As a consequence of emerging numbers of vulvovaginitis cases caused by azole-resistant and biofilm-forming Candida species, fast and efficient treatment of this infection has become challenging. The problem is further exacerbated by the severe side effects of azoles as long-term-use medications in the recurrent form.
KEYWORDS: Candida albicans, Neosartorya fischeri antifungal protein 2, antifungal mechanism, in vitro cytotoxicity, in vitro susceptibility, in vivo murine model, vulvovaginitis
ABSTRACT
As a consequence of emerging numbers of vulvovaginitis cases caused by azole-resistant and biofilm-forming Candida species, fast and efficient treatment of this infection has become challenging. The problem is further exacerbated by the severe side effects of azoles as long-term-use medications in the recurrent form. There is therefore an increasing demand for novel and safely applicable effective antifungal therapeutic strategies. The small, cysteine-rich, and cationic antifungal proteins from filamentous ascomycetes are potential candidates, as they inhibit the growth of several Candida spp. in vitro; however, no information is available about their in vivo antifungal potency against yeasts. In the present study, we investigated the possible therapeutic application of one of their representatives in the treatment of vulvovaginal candidiasis, Neosartorya fischeri antifungal protein 2 (NFAP2). NFAP2 inhibited the growth of a fluconazole (FLC)-resistant Candida albicans strain isolated from a vulvovaginal infection, and it was effective against both planktonic cells and biofilm in vitro. We observed that the fungal cell-killing activity of NFAP2 is connected to its pore-forming ability in the cell membrane. NFAP2 did not exert cytotoxic effects on primary human keratinocytes and dermal fibroblasts at the MIC in vitro. In vivo murine vulvovaginitis model experiments showed that NFAP2 significantly decreases the number of FLC-resistant C. albicans cells, and combined application with FLC enhances the efficacy. These results suggest that NFAP2 provides a feasible base for the development of a fundamental new, safely applicable mono- or polytherapeutic topical agent for the treatment of superficial candidiasis.
INTRODUCTION
Candida spp. belong to the normal human flora under the control of a sensitive and well-regulated balance mechanism between the fungus and the host defense system. If this mechanism is disturbed by physiological or nonphysiological changes, Candida can overgrow the dermal and mucosal surfaces in healthy individuals. One of these symptoms is vulvovaginal candidiasis (VVC), when Candida infects the surface of the vaginal and vulvar mucosa (1). VVC is estimated to be the most common fungal infection in a number of countries (2) and has been considered to be an important worldwide public health problem by the World Health Organization (3). VVC affects ∼75% of adult women at least once in their lifetime; ∼15% of the cases are asymptomatic, and ∼10% are recurrent (recurrent VVC [RVVC]), which means that there are more than four infection episodes per year in the absence of predisposing factors. Although VVC is not associated with mortality, it causes discomfort, pain, and social embarrassment, which impair sexual and affective relationships and work performance. Untreated VVC can lead to severe complications, such as vaginitis and penitis if it is transferred to the male partner, and as a consequence, pelvic inflammation, infertility, ectopic pregnancy, pelvic abscess, spontaneous abortion, and menstrual disorders can occur (1).
Candida albicans is still the most common VVC-associated yeast in most countries. However, epidemiology surveys from the last 15 years have demonstrated an increasing prevalence of nonalbicans Candida (NAC) species (1). The recommended treatment in the United States for uncomplicated C. albicans VVC is the vaginal application of nystatin or azole-based topical agents, but considering personal preference, a single oral dose of 150 mg fluconazole (FLC) is suggested alternatively. For severe acute cases, such as RVVC, 150 mg FLC, given every 72 h for a total of two or three doses, is recommended for 6 months (4, 5). This long-term FLC use may cause severe side effects in the host (e.g., liver toxicity) and promote the development of a resistance mechanism in the fungus (6). Susceptibility data indicate a continuous increase in the number of VVC-related and FLC-resistant C. albicans isolates (2, 7). The development of resistance mechanisms is connected to the biofilm-forming ability of the fungus. Namely, C. albicans is able to adhere to the surface of the vaginal epithelium and form a complex three-dimensional structure of fungal cell agglomerates with reduced susceptibility to azoles and less sensitivity to the killing mechanisms of the host immune system, resulting frequently in RVVC (4). Therefore, at present, fast and efficient treatment of RVVC is becoming more and more challenging, and novel, safely applicable antifungal strategies with high efficiency are needed against Candida biofilms.
In vitro susceptibility data suggest that the low-molecular-weight, cysteine-rich and cationic antifungal proteins (crAFPs) secreted by filamentous ascomycetes are potential therapeutic candidates to fight against Candida infections (8–13). In our previous study, we demonstrated that one of their representatives, Neosartorya fischeri antifungal protein 2 (NFAP2), effectively inhibits the growth of clinically relevant Candida spp. using the standardized Clinical and Laboratory Standards Institute (CLSI) document M27-A3 clinical susceptibility testing method and interacts synergistically with FLC in vitro (12). These observations indicate the in vivo efficacy and potential applicability of NFAP2 as a mono- or polytherapeutic agent in anti-Candida therapy.
To prove this assumption, in the present study, we investigated the in vivo applicability of NFAP2 for the treatment of VVC. First of all, we determined the in vitro cell-killing efficacy and antifungal mechanism of NFAP2 against a FLC-resistant and biofilm-forming C. albicans strain isolated from a human VVC case, before testing the in vitro cytotoxicity of NFAP2 on primary human keratinocytes (HKCs) and human dermal fibroblasts (HDFs). Based on the promising in vitro results, we successfully applied NFAP2 alone and in combination with FLC in an in vivo murine VVC model system.
RESULTS
In vitro susceptibility.
