Limited methods for colistin MIC determination are available to clinical microbiology laboratories. The purpose of this study was to evaluate the accuracy of the colistin broth disk elution (CBDE) test compared to that of broth microdilution (BMD) for identifying colistin MICs.
KEYWORDS: colistin broth disk elution, antimicrobial susceptibility testing, colistin, method
ABSTRACT
Limited methods for colistin MIC determination are available to clinical microbiology laboratories. The purpose of this study was to evaluate the accuracy of the colistin broth disk elution (CBDE) test compared to that of broth microdilution (BMD) for identifying colistin MICs. CBDE was compared to colistin BMD using a collection of Gram-negative bacilli tested at two U.S. microbiology laboratories. The isolates tested included 121 retrospective clinical isolates, 45 prospective clinical isolates, and 6 mcr-1-positive Escherichia coli isolates. CBDE was performed with four 10-ml cation-adjusted Mueller-Hinton broth tubes per isolate, to which 0, 1, 2, and 4 colistin 10-µg disks were added, generating final concentrations in the tubes of 0 (growth control), 1, 2, and 4 µg/ml, respectively. MICs were evaluated visually and interpreted using Clinical and Laboratory Standards Institute breakpoints. Site 2 also compared CBDE to the reference broth macrodilution (BMAD) method (n = 110 isolates). Overall, CBDE yielded a categorical agreement (CA) and essential agreement (EA) of 98% and 99%, respectively, compared to the results of colistin BMD. Very major errors occurred for mcr-1-producing strains, with MICs fluctuating from 2 to 4 µg/ml on repeat testing. The results for all other isolates were in CA with those of BMD. CBDE versus BMAD had an EA of 100% and a CA of 100%. Compared to currently used techniques, CBDE is an easy and practical method to perform colistin MIC testing. Some mcr-1-producing isolates yielded MICs of 2 µg/ml by CBDE and 4 µg/ml by BMD. As such, the results for isolates with colistin MICs of 2 µg/ml by CBDE should be confirmed by the reference BMD method, and isolates with MICs of ≥2 µg/ml should be evaluated for the presence of mcr genes.
INTRODUCTION
Colistin (polymyxin E) is a polymyxin antibiotic that is prescribed as an agent of last resort for the treatment of multidrug-resistant (MDR) Gram-negative bacterial infections, including those caused by carbapenem-resistant Enterobacteriaceae (CRE). It consists of a large polycationic peptide that binds to the negatively charged cell wall of Gram-negative bacteria, causing disruption of the outer membrane, eventually leading to cell death (1). Resistance to colistin may be intrinsic or acquired, with the most common mechanism of resistance to colistin resulting from mutations in genes encoding the two-component regulatory system responsible for the synthesis of lipid A. These mutations yield alterations in lipid A, reducing the overall net negative charge of the outer membrane and subsequently reducing the binding of colistin. In 2015, the first plasmid-mediated colistin resistance gene, mcr-1, was described. mcr-1 encodes a phosphoethanolamine transferase that adds a phosphoethanolamine group to lipid A, decreasing the net negative charge of the cell wall (2). The plasmid-mediated colistin resistance gene mcr-1 and its variants (mcr-2 to mcr-8) are public health concerns, given their potential to readily disseminate among clinical pathogens (3–10).
The clinical use of colistin has been fraught with challenges related to drug toxicity, limited pharmacokinetic-pharmacodynamic data, the lack of robust clinical outcomes studies, and issues with antimicrobial susceptibility testing (AST) of the drug (11). The issues surrounding AST testing are 2-fold: (i) the lack of clinical breakpoints for the Enterobacteriaceae and (ii) the physiochemical properties of the drug, which render in vitro AST challenging. Due to insufficient data to establish colistin clinical breakpoints for Enterobacteriaceae, epidemiological cutoff values (ECVs) for certain Enterobacteriaceae were established by the Clinical and Laboratory Standards Institute (CLSI) based on MIC distribution data (12). ECVs allow for the differentiation between isolates that have MICs above the wild-type distribution (i.e., those with acquired and/or mutational mechanisms of resistance to colistin) and those that have MICs within the wild-type distribution. As colistin is a large, positively charged molecule, it diffuses poorly through agar-based media and adsorbs to negatively charged plastics, such as pipette tips and polystyrene tubes and plates.
