Despite the availability of new antifungal compounds, invasive aspergillosis carries high morbidity and mortality in hematopoietic stem cell transplant recipients. In vitro studies and animal models suggest that the adoptive transfer of natural killer (NK) cells might be a promising immunotherapeutic option in this setting.
KEYWORDS: Aspergillus fumigatus, antifungal agent, human natural killer cell
ABSTRACT
Despite the availability of new antifungal compounds, invasive aspergillosis carries high morbidity and mortality in hematopoietic stem cell transplant recipients. In vitro studies and animal models suggest that the adoptive transfer of natural killer (NK) cells might be a promising immunotherapeutic option in this setting. As it is unclear whether the viability and function of human NK cells are affected by common antifungal agents, we analyzed the interaction of various concentrations of amphotericin B deoxycholate (AmB-D), liposomal amphotericin B, caspofungin, fluconazole, voriconazole, and posaconazole with human NK cells. When adding NK cells to therapeutic concentrations of antifungal agents, a significant increase in the antifungal effect was seen for caspofungin and voriconazole, whereas NK cells significantly decreased the hyphal damage of escalated doses of AmB-D. In contrast, therapeutic concentrations of all antifungal compounds tested did not have a negative effect on proliferation, viability, and the release of soluble immunomodulatory molecules of NK cells. These data indicate that therapeutic concentrations of the antifungal agents tested do not negatively affect the functional properties of human NK cells, which is a prerequisite for further studies evaluating NK cells as antifungal immunotherapy in immunocompromised patients suffering from invasive aspergillosis.
INTRODUCTION
Invasive fungal disease, in particular, invasive aspergillosis, carries high morbidity and mortality in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) (1). Unfortunately, the availability of new antifungal compounds and the development of immunotherapeutic strategies such as granulocyte transfusions and the administration of cytokines or interferons did not result in a significant improvement of outcome in this setting (2, 3). Recently, the effect of natural killer (NK) cells against a variety of fungal pathogen has been described in vitro (4–7), and animal models support the benefit of adoptively transferring NK cells to immunocompromised hosts suffering from invasive fungal infection (8). However, comparable to that with other cellular therapies, a sufficient number of vital and functionally active effector cells are a prerequisite for clinical efficacy (9). Whereas it is well described that some commonly used antifungal compounds alter the functional properties of phagocytes or cellular immunity (10–14), there is a lack of data on the effect on human NK cells. We therefore investigated the influence of various antifungal compounds on the proliferative capacity, on apoptosis and necrosis, on the release of interferon and cytokines, and on the direct antifungal activity of human NK cells, which is needed before evaluating the use of NK cells as antifungal immunotherapy in the clinical setting.