In our previous work, we observed that the antifungal efficacy and the MIC of NFAP2 depend on the applied test medium and the investigated Candida strain (10, 12). One of the major virulence factors of C. albicans is the ability to form a biofilm, which shows less susceptibility or intrinsic resistance to conventional antifungal agents. Furthermore, the formation of a biofilm plays a role in the colonization of mucosal surfaces (14). Hence, we determined the exact MICs of FLC and NFAP2 for planktonic and sessile biofilm cells of C. albicans 27700 in RPMI 1640 medium simulating the human extracellular environment in composition. MIC values of FLC proved to be 16 μg/ml and 512 μg/ml for planktonic and sessile cell populations, respectively. According to susceptibility breakpoints (15), C. albicans 27700 is resistant to FLC. Both cell types showed the same susceptibility to NFAP2, with MICs of 800 μg/ml. It is noteworthy that 400 μg/ml NFAP2 already caused a >50% decrease in turbidity and metabolic activity for planktonic cells. At this concentration, NFAP2 was inactive against the biofilm, and significant decreases in turbidity and in metabolic activity were not observed.
Anti-Candida mechanism.
Our previous observations applying the membrane-impermeant, red-fluorescent nuclear and chromosome stain propidium iodide (PI) suggested the prompt plasma membrane disruption ability of NFAP2 on yeast cells as the key factor of the antifungal effect (10, 12), but the exact mechanism of membrane disruption has not yet been investigated. First, we quantified the number of disrupted cells by fluorescence-activated cell sorter (FACS) analysis. This revealed that 38.20% ± 3.12% (P = 0.00007) of the FLC-resistant C. albicans 27700 cells have a PI-positive phenotype after 24 h of NFAP2 treatment at the MIC compared to the untreated control (3.26% ± 1.72%) (see Fig. S1 in the supplemental material). Scanning electron microscopy (SEM) images showed that NFAP2 forms pores in the plasma membrane, causing a loss of cell content, which finally results in cell death (Fig. 1). Several different molecular mechanisms of membrane disruption were proposed for antimicrobial peptides and proteins previously. Many such mechanisms (including pore formation) involve significant conformational changes and/or oligomerization of the membrane-acting proteins (16–18). This conformational change can be detected by electronic circular dichroism (ECD) spectroscopy (19). We observed that the ECD spectrum of NFAP2 in the presence of yeast cells is similar to that of the pure aqueous NFAP2 solution and demonstrates previously described spectral contributions emerging from the β-conformation (200 nm and 212 nm) and disulfide bridges (228 nm) (Fig. 2) (12). The presence of C. albicans 27700 cells did not induce any change in the secondary structure of the protein within 24 h of incubation. However, the number of CFU decreased significantly (P = 0.00062), from 6.10 × 106 ± 0.54 × 106 cells/ml to 2.49 × 106 ± 0.34 × 106 cells/ml in the samples during the 24-h time frame of ECD measurements. This suggests that while exposure to 100 mg/ml NFAP2 results in notable cell death, mechanisms of action accompanied by large-scale structural changes can be ruled out for NFAP2.
FIG 1.
Scanning electron microscopy of C. albicans 27700 cells after incubation in RPMI 1640 medium (A and B) and in RPMI 1640 medium supplemented with 800 μg/ml NFAP2 (C and D) for 24 h at 30°C with continuous shaking at 160 rpm. Framed regions in panels A and C are shown at higher magnification in panels B and D, respectively. Arrows indicate pore formation in the cell envelope and the loss of cell content after exposure to NFAP2. Bars, 1 μm.
FIG 2.
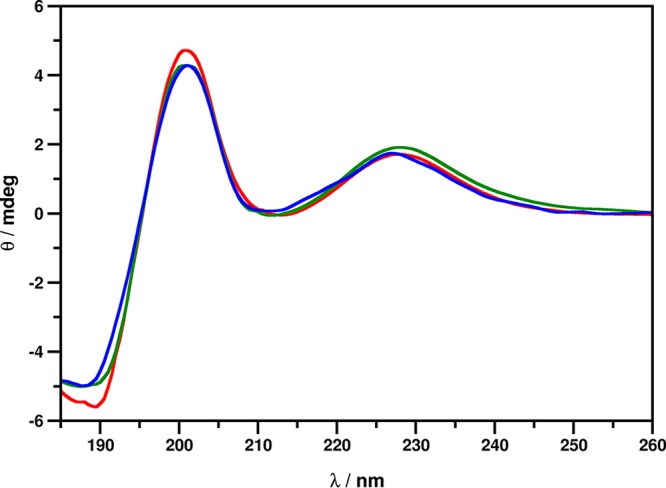
ECD spectra of NFAP2 in ddH2O (blue) and in the presence of C. albicans cells immediately after exposure to (red) and after 24 h of incubation with (green) 100 μg/ml NFAP2 at 30°C with continuous shaking at 160 rpm.
In vitro cytotoxicity.
In silico prediction showed a high binding affinity of NFAP2 for human serum albumin (HSA) (ΔG = −12.16 kcal/mol; Kd [dissociation constant] = 1.21e−09 M) (20); hence, its systemic application as an antifungal drug is debatable. However, NFAP2 is considered a potential candidate for a novel topical antifungal agent, and the most possible therapeutic application is in the treatment of superficial candidiasis (12). To verify this suggestion, it is necessary to elucidate the cytotoxic potential of the protein on HKCs and HDFs as the predominant cell types in the epidermis and the most common cells of connective tissue synthesizing the extracellular matrix and collagen, respectively. In vitro viability staining of primary HKCs and HDFs with PI after exposure to NFAP2 for 24 h revealed no change in the number of PI-positive cells even after treatment with twice the MIC (see Fig. S2 in the supplemental material).
In vivo application.
