Abstract
Porphyria is a group of metabolic disorders due to altered enzyme activities within the heme biosynthetic pathway. It is a systemic disease with multiple potential contributions to mitochondrial dysfunction and oxidative stress. Recently, it has become possible to measure mitochondrial function from cells isolated from peripheral blood (cellular bioenergetics) using the XF96 analyzer (Seahorse Bioscience). Mitochondrial respiration in these cells is measured with the addition of activators and inhibitors of respiration. The output is measured as the O2 consumption rate (OCR) at basal conditions, ATP linked, proton leak, maximal, reserve capacity, non-mitochondrial, and oxidative burst. We performed cellular bioenergetics on 22 porphyria (12 porphyria cutanea tarda (PCT), seven acute hepatic porphyria (AHP), and three erythropoietic protoporphyria (EPP)) patients and 18 age and gender matched healthy controls. Of porphyria cases, eight were active (2 PCT, 1 EPP, and 5 AHP) and 14 in biochemical remission. The OCR were decreased in patients compared to healthy controls. The bioenergetic profile was significantly lower when measuring proton leak and the non-mitochondrial associated OCR in the eight active porphyria patients when compared to 18 healthy controls. In conclusion, we demonstrate that the bioenergetic profile and mitochondrial activities assessed in porphyria patients and is different than in healthy control individuals. Further, our novel preliminary findings suggest the existence of a mitochondrial dysfunction in porphyria and this may be used as potential non-invasive biomarker for disease activity. This needs to be assessed with a systematic examination in a larger patient cohort. Studies are also suggested to examine mitochondrial metabolism as basis to understand mechanisms of these findings and deriving mitochondrial based therapies for porphyria.
Keywords: PCT, AIP, AHP, Protoporphyria, Mitochondrial
1. Introduction
Porphyria is a group of metabolic disorders, with eight different porphyria, each due to specific enzymatic defect within the heme biosynthetic pathway [1,2]. Based on the dominant site of the enzymatic defect, porphyria can be hepatic or erythroid, with dominant site of defect being the liver or the bone marrow respectively. Based on their clinical presentation, porphyria can be grouped as acute with neurovisceral manifestations or cutaneous with blistering or non-blistering photosensitivity. Of the porphyria disorders, porphyria cutanea tarda (PCT), acute intermittent porphyria (AIP), and erythropoietic protoporphyria (EPP) are most commonly encountered in clinical practice [1,2]. Both PCT and AIP are hepatic in origin and EPP is erythroid in nature. Clinically, AIP presents with acute neurovisceral features while other two are cutaneous porphyria.
Heme is needed for synthesis of hemoglobin, myoglobin, and cytochrome P450 enzyme, antioxidants such as catalase, and the respiratory cytochromes required for mitochondrial electron transport. Heme is also an essential component of the NADPH oxidase responsible for the production of superoxide in neutrophils and monocytes [3]. Although, the predominant sites of heme biosynthesis are bone marrow and the liver, it is ubiquitous and occurs in all tissues and cells including peripheral blood cells [4]. Over half of the heme biosynthetic pathway occurs within the mitochondria [5]. Heme is also an important factor for functioning of many mitochondrial complexes and electron transport needed for ATP generation.
Mitochondrial dysfunction with abnormalities of respiratory complexes has been described in AIP and these are associated with mitochondrial energetic failure and impairment of ATP generation [4,[6], [7], [8], [9], [10], [11]]. Aminolevulinic acid (ALA) and mitochondrial dysfunction have also been shown to be associated with increased oxidative stress in AIP [12]. Using cultured lymphoblasts from EPP patients, the mitochondrial iron transporter protein, mitoferrin located on the inner mitochondrial membrane is correlated with ferro chelatase enzymatic activity, the final enzyme in heme biosynthesis which catalyzes insertion of ferrous iron into the protoporhyrin molecule to form heme [4]. Similarly, oxidative stress and increased generation of free radicals has been shown in PCT patients, when associated with hepatitis C virus infection and/or alcohol abuse [8].
