Piperacillin-tazobactam has been proposed as an alternative to carbapenems for the treatment of infections caused by extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae. However, limited understanding of optimal dosing strategies for this combination may curtail its utility.
KEYWORDS: beta-lactamase inhibitors, beta-lactamases, pharmacodynamics, pharmacokinetics
ABSTRACT
Piperacillin-tazobactam has been proposed as an alternative to carbapenems for the treatment of infections caused by extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae. However, limited understanding of optimal dosing strategies for this combination may curtail its utility. In this study, we correlated various exposures of piperacillin-tazobactam to efficacy, using a modified pharmacokinetic/pharmacodynamic index. Using a clinical Klebsiella pneumoniae isolate expressing CTX-M-15, piperacillin MIC values were determined with increasing tazobactam concentrations and fitted to a sigmoid inhibitory maximum effect (Emax) model. A hollow-fiber infection model (HFIM) was used to evaluate the efficacy of escalating tazobactam dosing with a fixed piperacillin exposure. Simulated drug concentrations from the HFIM were incorporated in the Emax model to determine the percentage of free time above instantaneous MIC (%fT>MICi) associated with each experimental exposure. The target %fT>MICi associated with growth suppression was prospectively validated using an SHV-12-producing isolate of Escherichia coli and 2 other CTX-M-15-producing K. pneumoniae isolates. Based on our reference isolate, piperacillin-tazobactam exposures of %fT>MICi of ≥55.1% were associated with growth suppression. Despite underlying differences, these findings were consistent with prospective observations in 3 other clinical isolates. Our modeling approach can be applied relatively easily in the clinical setting, and it appeared to be robust in predicting the effectiveness of various piperacillin-tazobactam exposures. This modified pharmacokinetic/pharmacodynamic index could be used to characterize response to other β-lactam/β-lactamase inhibitor combinations.
INTRODUCTION
The production of β-lactamases is a commonly encountered mechanism of resistance in Gram-negative bacteria (1). In health care settings, extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae are of particular concern. The CDC has estimated that ESBL-producing Enterobacteriaceae account for 19% of health care-related infections annually; infections implicated by these bacteria are also associated with increased mortality and cost of care (2). ESBLs limit treatment options because they hydrolyze most β-lactams, including penicillins and third-generation cephalosporins (e.g., ceftazidime). Currently, carbapenems are the mainstay therapy for ESBL-producing Enterobacteriaceae infections, but their widespread use may have contributed to the rapid dissemination of carbapenem resistance (3). Consequently, there is growing interest in alternatives to carbapenem therapy, such as piperacillin-tazobactam and other β-lactam/β-lactamase inhibitor combinations (4).
Piperacillin-tazobactam is a β-lactam/β-lactamase inhibitor combination widely used in clinical practice. Piperacillin is a semisynthetic ureidopenicillin with antibiotic activity against both Gram-positive and Gram-negative pathogens (5). When administered alone, piperacillin is susceptible to inactivation by β-lactamases. To circumvent this problem, it is coadministered with tazobactam, a penicillanic acid β-lactamase inhibitor. While tazobactam lacks appreciable intrinsic antimicrobial activity, it helps to preserve the activity of piperacillin in the presence of both narrow and extended-spectrum (such as TEM-, SHV-, and CTX-M-type) β-lactamases (5, 6). Several clinical studies have suggested that piperacillin-tazobactam might be as efficacious as carbapenem therapy against ESBL-producing Enterobacteriaceae (7–10). Nonetheless, the utility of piperacillin-tazobactam in this context remains controversial in view of reports of treatment failure and poor characterization of the pharmacokinetics/pharmacodynamics (PK/PD) of the combination (11, 12).
Pharmacokinetic/pharmacodynamic indices, such as the maximum concentration of drug in serum divided by the MIC (Cmax/MIC), the area under the 24-h concentration-time curve divided by the MIC (AUC/MIC), and the free time above the MIC (fT>MIC) are commonly used to characterize killing profiles for various antibiotics. β-Lactams such as piperacillin are described as exhibiting time-dependent killing, and thus their efficacy is closely correlated to fT>MIC (13). While older inhibitors (such as tazobactam) lack intrinsic antimicrobial activity, they alter susceptibility (MIC) to the partner β-lactam in a concentration-dependent manner over the dosing interval. As a result, the conventional approach for establishing PK/PD indices is not directly applicable to these β-lactam/β-lactamase inhibitor combinations. Additionally, the rationale for clinically dosing piperacillin-tazobactam in a fixed ratio of 8:1 (piperacillin to tazobactam) remains unclear.