Based on the observed in vitro MIC values, NFAP2 is considered a monotherapeutic agent for the treatment of VVC caused by FLC-resistant strains. In vitro data have already suggested that NFAP2 could interact synergistically with FLC against C. albicans (12); hence, the in vivo antifungal effect of the NFAP2-FLC combination was also investigated to reveal a possible FLC resistance reversion. Results of the in vivo experiments are shown in Fig. 3. A single dose of 35-mg/kg of body weight and daily doses of 5-mg/kg of FLC could not reduce significantly (P > 0.05) the vaginal fungal burden compared to that in untreated mice. In comparison with the untreated group of animals, NFAP2 regimens of 800 μg/ml/day alone or in combination with 5 mg/kg/day FLC caused a significant reduction (P ≤ 0.05) in the number of living C. albicans cells from vaginal tissue. This reduction was more prominent when NFAP2 was applied in combination with FLC (P = 0.0017) than when it was applied as a monotherapeutic agent (P = 0.0177). Furthermore, the yeast-cell-number-decreasing activity of the NFAP2-FLC combination proved to be significantly more effective than that of FLC alone (P = 0.0001 and P = 0.0084 compared to 35-mg/kg single and 5-mg/kg daily doses, respectively). All significance values are indicated in Table S2 in the supplemental material.
FIG 3.
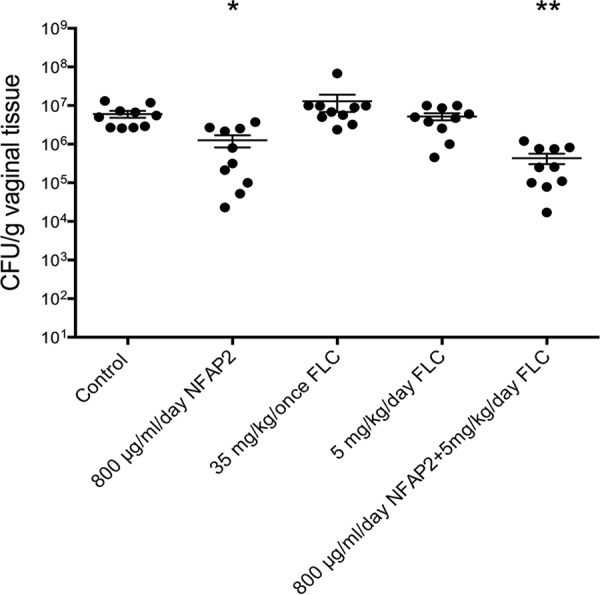
In vivo efficacies of NFAP2, fluconazole (FLC), and their combination in the murine vulvovaginitis model. The bars represent the means ± SEM (standard errors of the means) of the vaginal tissue burdens of BALB/c mice intravaginally infected with the FLC-resistant C. albicans 27700 isolate. Significant differences (P values) between numbers of CFU were determined based on comparison with the untreated controls. Other significance values existing between the different treatments are presented in Table S2 in the supplemental material. Levels of significant differences are indicated (*, P ≤ 0.05; **, P ≤ 0.005).
Histology.
Grocott-Gömöri methenamine-silver nitrate (GMS) staining revealed the presence of yeast and pseudohyphal forms of Candida cells in the vaginal tissues of infected mice (Fig. 4A to D). However, a decrease in the number of fungal cells was observable when the animals were treated with NFAP2 or the NFAP2-FLC combination (Fig. 4C and D) in comparison with the untreated and FLC-treated groups (Fig. 4A and B). An inflammatory reaction indicated by neutrophilic granulocytes was observable in all samples stained with hematoxylin and eosin (H&E) (Fig. 4), but it was more moderate in NFAP2- and NFAP2-FLC-treated animals (Fig. 4C and D) than in the untreated and FLC-treated groups (Fig. 4A and B). The vaginal inflammation detected in uninfected mice could have been the consequence of the prior estradiol-valerate treatment (Fig. 4E) (21).
FIG 4.
(A to D) Histological investigation of vaginal tissue from mice suffering from vulvovaginal candidiasis without treatment (A) and with topical treatment with 5 mg/kg/day FLC (B), 800 μg/ml NFAP2 (C), and the combination of 5 mg/kg/day FLC and 800 μg/ml NFAP2 (D). (E) Vaginal tissue of uninfected mice. Vaginal tissues were stained with GMS (left) and H&E (right). Blue arrows indicate the presence of C. albicans 27700 cells (left images) and neutrophilic granulocytes (right images). Bars, 50 μm.
DISCUSSION
crAFPs (such as the NFAP2-related Aspergillus giganteus antifungal protein [AFP] and Penicillium chrysogenum antifungal protein [PAF]) are of particular interest in the fight against fungal infections, as they show in vitro growth-inhibitory activity against fungal pathogens, and they are nontoxic to mammalian cells (22, 23). However, their in silico-predicted strong ability to bind to HSA (ΔG = −13.52 kcal/mol and Kd = 1.22e−10 M for AFP; ΔG = −11.09 kcal/mol and Kd = 7.33e−09 M for PAF) diminishes the expectations for systemic application (20). In this study, we provide for the first time information about the in vivo antifungal efficacy of a crAFP as a topical agent for the treatment of mucosal infection caused by C. albicans, an opportunistic human-pathogenic yeast.
NFAP2 represents a novel, phylogenetically distinct group of crAFPs and shows a unique high antiyeast activity in vitro (10, 12). The in vivo animal model experiments in our study required the determination of the in vitro MIC of NFAP2 against the microorganism applied for the infection and the investigation of the cell-killing ability under clinically approved test conditions. Previous studies demonstrated that the in vitro antifungal efficacy of crAFPs highly depends on the ion strength of the test medium (24, 25). According to this, NFAP2 shows higher MICs for the same Candida strain in the highly cationic RPMI 1640 medium than in a low-cationic medium (12). This feature is not exclusive to NFAP2; relatively high MICs were observed for PAF (26) and NFAP (27) when their activity was tested against different human-pathogenic filamentous fungi in RPMI 1640. RPMI 1640 is a standard medium recommended by the CLSI for clinical susceptibility tests, and it simulates the composition of the human extracellular environment. Our results showed that both planktonic and sessile biofilm cells of the tested FLC-resistant C. albicans isolate from a human VVC case are susceptible to NFAP2 in this medium. Biofilm formation of C. albicans isolates from hospitalized patients is directly related to virulence. C. albicans is more tolerant to antifungal drugs in this form than as planktonic cells, contributing to the pathogenesis of superficial and systematic candidiasis (28). Parallel to this observation, the sessile biofilm cells of the involved C. albicans isolate were less susceptible to FLC and NFAP2 than the planktonic cells. The applied CLSI M27-A3 method recommends 103 cells/ml as the inoculum for MIC determination. However, the detected MIC based on this method does not guarantee the same inhibitory efficacy against higher cell numbers (29). After 24 h of incubation, around one-third of the yeast cells were killed when the MIC of NFAP2 was applied against 107 cells/ml (see Fig. S1 in the supplemental material). This amount represents the number of yeast cells that was used for vaginal infection in the in vivo animal model experiments.