Mitochondrial health is maintained by the synthesis of new mitochondria (biogenesis) and degradation of damaged mitochondria through mitophagy, a specialized form of autophagy [13]. The fate of the cells ultimately depends upon a functioning healthy mitochondrial population. Systemic inflammation impairs mitochondrial function as recognized in sepsis, diabetes mellitus, and liver failure [[14], [15], [16]]. Systemic inflammation due to accumulated porphyrins and precursors in plasma of AIP patients has been described which is directly associated with increased circulating cytokines and increased disease activity [17]. The levels of plasma porphyrins in the cutaneous porphyria such as EPP are also directly associated with dermal photosensitivity and severity of the disease [18]. Similarly, plasma porphyrins are a biochemical marker for and diagnostic of PCT and levels normalize with on remission by treatment with low dose hydroxychloroquine or therapeutic phlebotomy [19].
It has been shown that circulating blood cells interact with various tissues and organs, and the mitochondrial health and bioenergetics of peripheral blood cells are a direct reflection of the disease state. This can be tested by measuring the cellular bioenergetics of the peripheral mononuclear, mitochondrial DNA content and mitochondrial damage [13,20]. In disease states including diabetes mellitus, sepsis, neurodegenerative diseases, and alcoholic liver disease mitochondrial bioenergetics in peripheral monocytes and platelets has been shown to reflect disease activity and severity [[21], [22], [23], [24], [25]].
Together these data suggest the existence of dynamic pathways that determine the activity and severity of disease states, including porphyria. We hypothesize that since mitochondria is the site of heme synthesis and electron transfer, translational measurements of mitochondrial function will reflect disease status in porphyria patients. To test this hypothesis, we performed this pilot study by measuring the bioenergetics and mitochondrial function on monocytes isolated from peripheral blood in patients with different types pf porphyria.
2. Methods
2.1. Study population
After obtaining informed consent, patients with AHP, EPP, or PCT were recruited from an ongoing IRB approved longitudinal study of the porphyria consortium. The type of porphyria was characterized based on standard criteria for diagnosis for each of this porphyria [1,2,18]. Activity of each of this porphyria was determined based on clinical symptoms and/or specific biochemical abnormalities for that specific porphyria. For example, patients with biochemical abnormalities with or without symptoms were classified as active porphyria. In contrast, patients known to have porphyria with a previous documented diagnosis or with genetic defect, who were asymptomatic and had no biochemical abnormalities, were defined as to be in remission. Patients with symptoms suggestive of porphyria but without characteristic biochemical abnormalities were diagnosed not to have porphyria. Age and gender matched healthy controls were also analyzed from the pool of healthy controls recruited for the other studies.
2.2. Data collection
Clinical and laboratory data was collected on patient demographics (age, gender, and race); clinical symptoms; and biochemical abnormalities. Porphyria patients were stratified to active status or those in remission as defined above.
2.3. Isolation of cells
Blood samples (20 mL) were collected from patients and processed within 15–30 min of collection. Monocytes and neutrophils were isolated from the buffy coat, and used to determine their bioenergetics, mitochondrial function and oxidative burst. Cell isolation procedures are designed to prevent activation of the cells. CD14+ monocytes and CD15+ neutrophils and granulocyte fractions were purified from peripheral blood mononuclear cells (PBMC) respectively by the magnetic-activated cell sorting technology (MACS, Milteneyi Biotec) using superparamagnetic iron-dextran microbeads-labeled CD14 antibodies which do not lead to activation or alter bioenergetics [26]. Similarly, neutrophils were purified by positive selection using magnetic bead labeled anti-CD15 antibodies. The purity of each fraction was assessed using FACS (fluorescence activated cell sorting) analysis. Over 95% of the isolated cells were viable as determined using trypan blue exclusion.
2.4. Measurements of bioenergetics and oxidative burst
XF96 analyzer from Seahorse Biosciences was used to determine the cellular bioenergetics, which measures oxygen consumption rate (OCR) in cell culture and cell populations prepared as we have recently described [[25], [26], [27], [28]]. Purified monocytes (150,000 cells/well) and neutrophils (75,000 cells/well) were suspended in XF assay buffer and plated in 75 μl on the CellTak (BD Biosciences) coated assay plates prior to assay. We have earlier shown that the cells prepared using this method is stable over a period of 5–6 h [26]. The system is capable of measuring 92 samples at a time, and is equipped with four injection ports per well to allow for injection of activators or inhibitors of mitochondrial respiration, that can aid in the elucidation of defects in individual cellular respiration pathways or enzymes [28]. Various components and key aspects of mitochondrial function, glycolysis and the oxidative burst in isolated monocytes were determined, as we have described previously [27,29]. After a stable baseline OCR is measured in the peripheral blood cells, oligomycin is injected, which results in a decrease in OCR which can be ascribed to ATP synthesis. Next FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) is added and this stimulates OCR as the mitochondria within the cell are uncoupled. Finally, antimycin is injected and the resulting OCR is ascribed to oxygen consuming processes which do not involve mitochondrial electron transport [25]. Injection of phorbol 12-myristate 13-acetate (PMA) was used to measure the oxidative burst in the plated cells [25].
