LETTER
Clostridioides difficile (formerly Clostridium difficile) infection (CDI) is one of the most common health care-associated infections in the United States, resulting in significant morbidity, deaths, and economic burdens (1). Whether a C. difficile nucleic acid amplification test (NAAT) alone or a two-step algorithm involving a toxin enzyme immunoassay (EIA) should be used for better diagnosis of CDI is still controversial (2). Here we describe the achievement of reducing the hospital-onset CDI (HO-CDI) rate by 61% by implementing a simple computerized support tool (CST) coupled with education to enforce preanalytical screening for C. difficile testing.
We conducted a retrospective study at four hospitals (total capacity of 1,048 beds) of the MultiCare Health System, reviewing the laboratory-identified (LabID) HO-CDI events defined by the National Healthcare Safety Network (NHSN) (onset on hospital day >3) and C. difficile test volumes before and after the introduction of a CST coupled with education. The preintervention period spanned 12 months (January 2015 to December 2015), the intervention period spanned 3 months (January 2016 to March 2016), and the postintervention period spanned 24 months (April 2016 to March 2018). The diarrheal episodes, laxatives administered, and treatment for the LabID HO-CDI events were analyzed. In this study, true HO-CDI was defined as clinically significant diarrhea (≥3 unformed stools within 24 h) with onset on hospital day >3, without laxatives administered within 48 h prior to C. difficile testing (3). The laboratory uses a standalone C. difficile NAAT (BD MAX Cdiff assay; Becton, Dickinson, Franklin Lakes, NJ) for unformed stool specimens rating 6 or 7 on the Bristol stool scale. The CST was built in our hospital information system (Epic, Verona, WI) for use prior to the placement of a C. difficile test order. The ordering provider is prompted to answer 2 or 3 questions, depending on the age of the patient. No C. difficile testing is allowed for children <1 year of age; therefore, such orders cannot be processed in Epic. For patients >2 years of age, the ordering provider must indicate (i) whether the patient had ≥3 unformed stools in the past 24 h and (ii) whether the patient has severe ileus or toxic megacolon. For patients 1 to 2 years of age, the ordering provider must answer a third question about obtaining test approval from an infectious diseases specialist, a gastroenterologist, or the director of microbiology. In addition to the mandatory questions, a best practice alert appears for all C. difficile test orders, reminding the provider not to test if the patient has received laxative or stool softener in the past 48 h. Data analyses were performed using the t test for continuous variables, the Z test for proportions, and the χ2 test for categorical variables. All statistical tests were 2 tailed, with P values of ≤0.05 being considered statistically significant.
