Candida glabrata is intrinsically less susceptible to azoles, and resistance to echinocandins and reduced susceptibility (RS) to amphotericin B (AMB) have also been detected. The molecular mechanisms of RS to AMB were investigated in C. glabrata strains in Kuwait by sequence analyses of genes involved in ergosterol biosynthesis.
KEYWORDS: Candida glabrata, ERG6, ERG2, and ERG11 mutations, reduced susceptibility, amphotericin B
ABSTRACT
Candida glabrata is intrinsically less susceptible to azoles, and resistance to echinocandins and reduced susceptibility (RS) to amphotericin B (AMB) have also been detected. The molecular mechanisms of RS to AMB were investigated in C. glabrata strains in Kuwait by sequence analyses of genes involved in ergosterol biosynthesis. A total of 1,646 C. glabrata isolates were tested by Etest, and results for 12 selected isolates were confirmed by reference broth microdilution. PCR sequencing of three genes (ERG2, ERG6, and ERG11) was performed for all isolates with RS to AMB (RS-AMB isolates) and 5 selected wild-type C. glabrata isolates by using gene-specific primers. The total cell sterol content was analyzed by gas chromatography-mass spectrometry. The phylogenetic relationship among the isolates was investigated by multilocus sequence typing. Wild-type isolates contained only synonymous mutations in ERG2, ERG6, or ERG11, and the total sterol content was similar to that of the reference strains. A nonsynonymous ERG6 mutation (AGA48AAA, R48K) was found in both RS-AMB and wild-type isolates. Four RS-AMB isolates contained novel nonsense mutations at Trp286, Tyr192, and Leu341, and 2 isolates contained a nonsynonymous mutation in ERG6 (V126F or C198F); and the sterol content of these isolates was consistent with ERG6 deficiency. Two other RS-AMB isolates contained a novel nonsynonymous ERG2 mutation (G119S or G122S), and their sterol content was consistent with ERG2 deficiency. Of 8 RS-AMB isolates, 1 fluconazole-resistant isolate also contained nonsynonymous Y141H plus L381M mutations, while 7 isolates contained only synonymous mutations in ERG11. All isolates with ERG6, ERG2, and ERG11 mutations were genotypically distinct strains. Our data show that ERG6 and ERG2 are major targets conferring RS-AMB in clinical C. glabrata isolates.
INTRODUCTION
Candida species are the most common cause of invasive fungal infections in seriously ill or immunocompromised hospitalized patients (1). Although Candida albicans is the most pathogenic species, infections by non-albicans Candida species have increased in recent years and are associated with high mortality rates (1–3). Candida glabrata is the second or third most commonly isolated yeast species causing candidemia and other invasive infections in critically ill older adult (>65 years) patients (1–3). C. glabrata is intrinsically less susceptible to azole antifungal drugs and causes mortality in nearly 50% of subjects with invasive infections (2–4). Recent years have also witnessed increasing reports of breakthrough C. glabrata infections in patients receiving systemic echinocandin (micafungin) or polyene treatment (5–7).
The major antifungal drugs used for invasive Candida infections include triazoles (such as fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole), echinocandins (such as caspofungin, anidulafungin, and micafungin), and polyenes (such as amphotericin B [AMB] and liposomal amphotericin B [LAMB]). In contrast to azoles and echinocandins, which disrupt ergosterol and glucan synthesis, respectively, polyenes were postulated, until recently, to intercalate directly with membrane ergosterol, forming ion channels, which permeabilize and kill yeast cells (3, 4). More recent studies, however, have shown that AMB forms extramembranous aggregates which extract ergosterol, a central molecule in yeast physiology, from phospholipid bilayers like a sterol sponge, and the removal of ergosterol kills yeast cells, while the contribution of ion channels is relatively minor (8). The sterol sponge model has also stipulated that the simultaneous extraction of cholesterol by AMB from mammalian cells is responsible for its toxicity, suggesting the possibility of separating cytocidal properties from membrane-permeabilizing activities (8). Further studies have indeed resulted in the synthesis of other derivatives of AMB (such as amphotericin B methyl urea and amphotericin B amino urea) that bind ergosterol with a much greater selectivity than cholesterol and, thus, are toxic to yeast cells but not to human cells (9).
Consistent with their mechanism of action, the resistance of Candida spp. to triazoles and echinocandins usually develops in a stepwise manner during prolonged therapy as a result of induced changes and mutations (3, 4, 10). Since extraction of ergosterol from yeast membranes by AMB affects all ergosterol-dependent cellular processes and membrane fluidity/hydrophobicity, the evolution of amphotericin B-resistant strains is expected to be highly unlikely due to the involvement of many different membrane proteins that directly bind to ergosterol as well as the blocking of transport processes by several essential transport proteins due to alterations in membrane properties (11). Thus, the emergence of Candida spp. exhibiting reduced susceptibility (RS) to AMB (RS-AMB) has generally been rare, despite >50 years of clinical use. The mutations in rare C. albicans strains with RS-AMB (RS-AMB strains) created defects in filamentation and tissue invasion and diverse stresses, resulting in hypersensitivity to oxidative stress, febrile temperatures, and neutrophil-mediated killing (12, 13). On the contrary, increasing reports of RS-AMB in C. glabrata, a haploid species, is a worrisome development (14–19).
Given the lower rates of susceptibility to azoles and the increasing incidence of breakthrough infections in patients on micafungin therapy, reduced susceptibility to polyenes will severely limit the choice of antifungal drugs to treat C. glabrata infections (3, 4). Since polyenes extract ergosterol from the cell membrane, changes in ergosterol content due to mutations in ERG genes involved in ergosterol biosynthesis alter susceptibility to polyenes, and the complete absence of ergosterol confers RS-AMB (17, 19, 20). A better understanding of the mechanisms that mediate reduced susceptibility to polyenes in C. glabrata is warranted. The first molecular mechanism describing RS-AMB in C. glabrata involved a missense mutation (C198F) or a nonsense mutation (at Gln332) in ERG6 encoding C-24 methyltransferase, which converts zymosterol to fecosterol in the ergosterol biosynthesis pathway (15, 17). Deletion of ERG11 or mutations in ERG1, ERG2, and ERG11 that are associated with RS-AMB in C. glabrata have also been described (18, 19, 21). A lack of ERG6- and ERG2-encoded enzyme activities leads to the accumulation of zymosterol and fecosterol, respectively. Zymosterol and fecosterol can support fungal cell growth, and the absence of ergosterol in the Candida cell membrane confers RS-AMB (15, 17, 19, 22).
Epidemiological cutoff values rather than clinical breakpoints are available for interpreting the MICs of AMB for Candida species, and yeasts with MICs of ≤2 μg/ml were classified as wild type (WT), while isolates with MICs of >2 μg/ml were defined as non-wild-type (non-WT) (23). Antifungal drug susceptibility testing (AST) data for clinical C. glabrata isolates collected from 2009 to 2016 in Kuwait by Etest identified 1 isolate as non-WT, while the remaining isolates were WT for AMB. However, among WT isolates, 7 strains exhibited reduced susceptibility (MIC ≥ 1 μg/ml) to AMB (RS-AMB). These isolates were used for sequence analyses of 3 ergosterol biosynthesis genes (ERG2, ERG6, and ERG11) to investigate the mechanisms responsible for RS-AMB. Here we describe novel missense/nonsense mutations in ERG6 and ERG2 in 6 and 2 C. glabrata isolates, respectively, that resulted in altered sterol content and RS-AMB.
