SCY-078, a fungicidal β-1,3-glucan synthesis inhibitor administered as intravenous or oral [14C]SCY-078 to rats, was distributed primarily into tissues associated with invasive fungal disease (kidney, lung, liver, spleen, bone marrow, muscle, vaginal tissue, and skin) to levels exceeding those in plasma. Oral fraction absorbed was ∼40%.
KEYWORDS: antifungal agents, mass balance
ABSTRACT
SCY-078, a fungicidal β-1,3-glucan synthesis inhibitor administered as intravenous or oral [14C]SCY-078 to rats, was distributed primarily into tissues associated with invasive fungal disease (kidney, lung, liver, spleen, bone marrow, muscle, vaginal tissue, and skin) to levels exceeding those in plasma. Oral fraction absorbed was ∼40%. Elimination was primarily via bile and feces (∼90%) and urine (∼1.5%). Mean half-time was ∼8 h. Quantitative whole-body autoradiography showed a rapid distribution at 8 h and elimination by 168 h postdose.
INTRODUCTION
SCY-078 has broad and potent in vitro activity against Candida spp. and Aspergillus spp., including azole-resistant and most echinocandin-resistant strains of Candida spp. (1–4). In vitro SCY-078 is fungicidal against Candida spp. (5) and met efficacy endpoints preclinically in murine animal models of invasive candidiasis (6, 7) and aspergillosis (8). In phase 2 clinical studies, SCY-078 demonstrated efficacy for invasive candidiasis and moderate to severe vulvovaginal candidiasis (9–11). This study characterizes the tissue distribution by quantitative whole-body autoradiography (QWBA), mass balance, and elimination after single intravenous (i.v.) and oral doses of SCY-078 in albino and pigmented rats.
Male albino Wistar Han (WH; Charles River, Raleigh, NC) (n = 38) or male (n = 18) and female (n = 3) pigmented Long-Evans (LE; Hilltop Lab Animals, Inc., Scottdale, PA) rats received [14C]SCY-078 by oral administration (15 mg/kg, ∼150 μCi/kg, in aqueous 0.5% methylcellulose) or i.v. administration (5 mg/kg, ∼108 μCi/kg, 7.5:1 molar ratio of Captisol:SCY-078 in saline) as a 1-h infusion (10 ml/kg/h). WH rats were used for mass balance and pharmacokinetic (PK) determinations after i.v. and oral doses, and both WH and LE rats were used for QWBA determinations. Dose levels were selected to reflect the clinically relevant 11.2-μg·h/ml target exposure for Candida spp. infections (6, 7). The concentration, homogeneity, radio purity, and stability of dosing formulations were confirmed to be acceptable before dosing.
The study was performed in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the U.S. Office of Laboratory Animal Welfare. The study was not intended to be in accordance with Good Laboratory Practices as defined in 21 CFR part 58; nonetheless, it was performed in accordance with the study protocol and QPS standard operating procedures. There were no protocol deviations that adversely affected the study.
For elimination studies, urine samples were collected predose (overnight) and at 0 to 8 h, 8 to 24 h, and every subsequent 24-h interval until 168 h postdose. Feces samples were collected 0 to 24 h and at 24-h intervals until 168 h postdose. Bile samples were collected from bile duct-cannulated (BDC) animals predose and at 0 to 4 h, 4 to 8 h, 8 to 24 h, and every subsequent 24-h interval until 72 h postdose. Cage washes were collected daily. Blood samples were collected at frequent intervals from time 0 to 168 h postdose for determination of plasma PK. Samples were typically collected from 3 animals per group per time point.
For QWBA whole-body sections (∼40 μm thick via Leica CM3600 cryomicrotome; Nussloch, Germany), where all major tissues, organs, and biological fluids were represented, sections were exposed for phosphor imaging (Fuji Biomedical, Stamford, CT) together with calibration standards. Animals were deeply anesthetized with isoflurane anesthesia and, after blood samples were obtained, were euthanized by freezing in a hexane/solid carbon dioxide bath for at least 15 min. The imaging plate was scanned with the GE Healthcare Typhoon FLA 9500 image acquisition system (GE/Molecular Dynamics, Sunnyvale, CA). Quantification was performed by image densitometry with MCID image analysis software (v. 7.0; Interfocus Imaging Ltd., Linton, Cambridge, UK), and a standard curve was constructed from the integrated response (molecular dynamics counts [MDC]/mm2) and the nominal concentrations of the 14C-calibration standards. The concentrations of radioactivity were expressed as [14C]SCY-078 μg equiv/g tissue. The lower limit of quantitation was 0.024 and 0.049 μg equiv/g of tissue for i.v. and oral doses of SCY-078, respectively.