In 2017, a CLSI (12) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) (13) joint working group recommended broth microdilution (BMD), without surfactant, as the reference method for testing colistin (rBMD). Although rBMD is an accurate method for colistin MIC determination, it can be resource intensive for clinical microbiology laboratories. Disk and gradient diffusion methods are not recommended by either CLSI or EUCAST for testing colistin due to unacceptably high error rates (14, 15), leaving microbiology laboratories without a practical method to identify colistin susceptibility. The objective of this study was to develop and compare the accuracy of a user-friendly colistin broth disk elution (CBDE) method to that of broth dilution methods for determining colistin MICs.
MATERIALS AND METHODS
Bacterial isolates.
Three sets of isolates were evaluated (Table 1). The first set (n = 56) was tested at UCLA Health in Los Angeles, CA (site 1). This collection encompassed 12 Acinetobacter baumannii, 20 Pseudomonas aeruginosa, and 24 Enterobacteriaceae retrospective clinical isolates, selected based on rBMD colistin MICs or resistance to carbapenems. The second set (n = 110 isolates) was tested at The Johns Hopkins Hospital (JHH; Baltimore, MD) (site 2). This collection encompassed 65 retrospective carbapenem-resistant Enterobacteriaceae (CRE) and 45 prospective clinical Gram-negative bacillus isolates (12 A. baumannii, 14 P. aeruginosa, and 19 carbapenem-resistant Enterobacteriaceae isolates). The JHH retrospective CRE were previously characterized by the Check-MDR CT103XL assay (Check-Points, Wageningen, The Netherlands) for carbapenemase production and included 36 non-carbapenemase-producing (non-CP) and 29 carbapenemase-producing (CP) CRE. Of the CP CRE, 23 were KPC producers, 3 were NDM producers, 2 were KPC and NDM producers, and 1 was an NDM and OXA-48 producer. The third set was obtained from the CDC-FDA Antimicrobial Resistance Isolate Bank (CDC AR Bank) and tested at both sites. Set 3 encompassed 6 mcr-1-producing Escherichia coli isolates. All retrospective isolates were stored at −70°C and subcultured twice on sheep’s blood agar prior to testing.
TABLE 1.
Summary of CBDE results compared to rBMD and BMD results in a two-site studyc
| Site and isolate | No. of isolates with the following BMD result: |
CA (%) | EA (%) | VME (%) | ME (%) | ||
|---|---|---|---|---|---|---|---|
| Total | S or WT (N) | R or NWT (N) | |||||
| Site 1 | |||||||
| Acinetobacter baumannii | 12 | 5 | 7 | 100 | 100 | 0 | 0 |
| Pseudomonas aeruginosa | 20 | 18 | 2 | 100 | 100 | 0 | 0 |
| Enterobacteriaceae | 24 | 10 | 14 | 100 | 100 | 0 | 0 |
| Site 2 | |||||||
| Retrospective CRE | 65 | 58 | 7 | 100 | 97a | 0 | 0 |
| A. baumannii | 12 | 12 | 0 | 100 | 100 | 0 | 0 |
| P. aeruginosa | 14 | 14 | 0 | 100 | 100 | 0 | 0 |
| Prospective CRE | 19 | 17 | 2 | 100 | 100 | 0 | 0 |
| Both sites, mcr-1-producing E. colib | 6 | 0 | 6 | 50 | 100 | 50 | 0 |
| Overall | 172 | 134 | 38 | 98 | 99 | 8 | 0 |
One Citrobacter freundii isolate had an MIC of ≤0.25 µg/ml by BMD and an MIC of 2 µg/ml by CBDE, and 1 Enterobacter cloacae isolate had an MIC of 0.5 µg/ml by BMD and an MIC of 2 µg/ml by CBDE.
Three mcr-1-positive E. coli isolates had MICs of 4 µg/ml by BMD and 2 µg/ml by CBDE on initial testing at both sites. These results were reproduced at the 2 sites.