RESULTS
Characterization of study isolates and antifungal susceptibility.
A total of 1,646 clinical C. glabrata isolates were received in the Mycology Reference Laboratory (MRL), Department of Microbiology, Kuwait University, from 2009 to 2016 for species-specific identification and AST as part of routine patient care. During AST by Etest, 1 isolate was identified as non-WT, while the remaining isolates were WT for AMB. However, among the WT isolates, 7 strains exhibited reduced susceptibility (MIC ≥ 1 μg/ml) to AMB (RS-AMB). Thus, 8 isolates exhibited RS-AMB and were included for further studies, while the remaining isolates were considered WT. Five isolates WT for susceptibility to AMB were analyzed for comparison purposes. Two other C. glabrata strains isolated in 2012 that required cholesterol (or other sterols) for growth (20) were not included as their susceptibility to AMB and total cell sterol content could not be determined accurately. All isolates were identified as C. glabrata by the Vitek 2 yeast identification system and as C. glabrata sensu stricto by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) and by PCR amplification of ribosomal DNA (rDNA) (data not shown). The results were further confirmed by PCR sequencing of the internal transcribed spacer (ITS) region of rDNA, which exhibited >99% identity with the corresponding sequences from two reference C. glabrata strains (ATCC 90030 and CBS138) and a well-characterized clinical C. glabrata isolate (Kw280/06) from Kuwait analyzed previously (16), as expected. The AST data for AMB and other antifungal drugs obtained by the reference broth microdilution method are presented in Table 1. Eight RS-AMB isolates identified by Etest also exhibited elevated MICs by the broth microdilution method. Other WT isolates showed MICs of ≤0.5 μg/ml for AMB. Only 1 RS-AMB isolate was resistant to fluconazole and also showed higher MICs for the other triazoles, while the remaining isolates were susceptible to triazoles. Interestingly, 4 RS-AMB isolates exhibited very low MICs (≤0.5 μg/ml) for fluconazole and other triazoles. Only 1 isolate detected as WT for AMB showed reduced susceptibility to echinocandins, while the remaining isolates were susceptible.
TABLE 1.
Source of isolation and MIC values of AMB by Etest and of AMB and other antifungal drugs by broth microdilution method for 13 C. glabrata isolatesb
| Isolate no. | Clinical specimen | AMB MIC (μg/ml) by Etest | MIC values (μg/ml) by broth microdilution method for: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMB | FLC | ITC | VOR | POS | ISA | ANI | MIC | |||
| ATCC 90030a | NA | 0.094 | 0.5 | 4 | 0.125 | 0.125 | 0.25 | 0.125 | 0.016 | <0.008 |
| Kw280/06a | Vaginal swab | 0.047 | 0.5 | 16 | 0.5 | 0.25 | 0.25 | 0.5 | 0.031 | <0.008 |
| Kw600/09 | Wound swab | 0.032 | ND | ND | ND | ND | ND | ND | ND | ND |
| Kw164/15 | Urine | 0.38 | 0.25 | 16 | 0.25 | 0.25 | 0.25 | 0.125 | 0.5 | 0.125 |
| Kw383/15 | Ascitic fluid | 0.19 | 0.5 | 2 | 0.125 | 0.031 | 0.125 | 0.031 | 0.016 | <0.008 |
| Kw590/15 | Sputum | 0.003 | 0.25 | 16 | 0.25 | 0.25 | 0.25 | 0.125 | 0.016 | <0.008 |
| Kw1421/16 | Bone | 0.25 | 0.5 | 1 | 0.063 | 0.031 | 0.063 | 0.016 | 0.031 | <0.008 |
| Kw844/10 | Urine | 1.5 | 1 | 2 | 0.125 | 0.031 | 0.125 | 0.031 | 0.031 | <0.008 |
| Kw861/13 | ET aspirate | 1 | 1 | >64 | 0.5 | 2 | 0.25 | 0.5 | 0.016 | <0.008 |
| Kw96/15 | Urine | 2 | 1 | 0.5 | 0.063 | <0.016 | 0.063 | <0.016 | 0.031 | <0.008 |
| Kw1856/15 | Urine | 1.5 | 1 | 0.25 | 0.125 | <0.016 | 0.125 | <0.016 | 0.016 | <0.008 |
| Kw2516/15 | Urine | 1.5 | 1 | 0.5 | 0.063 | 0.031 | 0.125 | 0.016 | 0.016 | <0.008 |
| Kw2813/15 | Urine | 4 | 1 | 0.25 | 0.031 | <0.016 | 0.031 | <0.016 | 0.016 | <0.008 |
| Kw3060/15 | Wound swab | 1.5 | 1 | 1 | 0.063 | <0.016 | 0.063 | <0.016 | 0.031 | <0.008 |
| Kw3357/15 | Urine | 2 | 1 | 2 | 0.5 | 0.125 | 0.25 | 0.016 | 0.031 | 0.016 |
C. glabrata strains ATCC 90030 and Kw280/06 were used as reference strains.
ET aspirate, endotracheal aspirate; AMB, amphotericin B; FLC, fluconazole; ITC, itraconazole; VOR, voriconazole; POS, posaconazole; ISA, isavuconazole; ANI, anidulafungin; MIC, micafungin; NA, not available; ND, not done.
Analysis of ERG2, ERG6, and ERG11 gene sequences.
Relative to the sequence of the ERG2 protein (C-8 sterol isomerase) from C. glabrata reference strain CBS138 (GenBank accession number CR380958), several nonsynonymous mutations were identified in the translated ERG2 protein among the clinical isolates analyzed in this study. These included the I207V mutation in all study isolates, the G122S mutation only in Kw844/10, and the G119S mutation only in Kw3060/15 (Table 2). The replacement of glycine with serine at Gly122 in C. glabrata Kw844/10 and at Gly119 in C. glabrata Kw3060/15 apparently impaired the ERG2 protein function, as ergosterol was totally absent in these isolates (Table 2). Additionally, some synonymous mutations within the coding region and a nucleotide insertion/deletion/substitution in the noncoding region of ERG2 were also detected in some isolates.
TABLE 2.