2.5. Data analyses
Comparison was made on OCR among a) healthy controls vs. porphyria patients and b) active porphyria vs, porphyria in remission. Student's t-test and non-parametric Wilcoxon Rank sum test were used for statistical analyses. P values <.05 were considered significant.
3. Results
A total of 22 porphyria patients and 18 age/gender matched healthy controls were recruited. Among the porphyria patients, 12 had PCT (10 in remission), 7 AHP (2 in remission), and 3 EPP (2 in remission), (Table 1). The demographics of age-matched healthy controls are shown in Table 2.
Table 1.
Demographic profile of patients with porphyria.
| S. No. | Age | Gender | Ethnicity | Diagnosis | Status |
|---|---|---|---|---|---|
| 1 | 63 | M | C | AHP | Remission |
| 2 | 32 | F | C | AHP | Active |
| 3 | 49 | M | C | PCT | Active |
| 4 | 65 | F | C | PCT | Remission |
| 5 | 26 | F | C | EPP | Remission |
| 6 | 61 | M | C | PCT | Remission |
| 7 | 43 | M | C | EPP | Active |
| 8 | 52 | F | C | AHP | Remission |
| 9 | 56 | F | C | AHP | Active |
| 10 | 51 | M | C | PCT | Remission |
| 11 | 65 | F | C | PCT | Remission |
| 12 | 34 | F | AA | AHP | Active |
| 13 | 32 | F | C | AHP | Active |
| 14 | 49 | M | C | PCT | Remission |
| 15 | 55 | M | C | PCT | Remission |
| 16 | 71 | M | C | PCT | Remission |
| 17 | 45 | F | C | PCT | Remission |
| 18 | 24 | F | C | EPP | Remission |
| 19 | 50 | M | C | PCT | Remission |
| 20 | 52 | M | C | PCT | Active |
| 21 | 39 | F | C | PCT | Remission |
| 22 | 32 | F | C | AHP | Active |
M: Male; F: Female; C: Caucasian; AA: African American; AHP: Acute hepatic porphyria; PCT: Porphyria cutanea tarda; EPP: Erythropoietic protoporphyria.
Table 2.
Demographics of healthy control subjects.
| No. | Age | Gender | Ethnicity |
|---|---|---|---|
| 1 | 67 | M | C |
| 2 | 59 | F | C |
| 3 | 33 | M | C |
| 4 | 58 | M | C |
| 5 | 48 | F | C |
| 6 | 55 | M | C |
| 7 | 28 | M | C |
| 8 | 44 | M | C |
| 9 | 32 | M | C |
| 10 | 45 | F | C |
| 11 | 38 | M | C |
| 12 | 43 | F | AA |
| 13 | 31 | M | C |
| 14 | 34 | F | C |
| 15 | 53 | M | C |
| 16 | 41 | F | C |
| 17 | 46 | F | C |
| 18 | 29 | F | C |
M: Male; F: Female; C: Caucasian; AA: African American.