Our laboratory previously proposed a modeling framework to account for the effect of β-lactamase inhibitors by using a novel PK/PD index, the time above instantaneous MIC (T>MICi) (14). Extending from this framework, the objective of this study was to discern the efficacy of alternative dosing strategies of piperacillin-tazobactam against ESBL-producing Enterobacteriaceae. Given the variability of clinical responses to piperacillin-tazobactam, we hypothesized that the conventional fixed dosing ratio might not be ideal against a diverse group of ESBL-producing Enterobacteriaceae. We anticipate that the outcomes of this study may provide insights to evaluate efficacy targets for β-lactam/β-lactamase inhibitor combinations and to guide rational dosing decisions.
RESULTS
Bacteria.
The susceptibility profiles and known mechanisms of resistance for the four isolates examined are shown in Table 1. All isolates were resistant to ceftazidime, and all but EcF65 were also resistant to piperacillin-tazobactam. Functional expression of the ESBL genes was confirmed. However, the rates of nitrocefin hydrolysis were dramatically different (data not shown), suggesting different enzyme expression levels of the isolates and/or the presence of other enzyme(s).
TABLE 1.
ESBL genes detected, susceptibility (MIC in μg/ml), inhibitory Emax parameter estimates, and model fit for clinical isolates
| Bacterial species (isolate) | ESBL gene | MICa |
Model estimates and fit |
|||||
|---|---|---|---|---|---|---|---|---|
| CAZ | PIP-TAZ | log2 (MIC0) | Imax | IC50 | H | r2 | ||
| K. pneumoniae (Kp3) | CTX-M-15 | 64b | 32/4 | 9.32 | 6.52 | 2.60 | 1.57 | 0.94 |
| K. pneumoniae (KpK91) | CTX-M-15 | 64 | 32/4 | 9.03 | 4.75 | 1.36 | 4.00 | 0.97 |
| K. pneumoniae (Kp2301) | CTX-M-15 | >512 | >512/4 | 9.09 | 6.23 | 35.25 | 2.67 | 0.97 |
| E. coli (EcF65) | SHV-12 | >512 | 4/4 | 8.67 | 6.99 | 2.71 | 3.41 | 0.98 |
CAZ, ceftazidime; PIP-TAZ, piperacillin-tazobactam. TAZ MIC values for all isolates were >256 μg/ml.
Boldface denotes resistant phenotype according to CLSI breakpoints.
Effect of inhibitor on MIC.
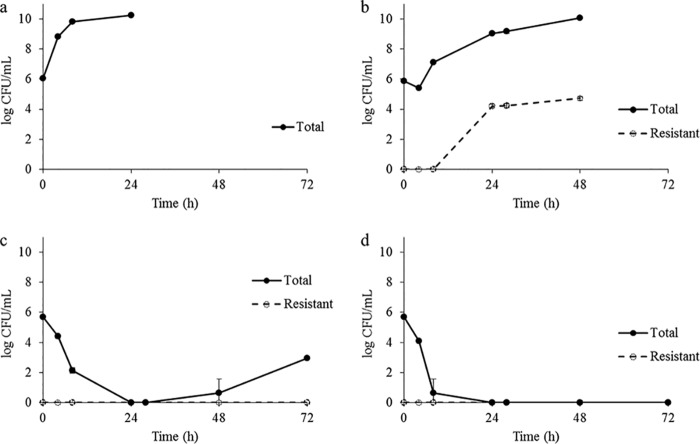
A tazobactam concentration-dependent reduction in piperacillin MIC was observed. The relationship between susceptibility and inhibitor concentrations was reasonably characterized by the sigmoid inhibitory maximum effect (Emax) model for all 4 isolates (r2 ≥ 0.94). The model best-fit parameter estimates (Table 1) illustrate differences in isolate sensitivity to tazobactam, with Kp3, KpK91, and EcF65 displaying lower 50% inhibitory concentration (IC50) values than that of Kp2301. Additionally, maximum effect conferred by the inhibitor (Imax) values indicate a more drastic reduction in MIC values for Kp3, EcF65, and Kp2301 than that for KpK91. A representative model fitting of the MIC data for Kp3, along with an associated MICi profile, is shown in Fig. 1.
FIG 1.
Representative model fit and instantaneous MIC profile. Model fit to piperacillin MIC data for Kp3 in the presence of escalating tazobactam concentrations (r2 = 0.94) (a). Open circles indicate experimental data, and the continuous line represents the best-fit model. MICi profile using 0.5 g tazobactam every 8 h is shown (b).
Pharmacokinetics and effect of drug exposures on bacterial burden.