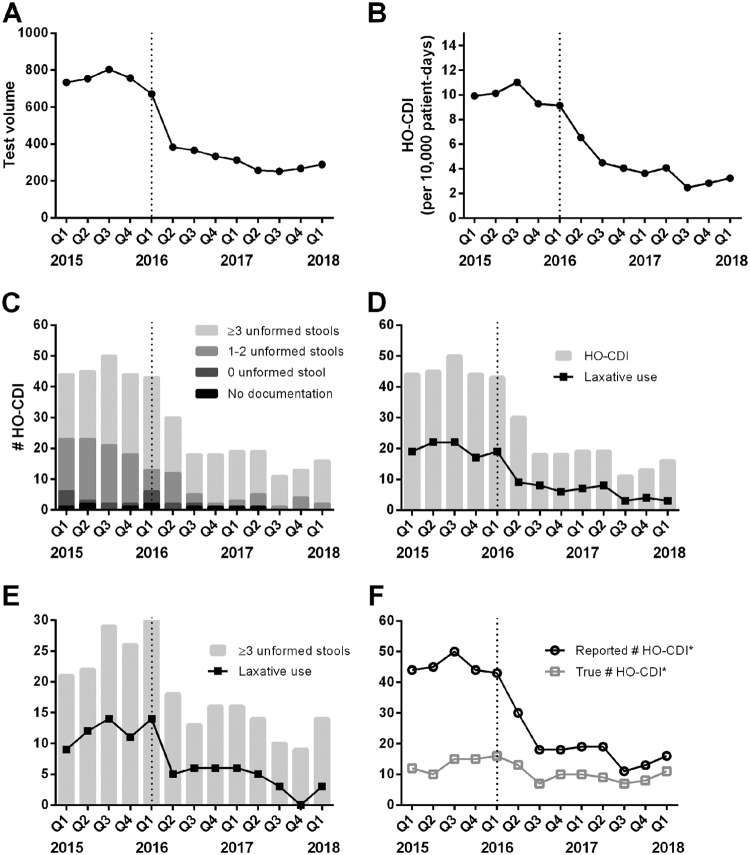
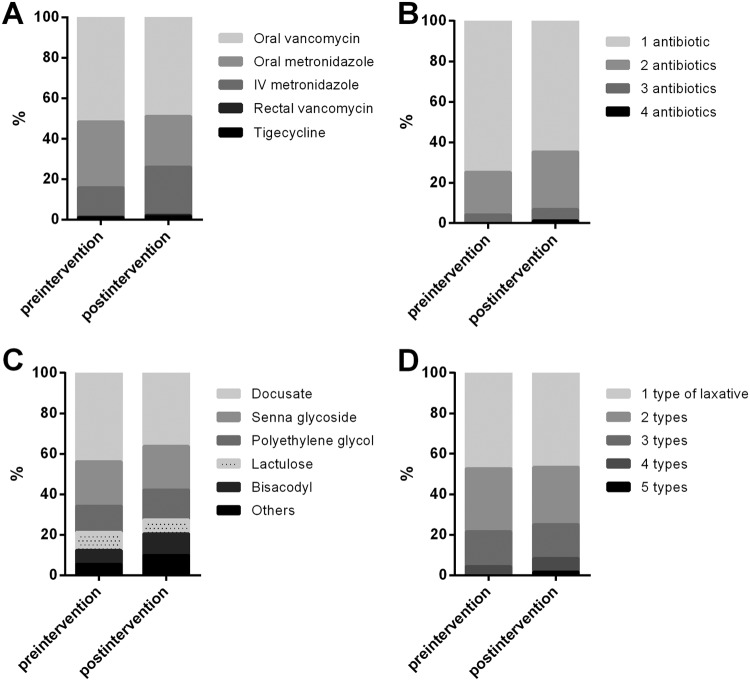
Between January 2015 and March 2018, a total of 377 LabID HO-CDI events were reported. Upon review, 7 events were excluded from this study; 3 events not meeting the NHSN criteria were misclassified as HO-CDI, and 4 events involved severe ileus or the use of a rectal tube, such that the typical definition of clinically significant diarrhea (≥3 unformed stools within 24 h) did not apply. During the study period, C. difficile test volumes decreased by 60% (mean ± standard deviation, 762 ± 30 tests/quarter preintervention versus 308 ± 50 tests/quarter postintervention; P < 0.001) (Fig. 1A). The HO-CDI rate decreased by 61% (10.1 cases/10,000 patient days preintervention versus 3.9 cases/10,000 patient days postintervention; P < 0.001) (Fig. 1B). Tests in cases with ≥3 unformed stools increased from 53% to 78% (from 98 of 183 cases to 110 of 144 cases; P < 0.001) (Fig. 1C). Tests in cases with prior laxative use decreased from 44% to 33% (from 80 of 183 cases to 48 of 144 cases; P = 0.003) (Fig. 1D). Among the events with clinically significant diarrhea, the percentage of cases with prior laxative use decreased from 47% to 31% (from 46 of 98 cases to 34 of 110 cases; P = 0.002) (Fig. 1E). After removal of events with insignificant diarrhea (<3 unformed stools within 24 h) and pretest laxative use, the numbers of true HO-CDI events were 13.0 ± 2.4 events/quarter preintervention and 9.4 ± 2.1 events/quarter postintervention (P = 0.02) (Fig. 1F). During the preintervention period, there was a 71% difference in the overall number of reported HO-CDI cases, compared to true HO-CDI cases; this difference was reduced by 23% postintervention (P < 0.001) (Fig. 1F). The percentage of patients who received treatment increased insignificantly, from 93% to 98% (from 171 of 183 patients to 141 of 144 patients; P = 0.1), whereas 95% of patients with inappropriate testing were treated. The variety and type of antibiotics that patients received did not change significantly during the study period (P > 0.1) (Fig. 2A and B). The variety and type of laxatives showed no significant changes before versus after the intervention (P > 0.2) (Fig. 2C and D).
FIG 1.