Excretion of radioactivity.
The primary elimination route for radioactivity following i.v. administration of SCY-078 was via the feces (87.9% dose, >60% during 0 to 24 h) (Fig. 1) with little elimination in urine (1.4% dose). For BDC animals, the primary elimination route was in bile (49.9% dose, >45% by 48 h). An average of 0.6% and 32.3% of the administered dose was recovered in the urine and feces, respectively. The presence of radioactivity in feces in the BDC rats after i.v. administration indicates potential for intestinal secretion as a route of elimination.
FIG 1.
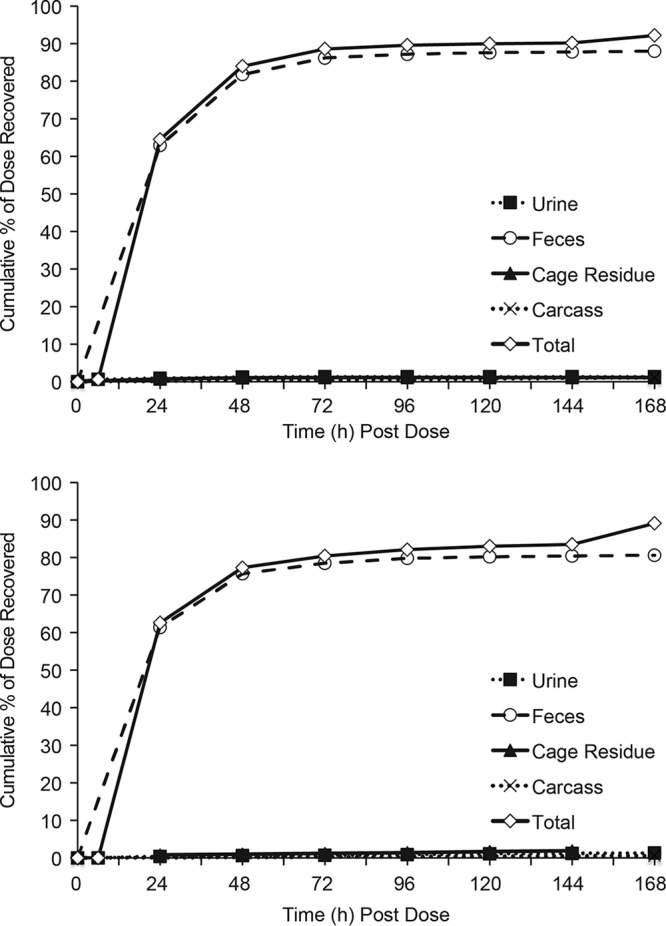
Time course of excretion of radioactivity for group 1 male rats after single a 5-mg/kg 1-h i.v. infusion or 15-mg/kg oral dose of [14C]SCY-078.
After oral administration, elimination was primarily in feces (80.5% dose, >60% during 0 to 24 h) with little elimination in urine (1.3% dose). For BDC animals, the primary elimination route was in feces (68.2% dose, >50% by 24 h). An average of 0.1% and 20.3% of the administered dose was recovered in the urine and bile, respectively. Of the administered dose (i.v. and oral groups), recovery in the cage rinse, cage wash, cage wipe, and carcass generally averaged ∼1% or less, with up to 10% for carcass in i.v.-dosed BDC rats. Total recovery of radioactivity (mass balance) was 92.2% and 91.8% of the administered i.v. and oral doses, respectively.
Plasma pharmacokinetics.
