Breakthrough mucormycosis in patients receiving isavuconazole prophylaxis or therapy has been reported. We compared the impact of isavuconazole and voriconazole exposure on the virulence of clinical isolates of Aspergillus fumigatus and different Mucorales species in a Drosophila melanogaster infection model.
KEYWORDS: Aspergillus fumigatus, Drosophila melanogaster, Rhizopus arrhizus, isavuconazole
ABSTRACT
Breakthrough mucormycosis in patients receiving isavuconazole prophylaxis or therapy has been reported. We compared the impact of isavuconazole and voriconazole exposure on the virulence of clinical isolates of Aspergillus fumigatus and different Mucorales species in a Drosophila melanogaster infection model. In contrast to A. fumigatus, a hypervirulent phenotype was found in all tested Mucorales upon preexposure to either voriconazole or isavuconazole. These findings may contribute to the explanation of breakthrough mucormycosis in isavuconazole-treated patients.
INTRODUCTION
Invasive mold infections (IMI) remain a major cause of morbidity and mortality in immunocompromised patients. Whereas invasive aspergillosis, mostly caused by Aspergillus fumigatus, accounts for the majority of these infections, an epidemiological shift toward an increased incidence of mucormycosis has been observed, with Rhizopus arrhizus (Rhizopus oryzae) being the most frequent causative agent (1, 2). Several studies have linked this epidemiological shift to the widespread use of voriconazole (VRC), an azole with good Aspergillus but no Mucorales activity (1, 3). Isavuconazonium sulfate (ISAV) is the most recently introduced triazole exhibiting activity against a wide range of medically important fungi and a favorable toxicity profile compared to other triazoles (4). A recent study examining breakthrough mold infections in leukemia patients who were receiving oral or intravenous ISAV for prophylaxis or therapy reported that most microbiologically documented breakthrough mold infections were caused by Mucorales (5).
Although antifungal therapy may select for breakthrough infections caused by more resistant pathogens through direct selection pressure, drug-induced changes in pathogen virulence may be encountered (6, 7). We previously found evidence of increased virulence, angioinvasion, and inflammatory host response in invertebrate and mammalian models of mucormycosis when animals were infected with a strain of R. arrhizus that was passaged on VRC-containing agar but not posaconazole (7). The impact of ISAV on the virulence of A. fumigatus and Mucorales has not yet been characterized. Using a well-validated Drosophila melanogaster infection model (8), we sought to evaluate virulence changes of A. fumigatus and Mucorales serially passaged on agar supplemented with ISAV or VRC.
Two A. fumigatus isolates (internal numbers 6058 and 8171) and four Mucorales isolates (Rhizopus arrhizus 749 and 969, Rhizomucor pusillus 449, and Mucor circinelloides 518) were obtained from patients at the University of Texas M.D. Anderson Cancer Center. Preexposure of these pathogens to VRC and ISAV by serial passage on antifungal-supplemented yeast extract-agar-glucose plates was performed as previously described (7). Four different concentrations of each antifungal were tested (0.0625, 0.125, 0.25, and 0.5× MIC as determined according to the CLSI M38-A2 reference method) in order to define the highest fraction of MIC still yielding confluent growth after 48 h at 37°C. This concentration (usually 0.25× MIC) was used for subsequent passages (Mucorales: 4 μg/ml VRC, 0.5 μg/ml ISAV; A. fumigatus: 0.5 μg/ml VRC, 0.125 μg/ml ISAV). Controls were prepared by serial passaging of each fungus on yeast extract-agar-glucose plates not supplemented with antifungals.
For Mucorales infections, the dorsal side of the thorax of female OregonR wild-type (WT) flies was pricked with a needle dipped in a 1 × 107/ml spore solution as previously described (8). A. fumigatus infections (inoculum concentration, 5 × 107/ml) were performed in Tlr632/TlI-RXA Drosophila mutant flies generated by crossing thermosensitive allele of Toll (Tlr632) flies with a null allele of Toll (TlI-RXA) flies. Survival was monitored daily for 7 days postinfection. For each isolate, two independent experiments were performed (n = 47 to 60 per cohort). Statistical comparison of survival rates was performed using a log rank test (GraphPad Prism 7.03). A P value of <0.05 was considered significant.