The potential in vivo application of a drug candidate for the treatment of mycotic infections highly depends on its fungal selectivity, namely, the antifungal mechanism exerted on the pathogenic fungi and the cytotoxic effects on the host cells. Antifungal plant defensins with features similar to those of crAFPs (such as a disulfide bond-stabilized tertiary structure, positive net charge, and amphipathic surface) are nontoxic to human cells, and they bind to specific fungal membrane components of yeast cells, causing membrane permeabilization and/or disruption (30). These actions may require the conformational change of the antifungal plant defensin (31). Our results show that the yeast cell-killing activity of NFAP2 is realized by pore formation in the fungal plasma membrane without any changes in the secondary structure (Fig. 1 and 2). These observations together with the lack of in vitro toxicity (even at twice the MIC [Fig. S2]) on primary HKCs and HDFs suggest the fungal selectivity of NFAP2 for yeast cells. Furthermore, based on the reported antifungal mode of action of membrane-destructive plant defensins (30), we hypothesize that the presence of a fungus-specific plasma membrane target may be involved in the antifungal mechanism of NFAP2. Revealing the nature of this target awaits further investigations.
Membrane-disrupting antifungal peptides are considered a potential new class of antifungals to treat FLC‐resistant VVC; however, their in vivo antifungal potency in this infection and their impact on the host body have not yet been tested (32, 33). Our above-discussed in vitro results proposed the in vivo therapeutic potency of NFAP2 as a topical agent for the treatment of VVC caused by FLC-resistant C. albicans. Considering the fact that biofilm formation is involved in C. albicans colonization of mucosal surfaces (14), one dose of NFAP2 in the in vivo murine VVC model corresponded to the determined in vitro MIC. However, total recovery from infection was not reached at this dosage (Fig. 4C). Instead, the daily application of NFAP2 significantly decreased the number of cells of the FLC-resistant C. albicans strain in the vagina, in contrast to FLC (Fig. 3). This result proves the potential effectiveness of NFAP2 monotherapy in the treatment of superficial yeast infections. Until now, the in vivo applicability of crAFPs as antifungal agents was investigated only with PAF (34, 35). Since PAF effectively inhibits the growth of human-pathogenic filamentous fungi (23), its therapeutic potential was tested by Palicz et al. in a murine pulmonary aspergillosis model (35). Intraperitoneal application of PAF twice a day was ultimately not able to overcome fungal invasion; however, it could prevent the spread of Aspergillus fumigatus in the lung tissue in the first days and prolonged the survival of the animals by 1 day (35).
Before the present study, the previously described in vitro synergistic interaction between NFAP2 and FLC against Candida isolates suggested the polytherapeutic potential of the protein (12). Our results from in vivo murine VVC model experiments clearly corroborate that the combined application of NFAP2 and FLC is more effective against the involved FLC-resistant C. albicans isolate than treatment with the two compounds alone (Fig. 3). This result suggests a positive in vivo interaction between them in the vaginal tissue and the reversion of FLC resistance. Similarly to our findings, a better outcome was observed in a murine pulmonary aspergillosis model when PAF was combined with amphotericin B (AMB); namely, the PAF-AMB combination prolonged the survival of the animals and decreased the lung injury score compared to monotherapeutic application (35).
Intranasal application of PAF in mice did not alter the important physiological parameters of the animals and did not cause morphological changes in the affected organs. Furthermore, an inflammatory response of the skin following PAF application was not observed (34). Based on these and other in vivo toxicity results, PAF is considered a safely applicable antifungal compound (34, 35). Our histological examinations indicated that NFAP2 could also be safely used for topical therapy since it did not cause morphological alterations and serious pathological reactions of the vaginal and vulvar tissues (Fig. 4), and it did not change the macromorphology of the affected organs (data not shown). The presence of neutrophilic granulocytes after NFAP2 application indicates that they are recruited to the site of the infection to kill the fungal pathogen (Fig. 4C and D), and NFAP2 does not inhibit this process. However, fungal infection was still present in the vagina after treatment with NFAP2 or the NFAP2-FLC combination (Fig. 4C and D); a significant decrease in the number of viable C. albicans cells was observed in comparison with the untreated group of animals (Fig. 3). As NFAP2 did not show any cytotoxic effects even at twice the MIC (Fig. S2), the protein should be administered in higher doses than the in vitro MIC dose applied in our experiments to reach full recovery from infection.
Considering our in vivo results presented in this study and the fact that recombinant NFAP2 can be produced in large amounts by the generally regarded as safe (GRAS) microorganism P. chrysogenum (12), this protein provides a feasible base to develop a novel topical agent for the treatment of superficial candidiasis caused by drug-resistant Candida strains.
MATERIALS AND METHODS
Strains and media.
The previously well-characterized FLC-resistant and biofilm-forming C. albicans 27700 strain, isolated from a case of human vulvovaginal candidiasis, was used in the experiments (36). It was maintained on yeast extract-glucose agar slants with KH2PO4 (YEGK) at 4°C. Primary HKC and HDF cells were isolated and grown in CellnTec basal medium (CnT-BM.1; CellnTec, Bern, Switzerland) and R10 medium, respectively, as described previously (37). CFU were determined on yeast extract-peptone-dextrose (YPD) and Sabouraud dextrose (SD) agar plates. In vitro antifungal susceptibility tests were performed in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 0.03% (wt/vol) l-glutamine and buffered to pH 7.0 with 0.165 M 4-morpholinopropanesulfonic acid (Sigma-Aldrich, St. Louis, MO, USA). Medium compositions are listed in Table S1 in the supplemental material.