The targeted piperacillin and tazobactam pharmacokinetic profiles were reasonably well simulated in the hollow-fiber infection model. Typical profiles for 4 g piperacillin and 0.5 g tazobactam are shown in Fig. 2. For the reference isolate (Kp3), the bacterial burden declined initially for all treatment exposures. The clinical regimen of 4 g piperacillin and 0.5 g tazobactam was associated with a %fT>MICi of 39.6 and resulted in bacterial regrowth after 8 h. An escalated tazobactam exposure of 1.5 g every 8 h resulted in a %fT>MICi of 55.1 and in growth suppression. For the other isolates, the estimated %fT>MICi and predicted outcomes associated with different dosing exposures are summarized in Table 2.
FIG 2.
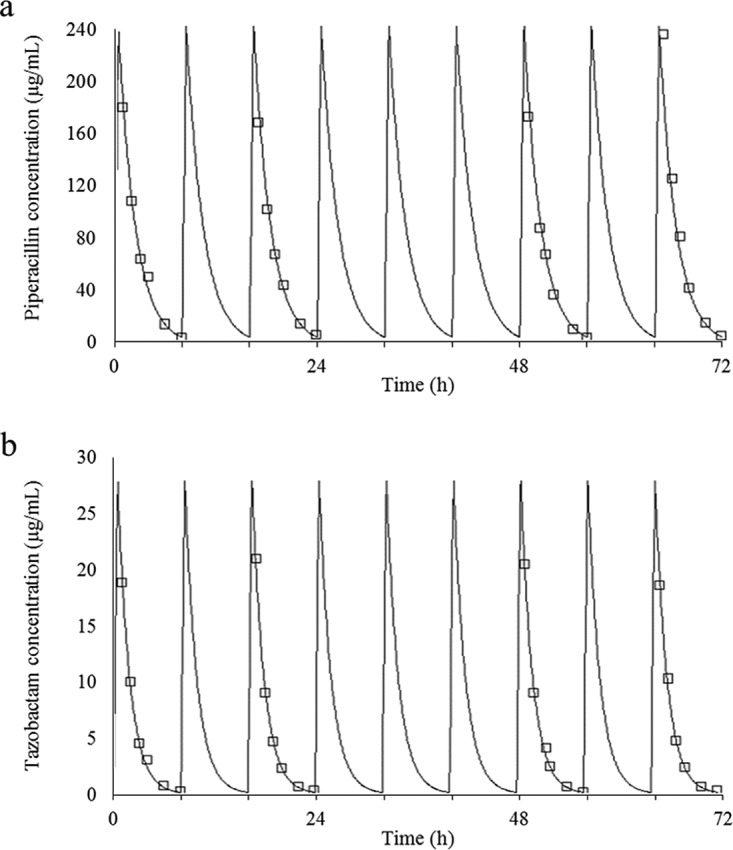
Typical simulated pharmacokinetic profile of 4 g piperacillin (target Cmax = 240 μg/ml; r2 = 0.97) (a) and 0.5 g tazobactam (target Cmax = 30 μg/ml; r2 = 0.99) (b) administered every 8 h. An elimination half-life of 1 h was simulated for both agents. Open squares represent observed concentrations, and continuous lines represent the best-fit model.
TABLE 2.
Predicted and observed outcomes associated with different piperacillin-tazobactam exposures
| Isolate | Tazobactam dose (g)a | %fT>MICi | Outcomeb |
|
|---|---|---|---|---|
| Predicted | Observed | |||
| Kp3 | 0.5 | 39.6 | NA | Regrowth |
| 1 | 51.6 | NA | Regrowth | |
| 1.5 | 55.1 | NA | Suppression | |
| 2 | 58.6 | NA | Suppression | |
| EcF65 | 0.5 | 43.8 | Regrowth | Regrowth |
| 1 | 60.0 | Suppression | Suppression | |
| 1.5 | 65.0 | Suppression | Suppression | |
| KpK91 | 0.5 | 44.5 | Regrowth | Regrowth |
| 1 | 50.9 | Regrowth | ND | |
| 1.5 | 50.9 | Regrowth | ND | |
| 2 | 50.9 | Regrowth | ND | |
| 4 | 50.9 | Regrowth | Regrowth | |
| Kp2301 | 0.5 | 13.5 | Regrowth | Regrowth |
| 1 | 19.9 | Regrowth | ND | |
| 1.5 | 25.5 | Regrowth | ND | |
| 2 | 29.8 | Regrowth | ND | |
| 4 | 36.8 | Regrowth | Regrowth | |
Coadministered with piperacillin, 4 g every 8 h.
NA, not applicable; ND, not determined.
Experimental validations.
