Abstract
Early recognition of rare mitochondrial respiratory chain defects has become readily available with the routine use of whole exome sequencing. Patients with oxidative phosphorylation defects present with a heterogenous phenotype, often rapidly progressive, and lethal. Clinicians aim for prompt identification of the specific molecular defect to provide timely management, decrease morbidity, and potentially improve survival rates. More recently, bi-allelic pathogenic variants in the TRMU gene responsible for encoding the mitochondrial tRNA-specific 2-thiouridylase were found in a syndrome characterized by infantile hepatopathy due to a mitochondrial translation defect (OMIM# 613070). This nuclear encoded enzyme catalyzes the addition of a sulfur-containing thiol group to the wobble position of mitochondrial specific tRNAs. TRMU deficiency is characterized by a combined respiratory chain deficiency without associated mitochondrial DNA depletion. This mitochondrial tRNA-modifying enzyme requires sulfur for its activity. Previous cellular models have suggested supplementation with cysteine, one of the sulfur containing amino acids, may play a role in increasing thiouridylation levels of mt-tRNAs by increasing sulfur availability for TRMU activity. Cysteine is considered a conditional essential amino acid due to limited availability in infants caused by immature cystathionine gamma-lyase (cystathionase) enzyme activity. The potential benefit of L-cysteine supplementation in TRMU deficiency has been previously proposed to ameliorate the severity and insidious course of the disease. Here we report the clinical, biochemical, and genetic findings of two siblings presenting with hepatopathy associated with hyperlactatemia due to bi-allelic pathogenic variants in TRMU. One patient died due to related complications. The other case was diagnosed prenatally allowing early implementation of L-cysteine supplementation, recovery of liver function, and avoidance of liver transplantation.
Keywords: TRMU, mitochondrial, cysteine, hepatopathy, liver transplant
1. Background/Introduction
Mitochondrial diseases are a clinically and genetically heterogeneous group of disorders resulting from dysfunction of the mitochondrial electron transport chain (ETC) and oxidative phosphorylation due to pathogenic variants in mitochondrial DNA (mtDNA) or nuclear DNA (nDNA) encoding mitochondrial proteins [1]. Efficient mDNA translation is essential to maintain the production of cellular energy and the assembly of oxidative phosphorylation (OXPHOS) enzymes. Besides the overall cellular energy status, mitochondrial gene expression is tightly regulated by different signaling pathways involving diverse mechanisms including reactive oxygen species (ROS) production and calcium homeostasis. While the majority of OXPHOS components, (complexes I–IV), the ATP synthase (complex V), and various elements required for mtDNA maintenance (replication, transcription, copy number control) are encoded within the nucleus, 13 polypeptides, two ribosomal RNAs (mt-rRNAs), and 22 transfer RNAs (mt-tRNAs) are encoded within the mtDNA [2]. The pathological mechanism of mitochondrial disease reflects the complexity of this organelle and its vital role in normal physiology. Energy deficiency in various organs results in multiorgan dysfunction leading to the variable manifestations observed in mitochondrial diseases including cognitive impairment, epilepsy, cardiac and skeletal myopathies, nephropathies, hepatopathies, and endocrinopathies. Despite deeper understanding of the clinical features of mitochondrial diseases, as a group, these disorders are often diagnosed late. Presently, pathogenic variants in mDNA or nDNA encoding mitochondrial proteins have been promptly identified in the pediatric population with the routine use of advanced diagnostic tools such as whole exome sequencing [3,4].
To date, several nuclear genes have been linked to abnormal mitochondrial translation and disease. Specific gene defects can be sub-divided as follows: (a) defects of mtDNA transcription (TFAM); (b) pre-RNA processing defects (MRPP1, MRPP2, ELAC2); (c) mRNA processing defects (FASTKD2, MTPAP, LRPPRC, PNPT1); (d) mt-tRNA modification defects (TRMU, MTO1, GTPBP3, NSUN3, TRMT5, TRIT1, PUS1, TRNT1, MTFMT); (e) mt-tRNA synthetases deficiencies (17 ARS2 genes, GARS, KARS, QRSL1); (f) defects of mitoribosome structure and assembly (MRPL3, MRPS16, MRPS22, MRPL12, MRPL44, MRPS34, ERAL1, MRM2); (g) initiation and elongation defects (GFM1, TUFM, TSFM, RMND1); (h) deficient releasing factors (C12orf65); and (i) deficiency of translational activators (TACO1) [5].