After i.v. dosing, mean plasma concentrations were quantifiable through 48 h postdose, at which time the concentration was 0.011 μg equiv/ml. Mean maximum concentration of drug in serum (Cmax) was 1.8 μg equiv/ml, mean plasma area under the curve from time 0 to the last quantifiable concentration (AUClast) was 11.69 μg equiv·h/ml, mean plasma half-life (t½) of total radioactivity was 7.6 h, and mean time to maximum concentration of drug in serum (Tmax) was at the end of infusion (Table 1, Fig. 2). After oral dosing, mean plasma concentrations were quantifiable through 48 h postdose, at which time the concentration was 0.041 μg equiv/ml. Mean Cmax was 0.716 μg equiv/ml, mean plasma AUClast was 14.42 μg equiv·h/ml, mean plasma t½ of total radioactivity was 9.8 h, and mean Tmax was 4 h (Table 1, Fig. 2).
TABLE 1.
Mean PK parameters after single i.v. or oral doses of [14C]SCY-078 in rats
| Parameter | [14C]SCY-078 dose |
|
|---|---|---|
| 5 mg/kg i.v. (n = 3) | 15 mg/kg PO (n = 3) | |
| t1/2 (h) | 7.5 | 9.8 |
| Tmax (h) | 1.0 | 4.0 |
| Cmax (μg equiv/ml) | 1.80 | 0.72 |
| AUClast (μg equiv · h/ml) | 11.69 | 14.42 |
| AUCinf (μg equiv · h/ml) | 11.81 | 15.00 |
FIG 2.
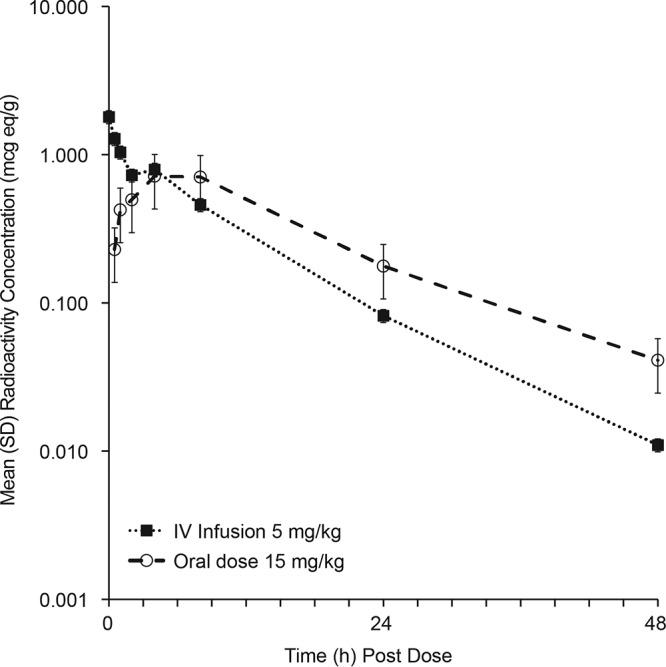
Time course of mean combined-sex plasma radioactivity concentrations after a 5-mg/kg 1-h i.v. infusion or 15-mg/kg oral dose of [14C]SCY-078 in WH rats.
Tissue distribution.