RESULTS
Interplay between antifungal agents and human NK cells in the damage of Aspergillus fumigatus hyphae.
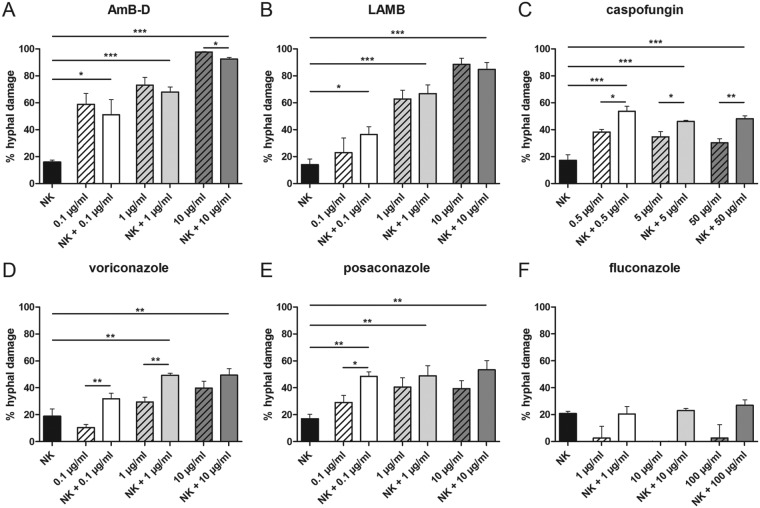
NK cells alone as well as therapeutic dosages of all mold-active antifungal compounds tested produced significant damage of A. fumigatus hyphae (Fig. 1). When adding NK cells to therapeutic concentrations of antifungal agents tested, a significant increase of the antifungal effect was seen for caspofungin (mean ± standard error of the mean [SEM]: 46.1% ± 0.9% versus 34.7% ± 3.9%; P = 0.029) and voriconazole ([mean ± SEM] 49.3% ± 1.5% versus 29.4% ± 3.6%; P = 0.007) (Fig. 1). Adding NK cells to subtherapeutic doses of caspofungin, voriconazole, and posaconazole significantly increased the antifungal damage compared to the antifungal compound alone ([mean ± SEM] respectively, caspofungin, 53.6% ± 3.9% versus 38% ± 2%, P = 0.012; voriconazole, 31.9% ± 4% versus 10.5% ± 2.3%, P = 0.001; and posaconazole, 48.6% ± 3.4% versus 29.1% ± 5.3%, P = 0.036). Adding NK cells to escalated doses of an antifungal compound only significantly increased the hyphal damage of caspofungin (48.1% ± 2.1% versus 30.3% ± 3.1%, P = 0.003). In contrast to all other antifungal compounds tested, NK cells decreased the hyphal damage of amphotericin B deoxycholate (AmB-D), which was seen for all dosages assessed, but was significant for the escalated dose only (97.8% ± 0.4% versus 92.5% ± 1.2%, respectively; P = 0.014). The mold-inactive antifungal compound fluconazole did not affect the direct antifungal effect of NK cells at any dosage tested (Fig. 1F).
FIG 1.
Damage of A. fumigatus hyphae induced by antifungal agents and human NK cells alone or in combination. Fungal damage was assessed by means of the XTT assay. Shown are the means ± standard errors of the means (SEMs) from at least 3 independent experiments. AmB-D, amphotericin B deoxycholate; LAMB, liposomal amphotericin B; NK, natural killer cell; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effect of antifungal compounds on the proliferative capacity, survival, and number of human NK cells.
As the effector-to-target ratio has an important impact on the antifungal activity of NK cells (4), we analyzed the apoptosis and necrosis of NK cells as the two major modes of cell death as well as the proliferative capacity in the presence of various antifungal compounds.
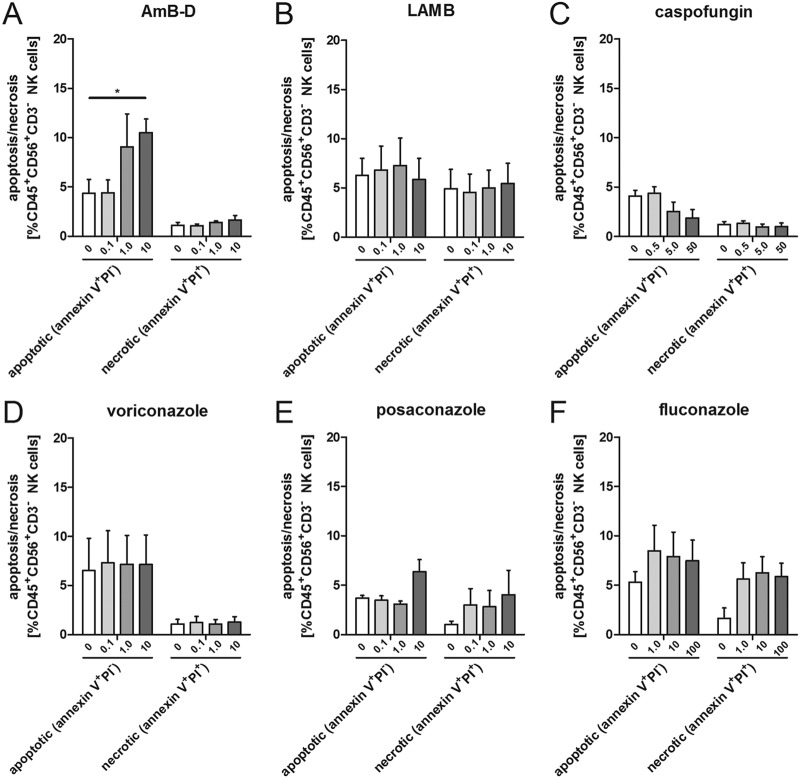
Adding escalated dosages of AmB-D significantly increased the percentage of apoptotic NK cells at 24 h (10.5% ± 1.4% versus 4.4% ± 1.4% in the control, P = 0.035), whereas this was not seen for therapeutic and subtherapeutic dosages of AmB-D and the other antifungal compounds at any concentration tested (Fig. 2). Both posaconazole and fluconazole increased the percentage of necrotic NK cells at 24 h compared to the respective controls, although none of the alterations reached statistical significance. However, when combining the percentage of apoptotic and necrotic cells, posaconazole at escalated doses resulted in a significantly higher percentage of dead cells than in the controls ([mean ± SEM] 4.4% ± 0.6% versus 9.8% ± 2.1%, respectively, P = 0.04).