Susceptibility to AMB, mutations detected in ERG2, ERG6, and ERG11 genes, fingerprinting by ITS-ERG2-ERG6-ERG11 sequence-based profile and by 6-locus-based sequence types, and content of ergosterol, fecosterol, zymosterol, and lanosterol as a percentage of the total cell sterol content in 13 C. glabrata isolatesc
| Isolate no. | Clinical specimen | Susceptibility to AMBa | Nonsynonymous mutation(s) detected in: |
ITS-ERG2-ERG6-ERG11-based profile | MLST-based STs | % of total cell sterol detected asd: |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ERG2 | ERG6 | ERG11 | Ergosterol | Fecosterol | Zymosterol | Lanosterol | |||||
| ATCC 90030 | NA | WT | Referenceb | Referenceb | Referenceb | Referenceb | ND | 93.0 + 0.1 | 1.0 + 0.0 | 0 | 1.3 + 0.1 |
| Kw280/06 | Vaginal swab | WT | I207V | R48K | None | Unique | ST151 | 84.8 + 2.8 | 2.0 + 0.3 | 2.5 + 0.6 | 3.2 + 0.3 |
| Kw600/09 | Wound swab | WT | I207V | None | None | Unique | ND | ND | ND | ND | ND |
| Kw164/15 | Urine | WT | I207V | None | None | Unique | ND | ND | ND | ND | ND |
| Kw383/15 | Ascitic fluid | WT | I207V | R48K | None | Unique | ND | ND | ND | ND | ND |
| Kw590/15 | Sputum | WT | I207V | R48K | None | Unique | ND | ND | ND | ND | ND |
| Kw1421/16 | Bone | WT | I207V | None | None | Unique | ND | 89.5 + 7.2 | 2.1 + 1.6 | 0 | 0.2 + 0.2 |
| Kw844/10 | Urine | RS-AMB | I207V + G122S | None | None | Unique | ND | 0 | 14.9 + 2.2 | 0 | 2.5 + 0.1 |
| Kw861/13 | ET aspirate | RS-AMB | I207V | Δnt 1021, L341* | Y141H + L381M | Unique | ST152 | 0 | 0 | 21.4 + 0.8 | 11.6 + 1.0 |
| Kw96/15 | Urine | RS-AMB | I207V | V126F | None | Unique | ST46 | 0 | 0 | 14.5 + 0.5 | 0.6 + 0.1 |
| Kw1856/15 | Urine | RS-AMB | I207V | W286* | None | Unique | ST46 | 0 | 0 | 42.5 + 1.3 | 3.3 + 0.3 |
| Kw2516/15 | Urine | RS-AMB | I207V | R48K + C198F | None | Unique | ST153 | 0 | 0 | 35.5 + 0.6 | 1.3 + 0.2 |
| Kw2813/15 | Urine | RS-AMB | I207V | Y192* | None | Unique | ST154 | 0 | 0 | 42.7 + 0.4 | 2.4 + 0.1 |
| Kw3060/15 | Wound swab | RS-AMB | I207V + G119S | None | None | Unique | ND | 0 | 18.9 + 2.3 | 0 | 1.2 + 0.3 |
| Kw3357/15 | Urine | RS-AMB | I207V | W286* | None | Unique | ND | 0 | 0 | 45.4 + 1.0 | 2.1 + 0.3 |
C. glabrata isolates with MIC values of ≤0.5 μg/ml were classified as wild type for AMB, while those with MIC values of ≥1 μg/ml were classified as isolates with reduced susceptibility to AMB (RS-AMB).
C. glabrata strain ATCC 90030 was used as the reference strain for susceptibility to amphotericin B (AMB), and strain CBS138 was used as the reference strain for ERG gene sequencing.
*, nonsense mutation; nt, nucleotide; ST, sequence type; STKN, new sequence type detected in Kuwait; ND, not done.
Sterol values (means for 3 replicates with standard deviations) are a percentage of the total sterol content, and values of >5% of the total cell sterol content are shown in bold.
The results of PCR sequencing of ERG6 were very interesting, as 3 isolates (Kw1856/15, Kw2813/15, and Kw3357/15) exhibiting RS-AMB contained a nonsense mutation and another RS-AMB isolate (Kw861/13) contained a deletion of 1 nucleotide (nucleotide 1021) which resulted in the premature termination of the translated protein at codon 341 (Table 2). Although a nonsynonymous mutation (R48K) was present in some WT and some RS-AMB isolates, 2 other nonsynonymous mutations (V126F in isolate Kw96/15 and C198F in isolate Kw2516/15) were detected in only 2 RS-AMB isolates. Interestingly, 5 of 6 RS-AMB isolates cultured in 2015 contained a mutation in ERG6. Compared to the sequence of the ERG6 protein (C-24 methyltransferase) from C. glabrata reference strain CBS138 (GenBank accession number CR380954), some synonymous ERG6 mutations were also detected among the study isolates, and the 2 RS-AMB isolates (Kw1856/15 and Kw3357/15) with the same ERG6 mutation (W286*) were genotypically very different strains. Consistent with the PCR sequencing results, all 6 RS-AMB isolates with ERG6 mutations lacked ergosterol and accumulated zymosterol, the substrate for ERG6.
Relative to the sequence of the ERG11 protein (sterol 14α-demethylase) from C. glabrata reference strain CBS138 (GenBank accession number CR380951), few synonymous mutations were identified in the translated ERG11 protein among all clinical isolates analyzed in this study, while two nonsynonymous mutations (Y141H plus L381M) were detected in one RS-AMB isolate (Kw861/13) (Table 2) that was also resistant to fluconazole (Table 1). Isolate Kw861/13 also contained an ERG6 mutation (deletion of nucleotide 1021, which resulted in the creation of a termination codon at Leu341) and no detectable ergosterol but higher levels of lanosterol in the cell (Table 2). None of the other WT or RS-AMB isolates contained a nonsynonymous mutation in ERG11 (Table 2). All mutations were confirmed by reextraction of DNA from fresh cultures of C. glabrata isolates and PCR sequencing of the respective ERG genes.
Total cell sterol composition.
The total cell sterol composition of one isolate WT for AMB was very similar to the sterol composition of the reference strains ATCC 90030 and Kw280/06, with ergosterol accounting for nearly 90% of total cell sterol. All 6 RS-AMB isolates with ERG6 mutations showed marked differences in sterol content from the isolates WT for AMB, as ergosterol was not detectable, while cholesta-type sterols (including zymosterol) accumulated in the mutants (Table 3). Differences in the abundance of various cholesta-type sterols were also apparent in the 6 ERG6 mutants. Among the 4 isolates with nonsense mutations resulting in a truncated ERG6 protein, the cholesta-8,24-dienol (zymosterol) was the more abundant sterol in 3 isolates (Kw1856/15, Kw2813/15, and Kw3357/15), while cholesta-5,7,24-trienol was the major accumulating sterol and cholesta-5,7,22,24-tetraenol was barely detectable in Kw861/13 (Table 3). The cholesta-5,7,24-trienol was also the major accumulating cholesta-type sterol in 2 isolates (Kw96/15 and Kw2516/15) containing nonsynonymous ERG6 mutations; however, these isolates also accumulated cholesta-5,7,22,24-tetraenol (Table 3). Isolate Kw844/10 with a nonsynonymous mutation (G122S) and isolate Kw3060/15 with another nonsynonymous mutation (G119S) in ERG2 also lacked ergosterol but accumulated ergosta-type (Δ8) sterols, including fecosterol [ergosta-8,24(28)-dienol] (Table 3).
TABLE 3.