Protein production and purification.
Recombinant NFAP2 was produced by Penicillium chrysogenum and purified by cation-exchange chromatography as described previously (12). To exclude the effects of any contaminating compounds during the experiments, NFAP2 was further purified by semipreparative reversed-phase high-performance liquid chromatography (RP-HPLC) on a Shimadzu-Knauer (Kyoto, Japan) apparatus to reach 100% purity (see Fig. S3 in the supplemental material). The following solvent system was applied: 0.1% (vol/vol) trifluoroacetic acid (TFA) (solvent A) and 80% (vol/vol) acetonitrile plus 0.1% (vol/vol) TFA (solvent B). A linear gradient from 0% to 30% (vol/vol) solvent B over 60 min was used at a flow rate of 4 ml/min. Peaks were detected at 220 nm. The purity of NFAP2 was checked by analytical RP-HPLC on an Agilent 1200 series HPLC instrument (Agilent Technologies, Santa Clara, CA, USA) using the same solvent system as the one for purification from 15% to 30% (vol/vol) solvent B over 15 min at a 1-ml/min flow rate.
In vitro susceptibility testing.
Testing of susceptibility of C. albicans 27700 planktonic cells to FLC and NFAP2 was performed using the broth microdilution method in accordance with the CLSI-approved standard M27-A3 protocol (38). The final drug concentrations ranged from 25 to 1,600 μg/ml and from 2 to 1,024 μg/ml for NFAP2 and FLC (Sigma-Aldrich, St. Louis, MO, USA), respectively. Susceptibility of sessile biofilm C. albicans 27700 cells to FLC and NFAP2 was determined by a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay according to the protocol described previously by Pierce et al. (39), with slight modifications. Briefly, aliquots of 100 μl of a standardized C. albicans 27700 suspension (1 × 106 CFU/ml) in RPMI 1640 were inoculated into wells of polystyrene flat-bottom 96-well microtiter plates (TPP, Trasadingen, Switzerland) and incubated statically for 24 h at 37°C to allow biofilm formation. The 1-day-old biofilms were washed three times with 200 μl saline in order to remove the nonattached fungal cells, and the final concentrations of NFAP2 (25 to 1,600 μg/ml) and FLC (8 to 512 μg/ml) were pipetted onto them. After 24 h of incubation at 37°C, metabolic activity was quantified. Briefly, wells were filled with 100 μl of a 0.5-mg/ml XTT–1 μM menadione solution (both from Sigma-Aldrich, St. Louis, MO, USA), and the plates were then covered with aluminum foil and incubated for 2 h at 37°C. After this incubation period, the absorbance (A492) of 80 μl of the supernatant was measured in flat-bottom 96-well microtiter plates. The MIC for planktonic and sessile biofilm cells was defined as the lowest protein or drug concentration at which ≥90% reductions in turbidity and metabolic activity were detected in comparison with the untreated control. The percent change in turbidity and metabolic activity was calculated on the basis of absorbance (A492) as 100% × (Awell − Abackground)/(Adrug-free well − Abackground). Abackground corresponds to the absorbance of fungus-free and drug-free wells. The susceptibility of C. albicans 27700 was tested in three independent experiments.
FACS analysis.
FACS, SEM, and ECD investigations (see below) were performed on mid-log-phase C. albicans 27700 cells grown in RPMI 1640 medium at 30°C under continuous shaking at 160 rpm. The proportion of dead cells after NFAP2 treatment was determined by applying the membrane-impermeant, red-fluorescent nuclear and chromosome stain PI (Sigma-Aldrich, St. Louis, MO, USA). The yeast cells (1 × 107 cells) were incubated in the presence of NFAP2 at the MIC (800 μg/ml) in RPMI 1640 for 24 h at 30°C with continuous shaking at 160 rpm. After incubation, cells were collected by centrifugation (17,000 × g for 2 min), washed with phosphate-buffered saline (PBS) (pH 7.4), stained with 5 μg/ml PI for 10 min at room temperature in the dark, and finally washed again with PBS (pH 7.4), before resuspending them in PBS (pH 7.4). The number of PI-positive cells was counted and analyzed using a FlowSight imaging flow cytometer (Amins, Merck Millipore, Billerica, MA, USA) and the related Image Data Exploration and Analysis software (IDEAS; Amins, Millipore, Billerica, MA, USA). Twenty thousand cells were screened, and the FACS analysis was repeated in three independent experiments. Cells treated with 70% (vol/vol) ethanol for 10 min at 4°C were used as positive staining controls. Untreated cells (RPMI 1640 without NFAP2) were used as natural death controls. FACS analyses were achieved in three independent experiments.
SEM.
C. albicans 27700 cells (1 × 107 cells) were treated with NFAP2 at the MIC (800 μg/ml) as described above for the FACS analysis. Untreated cells served as positive phenotype controls. Eight microliters of the cell suspensions in PBS was spotted onto a silicon disc coated with 0.01% poly-l-lysine (Merck Millipore, Billerica, MA, USA), and the cells were then fixed by gently adding 2.5% (vol/vol) glutaraldehyde and 0.05 M cacodylate buffer (pH 7.2) in PBS (pH 7.4) for 1 h. After that, the discs were washed twice with PBS (pH 7.4) and dehydrated with a graded ethanol series (30%, 50%, 70%, 80%, and 100% [vol/vol] ethanol, each for 15 min at room temperature). The samples were dried with a Quorum K850 critical-point dryer (Quorum Technologies, Laughton, East Sussex, UK), followed by 12-nm gold coating, and observed under a JEOL JSM-7100F/LV scanning electron microscope (JEOL Ltd., Tokyo, Japan).
ECD spectroscopy.