FIG 2.
Effect of antifungal agents on the viability of human NK cells. Apoptosis and necrosis were assessed by means of annexin V and propidium iodide (PI) staining. Shown are the means and SEMs from at least 3 different experiments. AmB-D, amphotericin B deoxycholate; LAMB, liposomal amphotericin B; *, P < 0.05.
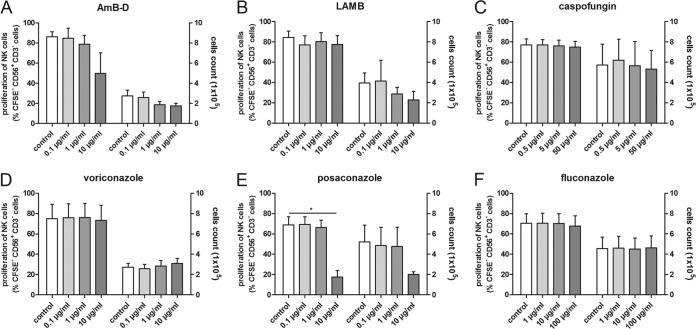
The proliferation of human NK cells, which was assessed at day 7, was significantly inhibited by posaconazole only at the highest dosage compared to that in the control (NK cells incubated alone) ([mean ± SEM] 68.9% ± 7.9% versus 17.5% ± 6.5%, respectively, P = 0.0024) (Fig. 3E).
FIG 3.
Effect of antifungal compounds on the proliferation of human NK cells and total cell number on day 7. The proliferation of human NK cells was assessed by means of CFSE assay and is shown on the left y axes. The absolute numbers of NK cells at day 7 are depicted on the right y axes. Shown are the means and SEMs from at least 3 different experiments. *, P < 0.05.
Effect of antifungal agents on the release of cytokines and gamma interferon by human NK cells in the absence or presence of A. fumigatus.
As human NK cells play an important role in the modulation of the immune system by secreting cytokines and interferons, we assessed whether antifungal compounds have an impact on the concentrations of these soluble factors measured in the supernatant. When NK cells were incubated with both A. fumigatus and an antifungal compound, no significant effects on the concentrations of gamma interferon (IFN-γ) (see Fig. S1 in the supplemental material), granulocyte-macrophage colony-stimulating factor (GM-CSF) (see Fig. S2), RANTES (see Fig. S3), or macrophage inflammatory protein 1 alpha (MIP-1α) (data not shown) were observed.
DISCUSSION
Despite the availability of new antifungal drugs, invasive aspergillosis is still a major cause of morbidity and mortality in allogeneic HSCT recipients (1–3). Previous studies have demonstrated that NK cells kill A. fumigatus in vitro and that the transfer of activated NK cells helps to clear pulmonary aspergillosis in immunocompromised mice (4, 5, 8). However, before adoptive antifungal immunotherapy using human NK cells can be evaluated in clinical studies, a number of open questions have to be addressed. In this regard, the potential interaction of antifungal compounds, which are administered as prophylaxis or therapy, and the functional activity of NK cells are of special interest.
