HD-BMSCs or without previous co-culture with BMSCs, suggesting that prior physical interaction of HSPCs with AML-BMSCs light help to preserve long-term hematopoietic reconstituting potential (Figure 2D). Although in our experimental setting BMSCs were not as supportive of HSPCs as in previous studies,8 overall, these co-culture studies agree with recent studies9 and suggest that AML-BMSCs might have a similar capacity as HD-BMSCs to maintain CD34+ cells in vitro. However, different findings by others6,7 might be due to different experimental conditions and our conclusions might not hold true for endogenous BMSCs in AML, which have been suggested to poorly support normal haematopoiesis in vivo.10–12
Figure 2.
BMSCs from risk-stratified AML patients do not impair in vivo multilineage repopulating function of CB-CD34+ HSPCs. (A) Representative FACS analysis of the graft. The human graft is identified as the CD45+HLA-ABC+ fraction. The CD45+ human graft comprises B-lymphoid cells (CD19+), myeloid cells (CD33+) as well as immature CD34+ and CD34+CD38− cells. (B) Long-term multilineage hematopoietic reconstitution in the IT, CL, PB and spleen of NSG mice sacrificed 6-7 weeks after intra BM injection of CB-CD34+ HSPCs alone (n=5) or co-cultured for 4 days in BMSCs from HD (n=5) or AML patients (n=15; LR, n=5; IR, n=5; HR, n=5). n=90 mice, 4 per donor/patient. (C) Multilineage and multiorgan human chimerism demonstrating that AML-BMSCs do not negatively impact migration of HSPCs from the IT. No differences in the graft composition were found between CD34+ HSPCs co-cultured with BMSCs from HD or risk-stratified AML patients. (D) Long-term BM hematopoietic reconstitution assessed upon serial transplantation of primografted cells. IT: injected tibia; CL: contralateral tibia and femur; PB: peripheral blood.
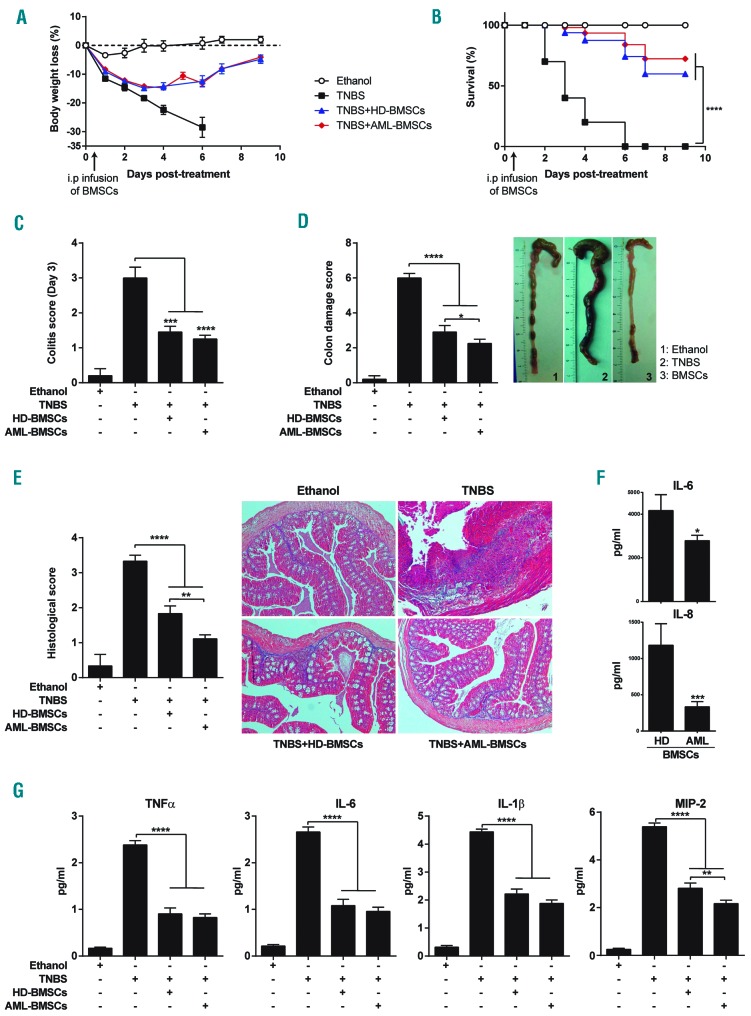
HD-BMSCs display robust immunomodulatory and anti-inflammatory properties both in vitro and in vivo. In our previous work, we have shown that AML-BMSCs not only retain their immune suppressive properties, but also that this function is enhanced compared to HD-BMSCs.5 Despite the fact that the anti-inflammatory properties of BMSCs could potentially contribute to the disease state in AML, to date, the in vivo anti-inflammatory properties of BMSCs from patients suffering from hematological malignancies remain unknown. We therefore investigated the in vivo anti-inflammatory properties of BMSCs derived from risk-stratified AML patients by taking advantage of a pre-clinical model of acute colitis that recapitulates