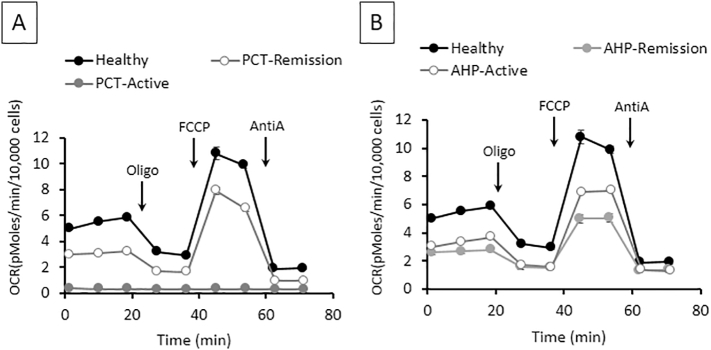
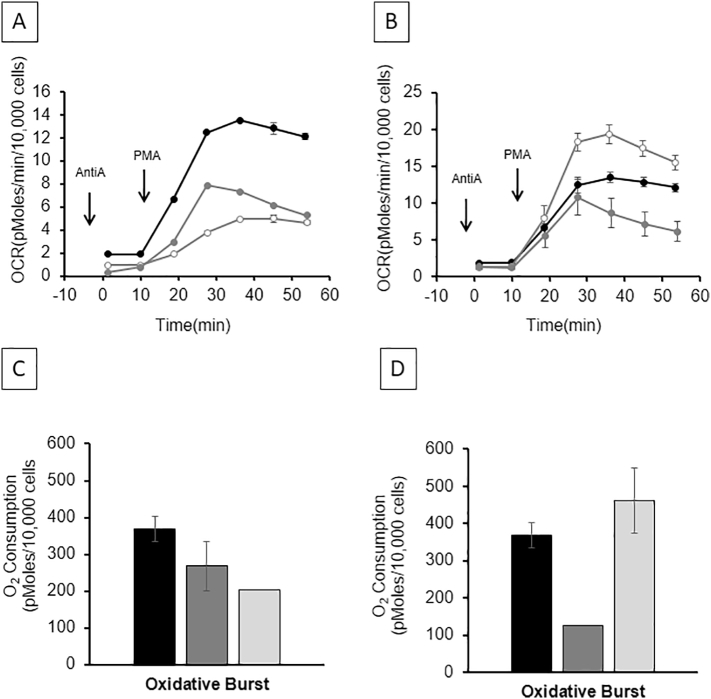
The bioenergetic profile on OCR in peripheral monocytes from PCT patients with active disease vs. PCT in remission vs. healthy control is shown in Fig. 1A. Active PCT patients have decreased bioenergetics when compared to healthy control, and these measurements tend to return to near normal when the PCT is in remission. Performing the same experiment in patients with AHP (Fig. 1B) showed that both active and AHP in remission were different than healthy controls. Following the mitochondrial stress test a PMA-stimulated oxidative burst-test was performed, as a measure of the NADPH oxidase activity in monocytes, the OCR was lower in active PCT compared to the healthy controls. This recovers in the patients that are in remission but remains lower than healthy control (Fig. 2A). Similarly, the oxidative burst in an active AHP patient was lower compared to healthy control and recovers to normal when the AHP is in remission (Fig. 2B).
Fig. 1.
Bioenergetics and glycolytic function alterations in porphyria cutanea tarda (PCT) and acute hepatic porphyria (AHP) monocytes as measured using the mitochondrial stress test. Representative bioenergetics (OCR) profiles of CD14+ monocytes of PCT (A) and that of AHP (B) as determined by injecting oligomycin, FCCP and antimycin A sequentially. Measurements are given as mean ± SEM from 3 to 6 assay replicates. Healthy controls shown in black, active porphyria in gray, and porphyria remission in open white circles.
Fig. 2.
Determination of oxidative burst in porphyria. Representative profiles of oxidative burst induced by phorbol myristate acetate (PMA) in monocytes isolated from patients with porphyria cutanea tarda or PCT (A) and acute hepatic porphyria or AHP (B). Measurements are given as mean ± SEM from 3 to 6 assay replicates. Panels C and D show mean ± SEM measurements on oxidative burst within 18 healthy controls, 12 PCT, and 7 AHP patients. Healthy controls shown in black, active porphyria in light gray, and porphyria remission in dark gray color.
While the number of subjects in each category of porphyria were small, subjects were grouped for further analyses, with OCR reported as mean ± SEM (Table 3).
Table 3.
Bioenergetic measurements (mean ± SEM) on oxygen consumption rate (pg. O2/10,000 cells) in peripheral monocytes isolated from healthy controls and patients with porphyria.