Clinical phenotypes associated with nuclear defects of mitochondrial translation are extremely variable with relatively strict tissue specificity. Overlapping features such as failure to thrive, global developmental delays, eye abnormalities, cardiomyopathy, and hypotonia are frequently observed [2]. Lactic acidosis is a common feature, as well as Leigh syndrome like lesions in the basal ganglia. These conditions usually have early-onset, and are associated with a severe, and often fatal outcome. Episodic metabolic decompensation and resultant developmental regression is frequently observed in the setting of increased metabolic stress during illness or prolonged fasting. Therapeutic interventions are very limited and include supportive measures during times of metabolic stress and palliative multidisciplinary care. While most mitochondrial translation defects are progressive conditions, there are rare forms involving mt-tRNA modification, which show notable spontaneous recovery such as combined oxidative phosphorylation deficiency 10 (MTO1, OMIM# 614702), and transient infantile liver failure (TRMU, OMIM# 613070) [6].
The TRMU gene encodes for the tRNA 5-methylaminomethyl-2-thiouridylate methyl-transferase. The encoded protein catalyzes the 2-thiolation of uridine on the wobble positions of tRNA(Lys), tRNA(Glu), and tRNA(Gln), resulting in the formation of 5-taurinomethyl-2-thiouridine moieties [7]. Pathogenic variants in the TRMU gene were first identified as a genetic modifier for sensorineural hearing loss caused by the homoplasmic m.1555G > A mutation in the mitochondrial 12 s rRNA gene (OMIM #580000) [8,9]. In 2009, Zaharia et al. reported 13 patients with autosomal recessive pathogenic variants in TRMU and reversible infantile liver failure [10]. To this date, at least 28 cases of TRMU deficiency and reversible infantile liver failure have been reported in the literature [[10], [11], [12], [13], [14]]. Described individuals presented with early onset hepatopathy. If patients survive this phase of liver failure, they improve spontaneously and recover fully by 2 or 3 years of age [2,6,12]. The disease course and age of manifestation in TRMU deficiency shows remarkable similarities to another defect of mitochondrial translation, m.14674T>C mutation in MT-TE also associated to a reversible phenotype in the form of infantile myopathy and COX deficiency (MTTE gene OMIM# 590025). Potential mechanisms for reversibility of these two conditions have been linked to decreased bioavailability of L-cysteine during the first 4 months of life. The TRMU protein requires sulfur for its activity, which is supplied by the cysteine desulfurase enzyme. Cysteine is an essential amino acid in infancy. The cystathionine gamma-lyase (cystathionase) is the rate-limiting enzyme for the synthesis of L-cysteine from L-methionine and appears to be regulated at the posttranscriptional level during development [15]. Therefore, the combination of temporally low cysteine and the presence of TRMU pathogenic variants in infants leads to low thiouridylation of mt-tRNAs, thereby interfering with mitochondrial protein translation and contributing to disease manifestations. Previous cellular models have suggested cysteine supplementation may play a role in increasing thiouridylation levels of mt-tRNAs by increasing sulfur availability for TRMU activity [15,16]. Here we present two siblings with TRMU deficiency and infantile liver failure. One of the patients received L-cysteine supplementation with remarkable improvement in liver function and avoidance of liver transplantation.