S, susceptible; R, resistant; WT, wild type; NWT, non-wild type; N, number of isolates; CA, categorical agreement; EA, essential agreement; VME, very major error; ME, major error; BMD, broth microdilution; rBMD, reference BMD. Site 1 performed rBMD and site 2 performed BMD. At site 1, the Enterobacteriaceae consisted of 8 Klebsiella pneumoniae isolates (3 were carbapenem resistant), 7 E. cloacae isolates, 4 Escherichia coli isolates, 2 Klebsiella (Enterobacter) aerogenes isolates, 1 C. freundii isolate, 1 Citrobacter koseri isolate, and 1 Enterobacter hermannii isolate. At site 2, the retrospective CRE consisted of 32 K. pneumoniae isolates, 15 E. cloacae isolates, 8 E. coli isolates, 4 C. freundii isolates, 3 Serratia marcescens isolates, 1 Proteus mirabilis isolate, 1 K. aerogenes isolate, and 1 Klebsiella oxytoca/Raoultella ornithinolytica isolate. At site 2, prospective carbapenem-resistant Enterobacteriaceae consisted of 13 Klebsiella pneumoniae isolates, 3 E. cloacae isolates, and 3 Escherichia coli isolates.
Colistin broth disk elution method.
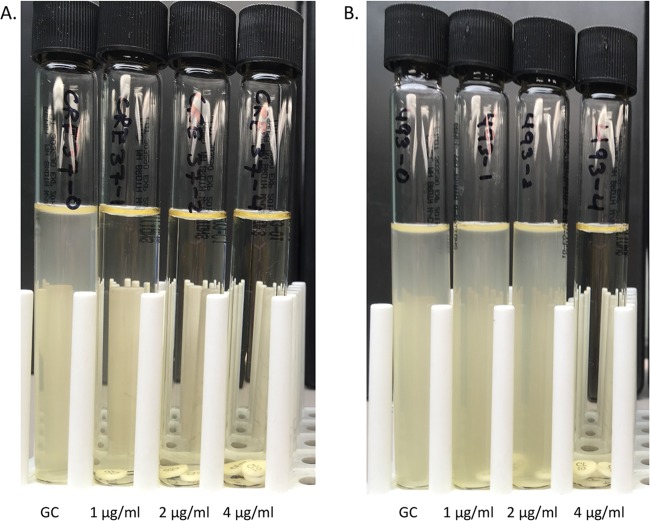
The CBDE method was performed with four 10-ml cation-adjusted Mueller-Hinton broth (CA-MHB; Remel, Lenexa, KS) tubes per isolate, to which 0, 1, 2, and 4 colistin disks (10 µg; BD, Sparks MD) were added, generating final concentrations of 0 (growth control), 1, 2, and 4 µg/ml, respectively (Fig. 1). The tubes were incubated at room temperature for 30 min to allow colistin to elute from the disks. Inocula were prepared by suspending fresh colonies from an overnight sheep’s blood agar plate in normal saline and standardizing the turbidity to match that of a McFarland 0.5 standard. A 50-µl aliquot of the standardized suspension was added to each tube, and the tubes were gently vortexed for a final inoculum of 7.5 × 105 CFU/ml, consistent with CLSI guidelines (16). Colistin MIC values were read visually, after a 16- to 20-h incubation at 35°C in ambient air. MICs were interpreted using CLSI breakpoints (for P. aeruginosa and A. baumannii) or ECVs (for Enterobacteriaceae). Quality control was performed with P. aeruginosa ATCC 27853 and an mcr-1-producing E. coli isolate from the CDC AR Bank (CDC AR Bank accession number 349) (expected MICs, 2 to 4 µg/ml).
FIG 1.
Colistin broth disk elution method. CBDE is performed with four 10-ml cation-adjusted Mueller-Hinton broth tubes per isolate, to which 0, 1, 2, and 4 colistin disks (10 µg) are added, generating final concentrations of 0 (growth control [GC]), 1, 2, and 4 µg/ml, respectively. (A) Tubes for a non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae isolate with a colistin MIC of ≤1 µg/ml. (B) Tubes for an mcr-1-producing Escherichia coli isolate (CDC AR Bank accession number 493) with a colistin MIC of 4 µg/ml.
Reference broth microdilution and macrodilution testing.