Total C. glabrata cell sterol composition in an isolate (Kw1421/16) wild type for AMB and 7 isolates with reduced susceptibility to AMB
| Type of sterol | % of each sterol as part of the total cell sterol composition of C. glabrata isolatea: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 90030 | Kw280/06 | Kw1421/16 | Kw844/10 | Kw861/13 | Kw96/15 | Kw1856/15 | Kw2516/15 | Kw2813/15 | Kw3060/15 | Kw3357/15 | |
| Ergosta-5,8,22,24-tetraenol | 0.9 + 0.1 | 3.4 + 1.2 | 0.7 + 0.1 | 1.5 + 0.0 | 1.2 + 0.3 | ||||||
| Unknown sterol | 1.4 + 0.1 | 1.4 + 0.0 | 0.9 + 0.1 | 1.2 + 0.0 | 1.0 + 0.1 | 0.9 + 0.0 | |||||
| Ergosta-5,8,22-trienol | 0.9 + 0.0 | 1.0 + 0.0 | 0.4 + 0.3 | 25.8 + 0.3 | 31.6 + 1.1 | ||||||
| Ergosta-8,22-dienol | 7.0 + 0.7 | 8.3 + 0.2 | |||||||||
| Ergosta-5,8,24-trienol | 8.2 + 0.5 | 8.0 + 1.4 | |||||||||
| Ergosta-5,8-dienol | 6.0 + 0.4 | 1.3 + 0.1 | |||||||||
| Cholesta-5,8,24-trienol | 5.1 + 0.3 | 9.0 + 0.3 | 5.2 + 0.5 | 9.5 + 0.5 | 6.0 + 0.4 | 6.5 + 0.1 | |||||
| Zymosterol (cholesta-8,24-dienol) | 2.5 + 0.6 | 21.4 + 0.8 | 14.5 + 0.5 | 42.5 + 1.3 | 35.5 + 0.6 | 42.7 + 0.4 | 45.4 + 1.0 | ||||
| Cholesta-5,7,24-trienol | 1.1 + 0.0 | 1.6 + 1.3 | 56.0 + 0.4 | 40.9 + 0.5 | 34.0 + 0.2 | 36.9 + 0.7 | 35.2 + 0.3 | 36.3 + 1.3 | |||
| Ergosterol | 93.0 + 0.1 | 84.8 + 2.8 | 89.5 + 7.2 | ||||||||
| Cholesta-7,24-dienol | 1.3 + 0.5 | 1.0 + 0.1 | 1.9 + 0.3 | 0.8 + 0.0 | 1.4 + 0.1 | 1.0 + 0.0 | |||||
| Cholesta-5,7,22,24-tetraenol | 0.1 + 0.2 | 26.7 + 0.9 | 11.5 + 0.8 | 13.6 + 1.6 | 10.3 + 0.5 | 6.8 + 0.4 | |||||
| 14-Methyl fecosterol | 1.0 + 0.0 | ||||||||||
| Fecosterol [ergosta-8,24(28)-dienol] | 1.0 + 0.0 | 2.0 + 0.3 | 2.1 + 1.6 | 14.9 + 2.2 | 18.9 + 2.3 | ||||||
| Ergosta-8-enol | 30.9 + 1.3 | 29.5 + 0.9 | |||||||||
| Ergosta-5,7-dienol | 1.3 + 0.2 | 1.8 + 0.1 | 2.2 + 1.7 | 0.3 + 0.3 | 5.9 + 0.3 | 0.4 + 0.1 | 0.6 + 0.1 | ||||
| Episterol [ergosta-7,24(28)-dienol] | 0.2 + 0.0 | 0.7 + 0.1 | 1.2 + 0.9 | ||||||||
| Unknown sterol | 2.1 + 0.3 | 0.7 + 0.2 | |||||||||
| 14-Methyl ergosta-8,24(28)-dien-3,6-diol | |||||||||||
| Lanosterol | 1.3 + 0.1 | 3.2 + 0.3 | 2.1 + 1.6 | 2.5 + 0.1 | 11.6 + 1.0 | 0.6 + 0.1 | 3.3 + 0.3 | 1.3 + 0.2 | 2.4 + 0.1 | 1.2 + 0.3 | 2.1 + 0.3 |
| 4,4-Dimethyl cholesta-8,24-dienol | 0.2 + 0.1 | 0.6 + 0.4 | 0.2 + 0.2 | 1.1 + 0.1 | 1.9 + 0.2 | 0.8 + 0.2 | 0.4 + 0.1 | 0.7 + 0.0 | 0.3 + 0.0 | ||
C. glabrata strains ATCC 90030 and Kw280/06 were used as the reference strains for determining the total cell sterol composition. Sterol values (means for 3 replicates with standard deviation) are the percentage of the total sterol composition, and values of >5% of the total cell sterol composition are shown in bold. AMB, amphotericin B.
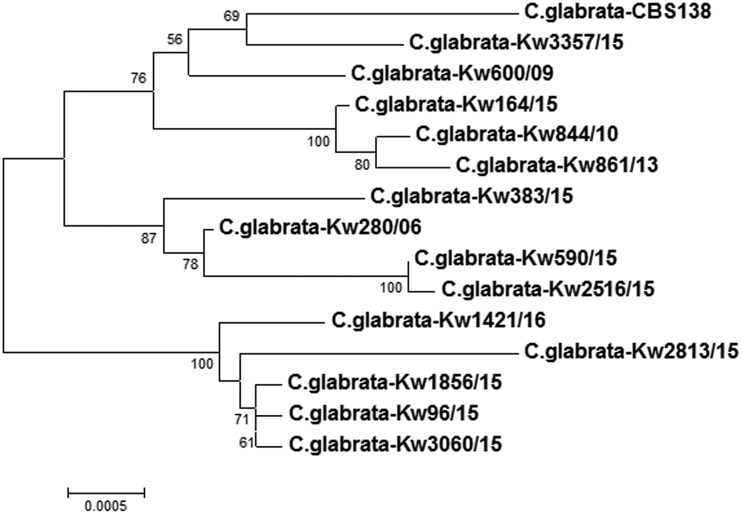
The genotypic relationship among the study isolates was also determined by constructing a phylogenetic tree based on concatenated sequences of the ITS region of rDNA, ERG2, ERG6, and ERG11, and the results are shown in Fig. 1. Both isolates with a missense mutation in ERG2 (Kw844/10 and Kw3060/15) and the two isolates with a missense mutation in ERG6 (Kw96/15 and Kw2516/15) were genotypically distinct strains. Furthermore, all four isolates with a nonsense mutation in ERG6 (Kw861/13, Kw1856/15, Kw2813/15, and Kw3357/15) were also unique strains (Fig. 1). Fingerprinting of selected isolates performed with 6-locus-based multilocus sequence typing (MLST) also showed that most of the isolates analyzed were unique strains (Table 2).
FIG 1.
Neighbor-joining phylogenetic tree based on combined sequence data for the ITS region of rDNA and the ERG2, ERG6, and ERG11 genes from 13 clinical C. glabrata isolates from Kuwait together with reference C. glabrata strains CBS138 and Kw280/06. The numbers on the node branches are bootstrap frequencies.