C. albicans 27700 cells were washed two times and resuspended in double-distilled water (ddH2O) or in an aqueous solution of NFAP2 (100 μg/ml) at a final concentration of 107 cells/ml. ECD spectroscopic measurements of these samples and an aqueous solution of NFAP2 (100 μg/ml) were performed in the 185- to 260-nm wavelength range using a JASCO-J815 spectropolarimeter (JASCO, Tokyo, Japan). Spectra were collected at 25°C with a scan speed of 100 nm/s using a 0.1-cm-path-length quartz cuvette. Spectra presented are accumulations of 10 scans for each sample. Spectrum acquisitions were done after 0 and 24 h of incubation of the samples at 30°C under continuous shaking at 160 rpm. After the spectroscopic measurements, CFU of the NFAP2-treated and untreated samples were determined. This experiment was repeated twice.
Determination of CFU.
Following ECD measurements, cells were collected by centrifugation (17,000 × g for 2 min) and washed two times with YPD medium, and 10-fold serial dilutions were then prepared in five steps in 1 ml YPD. One-hundred-microliter cell suspensions from the last three steps were spread onto YPD agar plates in three replicates. The number of colonies was counted after incubation for 24 h at 30°C.
In vitro cytotoxicity assay.
Fluorescence viability staining was performed on primary HKC and HDF cells grown in chambered cell culture slides (Falcon; Corning Life Sciences, Tewksbury, MA, USA). The cells (4 × 103 cells/well) were seeded and grown until they reached 70% to 80% confluence at 37°C with 5% CO2, NFAP2 at a concentration range of between 400 and 1,600 μg/ml was then added, and the plates were incubated for 24 h under the same conditions. After the incubation period, the cells were washed with PBS (pH 7.4), and the fluorescent dye PI (1 μg/ml) and 2′-(4-hydroxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-1H-benzimidazole trihydrochloride hydrate (Hoechst, 1 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) were added for 10 min in the dark. Untreated cells were used as living controls, and 50% ethanol-treated (for 10 min) cells were used as dead controls. The cells were washed three times with PBS (pH 7.4) and observed with a Zeiss Axioplan fluorescence microscope (Zeiss, Oberkochen, Germany) equipped with an AxioCam monomicroscope digital camera (Zeiss, Oberkochen, Germany) and excitation/emission filters at 365/420 nm for blue fluorescence and at 546/590 or 565/620 nm for red fluorescence. Image acquisition and editing were done with ZEN 2 (blue edition) microscope software (Zeiss, Oberkochen, Germany) and GIMP 2 (GNU Image Manipulation Program, version 2.8.10). The study with primary HKCs and HDFs was carried out in accordance with the recommendations of the Ethics Committee of the Medical University of Innsbruck (Innsbruck, Austria). The protocol was approved by the Ethics Committee of the Medical University of Innsbruck. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The in vitro cytotoxicity assay was repeated twice.
In vivo murine vulvovaginitis model.
Groups of 10 BALB/c immunocompetent female mice (weight, 20 to 22 g) were used in this study. The animals were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals (40); experiments were approved by the Animal Care Committee of the University of Debrecen (permission no. 12/2014). Mice were subcutaneously administered 50 μl estradiol-valerate (10 mg/ml prepared in sesame seed oil) 72 h prior to infection to establish VVC (41, 42). In accordance with our previous studies, mice were challenged intravaginally with 1 × 107 to 1.2 × 107 CFU of C. albicans 27700 in a final volume of 25 μl (36, 42). Mice were divided into the following five groups: (i) untreated controls; (ii) 800 μg/ml/day NFAP2; (iii) 35 mg/kg FLC once, which corresponds to the normal human dose of 150 mg based on the 24-h area under the concentration-time curve (AUC) value (43); (iv) 5 mg/kg/day FLC; and (v) 800 μg/ml/day NFAP2 plus 5 mg/kg/day FLC. All treatments were started after 24 h of infection, when the presence of C. albicans biofilm had become evident on the murine vaginal mucosa (44). FLC treatment was given intraperitoneally at a volume of 0.5 ml, while NFAP2 was administered intravaginally at a volume of 25 μl and 1 h after the FLC treatment when it was applied in combination with FLC. Untreated control mice were given 0.5 ml and/or 25 μl physiological saline intraperitoneally and intravaginally, respectively. At 4 days postinfection, the vaginal fungal burden was determined after sacrificing of animals. Whole vaginae were excised, weighed, and homogenized in 1 ml saline. Aliquots of 100 μl of the undiluted and diluted (1:10) homogenates were plated onto SD agar plates. The plates were incubated for 48 h at 35°C, and CFU were then determined. The lower limit of detection was 50 CFU/g tissue. All animal experiments were repeated two times, and five animals were involved in each group for each treatment.
Histology.
Vaginae of different but identically treated mice were involved in histological investigations as described above. Histopathological examination and histochemical staining were performed on routine formalin-fixed, paraffin-embedded mouse vaginal tissues. Serial 4-μm-thick sections were cut from paraffin blocks, and routine GMS and H&E staining was performed (45).
In silico analysis.
The ability of NFAP2 to bind to HSA (UniProt accession no. A0A1D0CRT2 and P02768, respectively [46]) was predicted by the PPA-Pred2 (Protein-Protein Affinity Predictor) server (20).
Statistical analyses.
FACS and CFU data after ECD experiments were analyzed using Microsoft Excel 2010 software (Microsoft, Edmond, WA, USA), and the two-sample t test was used to determine the significance values. Vaginal burden was analyzed using a Kruskal-Wallis test with Dunn’s posttest for multiple comparisons using GraphPad Prism version 6.05 software (GraphPad Software, San Diego, CA, USA). Significance was defined as a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
L.G. is financed by the Postdoctoral Excellence Programme (PD 120808) and the bilateral Austrian-Hungarian Joint Research Project (ANN 122833) of the Hungarian National Research, Development, and Innovation Office (NKFI Office). This work was supported by a grant from the Austrian Science Fund (I3132-B21) to F.M. The research was also supported by the European Union and cofinanced by the European Regional Development Fund under project GINOP-2.3.2-15-2016-00014 (to G.K.T.). Research of A.B. and L.G. has been supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The present work of L.G. was supported by the UNKP-18-4 New National Excellence Program of the Ministry of Human Capacities.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01777-18.