For the testing performed at UCLA, rBMD panels with colistin were produced at MicroScan (Beckman Coulter, Sacramento, CA), with testing being performed following CLSI recommendations (16). The rBMD frozen-form panels included two types of CA-MHB (BBL MH II; Difco), and each was tested in duplicate with colistin at concentrations of 0.25 to 8 µg/ml. The reference colistin MIC value assigned to each isolate represented the modal value obtained from the 4 readings. At JHH, the results of the CBDE method were compared to those obtained by broth macrodilution (BMAD) and by the use of RUO Sensititre GNX2F panels (Thermo Fisher), a BMD method that has been shown to perform comparably to rBMD (17). BMD was performed using Sensititre cation-adjusted Mueller-Hinton broth with TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid; Thermo Fisher] for setup of the RUO Sensititre GNX2F panels. BMAD was performed following the CLSI reference method using 16- by 100-mm acid-washed borosilicate glass tubes (16). Stock solutions of colistin sulfate (Sigma-Aldrich) were diluted in commercially prepared CA-MHB (Remel, Lenexa, KS) to obtain 1-ml volumes at final concentrations of 0.03 to 16 µg/ml colistin.
Testing strategy.
The CBDE and rBMD tests were performed in parallel at UCLA (site 1). The CBDE and BMAD tests were performed in parallel at JHH (site 2), and BMD was performed at a later date on both retrospective and prospective isolates that had been stored at −70°C. If any discrepant results were apparent between methods for an individual isolate (a ≥2-doubling-dilution difference), all 2 or 3 methods were repeated from the same subculture, and these results were used for the final analysis. Quality control for BMD and BMAD methods was performed using P. aeruginosa ATCC 27853 and E. coli ATCC 25922.
Reproducibility study.
A reproducibility study was completed by independently testing the 6 mcr-1-positive isolates on three separate days by the BMD, BMAD, and CBDE methods at one site (JHH).
Statistical analysis.
Colistin MIC results were truncated to the same concentration range for method comparisons using the smallest range for the methods being compared. The categorical agreement (CA; applied to both ECVs and clinical breakpoints) and essential agreement (EA; MIC ± 1 dilution) were evaluated, and major errors (ME) and very major errors (VME) were identified. The results were considered acceptable if CA and EA were ≥90% and VME and ME were <3%.
RESULTS
CBDE compared to rBMD and BMD.
Site 1 and 2 CBDE results compared to BMD and rBMD results are summarized in Table 1. Of the 172 isolates tested, 134 (78%) were susceptible/wild type and 38 (22%) were resistant/non-wild type by BMD methods. For site 1, a CA of 100% and an EA of 100% compared to the results of rBMD were achieved by CBDE. For 2 Enterobacter cloacae isolates, skipping was observed by rBMD, but this resolved on repeat testing. For the 65 retrospective CRE isolates tested at site 2, CBDE yielded a CA of 100% and an EA of 97%. One Citrobacter freundii isolate had a colistin MIC of ≤0.25 µg/ml by BMD and an MIC of 2 μg/ml by CBDE, and 1 Enterobacter cloacae isolate had a colistin MIC of 0.5 µg/ml by BMD and an MIC of 2 µg/ml by CBDE (Table 2). There were 2 E. cloacae isolates and 1 Klebsiella pneumoniae isolate that demonstrated skipping by BMD and a different E. cloacae isolate that demonstrated skipping by CBDE that resolved on repeat testing. For the 45 prospective clinical isolates at site 2, CBDE achieved a CA and an EA of 100% each. A single Pseudomonas aeruginosa isolate demonstrated skipping by BMD, but this resolved on repeat testing. For the 6 mcr-1-producing E. coli isolates, a CA and an EA of 50% and 100%, respectively, were obtained at both sites. The MICs of three of the mcr-1-producing E. coli isolates tested as 2 µg/ml (wild type) by CBDE and 4 µg/ml (non-wild type) on initial testing by BMD, resulting in a CA and a VME of 50% each, despite being within the acceptable EA for all 6 isolates (100%). Combining data from both sites, the CBDE had a CA and an EA of 98% and 99%, respectively, compared to the results of colistin rBMD or BMD for the 172 Gram-negative bacilli tested. The overall VME rate of 8% was due to the 3 mcr-1-producing E. coli isolates that tested 1 dilution lower by CBDE than by rBMD and BMD methods.
TABLE 2.