| Group | Basal | ATP linked | Proton leak | Maximal | Reserve | Non-mitochondrial |
|---|---|---|---|---|---|---|
| Healthy controls (N = 18) | 2.53 ± 0.17 | 2.01 ± 0.15 | 0.55 ± 0.05 | 6.39 ± 0.59 | 3.83 ± 0.50 | 1.32 ± 0.08 |
| All porphyria (N = 22) | 2.19 ± 0.24 | 1.60 ± 0.17 | 0.38 ± 0.06 | 4.94 ± 0.91 | 3.26 ± 0.64 | 1.34 ± 0.39 |
| Porphyria cutanea tarda (N = 12) | 2.04 ± 0.36 | 1.44 ± 0.25 | 0.43 ± 0.08 | 4.52 ± 1.19 | 2.89 ± 0.86 | 1.19 ± 0.26 |
| Acute hepatic porphyria (N = 7) | 2.05 ± 0.18 | 1.79 ± 0.21 | 0.25 ± 0.007 | 5.28 ± 0.39 | 3.22 ± 0.52 | 0.64 ± 0.02 |
| Active porphyria (N = 8) | 1.92 ± 0.47 | 1.66 ± 0.43 | 0.26 ± 0.08 | 4.44 ± 1.38 | 2.54 ± 1.00 | 0.60 ± 0.55 |
| Student's t-Test P* | 0.19 | 0.015 | 0.14 | 0.06 | 0.25 | 0.90 |
| Student's t-Test P** | 0.39 | 0.014 | 0.025 | 0.0002 | 0.009 | 0.34 |
| Wilcoxon Rank Sum P* | 0.15 | 0.013 | 0.09 | 0.018 | 0.06 | 0.035 |
| Wilcoxon Rank Sum P** | 0.25 | 0.015 | 0.018 | 0.002 | 0.012 | 0.27 |
*Healthy controls vs. all porphyria and **Healthy controls vs. active porphyria.
3.1. Healthy controls vs. porphyria
The OCR in monocytes was lower in 22 porphyria patients compared to 18 healthy controls, however the results were only statistically significant for proton leak related OCR using the student's t-test or Wilcoxon Rank sum test. Analysis based on the Wilcoxon Rank sum nonparametric test, ATP mediated and maximal OCR were significantly lower in porphyria patients compared to healthy controls (Table 3). The bioenergetic profile was also similar when comparing healthy controls with the bioenergetics measurements in each of the three-specific porphyria (data not shown).
3.2. Healthy controls vs. porphyria based on disease activity
The measurements on OCR in monocytes in eight active porphyria patients compared to 18 healthy controls were significantly lower for proton leak and non-mitochondrial components using the student's t-test. Analysis based on Wilcoxon rank sum test, except for basal and non-mitochondrial, most other components of OCR were lower in active porphyria patients compared to healthy controls (Table 3). Statistical comparisons comparing active vs. remission was not performed for porphyria type given small number of patients (1 active EPP, 2 active PCT, and 5 active AHP).
Oxidative burst related OCR measurements showed a similar pattern based on disease activity in PCT and AHP (Fig. 2 A–B). Pooled analysis PCT patients showed similar pattern on oxidative burst related OCR (Fig. 2C). However, pooled analysis of AHP patients for this analysis showed lowest activity during remission compared to active AHP and this was statistically significant (Figs. 2C–D).
ECAR measurements, which represent the net pH change in the medium due to the combined effects of glycolysis and the TCA cycle showed no marked differences between any of the groups (result not shown).
4. Discussion
The main finding of this pilot study demonstrates that a) presence of mitochondrial abnormalities in porphyria patients during active disease and less so during remission, and b) use bioenergetic measurements from monocytes isolated from peripheral blood of porphyria patients can be potentially used as biomarkers of disease.
The observed decreased OCR in porphyria subjects may be due to oxidative stress mediated increased calcium cycling with decreased efficiency of the mitochondrial ATP generation [13,30,31]. Remarkably, given the extent of the limited ability to synthesize heme in these patients, they appear to be reasonably well compensated in terms of the metabolic requirements for mitochondrial function. This could be achieved by several mechanisms such as decreasing the turnover of mitochondria in the cells by suppressing mitophagy or the mitochondrial proteolytic system. It may also be due to decreased turnover of mitochondrial proteins necessary for ATP production. Indeed, it has been reported that there is a feedback loop between the mitochondrial protease system and heme [32]. The impact of this pathway on the turnover of mitochondrial proteins in porphyria remains unknown.