Our model reliably predicted the outcomes of various piperacillin-tazobactam exposures for each validation isolate (EcF65, KpK91, and Kp2301), as shown in Table 2. The clinical dosing regimen of 4 g piperacillin and 0.5 g tazobactam was predicted to result in regrowth of all 3 isolates. For EcF65 (which was considered susceptible to piperacillin-tazobactam by the standard susceptibility testing method), the clinical regimen would achieve a %fT>MICi of 43.8; regrowth was noted by 8 h, and the development of resistance was observed over time, as shown in Fig. 3b. Instead, an elevated exposure equivalent to 4 g piperacillin and 1.0 g tazobactam (%fT>MICi = 60) was necessary to suppress growth below the starting inoculum (Fig. 3c). Further reduction of bacterial density (below the limit of detection) was observed with 4 g piperacillin and 1.5 g tazobactam (%fT>MICi = 65), as shown in Fig. 3d. For KpK91, the clinical dosing regimen achieved a %fT>MICi of 44.5, and bacterial regrowth was observed. This isolate would be considered equally susceptible to piperacillin-tazobactam compared to the reference isolate Kp3, but owing to the attenuated Imax value for this isolate, higher exposures of tazobactam were predicted to be insufficient to suppress growth. At the highest tazobactam exposure evaluated (4 g every 8 h), the %fT>MICi (50.9) remained below the target exposure threshold and regrowth was seen. Finally, for Kp2301, the efficacy threshold was predicted to be unattainable at the doses explored, due to the high IC50 value of the isolate. Simulated dosing regimens as high as 4 g piperacillin with 4 g tazobactam every 8 h (%fT>MICi = 36.8) resulted in regrowth (data not shown).
FIG 3.
Killing profiles for EcF65. Shown are placebo control (a) and with killing profiles for 4 g piperacillin and 0.5 g tazobactam (%fT>MICi = 43.8) (b), 4 g piperacillin and 1.0 g tazobactam (%fT>MICi = 60.0) (c), and 4 g piperacillin and 1.5 g tazobactam (%fT>MICi = 65.0) (d). Data are displayed as means ± standard deviation (SD).
DISCUSSION
Limited options for the treatment of ESBL-producing Enterobacteriaceae infections have prompted growing interest in reevaluating β-lactam/β-lactamase inhibitor combinations. ESBL enzymes are inhibited in vitro by β-lactamase inhibitors, such as tazobactam. Thus, ESBL producers may be susceptible to the combination of piperacillin with tazobactam. However, in vitro susceptibility may not always correlate to clinical efficacy, especially for severe nosocomial infections. This is due (at least in part) to technical limitations in susceptibility testing. Currently, MIC testing recommendations involve use of a single inhibitor concentration (e.g., 4 μg/ml of tazobactam), which lacks adequate correlation to the fluctuating inhibitor concentrations observed in vivo. Reservations regarding the use of combinations like piperacillin-tazobactam also stem from observations of reduced bactericidal activity in the presence of high inocula (>1 × 107 CFU/ml) (15). Similarly, overexpression of β-lactamases by some Enterobacteriaceae at the standard inoculum may also overcome the inhibitor, and hence conventional dosing may not reliably achieve efficacious drug exposures. Additionally, characterization of inhibitor activity and subsequent dose optimization remain a challenge because traditional PK/PD indices are not directly applicable. For these reasons, there is a dire need for a more robust platform to optimize the dosing of β-lactam/β-lactamase inhibitor combinations.
Several clinical studies have highlighted discrepancies with the classical concerns regarding β-lactam/β-lactamase inhibitor combinations. In a pivotal post hoc analysis of bloodstream infections due to ESBL-producing Escherichia coli, piperacillin-tazobactam and amoxicillin-clavulanic acid were comparable in efficacy to carbapenems when isolates were susceptible in vitro (10). It is, however, noteworthy that a majority of the bloodstream infections were secondary to urinary or biliary infections, which are considered to be low-inoculum infections. These findings were supported by a recent meta-analysis of ESBL-producing Enterobacteriaceae bloodstream infections (of different sources), in which there was no statistically significant difference in mortality between patients treated with carbapenems and β-lactam/β-lactamase inhibitor combinations (16). Nonetheless, there was apparent variability in response to β-lactam/β-lactamase inhibitor therapy based on the type of pathogen and severity of illness.