Colistin MIC results by BMD compared to those by CBDE at site 2b
| Colistin MIC (µg/ml) by BMD | No. of isolates with the following colistin MIC (µg/ml) by CBDE: |
|||
|---|---|---|---|---|
| ≤1 | 2 | 4 | >4 | |
| ≤1 | 99 | 2a | ||
| 2 | ||||
| 4 | ||||
| >4 | 9 | |||
One C. freundii isolate had an MIC of ≤0.25 µg/ml by BMD, and 1 E. cloacae isolate had an MIC of 0.5 µg/ml by BMD.
Categorical agreement was 100% and essential agreement was 98%, with no very major or major errors. CBDE, colistin broth disk elution; BMD, broth microdilution.
CBDE compared to broth macrodilution.
Site 2 compared CBDE to BMAD, as summarized in Table 3. A single P. aeruginosa isolate demonstrated an MIC of 0.5 µg/ml by BMAD but had an MIC of 2 µg/ml by CBDE on initial testing. On repeat testing from the same subculture, the MICs by both BMAD and BMD were 0.5 µg/ml and the MIC by CBDE was consistent with that MIC with an MIC of ≤1 µg/ml. After discordant analysis, CBDE and BMAD demonstrated an EA of 100% and a CA of 100%.
TABLE 3.
Colistin MIC results by BMAD compared to those by CBDE at site 2a
| Colistin MIC (µg/ml) by BMAD | No. of isolates with the following colistin MIC (µg/ml) by CBDE: |
|||
|---|---|---|---|---|
| ≤1 | 2 | 4 | >4 | |
| ≤1 | 99 | |||
| 2 | 1 | 1 | ||
| 4 | ||||
| >4 | 9 | |||
Categorical agreement was 100%, and essential agreement was 100%, with no very major or major errors. CBDE, colistin broth disk elution; BMAD, broth macrodilution.
Reproducibility study.
Table 4 summarizes the results of the reproducibility study comparing the methods to determine colistin MICs for 6 mcr-1-positive E. coli isolates over 3 days of testing. All methods were within acceptable EA for the 3 replicates. The MICs for one isolate (CDC AR Bank accession number 349) fluctuated between 2 and 4 µg/ml when tested by BMD and CBDE, and the MIC consistently tested as 2 µg/ml by BMAD. Another isolate (CDC AR Bank accession number 350) demonstrated an MIC of 2 µg/ml by BMD on day 1 of reproducibility testing. The MICs for the remaining four mcr-1-positive isolates tested as ≥4 µg/ml (non-wild type) during the reproducibility study by all methods.
TABLE 4.
Summary of reproducibility of the various methods to determine colistin MICs for mcr-1-positive Escherichia coli isolates
| CDC AR Bank accession no. | Colistin MIC (µg/ml) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDC result | Broth microdilution |
Broth macrodilution |
CBDE |
||||||||||
| Day 1 | Day 2 | Day 3 | Mode | Day 1 | Day 2 | Day 3 | Mode | Day 1 | Day 2 | Day 3 | Mode | ||
| 346 | 4 | 4 | >4 | >4 | >4 | 8 | 8 | 8 | 8 | 4 | 4 | 4 | 4 |
| 349a | 2–4 | 2 | 4 | 4 | 4 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 4 |
| 350a | 4 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 493 | 8 | >4 | >4 | >4 | >4 | 8 | 8 | 8 | 8 | 4 | 4 | 4 | 4 |
| 494 | 8 | >4 | >4 | >4 | >4 | 8 | 8 | 8 | 8 | 4 | 4 | 4 | 4 |
| 495a | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
Isolates that demonstrated MICs of 2 µg/ml by colistin broth disk elution on initial testing at both sites.