Mitochondrial defects have been shown in rodent models of AIP and patients with AIP. For example, in a mouse model of AIP (hydroxymethylbilane synthase or HMBSM/M), the study showed reduced activity of respiratory complexes I and II in mitochondria isolated from skeletal muscle and four of the respiratory complexes in brain also showed significant decreases [9]. These abnormalities were associated with bioenergetics failure and a defect in oxidative phosphorylation within the mitochondria [9]. In another study from the same group, these respiratory complexes abnormalities were also observed in the HMBSM/M mice and were associated with reduced activities of the intermediates of the tricarboxylic acid cycle. The authors describe this state as cataplerosis of the respiratory cycle. In humans, a translational study reported higher urinary concentration of metabolites of acetate, citrate, and pyruvate in asymptomatic AIP patients [11]. In EPP patients there is indirect evidence of possible mitochondrial defects, based on reported abnormalities of the iron transporter, mitoferrin, which is colocalized with ferro chelatase and other proteins on the matrix side of the inner mitochondrial membrane. The activity and concentration of mitoferrin in cultured lymphoblasts which were obtained from patients with EPP had a direct correlation with the levels and enzymatic activity of ferro chelatase, the last enzyme in the heme pathway. Minimal data exists on assessment of mitochondrial defects in PCT. In a translational study on asymptomatic porphyria patients, the concentration of urinary glycolytic intermediates was higher in AIP patients as compared to PCT patients. Although, this study suggests more severe disturbance in mitochondrial activity in AIP, it does not rule out presence of similar abnormalities in PCT [11].
Interactions between the heme biosynthetic pathway and the tricarboxylic acid cycle may explain these mitochondrial abnormalities and account for the observed bioenergetics failure in AIP. In a mouse model of AIP, induction of ALA synthase enzyme (the rate limiting enzyme of the heme biosynthesis pathway) resulted in excessive utilization of succinyl coenzyme A, leading to cataplerosis of the tricarboxylic acid cycle with reduced levels of the tricarboxylic acid cycle intermediates [6]. Further evidence of this mechanistic basis is that the mitochondrial abnormalities were restored with downregulation of ALA synthase enzyme activity by supplementation of heme in these mice [6]. Systemic inflammation in any disease state can affect the mitochondrial function and its bioenergetics, as exemplified in certain disease states including diabetes mellitus, sepsis, and alcoholic liver disease [21,22,25]. In a case control study on 50 AIP patients and matched healthy controls, levels of cytokines, chemokines, and growth factors were elevated in AIP compared to healthy controls, suggesting existence of systemic inflammation in AIP [17]. Circulating elevated ALA, PBG, and porphyrins in porphyria patients may cause tissue damage with release of damage associated molecular patterns or DAMPs, with consequent inflammatory signaling and immune cell activation with release of cytokines resulting in systemic inflammatory state [33,34]. Although, there are no reported studies evaluating inflammation and cytokines in PCT and EPP, there is indirect evidence of systemic inflammation in these patients with a) elevated plasma porphyrins in PCT and EPP [2,35,36], b) clinical features marked by photosensitivity and inflammation [2,35,36], and c) immune and complement activation around dermal capillaries in PCT from photodynamic activation of circulating uroporphyrins [37].
This is the first study examining cellular bioenergetics and oxygen consumption rates in peripheral blood cells in patients with different types of porphyria. Although, limited by small sample size, our study does illustrate the potential of relatively non-invasive tests for bioenergetics function in patients with porphyria. Another limitation of the study confounded by the small size is inability to correlate the OCR measurements in peripheral monocytes to the plasma porphyrin concentration. Future large multicenter studies are needed to overcome these limitations as basis for noninvasive biomarker to follow porphyria patients. Further, mechanistic studies are also needed using mitochondrial metabolomics as an approach to localize the defects in mitochondria, as basis for developing therapeutic targets and newer mitochondrial based therapies for patients with porphyria.