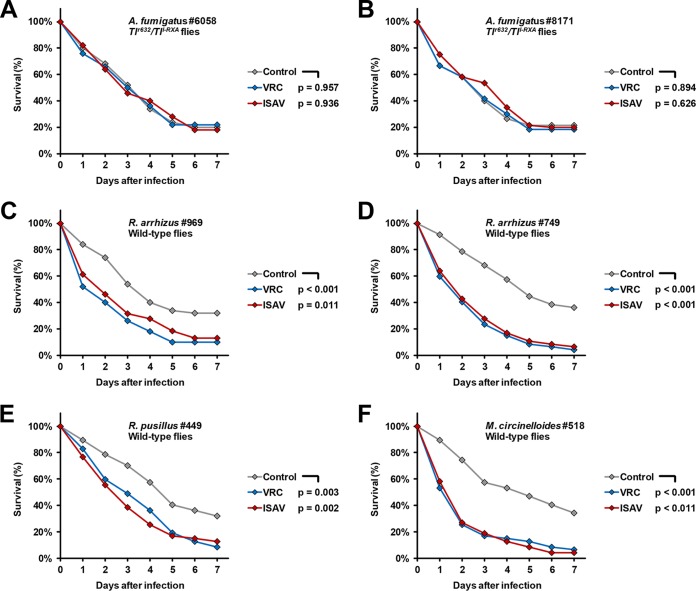
For both A. fumigatus isolates tested, preexposure to VRC or ISAV for four passages did not result in significant differences in survival curves, with 7-day survival rates of 20 to 22% (control), 18 to 22% (preexposed to VRC), and 18 to 20% (preexposed to ISAV), respectively. The median survival times (MSTs) of Toll-deficient flies infected with A. fumigatus strain 6058 were 4 days (control), 3.5 days (preexposure to VRC), and 3 days (preexposure to ISAV), respectively (Fig. 1A). Upon infection with strain 8171, 3-day (no preexposure or preexposure to VRC) and 4-day (preexposure to ISAV) MSTs were observed (Fig. 1B).
FIG 1.
Survival rates of A. fumigatus- and Mucorales-infected D. melanogaster flies depending on fungal preexposure to azoles. Clinical isolates of A. fumigatus and four different Mucorales isolates underwent four serial passages on ISAV-supplemented, VRC-supplemented, or nonsupplemented (Control) yeast extract-agar-glucose plates. Tlr632/TlI-RXA Drosophila mutant flies and OregonR WT flies were infected with A. fumigatus and Mucorales, respectively. The survival of the flies was monitored for 7 days postinfection. Two independent experiments were performed, and survival rates were calculated based on cumulative data.
In line with earlier findings (7, 9, 10), four passages of R. arrhizus 969 on VRC-supplemented plates led to reduced MST (2 days versus 4 days) and 7-day survival (10% versus 32%, P < 0.001) of infected WT flies compared to infection with the nonexposed control (Fig. 1C). Similarly, preexposure of R. arrhizus 969 to ISAV resulted in a 2-day MST and 87% mortality of infected flies during the 7-day observation period (P = 0.011). To preclude a strain-specific phenotype, these findings were corroborated using a second isolate (isolate 749), revealing an even more prominent decline in MST (2 versus 5 days) and 7-day survival rates (4 and 6% versus 36%) of infected flies upon fungal preexposure to VRC or ISAV (Fig. 1D). In addition, two Mucorales isolates from other genera were used to evaluate intergenus differences in VRC- and ISAV-induced hypervirulence. In R. pusillus 449, preexposure to either drug reduced the MST from 5 to 3 days, and the 7-day survival rates dropped from 32 to 9% (VRC) and 12% (ISAV), respectively (Fig. 1E). Similarly, VRC- and ISAV-exposed M. circinelloides caused significantly declined MST (2 versus 5 days) and a 7-day survival compared to the nonexposed serially passaged control (Fig. 1F).
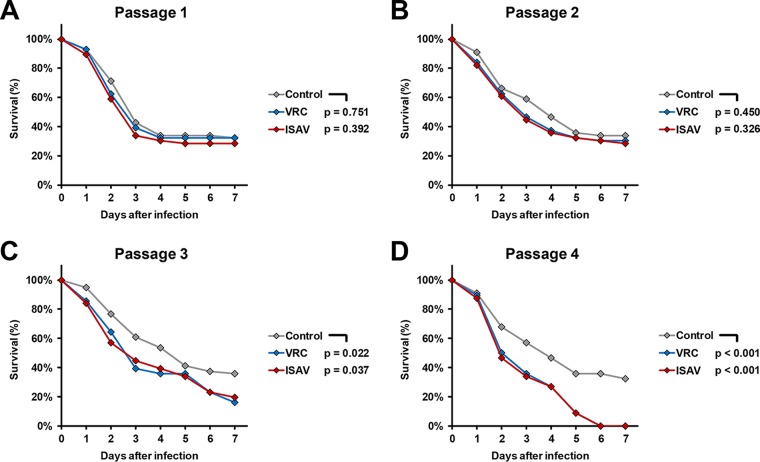
To assess whether ISAV and VRC differ in their potential to cause hypervirulence depending on the duration of exposure, we infected WT flies with serially passaged R. arrhizus 749 after each of four subsequent passages. Exposure to both drugs gradually increased the gap in 3- and 7-day survival rates compared to nonexposed R. arrhizus 749, reaching statistical significance after three passages and 100% 7-day mortality after four passages (Fig. 2).