DISCUSSION
This is the first study identifying the CBDE method as a practical approach for clinical laboratories to perform colistin AST for Gram-negative bacilli. The method is performed using readily available and affordable supplies. CBDE applies the same principle that was used to determine anaerobe antimicrobial susceptibility in 1973, where antimicrobial disks of a known concentration were eluted in a set volume of broth, to obtain standard doubling dilutions to determine MICs (18). CLSI and EUCAST currently recommend that colistin AST be performed by rBMD without surfactant, a method few laboratories have access to (12, 13). Some laboratories may choose to perform labor-intensive approaches, such as agar dilution or BMAD testing, but these require significant technologist time for test preparation and setup and are not currently recommended by CLSI and EUCAST for colistin testing. Furthermore, despite the advice of standards-setting organizations (CLSI/EUCAST) and the high error rates reported for disk and gradient diffusion methods (the VME rate is reported to be >40%), some laboratories continue to use these approaches due to the low cost and availability of necessary materials (14, 15). Alternatively, labs may decide to outsource colistin MIC testing, but prolonged turnaround times can lead to substantial delays in the time to optimal therapy for critically ill patients. CBDE overcomes many of the barriers that exist with the available approaches for colistin MIC testing and offers a practical, albeit still accurate and reproducible, alternative for colistin testing for laboratories of all sizes.
Other simplified approaches for colistin antimicrobial susceptibility determination have been described. The Polymyxin NP is a 2-h colorimetric assay, similar to the Carba NP, that detects glucose metabolism related to bacterial growth in the presence of a pH indicator with a set concentration of colistin (3.75 µg/ml/well). It has a sensitivity and a specificity of upwards of 95% (19). Due to the requirement of glucose metabolism, the test is limited for use with the Enterobacteriaceae, unlike the CBDE method, which can also be used for glucose-nonfermenting organisms (e.g., P. aeruginosa, A. baumannii). Furthermore, the Polymyxin NP assay requires the preparation of specialized reagents and interpretation of color changes, occasionally yielding subjective results. The Polymyxin NP assay does, however, appear to have excellent accuracy when evaluating mcr-positive isolates. Using the CBDE method, we identified VME for mcr-1-producing strains with MICs that fluctuated between 2 and 4 µg/ml. The Polymyxin NP assay was found to have a sensitivity and a specificity of 100% for detecting colistin resistance mediated by mcr-1 or mcr-2 in 70 unique Enterobacteriaceae isolates with MICs ranging from 4 to 16 μg/ml (20). This difference might be attributable to a concentration of 3.75 µg/ml/well in the Polymyxin NP assay compared to one of 4 µg/ml/tube in the CBDE method and the lack of isolates with MICs of 2 μg/ml.
Another testing approach with colistin (3.5 μg/ml)-containing SuperPolymyxin (ELITechGroup, Puteaux, France) medium uses a growth-versus-no growth result to identify colistin-resistant/non-wild-type Gram-negative organisms. A study evaluating 36 Enterobacteriaceae isolates identified this approach to be associated with a sensitivity and a specificity of 100%. This study included isolates with acquired resistance to polymyxins, including 3 mcr-1-containing E. coli isolates with colistin MICs of 4, 8, and 16 μg/ml by rBMD (21). The fact that MICs in the 2- to 4-μg/ml range are frequently observed for mcr-expressing Enterobacteriaceae raises concerns as to whether the ECV should be reevaluated, as it was established prior to the mcr resistance mechanism being widely described. Furthermore, laboratories should be cognizant of the possibility of the presence of mcr in isolates with colistin MICs of ≥2 μg/ml. We recommend that colistin MICs of 2 µg/ml by the CBDE method be confirmed by rBMD (the results for two isolates [1%] from this study would have required confirmation), and consideration should be taken to test those with MICs of ≥2 µg/ml for mcr genes, especially among the Enterobacteriaceae, in which mcr genes have mostly been reported (3–10). However, due to the mobility of the plasmid-mediated gene and a report of a chromosomally encoded mcr-5 gene in a colistin-nonsusceptible P. aeruginosa isolate (MIC 4 µg/ml), further confirmation testing of nonfermenters may be warranted (22).
A limitation of the present study is that not all testing methods were performed simultaneously at the JHH site. However, repeat testing of discrepant isolates was conducted for all methods using the same subculture. Additionally, different cohorts of isolates were tested between the two study sites. A larger multicenter study utilizing the same isolates and a larger number of non-wild-type/resistant isolates tested across various sites is required to confirm the accuracy and reproducibility of the CBDE method. Lastly, the concentration of colistin eluted from the disks in CA-MHB was assumed and not confirmed. However, the concordance with broth dilution methods suggests that the concentrations of colistin eluted were sufficient.