clinical, histopathological, and immunological features similar to those of the human Crohn’s disease.13–15 Inflammatory colitis was induced by intracolonic administration of TNBS, and mice were i.p. infused with either 106 HD- or AML-BMSCs 12 hours after TNBS instillation. TNBS-treated mice developed a severe acute illness characterized by substantial (>20%) and sustained body weight loss (Figure 3A), bloody diar-rhea, rectal prolapse, and pancolitis accompanied by extensive wasting syndrome (Figure 3B), that resulted in 100% mortality within 6 days (Figure 3C). Macroscopic examination of colons revealed profound signs of inflammation, hyperemia, ulceration and shortening (Figure 3D). In contrast, mice treated with either HD- or AML-BMSCs were largely protected against colitis showing a significant recovery of their body weight loss within 9 days, with a significant increase in survival rate (60% for HD-BMSCs and 72% of AML-BMSCs) (Figure 3 A,C) that was accompanied by the regain of a healthy appearance, indistinguishable from ethanol-treated control mice. Furthermore, the wasting syndrome and the signs of colon inflammation were improved in BMSCs-transplanted mice, leading to a significant decrease in both the day 3 colitis scores and the colon damage scores (Figure 3 B,D). Interestingly, colitis and colon damage scores in mice treated with AML-BMSCs were significantly lower than those treated with HD-BMSCs suggesting that, in line with previous immunological properties in vitro,5 BMSCs from AML patients may have greater anti-inflammatory properties in vivo. Colitis and colon score data were directly supported by histological colonic examination which showed that AML-BMSCs significantly reduced TNBS-induced transmural inflammation, depletion of mucin-producing goblet cells, epithelial ulceration, infiltration of inflammatory cells in the lamina pro-pria, and focal loss of crypts (Figure 3E). To further confirm the in vivo anti-inflammatory phenotype of AML-BMSCs, we measured the levels of master pro-inflammatory cytokines/chemokines in the serum of colitis mice treated with either HD- or AML-BMSCs, and found a slightly pronounced decrease in the levels of TNFα, IL6, IL1β and MIP-2 in AML- as compared with HD-BMSC-treated mice (Figure 3F). In line with this, in vitro expanded AML-BMSCs also displayed a significantly lower production of IL6 and IL8 than HD-BMSCs (Figure 3G). Collectively, our results show that AML-BMSCs are capable of suppressing inflammation in vivo to largely protect mice against acute colitis in a TNBS-induced disease model.
Figure 3.