As the antifungal effect of NK cells in vitro depends on the effector-to-target ratio and increases with higher ratios (4), it is important to evaluate the impact of antifungal compounds on NK cell proliferation and viability. Posaconazole in escalated concentrations significantly increased the number of dead cells and significantly decreased the proliferation of NK cells, both of which led to a decreased absolute number of NK cells on day 7. Similarly, AmB-D in escalated doses significantly increased the number of apoptotic NK cells. Our data corroborate previous studies in other cell types, where posaconazole at a higher dosage decreased the proliferation of antigen-specific CD4+ T cells (15), and both AmB-D and liposomal amphotericin B (LAMB) at higher dosages had a negative impact on the proliferation of freshly rat lymphocytes (16). However, it is important to note that none of the antifungal compounds tested at therapeutic concentrations exhibited a negative impact on the proliferation or viability of human NK cells. To increase the effector-to-target ratio, a number of protocols have been established to generate a high number of functionally active NK cells for the use of primary human NK cells or NK cell lines as immunotherapy for cancer (17–19), and dosages of up to 2 × 108/kg body weight have been well tolerated (20, 21). On the other hand, it has to be noted that it is not possible to determine the effector-to-target (E:T) ratio needed in an individual patient suffering from invasive aspergillosis, and the risk of adverse events in patients suffering from fungal infection might be increased by the release of proinflammatory cytokines which could result in tissue damage (19).
NK cells are able to produce a number of soluble immunoregulatory factors such as GM-CSF, IFN-γ, or RANTES, by which they modulate different arms of the immune system (22–26). When adding antifungal compounds to NK cells and A. fumigatus, none of the agents significantly altered the concentration of the soluble factors in the supernatant. In contrast and consistent with our previous work, A. fumigatus itself decreases the levels of these molecules in the supernatant, as it inhibits their release from NK cells, which leads to intracellular accumulation (27). In contrast to our findings, LAMB has been shown to suppress IFN-γ production in CD8+ T cells, (12), whereas AmB-D enhanced IFN-γ production in murine NK cells in combination with interleukin 18 (IL-18) (28). However, it only can be speculated whether these effects are ultimately beneficial or harmful for the host, as an increase in the proinflammatory host response might help in the elimination of a pathogen but, at the same time, might lead to hyperinflammation causing tissue damage associated with a worse outcome (29).
In addition to the immunoregulatory functions, NK cells are able to directly kill A. fumigatus, but the exact mechanism of this damage is a matter of ongoing controversy (4, 5). When coincubating A. fumigatus hyphae with therapeutic concentrations of antifungal compounds, the addition of NK cells increased the hyphal damage, which was significant for caspofungin and voriconazole. Similarly, additive effects were seen when using subtherapeutic and/or escalated doses of caspofungin, voriconazole, and posaconazole. As in our experimental setting, all antimold-active compounds tested alone resulted in higher antifungal damage than with NK cells alone, it was not possible to investigate the effect of antifungal compounds on the direct hyphal damage of NK cells. However, the mold-inactive agent fluconazole did not affect the direct antifungal activity of NK cells.
In conclusion, our in vitro data demonstrate that NK cells exhibit an additive effect with most antifungals at therapeutic concentrations and that antifungal compounds at therapeutic dosages did not have a negative effect on proliferation, viability, and cytokine release of human NK cells. Although we cannot exclude that different strains of Aspergillus might lead to different results, our data are a prerequisite for further evaluating human NK cells as antifungal immunotherapy in immunocompromised patients suffering from invasive aspergillosis and may ultimately help to improve outcome.
MATERIALS AND METHODS
Isolation and cultivation of primary human NK cells.
Primary human NK cells were isolated from peripheral blood from different healthy volunteers by negative selection using the EasySep Human NK cell enrichment kit (StemCell Technologies, Grenoble, France) according to the manufacturer’s instructions. The viability and purity of the isolated CD56+ CD3− NK cells were ≥90% as determined by flow cytometry (Canto II; Becton Dickinson, San Jose, CA, USA). Isolated NK cells were cultivated for up to 10 days in RPMI medium (Gibco, Paisley, UK) supplemented with 5% human frozen plasma (German Red Cross Blood Donor Service, Baden-Wuerttemberg-Hessen, Frankfurt, Germany) and stimulated with recombinant human interleukin 2 (rhIL-2) (1000 U/ml; Novartis, Basel, Switzerland) every 3 days. The protocol was approved by the local ethics committee.