In summary, we describe an accurate, simple, and practical method for determining colistin MICs that overcomes many of the current limitations to colistin AST. Based on its underestimation of MICs for mcr-positive isolates, we recommend that colistin MICs of 2 µg/ml by the CBDE method be confirmed by rBMD and that isolates with MICs of ≥2 µg/ml be evaluated for the presence of mcr genes. The need for an improved approach to colistin MIC determination is timely as we continue to witness an increasing prevalence of multidrug-resistant Gram-negative bacterial infections with limited treatment options.
ACKNOWLEDGMENTS
The work was supported by funding from The Sherrilyn and Ken Fisher Center for Environmental Diseases (to P.J.S.) and National Institutes of Health grants R21AI130608, awarded to P.J.S., and K23AI127935, awarded to P.D.T.
The rBMD panels used at UCLA were supplied by Beckman Coulter.
REFERENCES
- 1.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired, and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 3.AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, Randall LP, Lemma F, Crook DW, Teale C, Smith RP, Anjum MF. 2017. mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother 72:2745–2749. doi: 10.1093/jac/dkx286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. 2017. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother 72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 5.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, Pezzotti G, Magistrali CF. 2017. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill 22(31):pii=30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, van Dorp L, Shaw LP, Bradley P, Wang Q, Wang X, Jin L, Zhang Q, Liu Y, Rieux A, Dorai-Schneiders T, Weinert LA, Iqbal Z, Didelot X, Wang H, Balloux F. 2018. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat Commun 9:1179. doi: 10.1038/s41467-018-03205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, Zhang S, Shen J, Shen Z, Wang Y. 2018. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect 7:122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, Malhotra-Kumar S. 2016. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia, Belgium, June 2016. Euro Surveill 21(27):pii=30280. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 9.Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. 2018. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother 73:1791–1795. doi: 10.1093/jac/dky111. [DOI] [PubMed] [Google Scholar]
- 10.Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, Zhang R, Walsh TR, Shen J, Wang Y. 2017. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia. mBio 8:e00543-17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogue JM, Ortwine JK, Kaye KS. 2017. Clinical considerations for optimal use of the polymyxins: a focus on agent selection and dosing. Clin Microbiol Infect 23:229–233. doi: 10.1016/j.cmi.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing; 28th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.EUCAST. 2017. Breakpoint tables for interpretation of MICs and zone diameters, version 8.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf. Accessed 6 December 2018.
- 14.Dafopoulou K, Zarkotou O, Dimitroulia E, Hadjichristodoulou C, Gennimata V, Pournaras S, Tsakris A. 2015. Comparative evaluation of colistin susceptibility testing methods among carbapenem-nonsusceptible Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 59:4625–4630. doi: 10.1128/AAC.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol 51:1678–1684. doi: 10.1128/JCM.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Matuschek E, Ahman J, Webster C, Kahlmeter G. 2017. Antimicrobial susceptibility testing of colistin—evaluation of seven commercial MIC products against standard broth microdilution for Escherichia, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin Microbiol Infect 24:865–870. doi: 10.1016/j.cmi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins TD, Thiel T. 1973. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother 3:350–356. doi: 10.1128/AAC.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordmann P, Jayol A, Poirel L. 2016. Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerg Infect Dis 22:1038–1043. doi: 10.3201/eid2206.151840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel L, Larpin Y, Dobias J, Stephan R, Decousser JW, Madec JY, Nordmann P. 2018. Rapid Polymyxin NP test for the detection of polymyxin resistance mediated by the mcr-1/mcr-2 genes. Diagn Microbiol Infect Dis 90:7–10. doi: 10.1016/j.diagmicrobio.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Nordmann P, Jayol A, Poirel L. 2016. A universal culture medium for screening polymyxin-resistant Gram-negative isolates. J Clin Microbiol 54:1395–1399. doi: 10.1128/JCM.00446-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snesrud E, Maybank R, Kwak YI, Jones AR, Hinkle MK, McGann P. 2018. Chromosomally encoded mcr-5 in colistin-nonsusceptible Pseudomonas aeruginosa. Antimicrob Agents Chemother 62:e00679-18. doi: 10.1128/AAC.00679-18. [DOI] [PMC free article] [PubMed] [Google Scholar]