To resolve the inconsistencies between observed in vitro effects and clinical outcomes, Nicasio et al. delineated the PK/PD index that best predicts the efficacy of tazobactam as the time above a threshold concentration (%time>threshold) (17). This threshold signified a critical concentration at which enzyme inhibition was maximized, and was shown to rise with increasing enzyme transcription levels. Notably, these findings suggested a need to customize tazobactam exposures (based on differences in enzyme expression) to achieve efficacy targets. However, by overlooking inhibitor effects below and above this critical value, this approach was subject to inherent limitations similar to those of the current paradigm. Other investigators have used a semimechanistic model to describe the combined activities of aztreonam-avibactam, another β-lactam/β-lactamase inhibitor combination. In their approach, Sy et al. incorporated data from time-kill studies to develop a model that characterized bacterial killing with varying β-lactamase inhibitor and β-lactam concentrations (18). Although informative, this model is limited in its application, since its implementation is dependent on time-kill data that is not readily available in clinical settings. Additionally, the model validations were limited to only 24 h, and thus the effect of β-lactam/β-lactamase inhibitor exposures during an extended time frame (beyond that of the initial experimentation) was not explored.
In our previous work, we captured the fluctuations in pathogen susceptibility associated with intermittent dosing of a β-lactamase inhibitor (14). A similar trend in pathogen susceptibilities was observed for tazobactam and the isolates in this study. Modeling of the susceptibility reversibility profiles revealed unique characteristics related to inhibitor affinity and the maximum inhibition achievable for each unique inhibitor-pathogen combination. Based on conventional susceptibility breakpoints, Kp3, KpK91, and Kp2301 were all resistant to piperacillin-tazobactam, and thus clinical dosing regimens would be expected to yield inadequate exposures. However, we demonstrated that each isolate responded distinctly to escalating tazobactam exposures, and a tailored tazobactam dosing approach could facilitate meeting the efficacy target. For instance, Kp3 and KpK91 shared an identical piperacillin MIC (using 4 μg/ml of tazobactam), and hence would be expected to respond similarly to piperacillin-tazobactam. However, a more nuanced effect was observed and could be attributed to differences in Imax values. Consequently, growth suppression was achieved with a more aggressive dosing approach for Kp3, but it was unattainable for KpK91 (using ≤4 g tazobactam). Consistent with our expectations, the efficacy threshold was also unattainable for Kp2301, due to high level of enzymatic activity, as reflected by the comparatively high IC50 for this isolate. With EcF65, our model further illustrated the shortcomings of predicting efficacy with a fixed tazobactam concentration. Although regarded as susceptible by current interpretation criteria, our findings indicated that dosing with 4 g piperacillin and 0.5 g tazobactam every 8 h would be inadequate against this isolate. Instead, a higher tazobactam exposure (equivalent to 1.0 g) was needed to achieve the efficacy target.
Our approach is novel for the following two reasons: (i) we attempted to address the drawbacks in conventional susceptibility testing with limited efficacy predictions, and (ii) we explored the adequacy of the standard 8:1 dosing ratio of piperacillin to tazobactam for different scenarios. Instead of characterizing the activity of piperacillin-tazobactam based on a single tazobactam concentration, we described this relationship more comprehensively using a range of concentrations. This approach was more informative, as it better reflected the changing β-lactamase inhibitor concentrations observed in vivo. It resulted in a more robust model framework in assessing the efficacy of various piperacillin-tazobactam dosing regimens against commonly encountered clinical isolates of Klebsiella pneumoniae and E. coli that produced ESBL enzymes. Our proof-of-concept data illustrated that a fixed dosing ratio may not be appropriate in all scenarios, and we advocate the development of multiple piperacillin-tazobactam dose ratio formulations, as seen with amoxicillin-clavulanic acid.
There were several limitations to our study. First, our model only focused on ESBL-mediated resistance, and the effects of additional mechanisms of resistance (including porin deficiency) in our isolates were not explored. Our proposed method is most relevant in strains for which the production of β-lactamases contributes significantly to the observed resistance phenotype. If other mechanisms of resistance coexist alongside the production of β-lactamases, optimized dosing of the β-lactamase inhibitor alone may not enhance the efficacy of the β-lactam. The hollow-fiber studies were limited to 72 h, and thus the predictive value of the model for longer durations of exposure is unknown. Additionally, since we only investigated the effect of piperacillin-tazobactam exposure on a moderate inoculum (∼1 × 106 CFU/ml), the impact on a high inoculum remains unclear. Given our limited sample size, our model warrants further validation against a larger collection of clinical isolates. Last, since the study was confined to combining escalating tazobactam exposures with a fixed piperacillin backbone regimen, further investigations are required to determine generalizability to other dosing options (e.g., prolonged/continuous piperacillin infusion or escalating piperacillin exposures with a fixed tazobactam backbone regimen) and β-lactam/β-lactamase inhibitor combinations. Future studies will evaluate whether a threshold for efficacy may be proposed across bacterial strains harboring other β-lactamases, and for other β-lactam/β-lactamase inhibitor combinations. Evaluation of the safety of elevated tazobactam dosing in humans should also be undertaken.