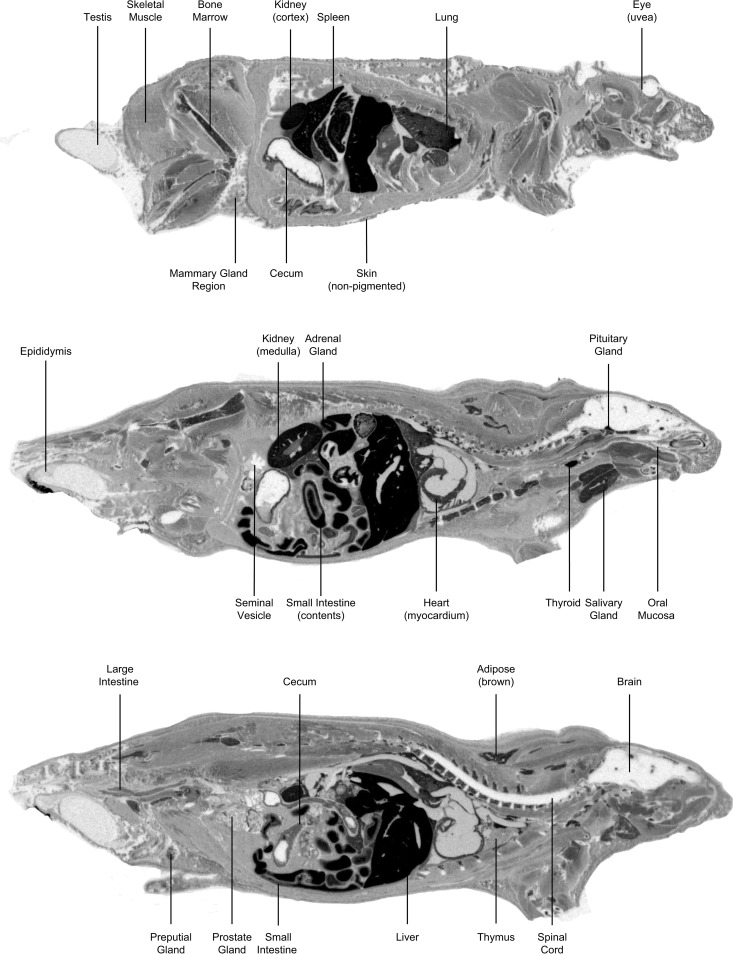
Intravenous [14C]SCY-078 in male LE rats was rapidly and widely distributed over 8 h to key tissues associated with fungal infections achieving total exposures exceeding that of blood (Table 2, Fig. 3). QWBA images showed distribution of radioactivity 5 min after completion of the i.v. infusion (Tmax), when metabolism was minimal and thereby demonstrated distribution of primarily parent [14C]SCY-078, and 4-h after oral dosing, which corresponded most closely to the plasma Tmax for oral SCY-078 in rat (Fig. 3). Radioactivity was mostly eliminated (42 of 44 tissues) by 936 h. Exceptions were seen in spleen and pigmented uvea of the eye, which had concentrations of 0.024 and 0.141 μg equiv/g, respectively. Intravenous Cmax in tissues (22 of 44) was at 5 min postdose (Tmax). In general, i.v. distribution in albino WH rats was similar.
TABLE 2.
Tissue-to-blood AUC ratios of total radioactivity after administration of [14C]SCY-078 to male pigmented LE rats
| Sample, by dosing method | Cmax (μg equiv/g) | Tmax (h) | t1/2 (h) | AUCall (μg equiv · h/g) | Tissue:blood AUC |
|---|---|---|---|---|---|
| i.v., 5 mg/kg 1 h infusion | |||||
| Adipose (brown) | 11.79 | 0.083 | 138.0 | 113.702 | 17.148 |
| Adipose (white) | 3.145 | 0.083 | 32.8 | 32.928 | 4.966 |
| Adrenal gland | 25.345 | 0.083 | 155.2 | 321.126 | 48.431 |
| Blood (cardiac) | 0.83 | 0.083 | 7.1 | 6.631 | 1.000 |
| Bone | 0.486 | 0.083 | 44.9 | 9.022 | 1.361 |
| Bone marrow (femur) | 12.033 | 8.0 | 13.7 | 238.388 | 35.953 |
| Brain (cerebellum) | 0.043 | 4.0 | NDa | 0.219 | 0.033 |
| Brain (cerebrum) | 0.059 | 8.0 | ND | 0.727 | 0.110 |
| Brain (medulla) | 0.028 | 2.0 | 38.1 | 0.136 | 0.020 |
| Cecum | 7.653 | 4.0 | 17.9 | 83.542 | 12.600 |
| Epididymis | 0.711 | 8.0 | 81.4 | 75.355 | 11.365 |
| Esophagus | 3.699 | 0.83 | 5.8 | 41.430 | 6.248 |
| Exorbital gland | 8.561 | 2.0 | 75.1 | 523.676 | 78.980 |
| Eye (lens) | 0.028 | 4.0 | ND | 0.084 | 0.013 |
| Eye (uvea) | 4.522 | 8.0 | 286.1 | 777.035 | 117.191 |
| Harderian gland | 7.483 | 8.0 | 60.3 | 927.358 | 139.862 |