DISCUSSION
Invasive fungal infections are difficult to treat due to the availability of only a few classes of antifungal agents. Among invasive Candida infections, C. glabrata infections are more common among elderly (≥65-year-old) hospitalized patients, who usually have several debilitating conditions, and so these infections are generally associated with higher mortality (24–26). Although the resistance of Candida spp. to azole antifungal drugs is common and resistance to echinocandins also appeared soon after their introduction in clinical practice nearly 15 years ago, RS-AMB is uncommon, despite >50 years of clinical use, as the sequestering of ergosterol by extramembranous aggregates of amphotericin B deprives phospholipid membranes of a sterol essential for many different aspects of yeast physiology (8, 9, 11). In diploid C. albicans, RS-AMB is rare due to fitness trade-offs which abrogate fungal virulence (9, 10). However, there have been several reports of RS-AMB in haploid C. glabrata, and breakthrough infections in patients receiving systemic LAMB is a worrisome development for the effective management of invasive C. glabrata infections (7, 14, 15, 17–19).
Although the molecular mechanisms involved in azole resistance are well recognized, those involved in conferring RS-AMB are poorly defined (3, 4, 24, 26). Results from this study on 8 C. glabrata isolates with RS-AMB showed that 4 isolates contained a nonsense mutation at codon Tyr192, Trp286, or Leu341, resulting in the premature translational termination of ERG6 transcripts. The mutant cells accumulated cholesta-type sterols (including cholesta-8,24-dienol, or zymosterol), indicating that the truncated ERG6 proteins were inactive. Since polyenes act as a sterol sponge and extract ergosterol from the phospholipid bilayer (8, 9), the absence of ergosterol in the yeast cell membrane was likely responsible for RS-AMB in these isolates. The nonsense mutations at codon Tyr192, Trp286, or Leu341 in ERG6 described in this study are novel mutations described for the first time. Premature termination of the ERG6 protein due to another nonsense mutation at codon Gln332 was recently described in a C. glabrata isolate with RS-AMB; the mutant cells lacked ergosterol but accumulated ergosterol pathway intermediates, and wild-type properties were restored in complementation studies with a wild-type copy of the ERG6 gene (17). The total cell sterol analysis of our isolates also showed differences in the accumulation of individual cholesta-type sterols in the 4 mutant strains. The mutation in isolate Kw861/13 introduced the stop codon only 32 amino acids before the C-terminal end of C-24 sterol methyltransferase, encoded by ERG6, while nonsense mutations in the other 3 isolates (Kw1856/15, Kw2813/15, and Kw3357/15) resulted in shortening of the ERG6 protein by 87 or more amino acids at the C-terminal end. Previous studies have shown the presence of two conserved domains in methyltransferases located between amino acid positions 134 and 222 and the second domain between amino acid position 231 and the C-terminal end (27, 28). The second domain was only 32 amino acids shorter in isolate Kw861/13. As the ERG6-encoded enzyme catalyzes the conversion of zymosterol into fecosterol by C-24 methylation, isolate Kw861/13, like the other 3 isolates, lacked fecosterol but, unlike the other 3 isolates, contained a low level of 14-methyl fecosterol, suggesting modification of C-24 sterol methyltransferase activity in Kw861/13. Furthermore, isolate Kw861/13, in addition to exhibiting RS-AMB, also exhibited resistance to fluconazole and other azoles, contained two nonsynonymous mutations (Y141H and L381M) in ERG11, and accumulated lanosterol, suggesting the loss of sterol 14α-demethylase activity, which conferred resistance to azoles. Fluconazole-resistant clinical C. glabrata isolates usually contain gain-of-function mutations in the gene encoding transcription factor C. glabrata PDR1 (CgPDR1), which results in the upregulation of drug efflux transporters encoded by the CgCDR1 and CgCDR2 genes and, to a lesser extent, CgCNQ2 (3, 4). Only a few studies have reported azole resistance-conferring mutations in ERG11, the main target of azoles in C. albicans and some other non-albicans Candida species (3, 4, 18, 29). A clinical C. glabrata isolate (CG156) with a nonsynonymous mutation (G315D) in the substrate recognition site of ERG11 that was resistant to triazoles (fluconazole and voriconazole) and AMB and that accumulated lanosterol due to the complete loss of sterol 14α-demethylase activity has been described previously (18). The mutated CG156 Erg11p failed to complement the function of a doxycycline-regulatable Saccharomyces cerevisiae erg11 strain, while wild-type C. glabrata Erg11p fully complemented the function, supporting a role of the ERG11 mutation in conferring resistance to fluconazole. Another recent study from Iran has also suggested the involvement of another nonsynonymous mutation (G236V) in ERG11 as the main mechanism conferring resistance to azoles in a clinical C. glabrata isolate (R1), even though several other nonsynonymous mutations were also detected in this and other isolates (29). However, the role of the G236V mutation in altering the function of ERG11 was speculated solely based on homology modeling studies but was not confirmed by sterol analysis or by gene replacement studies (29). It will be interesting to see if resistance-conferring ERG11 mutations are also found in other fluconazole-resistant C. glabrata isolates in Kuwait.
Two other RS-AMB isolates contained two different nonsynonymous mutations in ERG6. Of these, the V126F mutation in Kw96/15 is a novel mutation, while the C198F mutation, found in isolate Kw2516/15, has been described previously in an RS-AMB C. glabrata isolate (isolate no. 21229) that lacked ergosterol in the cell membrane, exhibited pseudohyphal growth, accumulated late sterol intermediates, and overexpressed genes encoding enzymes involved in late steps of the ergosterol biosynthesis pathway (15). Complementation studies with a wild-type copy of ERG6 gene restored the WT pattern of AMB susceptibility for isolate 21229, demonstrating the role of the ERG6 protein in conferring resistance to polyenes (15). Consistent with these observations, isolates Kw96/15 and Kw2516/15 also lacked ergosterol and accumulated cholesta-type sterols. As described above with different nonsense mutations, isolates Kw96/15 and Kw2516/15 also showed variations in the accumulation of individual intermediates, with zymosterol (cholesta-8,24-dienol) and cholesta-5,7,22,24-tetraenol levels varying by nearly 2-fold and with the accumulation of detectable levels of ergosta-5,7-dienol in isolate Kw96/15 but not in isolate Kw2516/15. The findings are consistent with observations that the enzymes encoded by various ERG genes and involved in the ergosterol biosynthesis pathway may act on similar substrates, leading to the formation of several sterol intermediates (15, 22, 30). Furthermore, similar to isolate 21229 with an ERG6 mutation (15) and an erg1 mutant of C. glabrata (31), both Kw96/15 and Kw2516/15 also exhibited increased susceptibility to azoles. Similarly, 2 isolates (Kw1856/15 with W286* and Kw2813/15 with Y192*) with nonsense mutations also exhibited increased susceptibility to azoles, while another isolate (Kw3357/15 with W286*) exhibited a slightly higher MIC value for fluconazole. It has been suggested that isolate 21229 with the C198F mutation in ERG6 becomes more susceptible to azoles due to the presence of Δ5,7-dienols (cholesta-type sterols), which maintain cell viability but do not completely replace ergosterol functionally (15). Another consequence of an altered sterol composition in mutant cells is the disturbance of protein trafficking, preventing targeting of ABC transporters (CgCDR1p and CgCDR2p) to the plasma membrane, and the decreased efflux capacity likely contributes to increased sensitivity to azoles (15, 32).