Preparation of A. fumigatus.
The A. fumigatus strain AF4215 (MYA 1163; American Type Culture Collection) was grown on Sabouraud glucose agar (BD Biosciences, San Jose, CA, USA) at 37°C for 2 to 3 days. Conidia were harvested by gently scraping the surface of the slants with a sterile cotton stick, which was then washed in Hanks’ balanced saline solution (Gibco). The suspension was filtered through sterile gauze, and the number of the conidia was estimated in a Neubauer slide (LO–Laboroptik, Friedrichsdorf, Germany). Resting conidia were immediately used or stored at 4°C for a maximum of 1 week. For the preparation of A. fumigatus hyphae, conidia were plated in flat-bottom cell culture plates (Nunc, Langenselbold, Germany) and incubated in yeast nitrogen base (Sigma-Aldrich, Taufkirchen, Germany) medium at 37°C for 17 h to allow formation of mycelium.
Antifungal agents.
The antifungal compounds amphotericin B deoxycholate (Bristol-Myer Squibb, Munich, Germany), LAMB (Gilead Sciences, San Dimas, CA, USA), voriconazole (Pfizer, Groton, CT, USA), and caspofungin and posaconazole (MSD/Merck, Kenilworth, NJ, USA) were commercially obtained. Fluconazole (Pfizer) was included in the experiments to evaluate the effect of a mold-inactive azole on the function of human NK cells. The three concentrations tested for each antifungal compound spanned a wide range and were chosen according to the calculated serum levels from the approved and clinically applied dosages for the respective antifungal drugs, which were designated “therapeutic” dose as well as “subtherapeutic” and “escalated” doses, for ten-times lower and a ten-times higher concentrations, respectively (15).
Assessment of the direct antifungal activity of NK cells.
The direct activity of human NK cells alone or in combination with antifungal compounds against A. fumigatus hyphae was analyzed by means of a colorimetric assay using 2,3-bis(2-methoxy-4-nitro-5-sulphenyl)-2H-tetrazolium-5-carboxyanilide sodium salt (XTT; Sigma, Steinheim, Germany) plus coenzyme Q0 (2,3-dimethoxy-5methyl-1,4-benzoquinone; Sigma) as previously described (30). In brief, NK cells and antifungal agents, alone or in combination, were added to A. fumigatus hyphae at an E:T ratio of 20:1 based on the number of Aspergillus conidia used for the formation of hyphae and the number of NK cells and were incubated for 6 h. After lysis of NK cells with sterile aqua dest. (distilled water), hyphae were incubated at 37°C for 1 h in the presence of XTT (0.25 mg/ml) and coenzyme Q0 (40 μg/ml). Then, the absorbance of the supernatant was assessed spectrophotometrically at 450 nm using a 690-nm reference. Antifungal activity was calculated according to the following formula: percent hyphal damage = (1 − X/C) × 100, where X is the absorbance of experimental wells and C is the absorbance of control wells with hyphae only.
Assessment of NK cell proliferation and cell numbers.
The effect of antifungal agents on the proliferative capacity of human NK cells was assessed by means of the carboxy-fluorescein-diacetate-succinimidyl-ester labeling assay (CFSE; Molecular Probes, Eugene, OR, USA). NK cells cultivated in the absence of antifungal drugs served as the control. The percentage of CFSE-negative CD56+ CD3− NK cells representing proliferating cells was assessed on days 0, 1, 3, and 7 by flow cytometry (Canto II; Becton Dickinson). The number of NK cells was estimated on day 7 in a Neubauer slide (LO-Laboroptik) by means of trypan blue staining.
Assessment of NK cell viability.
The effect of antifungal agents on the apoptosis and necrosis of human NK cells was assessed by means of annexin V and propidium iodide (PI) staining (BD Biosciences). After 24 h, NK cells were labeled according to the manufacturer’s instructions, and the percentages of annexin+ PI− (apoptotic) and annexin+ PI+ (necrotic) NK cells were assessed by flow cytometry (Canto I; Becton Dickinson).