BMSCs from AML patients are highly anti-inflammatory in vivo in an experimental model of acute severe colitis. Colitis was induced by intracolonic administration of TNBS and mice were treated i.p. with PBS (TNBS group), HD-BMSCs or AML-BMSCs (106 cells) 12 hours after TNBS injection. Control mice received 50% ethanol. Clinical evolution was monitored by determining the daily body weight loss (A) as well as measuring the colitis score at day 3 (B), survival (C) the macroscopic colonic damage score (D), and the histopathologic score at day 3 (E). Data are expressed as mean±s.e.m. n=10 mice per group in a-d. n=5 mice per group in e. Statistical differences between groups were calculated as described in the methods section. (F) Serum levels on the indicated cytokines in colitis mice treated with either HD- or AML-BMSCs. (G) Levels of IL6 and IL8 produced by in vitro expanded HD- and AML-BMSCs. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
This study is the first to assess and show the in vivo anti-inflammatory properties of BMSCs from patients suffering from hematological malignancies. The finding that AML-BMSCs are fully anti-inflammatory in vitro and in vivo is of clinical relevance not only to our understanding of the pathobiology of AML, but to our greater understanding of how these cells retain their native properties despite severe histopathologic perturbations in situ. Further studies are warranted to determine whether BMSCs from hematological malignancies other than AML and from solid malignances equally retain their native phenotype(s).
Supplementary Material
Footnotes
Funding: financial support for this work was obtained from: Health Canada (H4084-112281) to PM and MR-M. The European Research Council (CoG-2014-646903) to PM. The Spanish Ministry of Economy and Competitiveness (SAF-SAF2013-43065), to PM. The Generalitat de Catalunya (SGR330 and PERIS 2017-2019 Program) to P.M. The Asociación Española Contra el Cáncer (AECC-CI-2015) and Fero Foundation to CB. The “Fundación Hay Esperanza” to E.A. The Health Institute Carlos III (ISCIII/FEDER/PI14-01191) to CB. PM also acknowledges the institutional support from The Obra Social La Caixa-Fundaciò Josep Carreras. PM is investigator of the Spanish Cell Therapy cooperative network (TERCEL).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Grimwade D, Lo Coco F. Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002;16(10):1959–1973. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Castro J, Trigueros C, Madrenas J, Perez-Simon JA, Rodriguez R, Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008;12(6B):2552–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner AK, Nepstad I, Bruserud O. Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol. 2017;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito S, Barrett AJ, Dutra A, et al. Long term maintenance of myeloid leukemic stem cells cultured with unrelated human mesenchymal stromal cells. Stem Cell Res. 2015;14(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz de la Guardia R, Lopez-Millan B, Lavoie JR, et al. Detailed characterization of mesenchymal stem/stromal cells from a large cohort of AML patients demonstrates a definitive link to treatment outcomes. Stem Cell Reports. 2017;8(6):1573–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran P, Le Y, Li Y, et al. Mesenchymal stromal cells from patients with acute myeloid leukemia have altered capacity to expand differentiated hematopoietic progenitors. Leuk Res. 2015;39(4):486–493. [DOI] [PubMed] [Google Scholar]
- 7.Geyh S, Rodriguez-Paredes M, Jager P, et al. Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia. 2016;30(3):683–691. [DOI] [PubMed] [Google Scholar]
- 8.Isern J, Martin-Antonio B, Ghazanfari R, et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep. 2013;3(5):1714–1724. [DOI] [PubMed] [Google Scholar]
- 9.Desbourdes L, Javary J, Charbonnier T, et al. Alteration analysis of bone marrow mesenchymal stromal cells from de novo acute myeloid leukemia patients at diagnosis. Stem Cells Dev. 2017;26(10):709–722. [DOI] [PubMed] [Google Scholar]
- 10.Glait-Santar C, Desmond R, Feng X, et al. Functional niche competition between normal hematopoietic stem and progenitor cells and myeloid leukemia cells. Stem Cells. 2015;33(12):3635–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanoun M, Zhang D, Mizoguchi T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao P, Sandhow L, Heshmati Y, et al. Distinct roles of mesenchymal stem and progenitor cells during the development of acute myeloid leukemia in mice. Blood Adv. 2018;2(12):1480–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–939. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez R, Rosu-Myles M, Arauzo-Bravo M, et al. Human bone marrow stromal cells lose immunosuppressive and anti-inflammatory properties upon oncogenic transformation. Stem Cell Reports. 2014;3(4):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Millan B, Diaz de la Guardia R, Roca-Ho H, et al. Therapeutic effect of the immunomodulatory drug lenalidomide, but not pomalidomide, in experimental models of rheumatoid arthritis and inflammatory bowel disease. Exp Mol Med. 2017;49(2):e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.