Although a nonsynonymous mutation (I207V) was found in ERG2 in all 13 C. glabrata isolates from Kuwait, this alteration represents a genetic polymorphism not associated with RS-AMB since it has also been described previously in C. glabrata isolates WT for AMB as well as in isolates with RS-AMB (19). However, 2 RS-AMB C. glabrata isolates from Kuwait (Kw844/10 and Kw3060/15) contained novel nonsynonymous mutations (G122S and G119S) in ERG2. Both isolates (Kw844/10 and Kw3060/15) lacked ergosterol and accumulated ergosta-type sterols, including fecosterol (Δ8 sterols). Two RS-AMB C. glabrata isolates containing nonsynonymous mutations at ERG2 codon Thr121 (T121I and T121V) have been described recently (19). Both ERG2 mutant isolates (CG852 and CG872) in that study (19) also lacked ergosterol and accumulated Δ8 sterols, indicating an impaired function of the ERG2 protein. Thr121 in the ERG2 protein is likely involved in the binding of the sterol substrate, as the corresponding amino acid (Thr119) in S. cerevisiae is involved in sterol Δ8-Δ7 isomerization (19, 33). Since Gly119 and Gly122 are located on either side of Thr121, they may be critical in maintaining the structure of the active site, and the extended region (codons 119 to 122) may constitute the ERG2 protein region conferring RS-AMB.
An intriguing observation of our study pertains to the fact that despite the complete absence of ergosterol from mutant C. glabrata cells, the increase in MIC values for AMB were only modest, as they were still categorized within the WT category for susceptibility to AMB. These observations and the presence of significant amounts of various ergosta-type and cholesta-type sterols in mutant C. glabrata cells suggest that at least some of these sterols can also maintain fungal membrane fluidity and are also sensitive to removal by the extramembranous AMB sponge. It will be interesting to see how these mutant strains respond to other derivatives of AMB that have recently been synthesized to overcome the problem of the toxicity of AMB to mammalian cells (9, 34, 35).
Fingerprinting of the isolates, carried out by comparisons of the sequences of the ITS region of rDNA together with the ERG2, ERG6, and ERG11 sequences, showed that all study isolates were genotypically distinct strains. The 6-locus-based MLST carried out on selected RS-AMB isolates also showed genetic variations among the isolates, as they usually belonged to different sequence types (STs). These findings suggest that RS-AMB in C. glabrata isolates in Kuwait is not clonal.
In conclusion, 8 RS-AMB C. glabrata strains were isolated in Kuwait, and 6 of these 8 isolates were obtained in 2015. Six isolates contained a nonsense or nonsynonymous mutation in ERG6, while 2 isolates contained a nonsynonymous mutation in ERG2, and the total cell sterol contents were consistent with ERG6 or ERG2 deficiency. Fingerprinting studies showed that RS-AMB in C. glabrata isolates in Kuwait was not clonal. The data show that ERG6 and ERG2 are major targets conferring RS-AMB in clinical C. glabrata isolates.
MATERIALS AND METHODS
Yeast strains, culture conditions, and identification.
Reference strains of Candida glabrata (ATCC 90030, CBS138, and a well-characterized clinical isolate, Kw280/06) were used. From 2009 to 2016, 1,646 clinical C. glabrata isolates were received in the Mycology Reference Laboratory (MRL), Department of Microbiology, Faculty of Medicine, Kuwait University. The isolates were cultured in Bactec Plus blood culture bottles (Becton, Dickinson, Sparks, MD, USA) from various clinical specimens in different hospitals across Kuwait after obtaining informed verbal consent as part of routine patient care and diagnostic workup. The isolates were initially subcultured at MRL on Sabouraud dextrose agar (SDA) for species-specific identification and antifungal susceptibility testing (AST) as part of routine patient care (16). A few isolates failed to grow on SDA upon subculturing and required addition of cholesterol to SDA for their growth, as described in detail previously (20).
Phenotypic identification of the isolates was initially performed by use of a Vitek 2 yeast identification system (bioMérieux, Marcy-l’Etoile, France). The isolates were also identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS), as described previously (36). The genomic DNA from the reference strains and clinical isolates was extracted by using a Gentra Puregene yeast DNA extraction kit (Qiagen, Hilden, Germany) according to the kit instructions or by the rapid method using Chelex-100, as described previously (37). Species-specific identification was performed by PCR amplification of rDNA as described previously (38). The complete ITS region was also amplified by using primers ITS1 and ITS4, and the amplicons were sequenced by using primers ITS1FS, ITS2, ITS3, and ITS4RS, as described previously (39, 40).
Antifungal susceptibility testing.
The antifungal susceptibility testing (AST) of the C. glabrata isolates to AMB was initially carried out on SDA, and MICs were determined by the Etest procedure (AB Biodisk, Solna, Sweden) according to the manufacturer’s instructions and as described previously (41). The isolates were also tested against different antifungal drugs by the CLSI reference broth microdilution method as described previously (42, 43), and the susceptibility breakpoints for the different antifungal agents were those described earlier (23, 43, 44). C. glabrata ATCC 90030 was used as the reference strain during AST.
ERG gene sequencing.
The complete coding sequence and the flanking 5′ and 3′ untranslated regions of the ERG2, ERG6, and ERG11 genes for all study isolates were amplified from genomic DNA by using gene-specific primers. The ERG2 gene was amplified by using primers CgERG2F (5′-CTAAACGAGCTCGTAATTCTA-3′) and CgERG2R (5′-GCCTTAGAGTCTATCCTTGAA-3′) and the PCR amplification reaction and cycling conditions described previously (37, 39). The amplicons were purified by using a PCR product purification kit (Qiagen, Hilden, Germany) according to the kit instructions. Both strands of the purified amplicons were sequenced by using forward primers (CgERG2FS1, 5′-GCTCGTAATTCTATCGGTTTGA-3′; CgERG2FS2, 5′-TTGCAATGGTGTACTTGCCAA-3′; CgERG2FS3, 5′-GGTATGACCCATCATCTACA-3′) and reverse primers (CgERG2RS1, 5′-GATTCTGTAGAGGCACTAGCA-3′; CgERG2RS2, 5′-CCTTGAGCCAATTCTAGTGCGA-3′; CgERG2RS3, 5′-CCTTAGAGTCTATCCTTGAATTA-3′) with a BigDye Terminator (v3.1) cycle sequencing kit (Applied Biosystems, Austin, TX, USA) and an ABI 3130xl genetic analyzer by following the manufacturer’s instructions (Applied Biosystems) and as described previously (20, 37). The complete ERG2 sequences of ∼1,060 bp were assembled and were compared with the corresponding sequences from reference C. glabrata strain CBS138 by using the program Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
The ERG6 gene was amplified and sequenced as two overlapping fragments. The N-terminal fragment was amplified by using primers CgERG6F1 (5′-GATTTTCTCGTTTCGCCGAGAA-3′) and CgERG6R1 (5′-GATGATGTTACAGCCGGTGAA-3′), while the C-terminal fragment was amplified by using primers CgERG6F2 (5′-ACGAACAGTACTTGGCATACA-3′) and CgERG6R2 (5′-CATGTGGAATGAATTCAAGTGA-3′) and the PCR amplification reaction and cycling conditions described previously (37, 39). The amplicons were purified, and both strands were sequenced by using the gene-specific primers and the sequencing protocol described previously (20, 37). The sequencing primers for the N-terminal fragment included forward primer CgERG6FS1 (5′-CTCGTTTCGCCGAGAATTGTTA-3′) or CgERG6FS2 (5′-CAGTTTATTGTGCTCTTGACG-3′) and reverse primer CgERG6RS1 (5′-TCGGGAGAATTTCAATTCCTT-3′) or CgERG6RS2 (5′-GATGTTACAGCCGGTGAATCT-3′). The primers for the C-terminal fragment included forward primer CgERG6FS3 (5′-GAACAGTACTTGGCATACATGG-3′) or CgERG6FS4 (5′-TTTGAAGAACGTCGGTTTCG-3′) and reverse primer CgERG6RS3 (5′-GTACTTCCATTCTCCGGTCAA-3′) or CgERG6RS4 (5′-GTGGAATGAATTCAAGTGAACA-3′). The complete ERG6 sequences of ∼1,840 bp were assembled and were compared with the corresponding sequences from reference C. glabrata strain CBS138 by using the program Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
The ERG11 gene was amplified and sequenced from all study isolates as described previously (20). The complete ERG11 sequences of ∼1,920 bp were assembled and were compared with the corresponding sequences from reference C. glabrata strain CBS138 by using the program Clustal Omega.