Changes in Clostridioides difficile tests and CDI cases from January 2015 to March 2018, divided into quarters, with the dotted line indicating 2016 quarter 1 as the intervention period. (A) C. difficile test volumes for inpatients. (B) Reported HO-CDI rates. (C) Distribution of diarrheal episodes among LabID HO-CDI events. (D) Laxative use among LabID HO-CDI events. (E) Laxative use among LabID HO-CDI events with clinically significant diarrhea, defined as ≥3 unformed stools within 24 h prior to testing. (F) Numbers of reported HO-CDI events and true HO-CDI events, after removal of events with laxative use and/or insignificant diarrhea (<3 unformed stools within 24 h). The asterisks indicate the exclusion of 3 cases of non-HO-CDI events and 4 cases involving ileus or the use of a rectal tube throughout the study period.
FIG 2.
Treatment for cases with positive C. difficile results and laxative use preintervention (January 2015 to December 2015) and postintervention (April 2016 to March 2018). (A) Type and route of antibiotics for C. difficile treatment (preintervention, n = 221; postintervention, n = 270). IV, intravenous. (B) Number of different types/routes of antibiotics per patient (preintervention, n = 171; postintervention, n = 141). (C) Type of laxatives administered within 48 h prior to C. difficile testing (preintervention, n = 146; postintervention, n = 102). (D) Number of different types of laxatives, administered within 48 h prior to C. difficile testing, per patient (preintervention, n = 80; postintervention, n = 48).
This study demonstrates the effectiveness of a CST coupled with education to reduce HO-CDI. We highlight that testing of patients without clinically significant diarrhea or with prior laxative use accounted for as much as 70% of reported LabID HO-CDI events. Similar to findings others have reported (3), 95% of patients with inappropriate testing received treatment. Inappropriate testing of unformed stools from patients who received laxative therapy remains a challenge (4), as this clinical parameter is seldom accessible to laboratorians except using a real-time monitoring system (5). Therefore, it is crucial to educate care providers that testing patients with a low pretest probability of disease leads to false-positive test results, unnecessary treatment, and increased costs (6, 7). One caveat of the CST is that its effectiveness relies on the integrity of the ordering providers answering the questions in Epic. However, we anticipate the HO-CDI rate to decrease further with future implementation of a hard stop on test orders with laxative use within the previous 48 h, instead of simply having the reminder in the best practice alert.
ACKNOWLEDGMENTS
We acknowledge the work of the C. difficile Prevention Team, the Infection Prevention and Control Operations Committee, the microbiology laboratory at MultiCare Health System, Andrew Reichert, and Billy Daniels.
No financial support is reported.
All authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang FC, Polage CR, Wilcox MH. 2017. Point-counterpoint: what is the optimal approach for detection of Clostridium difficile infection? J Clin Microbiol 55:670–680. doi: 10.1128/JCM.02463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock C, Pana Z, Leekha S, Trexler P, Andonian J, Gadala A, Carroll KC, Maragakis LL. 2018. National Healthcare Safety Network laboratory-identified Clostridium difficile event reporting: a need for diagnostic stewardship. Am J Infect Control 46:456–458. doi: 10.1016/j.ajic.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubberke ER, Han Z, Bobo L, Hink T, Lawrence B, Copper S, Hoppe-Bauer J, Burnham CA, Dunne WM Jr.. 2011. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol 49:2887–2893. doi: 10.1128/JCM.00891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truong CY, Gombar S, Wilson R, Sundararajan G, Tekic N, Holubar M, Shepard J, Madison A, Tompkins L, Shah N, Deresinski S, Schroeder LF, Banaei N. 2017. Real-time electronic tracking of diarrheal episodes and laxative therapy enables verification of Clostridium difficile clinical testing criteria and reduction of Clostridium difficile infection rates. J Clin Microbiol 55:1276–1284. doi: 10.1128/JCM.02319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz K, Sickbert-Bennett E, Marx A, Weber DJ, DiBiase LM, Campbell-Bright S, Bode LE, Baker M, Belhorn T, Buchanan M, Goldbach S, Harden J, Hoke E, Huenniger B, Juliano JJ, Langston M, Ritchie H, Rutala WA, Smith J, Summerlin-Long S, Teal L, Gilligan P. 2018. Preventable patient harm: a multidisciplinary, bundled approach to reducing Clostridium difficile infections while using a glutamate dehydrogenase/toxin immunochromatographic assay/nucleic acid amplification test diagnostic algorithm. J Clin Microbiol 56:e00625-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen C, Holtom P, Butler-Wu SM, Wald-Dickler N, Shulman I, Spellberg B. 2018. Reducing Clostridium difficile colitis rates via cost-saving diagnostic stewardship. Infect Control Hosp Epidemiol 39:734–736. doi: 10.1017/ice.2018.51. [DOI] [PubMed] [Google Scholar]