REFERENCES
- 1.Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. 2016. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 42:905–927. doi: 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- 2.Sherry L, Kean R, McKloud E, O’Donnell LE, Metcalfe R, Jones BL, Ramage G. 2017. Biofilms formed by isolates from recurrent vulvovaginal candidiasis patients are heterogeneous and insensitive to fluconazole. Antimicrob Agents Chemother 61:e01065-17. doi: 10.1128/AAC.01065-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobel JD. 2007. Vulvovaginal candidosis. Lancet 369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 4.Muzny CA, Schwebke JR. 2015. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis 61:601–606. doi: 10.1093/cid/civ353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobel JD. 2013. Factors involved in patient choice of oral or vaginal treatment for vulvovaginal candidiasis. Patient Prefer Adherence 8:31–34. doi: 10.2147/PPA.S38984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand SR, Degenhardt TP, Person K, Sobel JD, Nyirjesy P, Schotzinger RJ, Tavakkol A. 2018. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 218:624.e1–624.e9. doi: 10.1016/j.ajog.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. 2012. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 120:1407–1414. doi: 10.1097/AOG.0b013e31827307b2. [DOI] [PubMed] [Google Scholar]
- 8.Gun Lee D, Shin SY, Maeng CY, Jin ZZ, Kim KL, Hahm KS. 1999. Isolation and characterization of a novel antifungal peptide from Aspergillus niger. Biochem Biophys Res Commun 263:646–651. doi: 10.1006/bbrc.1999.1428. [DOI] [PubMed] [Google Scholar]
- 9.Galgóczy L, Virágh M, Kovács L, Tóth B, Papp T, Vágvölgyi C. 2013. Antifungal peptides homologous to the Penicillium chrysogenum antifungal protein (PAF) are widespread among Fusaria. Peptides 39:131–137. doi: 10.1016/j.peptides.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Tóth L, Kele Z, Borics A, Nagy LG, Váradi G, Virágh M, Takó M, Vágvölgyi C, Galgóczy L. 2016. NFAP2, a novel cysteine-rich anti-yeast protein from Neosartorya fischeri NRRL 181: isolation and characterization. AMB Express 6:75. doi: 10.1186/s13568-016-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber A, Hajdu D, Bratschun-Khan D, Gáspári Z, Varbanov M, Philippot S, Fizil Á, Czajlik A, Kele Z, Sonderegger C, Galgóczy L, Bodor A, Marx F, Batta G. 2018. New antimicrobial potential and structural properties of PAFB: a cationic, cysteine-rich protein from Penicillium chrysogenum Q176. Sci Rep 8:1751. doi: 10.1038/s41598-018-20002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tóth L, Váradi G, Borics A, Batta G, Kele Z, Vendrinszky Á, Tóth R, Ficze H, Tóth GK, Vágvölgyi C, Marx F, Galgóczy L. 2018. Anti-candidal activity and functional mapping of recombinant and synthetic Neosartorya fischeri antifungal protein 2 (NFAP2). Front Microbiol 9:393. doi: 10.3389/fmicb.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonderegger C, Váradi G, Galgóczy L, Kocsubé S, Posch W, Borics A, Dubrac S, Tóth GK, Wilflingseder D, Marx F. 2018. The evolutionary conserved γ-core motif influences the anti-Candida activity of the Penicillium chrysogenum antifungal protein PAF. Front Microbiol 9:1655. doi: 10.3389/fmicb.2018.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati M, Nobile CJ. 2016. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect 18:310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D, CLSI Subcommittee for Antifungal Susceptibility Testing. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Cosentino K, Ros U, García-Sáez AJ. 2016. Assembling the puzzle: oligomerization of α-pore forming proteins in membranes. Biochim Biophys Acta 1858:457–466. doi: 10.1016/j.bbamem.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sani MA, Separovic F. 2016. How membrane-active peptides get into lipid membranes. Acc Chem Res 49:1130–1138. doi: 10.1021/acs.accounts.6b00074. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P, Kizhakkedathu JN, Straus SK. 2018. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 8:E4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avitabile C, D’Andrea LD, Romanelli A. 2014. Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci Rep 4:4293. doi: 10.1038/srep04293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yugandhar K, Gromiha MM. 2014. Protein-protein binding affinity prediction from amino acid sequence. Bioinformatics 30:3583–3589. doi: 10.1093/bioinformatics/btu580. [DOI] [PubMed] [Google Scholar]
- 21.Finking G, Brehme U, Bruck B, Wehrmann M, Hanke S, Kamenz J, Kern S, Lenz C, Hanke H. 1998. Does anti-atherogenic estradiol valerate treatment cause adverse effects on liver and uterus in NZW rabbits? Vet Hum Toxicol 40:136–140. [PubMed] [Google Scholar]
- 22.Meyer V. 2008. A small protein that fights fungi: AFP as a new promising antifungal agent of biotechnological value. Appl Microbiol Biotechnol 78:17–28. doi: 10.1007/s00253-007-1291-3. [DOI] [PubMed] [Google Scholar]
- 23.Hegedus N, Leiter E, Kovács B, Tomori V, Kwon NJ, Emri T, Marx F, Batta G, Csernoch L, Haas H, Yu JH, Pócsi I. 2011. The small molecular mass antifungal protein of Penicillium chrysogenum—a mechanism of action oriented review. J Basic Microbiol 51:561–571. doi: 10.1002/jobm.201100041. [DOI] [PubMed] [Google Scholar]