2. Case 1
The patient was a term Filipino female born at 40 weeks of gestation by vaginal delivery to a 22-year-old G1 P0 mother. She was discharged at 48 h of life after receiving phototherapy for neonatal unconjugated hyperbilirubinemia. She presented to the emergency department (ED) with increased respiratory rate and poor feeding at 4 days of age. At the time of arrival to the ED, her blood glucose was 16 mg/dL (60–110 mg/dL), which improved to 80 mg/dL after receiving a bolus of intravenous dextrose. Her initial venous blood gas showed metabolic acidosis (pH: 7.03, pCO2: 10, bicarbonate: 2.6 with a base excess of −25.8). She was admitted to the neonatal unit with a working diagnosis of sepsis and placed on empiric antibiotic therapy. Follow-up laboratory data showed a persistently elevated venous blood lactate of 14.8 (normal: 0.2–2 mmol/L) and ammonia of 162 (normal: 15–47 umol/L). With the presentation of hyperammonemia, and high anion gap metabolic acidosis, a possibility of an organic aciduria was considered, and she was placed on high dextrose infusion, intravenous carnitine therapy, and carglumic acid therapy. With the treatment, her lactate decreased to 6.5 mmol/L with normalization of ammonia to 44 umol/L and resolution of metabolic acidosis within 24 h of treatment. Her newborn screen #1 was normal and secondary biochemical testing was not indicative of an organic acidemia. Urine organic acids analysis was only abnormal for elevated lactate. Acylcarnitine profile showed numerous acylcarnitine species elevations but the pattern was not consistent with a specific defect of fatty acid oxidation. Similarly, plasma amino acids showed multiple elevations including alanine with a maximum value at 3305 mmol/L (148-420 mmol/L) and citrulline with a peak value at 99 mmol/L (2–41 mmol/L) suggesting the possibility of mitochondrial dysfunction. A ketogenic diet to treat the persistent lactic acidosis was initiated with the working diagnosis of congenital lactic acidosis due to pyruvate metabolism defect. Further genetics evaluation included concomitant chromosomal microarray analysis, critical trio whole exome sequencing (WES) and mitochondrial DNA sequencing (mitoNGS). In the interim, carnitine supplementation was discontinued, and oral feeds were reintroduced. Despite these interventions, lactate remained persistently elevated at 5–6 mmol/L throughout this time. At the age of 2 weeks, without obvious trigger, the patient had a second episode of tachypnea accompanied by metabolic acidosis, hypoglycemia (14 mg/dL), and acute on subacute rise in lactate to 17 mmol/L (Fig. 1). A second empiric sepsis work-up came back within normal limits. Caloric intake was increased from 110 kcal/kg/day to 143 kcal/kg/day and the metabolic decompensation resolved. Chromosomal microarray and mitoNGS were resulted as normal. However, critical trio WES showed compound heterozygous variants in the TRMU gene, c.117G>A (p.W39X) reported as a pathogenic variant and c.680G>C (p.R227T) reported as a variant of unknown significance. The ketogenic diet was discontinued. Plasma amino acids were repeated and did not show any deficiency of cysteine. At 6 weeks of age, with worsening of lactic acidosis, L-cysteine supplementation was started. She began to have seizure-like episodes for which treatment with antiepileptic medication was initiated. Vitamin E (200 IUdaily), vitamin C (25 mg daily), and selenium were started for their antioxidant properties. Around 8 weeks of life, the patient developed sepsis secondary to the placement of a central line catheter. Despite broad spectrum antibiotic coverage, she developed multiple organ failure including fulminant acute liver failure with severe coagulopathy, which did not respond to resuscitation measures with crystalloid and blood products. She succumbed to death at 8 weeks of life.
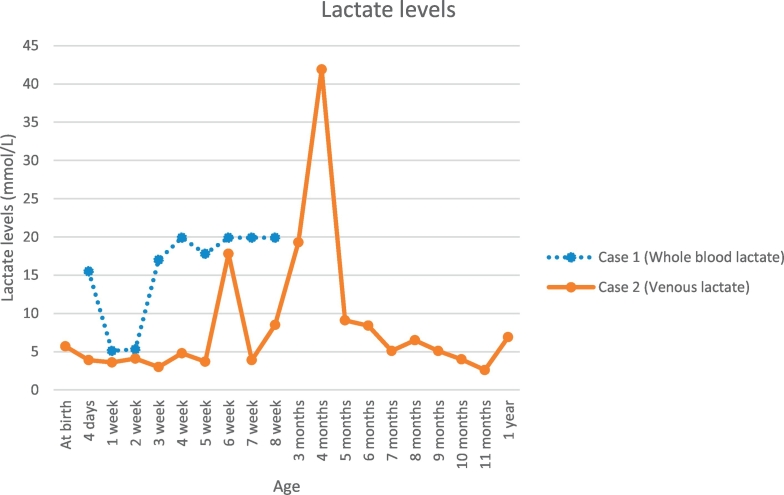
Fig. 1.
Lactate levels in cases 1 & 2.
Case 1 Whole blood lactate levels were measured with normal reference value of 0.2–1.7 mmol/L. Unable to calibrate the exact levels when 19.9 mmol/L and above. Peak lactate >19.9 mmol/L.
Case 2 Venous lactate levels were measured with a peak value of 41.9 mmol/L (normal reference value 0.2–2 mmol/L).