Assessment of soluble molecule concentrations in the supernatant.
Supernatants were collected from NK cells incubated for 6 h with antifungal agents and A. fumigatus alone or in combination. Levels of RANTES (level of detection, 0.002 pg/ml), GM-CSF (0.2 pg/ml), IFN-γ (0.8 pg/ml), and MIP-1α (0.2 pg/ml) were assessed by means of a cytometric bead array (CBA; BD Biosciences) according to the manufacturer’s instructions.
Statistical analyses.
Data were analyzed using GraphPad Prism (version 5.04; GraphPad Software, La Jolla, CA, USA) and compared by Student’s t tests. Additive and synergistic effects were used as defined previously (31). A two-sided P value of less than 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Frauke Röger and Asuman Demir for technical assistance.
This work was supported by an unrestricted grant of Gilead Sciences.
T.L. has received research grants from Gilead Sciences, is a consultant to Astellas, Basilea, Gilead Sciences, and Merck/MSD, and served at the speaker’s bureau of Astellas, Gilead Sciences, Merck/MSD, and Pfizer.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01993-18.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Bitar D, Lortholary O, Strat YL, Nicolau J, Coignard B, Tattevin P, Che D, Dromer F. 2014. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis 20:1149–1155. doi: 10.3201/eid2007.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehrnbecher T, Kalkum M, Champer J, Tramsen L, Schmidt S, Klingebiel T. 2013. Immunotherapy in invasive fungal infection - focus on invasive aspergillosis. Curr Pharm Des 19:3689–3712. doi: 10.2174/1381612811319200010. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt S, Tramsen L, Hanisch M, Latgé J-P, Huenecke S, Koehl U, Lehrnbecher T. 2011. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis 203:430–435. doi: 10.1093/infdis/jiq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouzani M, Ok M, McCormick A, Ebel F, Kurzai O, Morton CO, Einsele H, Loeffler J. 2011. Human NK cells display important antifungal activity against aspergillus fumigatus, which is directly mediated by IFN-γ release. J Immunol 187:1369–1376. doi: 10.4049/jimmunol.1003593. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt S, Tramsen L, Perkhofer S, Lass-Flörl C, Hanisch M, Roger F, Klingebiel T, Koehl U, Lehrnbecher T. 2013. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology 218:939–944. doi: 10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt S, Schneider A, Demir A, Lass-Flörl C, Lehrnbecher T. 2016. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses 59:34–38. doi: 10.1111/myc.12431. [DOI] [PubMed] [Google Scholar]
- 8.Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. 2009. Early NK cell-derived IFN-γ is essential to host defense in neutropenic invasive aspergillosis. J Immunol 182:4306–4312. doi: 10.4049/jimmunol.0803462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. 2013. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Vuddhakul V, Mai GT, McCormack JG, Seow WK, Thong YH. 1990. Suppression of neutrophil and lymphoproliferative responses in vitro by itraconazole but not fluconazole. Int J Immunopharmacol 12:639–645. doi: 10.1016/0192-0561(90)90101-R. [DOI] [PubMed] [Google Scholar]
- 11.Reyes E, Cardona J, Prieto A, Bernstein ED, Rodríguez-Zapata M, Pontes MJ, Alvarez-Mon M. 2000. Liposomal amphotericin B and amphotericin B-deoxycholate show different immunoregulatory effects on human peripheral blood mononuclear cells. J Infect Dis 181:2003–2010. doi: 10.1086/315517. [DOI] [PubMed] [Google Scholar]