- 24.Sonderegger C, Fizil Á, Burtscher L, Hajdu D, Muñoz A, Gáspári Z, Read ND, Batta G, Marx F. 2017. D19S mutation of the cationic, cysteine-rich protein PAF: novel insights into its structural dynamics, thermal unfolding and antifungal function. PLoS One 12:e0169920. doi: 10.1371/journal.pone.0169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galgóczy L, Borics A, Virágh M, Ficze H, Váradi G, Kele Z, Marx F. 2017. Structural determinants of Neosartorya fischeri antifungal protein (NFAP) for folding, stability and antifungal activity. Sci Rep 7:1963. doi: 10.1038/s41598-017-02234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galgóczy L, Papp T, Pócsi I, Hegedus N, Vágvölgyi C. 2008. In vitro activity of Penicillium chrysogenum antifungal protein (PAF) and its combination with fluconazole against different dermatophytes. Antonie Van Leeuwenhoek 94:463–470. doi: 10.1007/s10482-008-9263-x. [DOI] [PubMed] [Google Scholar]
- 27.Virágh M, Vörös D, Kele Z, Kovács L, Fizil Á, Lakatos G, Maróti G, Batta G, Vágvölgyi C, Galgóczy L. 2014. Production of a defensin-like antifungal protein NFAP from Neosartorya fischeri in Pichia pastoris and its antifungal activity against filamentous fungal isolates from human infections. Protein Expr Purif 94:79–84. doi: 10.1016/j.pep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira AV, Prado CG, Carvalho RR, Dias KS, Dias AL. 2013. Candida albicans and non-C. albicans Candida species: comparison of biofilm production and metabolic activity in biofilms, and putative virulence properties of isolates from hospital environments and infections. Mycopathologia 175:265–272. doi: 10.1007/s11046-013-9638-z. [DOI] [PubMed] [Google Scholar]
- 29.Cuenca-Estrella M, Díaz-Guerra TM, Mellado E, Rodríguez-Tudela JL. 2001. Influence of glucose supplementation and inoculum size on growth kinetics and antifungal susceptibility testing of Candida spp. J Clin Microbiol 39:525–532. doi: 10.1128/JCM.39.2.525-532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cools TL, Struyfs C, Cammue BP, Thevissen K. 2017. Antifungal plant defensins: increased insight in their mode of action as a basis for their use to combat fungal infections. Future Microbiol 12:441–454. doi: 10.2217/fmb-2016-0181. [DOI] [PubMed] [Google Scholar]
- 31.Valente AP, de Paula VS, Almeida FC. 2013. Revealing the properties of plant defensins through dynamics. Molecules 18:11311–11326. doi: 10.3390/molecules180911311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng SM, Yap YY, Cheong JW, Ng FM, Lau QY, Barkham T, Teo JW, Hill J, Chia CS. 2017. Antifungal peptides: a potential new class of antifungals for treating vulvovaginal candidiasis caused by fluconazole-resistant Candida albicans. J Pept Sci 23:215–221. doi: 10.1002/psc.2970. [DOI] [PubMed] [Google Scholar]
- 33.Ng SMS, Yap JM, Lau QY, Ng FM, Ong EHQ, Barkham T, Teo JWP, Alfatah M, Kong KW, Hoon S, Arumugam P, Hill J, Chia CSB. 2018. Structure-activity relationship studies of ultra-short peptides with potent activities against fluconazole-resistant Candida albicans. Eur J Med Chem 150:479–490. doi: 10.1016/j.ejmech.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Palicz Z, Jenes A, Gáll T, Miszti-Blasius K, Kollár S, Kovács I, Emri M, Márián T, Leiter E, Pócsi I, Csősz E, Kalló G, Hegedűs C, Virág L, Csernoch L, Szentesi P. 2013. In vivo application of a small molecular weight antifungal protein of Penicillium chrysogenum (PAF). Toxicol Appl Pharmacol 269:8–16. doi: 10.1016/j.taap.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Palicz Z, Gáll T, Leiter É, Kollár S, Kovács I, Miszti-Blasius K, Pócsi I, Csernoch L, Szentesi P. 2016. Application of a low molecular weight antifungal protein from Penicillium chrysogenum (PAF) to treat pulmonary aspergillosis in mice. Emerg Microbes Infect 5:e114. doi: 10.1038/emi.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bozó A, Domán M, Majoros L, Kardos G, Varga I, Kovács R. 2016. The in vitro and in vivo efficacy of fluconazole in combination with farnesol against Candida albicans isolates using a murine vulvovaginitis model. J Microbiol 54:753–760. doi: 10.1007/s12275-016-6298-y. [DOI] [PubMed] [Google Scholar]
- 37.Blunder S, Rühl R, Moosbrugger-Martinz V, Krimmel C, Geisler A, Zhu H, Crumrine D, Elias PM, Gruber R, Schmuth M, Dubrac S. 2017. Alterations in epidermal eicosanoid metabolism contribute to inflammation and impaired late differentiation in FLG-mutated atopic dermatitis. J Invest Dermatol 137:706–715. doi: 10.1016/j.jid.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 41.Fidel PL Jr, Cutright J, Steele C. 2000. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun 68:651–657. doi: 10.1128/IAI.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovács R, Czudar A, Horváth L, Szakács L, Majoros L, Kónya J. 2014. Serum interleukin-6 levels in murine models of Candida albicans infection. Acta Microbiol Immunol Hung 61:61–69. doi: 10.1556/AMicr.61.2014.1.6. [DOI] [PubMed] [Google Scholar]
- 43.Louie A, Banerjee P, Drusano GL, Shayegani M, Miller MH. 1999. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob Agents Chemother 43:2841–2847. doi: 10.1128/AAC.43.12.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Noverr MC. 2010. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156:3635–3644. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris GB, Ridgway EJ, Suvarna SK. 2018. Traditional stains and modern techniques for demonstration microorganisms in histology, p 254–279. In Suvarna K, Layton C, Bancroft J (ed), Bancroft’s theory and practice of histological techniques, 8th ed Elsevier, New York, NY. [Google Scholar]
- 46.UniProt Consortium. 2017. UniProt: the universal protein knowledgebase. Nucleic Acids Res 45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