3. Case 2
Patient 2 is the full sibling of case 1 who had confirmation of the known familial biallelic variants in the TRMU gene by prenatal diagnosis obtained using amniocentesis. He was born at term. Birth weight was 3.085 kg (25th centile; z score: −0.66), length was 51.8 cm (73rd centile; z score: 0.6) and head circumference of 33.5 cm (20th centile; z score: −0.83). Immediately after birth he was started on Similac Advance® + Hominex-1® (methionine free amino acid formula typically used for homocystinuria), which provided an increased concentration of cysteine compared to other formulas and breast milk. N-acetylcysteine, selenium, vitamin C and vitamin E supplementation was started (Table 1). Quantitative plasma amino acid evaluation showed low methionine and as a result cysteine supplementation from Hominex-1® was switched to L-cysteine amino acid powder at one month of age (430 mg/kg/day of L-cysteine). His liver enzymes were within normal limits and he was gaining weight appropriately. He was discharged from the hospital at 5 weeks of age. Around 6 weeks of age, the patient's liver enzymes and lactate levels started to rise around the same time. He was admitted at 8 weeks of age for multiple episodes of emesis. Lactic acid level peaked at a value of 41.9 mmol/L (0.2–2.0 mmol/L) (Fig. 1). He had elevated liver enzymes especially GGT with a maximum elevation at 624 U/L (10–160 U/L) during this admission. Citric acid/sodium citrate was started to correct his metabolic acidosis. A trial of ursodiol was started for raised GGT. Once the acidosis was corrected, citric acid/sodium citrate was discontinued, and he was discharged home. A week later, he had worsening metabolic acidosis and liver enzymes, which prompted admission to the pediatric intensive care unit. Management included continuous sodium bicarbonate infusion (maximum rate of 48 mEq/kg/day). Due to worsening of liver function tests including conjugated bilirubin (8.5 mg/dL), prothrombin time (PT) (23 s) and INR (peak 2.0) along with metabolic acidosis and electrolyte imbalances including iatrogenic hypernatremia due to sodium bicarbonate infusion (peak 173 mmol/L) and hyperammonemia, he was put on the waiting list for liver transplantation during this admission (Table 2). Dialysis was not recommended as it cannot filter lactate and would have removed the supplemental L-cysteine resulting in further sulfur deficiency, which could be lethal. The patient had intermittent hypoglycemia during this admission with negative work up for endocrine causes including growth hormone deficiency, hyperinsulinemia, or adrenal insufficiency. His hypoglycemia was treated with continuous nasograstrics feeds and considered to be likely related to liver dysfunction and reduced glycogen stores. The patient received multiple fresh frozen plasma and packed red blood cells transfusions. His metabolic abnormalities and liver dysfunction slowly improved over the the next 3 weeks. He was inactivated on the liver transplant list due to continued improvement of liver function and was discharged home on a combination of citric acid/sodium citrate and potassium citrate in addition to the cited L-cysteine supplementation and antioxidant supplements. Liver enzymes and lactate were monitored weekly and gradually decreased (Table 1).
Table 1.
Medication doses in case 1 & 2.
| Case | L-cysteine (mg/kg/day) | N-acetylcysteine (mg/kg/day) | Selenium (mcg) | Vitamin C (mg) | Vitamin E (IU) |
|---|---|---|---|---|---|
| 1 | – | 105 | – | 250 | 200 |
| 2 | 300 | 70 | 2 | 250 | 200 |
Table 2.
Abnormalities in liver enzymes detected in cases 1 & 2.
| Case 1 |
Case 2 |
||||
|---|---|---|---|---|---|
| Initial Valuea | Peakb | Initial Valuea | Peakc | Most recentd | |
| ALT (6-50 U/L) | 64 | 309 | 24 | 347 | 42 |
| AST (35-140 U/L) | 110 | 1228 | 64 | 540 | 102 |
| ALP (77-265 U/L) | 143 | 425 | 172 | 579 | 232 |
| GGT (34-263 U/L) | 73 | 861 | 112 | 624 | 57 |
ALT: Alanine aminotransferase. AST: Aspartate aminotransferase. ALP: Alkaline phosphatase. GGT: Gamma-glutamyl transferase.
At birth.
1–1.5 months of age.
2–4 months of age.
10 months of age.
The patient was seen last in the metabolic clinic when he was 8 months old. At the time of visit, his growth parameters were the following: (i) weight of 9.08 kg (60th centile; z score: 0.25); (ii) height of 45.5 cm (<1st centile, z score: −2.89); (iii) FOC of 45.5 cm (69th centile; z score: 0.48). His developmental milestones corresponded to 5 months of age. He continues to receive physical therapy and speech therapy twice a week. He continues to be treated with citric acid/sodium citrate, N-acetylcysteine, selenium and vitamin C. Vitamin E was discontinued. Markers of liver function including INR, blood glucose, and bilirubin levels normalized, and his lactate remains in the range of 3–5 mmol/L. He was weaned off continuous nasogastric tube feeds and is currently taking all nutrition by mouth. Interestingly, by 9 months of age, he developed pigmentary changes in his hair including hypopigmented areas. Plasma amino acids, plasma copper and plasma ceruloplasmin levels were checked to evaluate for protein deficiency with normal results.