- 12.Kretschmar M, Geginat G, Bertsch T, Walter S, Hof H, Nichterlein T. 2001. Influence of liposomal amphotericin B on CD8 T-cell function. Antimicrob Agents Chemother 45:2383–2385. doi: 10.1128/AAC.45.8.2383-2385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stergiopoulou T, Meletiadis J, Sein T, Papaioannidou P, Walsh TJ, Roilides E. 2011. Synergistic interaction of the triple combination of amphotericin B, ciprofloxacin, and polymorphonuclear neutrophils against Aspergillus fumigatus. Antimicrob Agents Chemother 55:5923–5929. doi: 10.1128/AAC.00548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil-Lamaignere C, Roilides E, Mosquera J, Maloukou A, Walsh TJ. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob Agents Chemother 46:2234–2237. doi: 10.1128/AAC.46.7.2234-2237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tramsen L, Schmidt S, Koehl U, Huenecke S, Latgé JP, Roeger F, Schubert R, Klingebiel T, Lehrnbecher T. 2013. No effect of antifungal compounds on functional properties of human antifungal T-helper type 1 cells. Transpl Infect Dis 15:430–434. doi: 10.1111/tid.12089. [DOI] [PubMed] [Google Scholar]
- 16.Boggs JM, Chang NH, Goundalkar A. 1991. Liposomal amphotericin B inhibits in vitro T-lymphocyte response to antigen. Antimicrob Agents Chemother 35:879–885. doi: 10.1128/AAC.35.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luhm J, Brand J-M, Koritke P, Höppner M, Kirchner H, Frohn C. 2002. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res 11:651–657. doi: 10.1089/15258160260194794. [DOI] [PubMed] [Google Scholar]
- 18.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. 2011. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt S, Tramsen L, Rais B, Ullrich E, Lehrnbecher T. 2018. Natural killer cells as a therapeutic tool for infectious diseases - current status and future perspectives. Oncotarget 9:20891–20907. doi: 10.18632/oncotarget.25058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, Favre G, Gratwohl A. 2004. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 18:1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 21.Choi I, Yoon SR, Park S-Y, Kim H, Jung S-J, Kang Y-L, Lee J-H, Lee J-H, Kim D-Y, Lee J-L, Park H-S, Choi E-J, Lee Y-S, Kang Y-A, Jeon M, Seol M, Baek S, Yun S-C, Kim HJ, Lee K-H. 2016. Donor-derived natural killer cell infusion after human leukocyte antigen-haploidentical hematopoietic cell transplantation in patients with refractory acute leukemia. Biol Blood Marrow Transplant 22:2065–2076. doi: 10.1016/j.bbmt.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Freund M, Kleine HD. 1992. The role of GM-CSF in infection. Infection 20:S84–S92. doi: 10.1007/BF01705024. [DOI] [PubMed] [Google Scholar]
- 23.Gil-Lamaignere C, Simitsopoulou M, Roilides E, Maloukou A, Winn RM, Walsh TJ. 2005. Interferon-γ and granulocyte-macrophage colony-stimulating factor augment the activity of polymorphonuclear leukocytes against medically important zygomycetes. J Infect Dis 191:1180–1187. doi: 10.1086/428503. [DOI] [PubMed] [Google Scholar]
- 24.Boehm U, Klamp T, Groot M, Howard JC. 1997. Cellular responses to interferon. Annu Rev Immunol 15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 25.Romani L. 2004. Immunity to fungal infections. Nat Rev Immunol 4:11–24. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 26.Appay V, Rowland-Jones SL. 2001. RANTES: a versatile and controversial chemokine. Trends Immunol 22:83–87. doi: 10.1016/S1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 27.Schneider A, Blatzer M, Posch W, Schubert R, Lass-Flörl C, Schmidt S, Lehrnbecher T. 2016. Aspergillus fumigatus responds to natural killer (NK) cells with upregulation of stress related genes and inhibits the immunoregulatory function of NK cells. Oncotarget 7:71062–71071. doi: 10.18632/oncotarget.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedges JF, Mitchell AM, Jones K, Kimmel E, Ramstead AG, Snyder DT, Jutila MA. 2015. Amphotericin B stimulates γδ T and NK cells, and enhances protection from Salmonella infection. Innate Immun 21:598–608. doi: 10.1177/1753425914567692. [DOI] [PubMed] [Google Scholar]
- 29.Romani L. 2011. Immunity to fungal infections. Nat Rev Immunol 11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 30.Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latgé JP, Klingebiel T, Einsele H, Lehrnbecher T. 2006. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood 107:2562–2569. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 31.Tallarida RJ. 2011. Quantitative methods for assessing drug synergism. Genes Cancer 2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.