In summary, we demonstrated that the efficacy of a β-lactam/β-lactamase inhibitor combination could be correlated to the concentration-response relationship between the inhibitor and the ESBL-producing bacteria using a modified PK/PD index. This platform is relatively easy to implement clinically and may be instrumental to the optimal dosing of old and newer β-lactam/β-lactamase inhibitor combinations.
MATERIALS AND METHODS
Antimicrobial agents, chemicals, and reagents.
Piperacillin was purchased from Sigma-Aldrich (St. Louis, MO). Tazobactam and ceftazidime were obtained from Chem-Impex International (Wood Dale, IL). Liquid chromatography-mass spectrometry (LC-MS)-grade water and acetonitrile were purchased from EMD Millipore Corporation (Billerica, MA). Stock solutions of piperacillin, tazobactam, and ceftazidime were prepared in sterile water, aliquoted, stored at −80°C, and thawed immediately before use.
Bacteria.
Four representative clinical isolates commonly encountered in serious nosocomial infections were studied. Two CTX-M-15-producing K. pneumoniae isolates (Kp3 and KpK91) and one SHV-12 producing E. coli isolate (EcF65) were obtained from a reference microbiology laboratory (Madrid, Spain). An additional CTX-M-15-producing K. pneumoniae isolate (Kp2301) was obtained from our local surveillance study (19). These isolates were selected based on their MICs and susceptibility reversibility profiles in the presence of tazobactam. From the modeling perspective, these diverse isolates would enhance the robustness (i.e., predictive ability) of our approach. The methods for the detection of resistance mechanisms in these isolates were detailed previously (20–23). β-Lactamase activity was assessed for all 4 isolates, using a nitrocefin degradation assay as described previously (19). The isolates were stored in Protect storage vials (Key Scientific Products, Round Rock, TX) at −80°C and subcultured twice on 5% blood agar plates (Hardy Diagnostics, Santa Maria, CA) for 24 h at 35°C before use.
Susceptibility and effect of inhibitor on MIC.
Initial piperacillin-tazobactam susceptibilities (MICs) were determined by the broth dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) (24). Piperacillin MICs were further determined for each isolate using escalating concentrations of tazobactam (0 to 256 μg/ml). All MIC experiments were performed in triplicate and repeated at least once on a different day. The MIC reductions in the presence of tazobactam were modeled using the following previously described sigmoid inhibitory Emax model (14):
wherein MIC represents the MIC with the addition of an inhibitor, MIC0 (intrinsic MIC) is the MIC in the absence of inhibitor, Imax represents the maximum effect conferred by the inhibitor, H describes the sigmoidicity coefficient, I represents the concentration of inhibitor, and IC50 represents the inhibitor concentration required for 50% of the maximal inhibitory effect. The Emax model was conditioned with fluctuating tazobactam concentrations associated with different dosing regimens (e.g., 0.5 g every 8 h). A theoretical instantaneous MIC (MICi) profile, which represents changing pathogen susceptibility in the presence of changing inhibitor concentrations (over a dosing interval), was then simulated using the best-fit parameter estimates for each isolate. Finally, each MICi profile was superimposed on a simulated free (unbound) piperacillin pharmacokinetic profile to determine the percentage of fT>MICi.
Hollow-fiber infection model.
The schematics of the experimental setup have been described previously (25). Fresh colonies of each isolate were inoculated in cation-adjusted Mueller-Hinton broth (CA-MHB) and grown to the late log phase. Based on the absorbance at 630 nm, the bacterial suspension was adjusted to ∼1 × 106 CFU/ml. Simulated unbound exposures of piperacillin and tazobactam (dosed over 30 min) were given every 8 h for up to 72 h. For both piperacillin and tazobactam, 30% protein binding and a 1-h elimination half-life were used to simulate the free-drug exposures (26). Serial samples were obtained from the internal circulating loop over 4 dosing intervals to verify the pharmacokinetic simulations. Once collected, samples were stored at −80°C until analysis. The simulations were deemed acceptable if the best-fit peak concentrations and elimination half-lives were both within 20% of target values. To determine changes to bacterial burden, serial samples (500 μl) were obtained from the bioreactor cartridges in duplicate. Samples were centrifuged at 10,000 × g at 4°C for 15 min, reconstituted with sterile saline to minimize drug carryover, and plated quantitatively on Mueller-Hinton agar plates. Additionally, drug-supplemented agar plates (using 3× the baseline piperacillin-tazobactam MIC) were used selectively (e.g., in the susceptible isolate) to detect regrowth associated with the development of resistance over time.