Fingerprinting of C. glabrata isolates.
The gene sequences were analyzed individually, or the nucleotide sequences of the ERG2, ERG6, and ERG11 genes, together with the nucleotide sequence of the ITS region of rDNA, were included in the combined analysis. Multiple-sequence alignments of concatenated sequence data were performed with the ClustalW Muscle program (https://www.ebi.ac.uk/Tools/msa/muscle/), and phylogenetic analysis was performed with Molecular Evolutionary Genetic Analysis (MEGA; version 6) software. The phylogenetic tree was constructed using the neighbor-joining method with the Kimura 2-parameter model. The DNA sequence data from C. glabrata strain CBS138 were used as a reference, and the robustness of the tree branches was assessed by bootstrap analysis with 1,000 replicates. The genotypic relationship among selected C. glabrata isolates was also studied by a 6-locus (FKS, LEU2, NMT1, TRP1, UGP1, and URA3)-based multilocus sequence typing (MLST) scheme, as described previously (45). The DNA sequences for each gene fragment were used to determine the allelic profile, and the combined data set was used to determine the sequence types (STs) (45).
Sterol analysis.
The total cell sterol content of the C. glabrata isolates was determined by inoculating 15-ml volumes of MOPS (morpholinepropanesulfonic acid)-buffered (0.165 M MOPS) RPMI medium (pH 7.0) with single colonies, and the cultures were grown for 24 h at 37°C. Cells were harvested by centrifugation and washed three times with sterile water before sterol extraction. Nonsaponifiable lipids were extracted by using alcoholic KOH as described previously (46). Samples were dried in a vacuum centrifuge (Heto) and were derivatized with trimethylsilane (TMS) by the addition of 100 μl of 90% N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA)–10% TMS (Sigma), 200 μl anhydrous pyridine (Sigma) and heating for 2 h at 80°C. The TMS-derivatized sterols were analyzed by using gas chromatography (GC)-mass spectrometry (MS) (a Thermo 1300 GC coupled to a Thermo ISQ mass spectrometer; Thermo Scientific, Loughborough, UK) and identified with reference to the retention times and fragmentation spectra for known standards (18, 46). The GC-MS data files were analyzed by using Xcalibur software (Thermo Scientific) to determine the sterol profiles for the study isolates and the integrated peak areas. The results of three replicates from each sample were used to calculate the mean percentage ± standard deviation for each sterol.
Patient samples.
The clinical specimens which yielded the C. glabrata isolates described in this study were obtained from different patients after obtaining informed verbal consent as part of routine patient care and diagnostic workup. The isolates were also analyzed in the Mycology Reference Laboratory in the Department of Microbiology, Faculty of Medicine, Kuwait University, for identification and antifungal susceptibility testing as part of routine patient care, and the results from deidentified samples are described in this paper.
Accession number(s).
The DNA sequence data reported in this study have been submitted to GenBank under accession numbers LS398111 to LS398136, LS398591 to LS398603, and LS399273 to LS399285.
REFERENCES
- 1.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 2.Guinea J. 2014. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 3.Sanguinetti M, Posteraro B, Lass-Flörl C. 2015. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 58:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 4.Kołaczkowska A, Kołaczkowski M. 2016. Drug resistance mechanisms and their regulation in non-albicans Candida species. J Antimicrob Chemother 71:1438–1450. doi: 10.1093/jac/dkv445. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JS, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. 2013. Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob Agents Chemother 57:4559–4561. doi: 10.1128/AAC.01144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraya T, Tanabe K, Araki K, Yonetani S, Makino H, Watanabe T, Tsujimoto N, Takata S, Kurai D, Ishii H, Miyazaki Y, Takizawa H, Goto H. 2014. Breakthrough invasive Candida glabrata in patients on micafungin: a novel FKS gene conversion correlated with sequential elevation of MIC. J Clin Microbiol 52:2709–2712. doi: 10.1128/JCM.03593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura M, Araoka H, Yamamoto H, Nakamura S, Nagi M, Yamagoe S, Miyazaki Y, Ogura S, Mitsuki T, Yuasa M, Kaji D, Kageyama K, Nishida A, Taya Y, Shimazu H, Ishiwata K, Takagi S, Yamamoto G, Asano-Mori Y, Uchida N, Wake A, Taniguchi S, Yoneyama A. 2017. Clinical and microbiological characteristics of breakthrough candidemia in allogeneic hematopoietic stem cell transplant recipients in a Japanese hospital. Antimicrob Agents Chemother 61:e01791-16. doi: 10.1128/AAC.01791-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD. 2014. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10:400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis SA, Vincent BM, Endo MM, Whitesell L, Marchillo K, Andes DR, Lindquist S, Burke MD. 2015. Nontoxic antimicrobials that evade drug resistance. Nat Chem Biol 11:481–487. doi: 10.1038/nchembio.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan Z, Ahmad S, Mokaddas E, Meis JF, Joseph L, Abdullah A, Vayalil S. 2018. Development of echinocandin resistance in Candida tropicalis following short-term exposure to caspofungin for empiric therapy. Antimicrob Agents Chemother 62:e01926-17. doi: 10.1128/AAC.01926-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Te Welscher YM, van Leeuwen MR, de Kruijff B, Dijksterhuis J, Breukink E. 2012. Polyene antibiotic that inhibits membrane transport proteins. Proc Natl Acad Sci U S A 109:11156–11159. doi: 10.1073/pnas.1203375109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, Jiang B, Ketela T, Lemieux S, Veillette K, Martel N, Davison J, Sillaots S, Trosok S, Bachewich C, Bussey H, Youngman P, Roemer T. 2007. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog 3:e92. doi: 10.1371/journal.ppat.0030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S. 2013. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11:e1001692. doi: 10.1371/journal.pbio.1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen D. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis 42:938–944. doi: 10.1086/500939. [DOI] [PubMed] [Google Scholar]
- 15.Vandeputte P, Tronchin G, Bergès T, Hennequin C, Chabasse D, Bouchara JP. 2007. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob Agents Chemother 51:982–990. doi: 10.1128/AAC.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan ZU, Ahmad S, Al-Obaid I, Al-Sweih NA, Joseph L, Farhat D. 2008. Emergence of resistance to amphotericin B and triazoles in Candida glabrata vaginal isolates in a case of recurrent vaginitis. J Chemother 20:488–491. doi: 10.1179/joc.2008.20.4.488. [DOI] [PubMed] [Google Scholar]