References
- 1.Bissell D.M., Anderson K.E., Bonkovsky H.L. Porphyria. N. Engl. J. Med. 2017;377:862–872. doi: 10.1056/NEJMra1608634. [DOI] [PubMed] [Google Scholar]
- 2.Arora S., Young S., Kodali S., Singal A.K. Hepatic porphyria: a narrative review. Indian J. Gastroenterol. 2016;35:405–418. doi: 10.1007/s12664-016-0698-0. [DOI] [PubMed] [Google Scholar]
- 3.Al Ghouleh I., Khoo N.K., Knaus U.G., Griendling K.K., Touyz R.M., Thannickal V.J., Barchowsky A., Nauseef W.M., Kelley E.E., Bauer P.M., Darley-Usmar V., Shiva S., Cifuentes-Pagano E., Freeman B.A., Gladwin M.T., Pagano P.J. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic. Biol. Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Langer N.B., Shaw G.C., Yang G., Li L., Kaplan J., Paw B.H., Bloomer J.R. Abnormal mitoferrin-1 expression in patients with erythropoietic protoporphyria. Exp. Hematol. 2011;39:784–794. doi: 10.1016/j.exphem.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajioka R.S., Phillips J.D., Kushner J.P. Biosynthesis of heme in mammals. Biochim. Biophys. Acta. 2006;1763(7):723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Homedan C., Laafi J., Schmitt C., Gueguen N., Lefebvre T., Karim Z., Desquiret-Dumas V., Wetterwald C., Deybach J.C., Gouya L., Puy H., Reynier P., Malthiery Y. Acute intermittent porphyria causes hepatic mitochondrial energetic failure in a mouse model. Int. J. Biochem. Cell Biol. 2014;51:93–101. doi: 10.1016/j.biocel.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Chung J., Anderson S.A., Gwynn B., Deck K.M., Chen M.J., Langer N.B., Shaw G.C., Huston N.C., Boyer L.F., Datta S., Paradkar P.N., Li L., Wei Z., Lambert A.J., Sahr K., Wittig J.G., Chen W., Lu W., Galy B., Schlaeger T.M., Hentze M.W., Ward D.M., Kaplan J., Eisenstein R.S., Peters L.L., Paw B.H. Iron regulatory protein-1 protects against mitoferrin-1-deficient porphyria. J. Biol. Chem. 2014;289:7835–7843. doi: 10.1074/jbc.M114.547778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura K., Taura K., Kodama Y., Schnabl B., Brenner D.A. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48:1420–1429. doi: 10.1002/hep.22486. [DOI] [PubMed] [Google Scholar]
- 9.Homedan C., Schmitt C., Laafi J., Gueguen N., Desquiret-Dumas V., Lenglet H., Karim Z., Gouya L., Deybach J.C., Simard G., Puy H., Malthiery Y., Reynier P. Mitochondrial energetic defects in muscle and brain of a Hmbs−/− mouse model of acute intermittent porphyria. Hum. Mol. Genet. 2015;24:5015–5023. doi: 10.1093/hmg/ddv222. [DOI] [PubMed] [Google Scholar]
- 10.Pereira B., Curi R., Kokubun E., Bechara E.J. 5-aminolevulinic acid-induced alterations of oxidative metabolism in sedentary and exercise-trained rats. J. Appl. Physiol. 1985;72(1992):226–230. doi: 10.1152/jappl.1992.72.1.226. [DOI] [PubMed] [Google Scholar]
- 11.Luck M., Schmitt C., Talbi N., Gouya L., Caradeuc C., Puy H., Bertho G., Pallet N. Urinary metabolic profiling of asymptomatic acute intermittent porphyria using a rule-mining-based algorithm. Metabolomics: Offl. J. Metabolomic Soc. 2018;14:10. doi: 10.1007/s11306-017-1305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M.L., Lane D.J., Richardson D.R. Mitochondrial mayhem: the mitochondrion as a modulator of iron metabolism and its role in disease. Antioxid. Redox Signal. 2011;15:3003–3019. doi: 10.1089/ars.2011.3921. [DOI] [PubMed] [Google Scholar]
- 13.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell'Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozlov A.V., Lancaster J.R., Jr., Meszaros A.T., Weidinger A. Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol. 2017;13:170–181. doi: 10.1016/j.redox.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa T.D., Pereira A.J., Brandt S., Vuda M., Djafarzadeh S., Takala J., Jakob S.M. Time course of blood lactate levels, inflammation, and mitochondrial function in experimental sepsis. Crit. Care. 2017;21:105. doi: 10.1186/s13054-017-1691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcala M., Calderon-Dominguez M., Bustos E., Ramos P., Casals N., Serra D., Viana M., Herrero L. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci. Rep. 2017;7:16082. doi: 10.1038/s41598-017-16463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storjord E., Dahl J.A., Landsem A., Fure H., Ludviksen J.K., Goldbeck-Wood S., Karlsen B.O., Berg K.S., Mollnes T.E., Nielson W., Brekke O.L. Systemic inflammation in acute intermittent porphyria: a case-control study. Clin. Exp. Immunol. 