Drug assay and pharmacokinetic modeling.
Piperacillin and tazobactam concentrations were assayed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The LC-MS/MS system consisted of a Waters Acquity ultraperformance liquid chromatograph (UPLC) with a Waters ethylene bridged hybrid (BEH) column (1.7 μm, 2.1 × 50 mm) and an API5500 Qtrap triple quadrupole mass spectrometer (Applied Biosystems/MDS SCIEX, Foster City, CA) equipped with a TurboIonSpray source. Each aliquot (5 μl) of sample was diluted with 895 μl LC-MS-grade water and 100 μl of ertapenem (320 ng/ml) as the internal standard. The resulting sample mixture was then injected (5 μl for piperacillin and 10 μl for tazobactam) into the LC-MS/MS system for analysis. The analytes were eluted by a gradient at 45°C consisting of mobile phase A (0.1% vol/vol formic acid in water) and mobile phase B (0.1% vol/vol formic acid in acetonitrile) at 0.35 ml/min. Tazobactam and piperacillin peaks were detected at 1.4 and 3.2 min, respectively. The linear range of quantification was 0.0625 to 128 μg/ml for both analytes. The intraday and interday variabilities for both agents were <9.4% and <13.4%, respectively. A one-compartment model with zero-order infusion input was fitted to the observed piperacillin and tazobactam concentration-time profiles using ADAPT 5 (27).
Identification of target exposure.
Using Kp3 as the (arbitrary) reference isolate, a clinical dosing regimen of 4 g piperacillin every 8 h was evaluated alongside escalating exposures of tazobactam (equivalent to 0.5 g, 1 g, 1.5 g, and 2 g) in our hollow-fiber model. With a %fT>MICi identified for each exposure, the threshold for efficacy was defined as the lowest %fT>MICi associated with bacterial growth suppression (i.e., bacterial burden below the baseline inoculum) at the end of the experiment. This threshold target exposure was subsequently validated in 3 other clinical isolates.
Experimental validations.
For the remaining isolates, predictions were made on the efficacy of different tazobactam exposures based on the corresponding %fT>MICi. Piperacillin-tazobactam regimens that exceeded the threshold value were expected to suppress growth. Conversely, exposures that failed to achieve the target %fT>MICi were predicted to result in bacterial regrowth over time. The %fT>MICi for 4 g piperacillin and 0.5 g tazobactam (every 8 h) was first determined for each isolate. If this initial exposure failed to achieve the threshold %fT>MICi, escalating experimental tazobactam exposures up to 4 g were then simulated to meet the target %fT>MICi (27). The hollow-fiber model was subsequently used to experimentally validate the predicted outcomes of the standard dosing regimen and either (i) exposure(s) that exceeded the target %fT>MICi or (ii) the highest exposure evaluated if the target %fT>MICi was unattainable.
ACKNOWLEDGMENT
This study was supported by MON4STRAT—Therapeutic Beta-Lactam Monitoring for Stratified Treatment of hospital-acquired pneumonia, improved dose-dependent efficacy, decreased treatment duration, and prevention of emergence of resistance, FP7-HEALTH-2013-INNOVATION-1, European Union’s Seventh Framework Programme funding for research, technological development, and demonstration, under grant agreement number 602906.