| Heart (myocardium) | 9.523 | 0.083 | 6.4 | 66.558 | 10.038 |
| Kidney (cortex) | 18.949 | 0.083 | 14.8 | 165.880 | 25.018 |
| Kidney (medulla) | 16.288 | 0.083 | 16.9 | 136.610 | 20.603 |
| Large intestine | 5.519 | 0.083 | 5.3 | 57.682 | 8.727 |
| Liver | 38.837 | 0.083 | 57.1 | 374.517 | 56.484 |
| Lung | 11.439 | 0.083 | 14.0 | 175.593 | 26.483 |
| Lymph node | 8.581 | 0.083 | 51.0 | 251.036 | 37.861 |
| Mammary gland region | 2.492 | 2.0 | 24.4 | 32.117 | 4.844 |
| Oral mucosa | 1.464 | 2.0 | 22.8 | 36.782 | 5.547 |
| Pancreas | 10.471 | 0.083 | 9.4 | 110.455 | 16.659 |
| Pituitary | 19.684 | 8.0 | 22.7 | 640-809 | 96.645 |
| Preputial gland | 7.574 | 2.0 | 136.9 | 962.901 | 145.222 |
| Prostate gland | 2.533 | 2.0 | 11.3 | 61.301 | 9.245 |
| Salivary gland | 11.867 | 0.083 | 8.5 | 149.193 | 22.501 |
| Seminal vesicle | 1.047 | 8.0 | 12.4 | 26.827 | 4.046 |
| Skeletal muscle | 2.265 | 2.0 | 5.2 | 28.304 | 4.269 |
| Skin (nonpigmented) | 3.656 | 8.0 | 47.1 | 74.884 | 11.294 |
| Skin (pigmented) | 2.358 | 8.0 | 50.1 | 110.249 | 16.627 |
| Small intestine | 15.463 | 0.083 | 5.0 | 141.607 | 21.357 |
| Spinal cord | 0.053 | 0.083 | ND | 0.759 | 0.115 |
| Spleen | 32.78 | 0.083 | 237.5 | 507.638 | 75.561 |
| Spleen (red pulp) | 38.408 | 0.083 | 131.2 | 431.011 | 65.004 |
| Spleen (white pulp) | 31.424 | 8.0 | 80.7 | 1381.359 | 208.333 |
| Stomach (gastric mucosa) | 22.381 | 0.083 | 16.5 | 181.074 | 27.309 |
| Testis | 0.361 | 8.0 | 100.8 | 64.972 | 9.799 |
| Thymus | 5.394 | 8.0 | 30.4 | 195.608 | 29.501 |
| Thyroid | 37.287 | 0.083 | 27.5 | 291.187 | 43.916 |
| Urinary bladder | 6.391 | 0.083 | 30.3 | 45.955 | 6.931 |
| Oral, 15 mg/kg | |||||
| Adipose (brown) | 6.139 | 4.0 | 6.6 | 85.515 | 11.070 |
| Adipose (white) | 1.472 | 4.0 | 22.5 | 29.845 | 3.864 |
| Adrenal gland | 19.499 | 4.0 | 8.8 | 380.527 | 49.261 |
| Blood (cardiac) | 0.466 | 4.0 | 6.5 | 7.725 | 1.000 |
| Bone | 0.507 | 8.0 | 17.9 | 11.399 | 1.476 |
| Bone marrow (femur) | 8.779 | 8.0 | 10.2 | 191.142 | 24.744 |
| Brain (cerebellum) | 0.000 | ND | ND | 0.000 | 0.000 |
| Brain (cerebrum) | 0.000 | ND | ND | 0.000 | 0.000 |