4. Discussion
The biological importance of sulfur-containing amino acids (methionine, cysteine, homocysteine, and taurine) is well recognized as serving a major role in cellular energy regulation. Methionine, an essential amino acid, is also a source of methyl groups for a number of methylation reactions such as methylation of nucleic acids, proteins, biogenic amines, phospholipids, etc. Methionine serves as a substrate for cysteine production, which is required for the synthesis of glutathione [17]. Cysteine plays a key role in a number of critical cellular processes, such as protein synthesis, oxidative stress response, iron‑sulfur (Fe—S) cluster biogenesis and regulatory and structural changes in proteins [18]. The transport of cysteine is important in regulating cellular cysteine biosynthesis as well as modulating the availability of sulfur for mitochondrial metabolism. Experimental evidence suggests cysteine can penetrate the lipid bilayer of the cell and can enter mitochondria through specific cellular cysteine uptake mechanisms [16]. All tRNA molecules require a wide variety of posttranscriptional modifications, which stabilize tRNA structure, enable efficient interaction with the ribosome, and fine-tune protein translation [19]. Interest in the metabolism of sulfur amino acids has remained high ever since it was observed that a key enzyme involved in the formation of cysteine from homocysteine (transsulfuration) could be low or absent in neonates [20,21]. It has been suggested neonates are unable to convert cystathionine to cysteine in significant quantities [22]. This concept has become more relevant as patients with transient infantile liver failure due to TRMU biallelic pathogenic variants have been diagnosed. Acute liver failure in infancy is a life-threatening condition manifested by poor feeding, jaundice, distended abdomen, hemorrhagic diathesis, irritability, and hypoactivity. Routine laboratory investigations reveal acute increases in liver transaminases, hypoglycemia, coagulopathy, hyperlactatemia, and direct/conjugated hyperbilirubinemia. The human TRMU gene encodes a 421-residue protein, which participates in the modification of mitochondrial tRNAs and is therefore critical for mitochondrial translation. Specifically, this mitochondrial enzyme is responsible for the 2-thiolation of the wobble position of the mitochondrial tRNA-Lys, tRNA-Gln, and tRNA-Glu [19]. The TRMU protein requires sulfur for its activity. Cysteine desulfurase, which transfers sulfur from cysteine to the TRMU ortholog, has been shown to be essential for the thio-modification of bacterial tRNAs. Most individuals with biallelic TRMU pathogenic variants present between two to four months of age. In contrast to most patients reported in the literature, both of our patients presented with laboratory abnormalities earlier, with case 1 presenting at birth and case 2 at two weeks of age. We postulate prior reported cases may have had abnormal liver function, which was not recognized until clinical symptoms manifested at a later period in infancy. Unfortunately, case 1 succumbed to overwhelming lactic acidosis and multiorgan failure due to sepsis from central line infection. Critical trio whole exome sequencing led to a rapid diagnosis in the proband and subsequent changes to management. Obtaining a molecular diagnosis in case 1 permitted prenatal molecular diagnosis for case 2 and time for the metabolic team to formulate an appropriate management strategy. Treatment with L-cysteine and N-acetylcysteine was initiated from birth. In retrospective, the evolution of case 2 has been typical when compared to previous literature cases. Most notably, he required a total dose of at least 300 mg/kg/day of L-cysteine to avoid increases in liver enzymes and lactic acidosis and/or hasten resolution of these lab abnormalities. Although further studies are necessary as more individuals are found to have TRMU deficiency, we propose early treatment with supplementation of L-cysteine powder and N-acetylcysteine should be considered in infants diagnosed with TRMU deficiency as it may slow disease progression, hasten recovery, and reduce the odds of liver transplantation.