REFERENCES
- 1.Paterson DL. 2006. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Med 119:S20 discussion S62-70. doi: 10.1016/j.amjmed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2014. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/threat-report-2013/index.html. [Google Scholar]
- 3.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Harris PNA, Tambyah PA, Paterson DL. 2015. β-Lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis 15:475–485. doi: 10.1016/S1473-3099(14)70950-8. [DOI] [PubMed] [Google Scholar]
- 5.Schoonover LL, Occhipinti DJ, Rodvold KA, Danziger LH. 1995. Piperacillin/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. Ann Pharmacother 29:501–514. doi: 10.1177/106002809502900510. [DOI] [PubMed] [Google Scholar]
- 6.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PN, Yin M, Jureen R, Chew J, Ali J, Paynter S, Paterson DL, Tambyah PA. 2015. Comparable outcomes for beta-lactam/beta-lactamase inhibitor combinations and carbapenems in definitive treatment of bloodstream infections caused by cefotaxime-resistant Escherichia coli or Klebsiella pneumoniae. Antimicrob Resist Infect Control 4:14. doi: 10.1186/s13756-015-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng TM, Khong WX, Harris PN, De PP, Chow A, Tambyah PA, Lye DC. 2016. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS One 11:e0153696. doi: 10.1371/journal.pone.0153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina J, Hernández A, Venditti M, Prim N, Origüen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh P-R, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. 2016. A multinational, preregistered cohort study of beta-lactam/beta-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4159–4169. doi: 10.1128/AAC.00365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Bano J, Navarro MD, Retamar P, Picon E, Pascual A. Extended-Spectrum Beta-Lactamases–Red Española de Investigación en Patologóa Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. 2012. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 11.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership G. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimhony O, Chmelnitsky I, Bardenstein R, Goland S, Hammer Muntz O, Navon Venezia S, Carmeli Y. 2006. Endocarditis caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: emergence of resistance to ciprofloxacin and piperacillin-tazobactam during treatment despite initial susceptibility. Antimicrob Agents Chemother 50:3179–3182. doi: 10.1128/AAC.00218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kays MB. 1999. Comparison of five beta-lactam antibiotics against common nosocomial pathogens using the time above MIC at different creatinine clearances. Pharmacotherapy 19:1392–1399. doi: 10.1592/phco.19.18.1392.30900. [DOI] [PubMed] [Google Scholar]
- 14.Bhagunde P, Chang KT, Hirsch EB, Ledesma KR, Nikolaou M, Tam VH. 2012. Novel modeling framework to guide design of optimal dosing strategies for beta-lactamase inhibitors. Antimicrob Agents Chemother 56:2237–2240. doi: 10.1128/AAC.06113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Cerero L, Picón E, Morillo C, Hernández JR, Docobo F, Pachón J, Rodríguez-Baño J, Pascual A. 2010. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin Microbiol Infect 16:132–136. doi: 10.1111/j.1469-0691.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 16.Muhammed M, Flokas ME, Detsis M, Alevizakos M, Mylonakis E. 2017. Comparison between carbapenems and beta-lactam/beta-lactamase inhibitors in the treatment for bloodstream infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: a systematic review and meta-analysis. Open Forum Infect Dis 4:ofx099. doi: 10.1093/ofid/ofx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicasio AM, VanScoy BD, Mendes RE, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2016. Pharmacokinetics-pharmacodynamics of tazobactam in combination with piperacillin in an in vitro infection model. Antimicrob Agents Chemother 60:2075–2080. doi: 10.1128/AAC.02747-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sy S, Zhuang L, Xia H, Beaudoin ME, Schuck VJ, Derendorf H. 2017. Prediction of in vivo and in vitro infection model results using a semimechanistic model of avibactam and aztreonam combination against multidrug resistant organisms. CPT Pharmacometrics Syst Pharmacol 6:197–207. doi: 10.1002/psp4.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abodakpi H, Chang K-T, Sánchez Díaz AM, Cantón R, Lasco TM, Chan K, Sofjan AK, Tam VH. 2018. Prevalence of extended-spectrum beta-lactamase and carbapenemase-producing bloodstream isolates of Klebsiella pneumoniae in a tertiary care hospital. J Chemother 30:115–119. doi: 10.1080/1120009X.2017.1399233. [DOI] [PubMed] [Google Scholar]
- 20.Endimiani A, Carias LL, Hujer AM, Bethel CR, Hujer KM, Perez F, Hutton RA, Fox WR, Hall GS, Jacobs MR, Paterson DL, Rice LB, Jenkins SG, Tenover FC, Bonomo RA. 2008. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob Agents Chemother 52:2680–2682. doi: 10.1128/AAC.00158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. 2007. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 115:1400–1408. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 22.Rasheed JK, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros AA, Tenover FC. 1997. Evolution of extended-spectrum beta-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother 41:647–653. doi: 10.1128/AAC.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Kelkar S, Wu W, Chen M, Quinn JP. 2003. Clinical isolates of Enterobacteriaceae producing extended-spectrum beta-lactamases: prevalence of CTX-M-3 at a hospital in China. Antimicrob Agents Chemother 47:790–793. doi: 10.1128/AAC.47.2.790-793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing: twenty-sixth informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Tam VH, Louie A, Fritsche TR, Deziel M, Liu W, Brown DL, Deshpande L, Leary R, Jones RN, Drusano GL. 2007. Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J Infect Dis 195:1818–1827. doi: 10.1086/518003. [DOI] [PubMed] [Google Scholar]
- 26.Pfizer Injectables. 2012. Piperacillin and tazobactam for injection, USP [package insert]. Pfizer Injectables, Philadelphia, PA.
- 27.D’Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. biomedical simulations resource. University of Southern California, Los Angeles, CA. [Google Scholar]