| Brain (medulla) | 0.056 | 8.0 | ND | 0,560 | 0.072 |
| Cecum | 9.002 | 8.0 | 19.6 | 165.065 | 21.368 |
| Epididymis | 0.704 | 24.0 | 112.3 | 132.872 | 17.201 |
| Esophagus | 3.608 | 8.0 | 4.3 | 54.758 | 7.089 |
| Exorbital gland | 7.996 | 8.0 | 37.4 | 455.299 | 58.940 |
| Eye (lens) | 0.06 | 8.0 | ND | 0.600 | 0.078 |
| Eye (uvea) | 3.646 | 24.0 | 430.2 | 681.587 | 88.234 |
| Harderian gland | 10.257 | 24.0 | 57.9 | 1415.862 | 183.289 |
| Heart (myocardium) | 4.693 | 4.0 | 17.9 | 67.335 | 8.717 |
| Kidney (cortex) | 10.092 | 4.0 | 13.0 | 162.648 | 21.055 |
| Kidney (medulla) | 10.458 | 4.0 | 6.1 | 148.741 | 19.255 |
| Large intestine | 2.822 | 8.0 | 12.1 | 47.846 | 6.194 |
| Liver | 22.076 | 4.0 | 67.6 | 387.337 | 50.142 |
| Lung | 12.462 | 8.0 | 8.7 | 241.411 | 31.252 |
| Lymph node | 6.176 | 8.0 | 9.9 | 181.554 | 23.503 |
| Mammary gland region | 1.95 | 4.0 | 19.3 | 40.604 | 5.256 |
| Oral mucosa | 1.021 | 8.0 | 23.5 | 30.481 | 3.946 |
| Pancreas | 6.60 | 4.0 | 6.2 | 102.103 | 13.218 |
| Pituitary | 12.532 | 8.0 | 23.1 | 357.973 | 46.341 |
| Preputial gland | 6.006 | 8.0 | 39.2 | 409.267 | 52.981 |
| Prostate gland | 1.0761 | 4.0 | 23.2 | 34.033 | 4.406 |
| Salivary gland | 8.409 | 8.0 | 6.3 | 143.875 | 18.625 |
| Seminal vesicle | 0.368 | 4.0 | 9.8 | 5.858 | 0.758 |
| Skeletal muscle | 2.12 | 4.0 | 5.3 | 29.752 | 3.852 |
| Skin (nonpigmented) | 1.765 | 4.0 | 20.0 | 93.490 | 12.103 |
| Skin (pigmented) | 1.502 | 4.0 | ND | 141.195 | 18.278 |
| Small intestine | 243.592 | 4.0 | 10.0 | 827.220 | 107.087 |
| Spinal cord | 0.065 | 8.0 | ND | 0.650 | 0.084 |
| Spleen | 16.663 | 4.0 | 99.8 | 418.910 | 54.230 |
| Spleen (red pulp) | 18.061 | 4.0 | 33.5 | 400.602 | 51.860 |
| Spleen (white pulp) | 9.445 | 4.0 | 91.8 | 825.024 | 106.803 |
| Stomach (gastric mucosa) | 13.046 | 4.0 | 5.3 | 168.163 | 21.769 |
| Testis | 0.442 | 24.0 | 146.8 | 104.051 | 13.470 |
| Thymus | 3.09 | 8.0 | 16.3 | 136.948 | 17.728 |
| Thyroid | 18.223 | 4.0 | 29.9 | 354.136 | 45.844 |
| Urinary bladder | 2.493 | 0.5 | 8.6 | 36.336 | 4.704 |
ND, not determined.
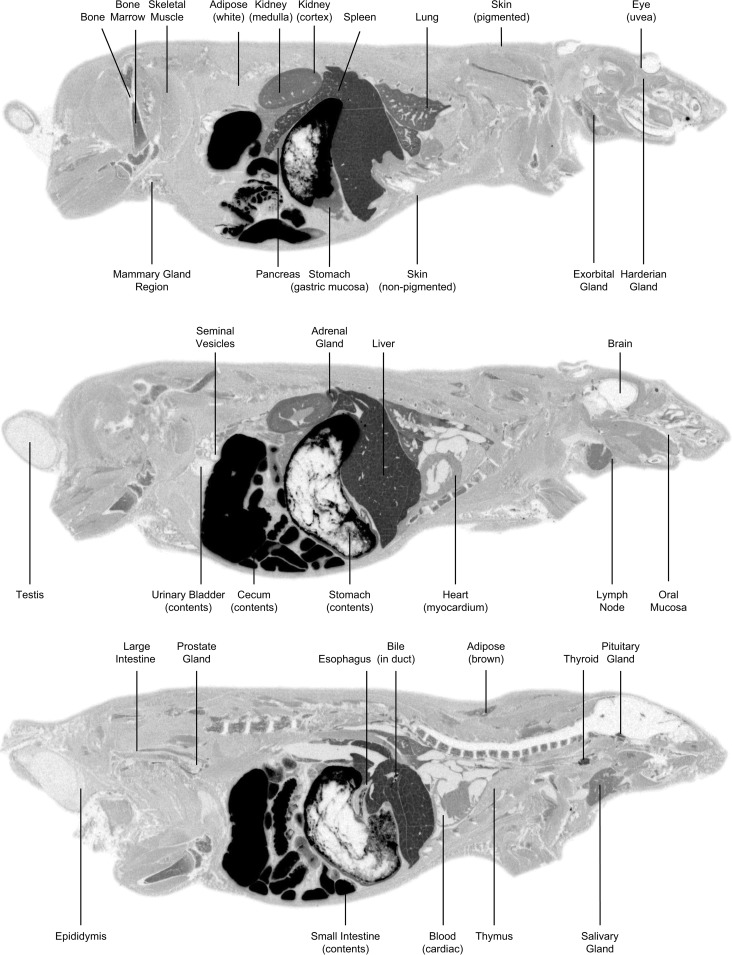
FIG 3.