References
- 1.Niyazov D.M., Kahler S.G., Frye R.E. Primary mitochondrial disease and secondary mitochondrial dysfunction: importance of distinction for diagnosis and treatment. Mol. Syndromol. 2016;7(3):122–137. doi: 10.1159/000446586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boczonadi V., Horvath R. Mitochondria: impaired mitochondrial translation in human disease. Int. J. Biochem. Cell Biol. 2014;48:77–84. doi: 10.1016/j.biocel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorman G.S. Mitochondrial diseases. Nat. Rev. Dis. Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 4.Taylor R.W. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA. 2014;312(1):68–77. doi: 10.1001/jama.2014.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boczonadi V., Ricci G., Horvath R. Mitochondrial DNA transcription and translation: clinical syndromes. Essays Biochem. 2018;62(3):321–340. doi: 10.1042/EBC20170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boczonadi V., Bansagi B., Horvath R. Reversible infantile mitochondrial diseases. J. Inherit. Metab. Dis. 2015;38(3):427–435. doi: 10.1007/s10545-014-9784-6. [DOI] [PubMed] [Google Scholar]
- 7.Sasarman F. The 2-thiouridylase function of the human MTU1 (TRMU) enzyme is dispensable for mitochondrial translation. Hum. Mol. Genet. 2011;20(23):4634–4643. doi: 10.1093/hmg/ddr397. [DOI] [PubMed] [Google Scholar]
- 8.Meng F. Biochemical evidence for a Nuclear Modifier Allele (A10S) in TRMU (Methylaminomethyl-2-thiouridylate-methyltransferase) Related to Mitochondrial tRNA Modification in the Phenotypic Manifestation of Deafness-associated 12S rRNA Mutation. J. Biol. Chem. 2017;292(7):2881–2892. doi: 10.1074/jbc.M116.749374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan M.X. Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations. Am. J. Hum. Genet. 2006;79(2):291–302. doi: 10.1086/506389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeharia A. Acute infantile liver failure due to mutations in the TRMU gene. Am. J. Hum. Genet. 2009;85(3):401–407. doi: 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaignard P. Mitochondrial Infantile Liver Disease due to TRMU Gene Mutations: three New cases. JIMD Rep. 2013;11:117–123. doi: 10.1007/8904_2013_230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schara U. Acute liver failure with subsequent cirrhosis as the primary manifestation of TRMU mutations. J. Inherit. Metab. Dis. 2011;34(1):197–201. doi: 10.1007/s10545-010-9250-z. [DOI] [PubMed] [Google Scholar]
- 13.Grover Z. Hepatic copper accumulation: a novel feature in transient infantile liver failure due to trmu mutations? JIMD Rep. 2015;21:109–113. doi: 10.1007/8904_2014_402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uusimaa J. Reversible infantile respiratory chain deficiency is a unique, genetically heterogenous mitochondrial disease. J. Med. Genet. 2011;48(10):660–668. doi: 10.1136/jmg.2011.089995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boczonadi V. Altered 2-thiouridylation impairs mitochondrial translation in reversible infantile respiratory chain deficiency. Hum. Mol. Genet. 2013;22(22):4602–4615. doi: 10.1093/hmg/ddt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsakoulia M. Cysteine Supplementation May be Beneficial in a Subgroup of Mitochondrial translation Deficiencies. J. Neuromuscul. Dis. 2016;3(3):363–379. doi: 10.3233/JND-160178. [DOI] [PubMed] [Google Scholar]
- 17.Metayer S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J. Nutr. Biochem. 2008;19(4):207–215. doi: 10.1016/j.jnutbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Tesseraud S. Role of sulfur amino acids in controlling nutrient metabolism and cell functions: implications for nutrition. Br. J. Nutr. 2009;101(8):1132–1139. doi: 10.1017/S0007114508159025. [DOI] [PubMed] [Google Scholar]
- 19.Umeda N. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J. Biol. Chem. 2005;280(2):1613–1624. doi: 10.1074/jbc.M409306200. [DOI] [PubMed] [Google Scholar]
- 20.Kalhan S.C., Bier D.M. Protein and amino acid metabolism in the human newborn. Annu. Rev. Nutr. 2008;28:389–410. doi: 10.1146/annurev.nutr.28.061807.155333. [DOI] [PubMed] [Google Scholar]
- 21.Zlotkin S.H., Anderson G.H. The development of cystathionase activity during the first year of life. Pediatr. Res. 1982;16(1):65–68. doi: 10.1203/00006450-198201001-00013. [DOI] [PubMed] [Google Scholar]
- 22.Riedijk M.A. Cysteine: a conditionally essential amino acid in low-birth-weight preterm infants? Am. J. Clin. Nutr. 2007;86(4):1120–1125. doi: 10.1093/ajcn/86.4.1120. [DOI] [PubMed] [Google Scholar]