2017;187:466–479. doi: 10.1111/cei.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balwani M., Naik H., Anderson K.E., Bissell D.M., Bloomer J., Bonkovsky H.L., Phillips J.D., Overbey J.R., Wang B., Singal A.K., Liu L.U., Desnick R.J. Clinical, biochemical, and genetic characterization of north American patients with Erythropoietic Protoporphyria and X-linked Protoporphyria. JAMA Dermatol. 2017;153:789–796. doi: 10.1001/jamadermatol.2017.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singal A.K., Kormos-Hallberg C., Lee C., Sadagoparamanujam V.M., Grady J.J., Freeman D.H., Jr., Anderson K.E. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin. Gastroenterol. Hepatol.: Offl. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012;10:1402–1409. doi: 10.1016/j.cgh.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell T., Johnson M.S., Ouyang X., Chacko B.K., Mitra K., Lei X., Gai Y., Moore D.R., Barnes S., Zhang J., Koizumi A., Ramanadham S., Darley-Usmar V.M. Dysfunctional mitochondrial bioenergetics and oxidative stress in Akita(+/Ins2)-derived beta-cells. Am. J Physiol. Endocrinol. Metab. 2013;305:E585–E599. doi: 10.1152/ajpendo.00093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widlansky M.E., Wang J., Shenouda S.M., Hagen T.M., Smith A.R., Kizhakekuttu T.J., Kluge M.A., Weihrauch D., Gutterman D.D., Vita J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Trans. Res.: J. Lab. Clin. Med. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Japiassu A.M., Santiago A.P., d'Avila J.C., Garcia-Souza L.F., Galina A., Castro Faria-Neto H.C., Bozza F.A., Oliveira M.F. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5′-triphosphate synthase activity. Crit. Care Med. 2011;39:1056–1063. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- 23.Ienco E.C., Simoncini C., Orsucci D., Petrucci L., Filosto M., Mancuso M., Siciliano G. May "mitochondrial eve" and mitochondrial haplogroups play a role in neurodegeneration and Alzheimer's disease? Int. J. Alzheimers Dis. 2011;2011:709061. doi: 10.4061/2011/709061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chacko B.K., Srivastava A., Johnson M.S., Benavides G.A., Chang M.J., Ye Y., Jhala N., Murphy M.P., Kalyanaraman B., Darley-Usmar V.M. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chacko B.K., Kramer P.A., Ravi S., Benavides G.A., Mitchell T., Dranka B.P., Ferrick D., Singal A.K., Ballinger S.W., Bailey S.M., Hardy R.W., Zhang J., Zhi D., Darley-Usmar V.M. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin. Sci. 2014;127:367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chacko B.K., Kramer P.A., Ravi S., Johnson M.S., Hardy R.W., Ballinger S.W., Darley-Usmar V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Invest. J Tech. Method Pathol. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer P.A., Chacko B.K., Ravi S., Johnson M.S., Mitchell T., Darley-Usmar V.M. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J. Vis. Exp. 2014;85 doi: 10.3791/51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L.Y., Chatham J.C., Hill B.G., Zhang J.H., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Rad. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill B.G., Dranka B.P., Zou L., Chatham J.C., Darley-Usmar V.M. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi S., Chacko B., Kramer P.A., Sawada H., Johnson M.S., Zhi D., Marques M.B., Darley-Usmar V.M. Defining the effects of storage on platelet bioenergetics: the role of increased proton leak. Biochim. Biophys. Acta. 2015;1852:2525–2534. doi: 10.1016/j.bbadis.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Q., Li T., Hou W., Zheng J., Schrum L.W., Bonkovsky H.L. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011;286:26424–26430. doi: 10.1074/jbc.M110.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singal A.P. World Scientific Publishing Co. Pte. Ltd.; Hackensack, NJ, USA: 2014. Porphyria Cutanea Tarda. [Google Scholar]
- 36.Bloomer J.R. Protoporphyria. Semin. Liver Dis. 1982;2:143–153. doi: 10.1055/s-2008-1040704. [DOI] [PubMed] [Google Scholar]
- 37.Meurer M., Schulte C., Weiler A., Goerz G. Photodynamic action of uroporphyrin on the complement system in porphyria cutanea tarda. Arch. Dermatol. Res. 1985;277:293–298. doi: 10.1007/BF00509083. [DOI] [PubMed] [Google Scholar]