FIG 2.
Survival rates of R. arrhizus 749-infected D. melanogaster flies depending on the number of passages of the fungus on azole-supplemented plates. R. arrhizus 749 was passaged four times on ISAV-supplemented, VRC-supplemented, or nonsupplemented (Control) yeast extract-agar-glucose plates. OregonR WT flies were infected after each passage. The survival of flies was monitored for 7 days postinfection. Two independent experiments were performed, and survival rates were calculated based on cumulative data.
In summary, these results indicate that, similar to VRC, ISAV induces a hypervirulent phenotype of Mucorales, whereas the virulence of A. fumigatus remains unaltered by ISAV. This observation, based on experiments in four Mucorales strains from three different species, may contribute to the explanation of breakthrough mucormycosis in patients receiving ISAV prophylaxis or therapy (5). Importantly, sufficient concordance of comparative virulence studies in the Drosophila model and mammalian hosts has been previously documented in experimental mucormycosis (7). The mechanism of VRC- and ISAV-associated hypervirulence of Mucorales remains to be determined. Based on the observation of attenuating hypervirulence upon termination of VRC exposure (7), complex epigenetic alterations, possibly associated with the plasticity of the ergosterol biosynthetic pathway, may be operative, resulting in accumulation of alternative sterols in the fungal cell membrane and leading to increased adherence to the extracellular matrix (7). In addition to the finding of enhanced virulence, further in vivo studies are warranted to explore whether exposure of Mucorales to ISAV results in a higher risk of diminished efficacy of subsequent therapy with other triazoles and other classes of antifungal drugs.
ACKNOWLEDGMENTS
This project was funded in part by an investigator initiated proposal by Astellas Pharma Global Development, Inc., to D.P.K. The sponsor had no role in the design or performance of experiments, data interpretation, or preparation of the manuscript. D.P.K. acknowledges a Texas 4000 Distinguished Professorship for Cancer Research and the NIH-NCI Cancer Center CORE Support grant 16672. D.P.K. reports research support from Astellas Pharma and honoraria for lectures from Merck & Co., Gilead, and United Medical. He has served as a consultant for Astellas Pharma, Cidara, Amplyx, Astellas, and Mayne and on the advisory board of Merck & Co. R.E.L. has received research support from Merck, Inc., and compensation for speaking or advisory boards by Gilead, Cidara, and Merck & Co. All other authors report no potential conflicts of interest.
REFERENCES
- 1.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 2.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. 2012. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 54(Suppl 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 3.Pongas GN, Lewis RE, Samonis G, Kontoyiannis DP. 2009. Voriconazole-associated zygomycosis: a significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin Microbiol Infect 15(Suppl 5):93–97. doi: 10.1111/j.1469-0691.2009.02988.x. [DOI] [PubMed] [Google Scholar]
- 4.Ordaya EE, Alangaden GJ. 2016. Real-life use of isavuconazole in patients intolerant to other azoles. Clin Infect Dis 63:1529–1530. doi: 10.1093/cid/ciw585. [DOI] [PubMed] [Google Scholar]
- 5.Rausch CR, DiPippo AJ, Bose P, Kontoyiannis DP. 2018. Breakthrough fungal infections in leukemia patients receiving isavuconazole. Clin Infect Dis 67:1610–1613. doi: 10.1093/cid/ciy406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontoyiannis DP, Lionakis MS, Lewis RE, Chamilos G, Healy M, Perego C, Safdar A, Kantarjian H, Champlin R, Walsh TJ, Raad II. 2005. Zygomycosis in a tertiary-care cancer center in the era of aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis 191:1350–1360. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 7.Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. 2009. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis 199:1399–1406. doi: 10.1086/597615. [DOI] [PubMed] [Google Scholar]
- 8.Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, Halder G, Kontoyiannis DP. 2008. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S A 105:9367–9372. doi: 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis RE, Liao G, Wang W, Prince RA, Kontoyiannis DP. 2011. Voriconazole pre-exposure selects for breakthrough mucormycosis in a mixed model of Aspergillus fumigatus-Rhizopus oryzae pulmonary infection. Virulence 2:348–355. doi: 10.4161/viru.2.4.17074. [DOI] [PubMed] [Google Scholar]
- 10.Bellanger AP, Albert ND, Lewis RE, Walsh TJ, Kontoyiannis DP. 2015. Effect of preexposure to triazoles on susceptibility and virulence of Rhizopus oryzae. Antimicrob Agents Chemother 59:7830–7832. doi: 10.1128/AAC.01583-15. [DOI] [PMC free article] [PubMed] [Google Scholar]