- 17.Vandeputte P, Tronchin G, Larcher G, Ernoult E, Bergès T, Chabasse D, Bouchara JP. 2008. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob Agents Chemother 52:3701–3709. doi: 10.1128/AAC.00423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hull CM, Parker JE, Bader O, Weig M, Gross U, Warrilow AG, Kelly DE, Kelly SL. 2012. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob Agents Chemother 56:4223–4232. doi: 10.1128/AAC.06253-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull CM, Bader O, Parker JE, Weig M, Gross U, Warrilow AG, Kelly DE, Kelly SL. 2012. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob Agents Chemother 56:6417–6421. doi: 10.1128/AAC.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan Z, Ahmad S, Joseph L, Al-Obaid K. 2014. Isolation of cholesterol-dependent, multidrug-resistant Candida glabrata strains from blood cultures of a candidemia patient in Kuwait. BMC Infect Dis 14:188. doi: 10.1186/1471-2334-14-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geber A, Hitchcock CA, Swartz JE, Pullen FS, Marsden KE, Kwon-Chung KJ, Bennett JE. 1995. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother 39:2708–2717. doi: 10.1128/AAC.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young LY, Hull CM, Heitman J. 2003. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47:2717–2724. doi: 10.1128/AAC.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glöckner A, Cornely OA. 2015. Candida glabrata: unique features and challenges in the clinical management of invasive infections. Mycoses 58:445–450. doi: 10.1111/myc.12348. [DOI] [PubMed] [Google Scholar]
- 25.Gabaldón T, Carreté L. 2016. The birth of a deadly yeast: tracing the evolutionary emergence of virulence traits in Candida glabrata. FEMS Yeast Res 16:fov110. doi: 10.1093/femsyr/fov110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arendrup MC, Patterson TF. 2017. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S445–S451. doi: 10.1093/infdis/jix131. [DOI] [PubMed] [Google Scholar]
- 27.Bouvier-Nave P, Husselstein T, Benveniste P. 1998. Two families of sterol methyltransferases are involved in the first and the second methylation steps of plant sterol biosynthesis. Eur J Biochem 256:88–96. doi: 10.1046/j.1432-1327.1998.2560088.x. [DOI] [PubMed] [Google Scholar]
- 28.Jensen-Pergakes KL, Kennedy MA, Lees ND, Barbuch R, Koegel C, Bard M. 1998. Sequencing, disruption, and characterization of the Candida albicans sterol methyltransferase (ERG6) gene: drug susceptibility studies in erg6 mutants. Antimicrob Agents Chemother 42:1160–1167. doi: 10.1128/AAC.42.5.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabili M, Abdollahi Gohar A, Badali H, Mohammadi R, Moazeni M. 2016. Amino acid substitutions in Erg11p of azole-resistant Candida glabrata: possible effective substitutions and homology modelling. J Glob Antimicrob Resist 5:42–46. doi: 10.1016/j.jgar.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Gaber RF, Copple DM, Kennedy BK, Vidal M, Bard M. 1989. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol 9:3447–3456. doi: 10.1128/MCB.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai HF, Bard M, Izumikawa K, Krol AA, Sturm AM, Culbertson NT, Pierson CA, Bennett JE. 2004. Candida glabrata erg1 mutant with increased sensitivity to azoles and to low oxygen tension. Antimicrob Agents Chemother 48:2483–2489. doi: 10.1128/AAC.48.7.2483-2489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umebayashi K, Nakano A. 2003. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol 161:1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahier A, Pierre S, Riveill G, Karst F. 2008. Identification of essential amino acid residues in a sterol 8,7-isomerase from Zea mays reveals functional homology and diversity with the isomerases of animal and fungal origin. Biochem J 414:247–259. doi: 10.1042/BJ20080292. [DOI] [PubMed] [Google Scholar]
- 34.Wilcock BC, Endo MM, Uno BE, Burke MD. 2013. C2'-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J Am Chem Soc 135:8488–8491. doi: 10.1021/ja403255s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis SA, Della Ripa LA, Hu L, Cioffi AG, Pogorelov TV, Rienstra CM, Burke MD. 2015. C3-OH of amphotericin B plays an important role in ion conductance. J Am Chem Soc 137:15102–15104. doi: 10.1021/jacs.5b05766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamal WY, Ahmad S, Khan ZU, Rotimi VO. 2014. Comparative evaluation of two matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) systems for the identification of clinically significant yeasts. Int J Infect Dis 26:167–170. doi: 10.1016/j.ijid.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 37.Asadzadeh M, Ahmad S, Hagen F, Meis JF, Al-Sweih N, Khan Z. 2015. Simple, low-cost detection of Candida parapsilosis complex isolates and molecular fingerprinting of Candida orthopsilosis strains in Kuwait by ITS region sequencing and amplified fragment length polymorphism analysis. PLoS One 10:e0142880. doi: 10.1371/journal.pone.0142880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad S, Mustafa AS, Khan Z, Al-Rifaiy AI, Khan ZU. 2004. PCR-enzyme immunoassay of rDNA in the diagnosis of candidemia and comparison with amplicon detection by agarose gel electrophoresis. Int J Med Microbiol 294:45–51. doi: 10.1016/j.ijmm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Khan ZU, Ahmad S, Mokaddas E, Chandy R, Cano J, Guarro J. 2008. Actinomucor elegans var. kuwaitiensis isolated from the wound of a diabetic patient. Antonie Van Leeuwenhoek 94:343–352. doi: 10.1007/s10482-008-9251-1. [DOI] [PubMed] [Google Scholar]
- 40.Khan ZU, Ahmad S, Hagen F, Fell JW, Kowshik T, Chandy R, Boekhout T. 2010. Cryptococcus randhawai sp. nov., a novel anamorphic basidiomycetous yeast isolated from tree trunk hollow of Ficus religiosa (peepal tree) from New Delhi, India. Antonie Van Leeuwenhoek 97:253–259. doi: 10.1007/s10482-009-9406-8. [DOI] [PubMed] [Google Scholar]
- 41.Asadzadeh M, Al-Sweih NA, Ahmad S, Khan ZU. 2008. Antifungal susceptibility of clinical Candida parapsilosis isolates in Kuwait. Mycoses 51:318–323. doi: 10.1111/j.1439-0507.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 42.Clinical and Laboratory Standards Institute. 2008. M27-A3. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.Clinical and Laboratory Standards Institute. 2012. Fourth informational supplement. M27-S4. Reference method for broth dilution antifungal susceptibility testing of yeasts. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 44.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodgson AR, Pujol C, Denning DW, Soll DR, Fox AJ. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J Clin Microbiol 41:5709–5717. doi: 10.1128/JCM.41.12.5709-5717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8,24(28)-dien-3β, 6α-diol. Biochem Biophys Res Commun 207:910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]