Whole-body autoradiograms in LE rats after 5 mg/kg i.v. administration 5 min postdose (top three images) or 15-mg/kg oral administration 4 h postdose (bottom three images) of [14C]SCY-078.
Oral [14C]SCY-078 administration in male LE rats achieved maximal radioactivity in most tissues (21 of 44) at 4 h postdose. The highest concentration in plasma was 0.621 μg equiv/g at 4 h postdose. Tissue concentration versus time profiles showed that most tissues had a rapid distribution phase over the first 8 h, followed by a 24- to 168-h elimination phase. Tissue concentrations decreased steadily, and elimination was observed in all tissues at 912 h postdose, except in the pigmented skin and uvea of the eye, which had concentrations of 0.126 and 0.288 μg equiv/g, respectively. In general, levels of radioactivity in female LE rats after a single oral dose of [14C]SCY-078 were similar to or higher than those observed in male LE rats. The highest concentration in plasma of female LE rats was 0.589 μg equiv/g at 4 h postdose, and key tissues had concentrations that were higher than that in blood at most time points. In female LE rats, the highest tissue concentrations were observed at 4 h postdose (Tmax) for 22 of 43 tissues. Concentrations in most tissues were still detectable at 24 h (39 of 43 tissues), whereas only brain and spinal cord concentrations were below the quantifiable limit at 24 h. In general, tissue distribution in albino male WH rats after a single oral dose of [14C]SCY-078 was lower at 2 h than that in pigmented male LE rats; however, it became comparable by 24 h (next sampling time for WH rats). The highest concentration in plasma of albino rats was 0.716 μg equiv/g at 4 h postdose, and key tissues had concentrations that were higher than that in blood at most time points. The highest tissue concentrations in albino rats were observed at 24 h postdose (Tmax) for 33 of 43 tissues.
Plasma concentrations were measurable at 48 h after both i.v. and oral doses of SCY-078. The elimination t½ for SCY-078 in this study of rats was ∼7 to 9 h, which was consistent with the previously reported t½ of ∼6 to 9 h in rats and ∼9 h in dogs (7). A previous study in healthy subjects reported a t½ of ∼20 h (12); results from both animal and human studies support once-daily dosing of SCY-078. Values for AUClast and AUC to infinity (AUCinf) were higher with oral than with i.v. administration, reflecting the higher dose. The amount of oral fraction absorbed based on total radioactivity (AUC) in plasma was 42.3%.
SCY-078 was widely distributed in tissues throughout the body after i.v. and oral administration. After oral delivery, the tissue-to-blood AUC ratios in the organs typically associated with fungal disease were as follows: spleen, 54×; liver, 50×; lung, 31×; bone marrow, 25×; kidney, 20×; skin, 12× nonpigmented and 18× pigmented; vaginal tissue, 9×; and skeletal muscle, 4×. Distribution to central nervous system tissues was low, with little or no radioactivity detected in brain and spinal cord. Distribution to adipose tissue was low, supporting the high volume of distribution reported in previous studies (7) and reflected distribution to tissues other than fat. Except for in skin, the distribution profile was similar between pigmented and nonpigmented animals.
An earlier study with SCY-078 in mice reported that tissue concentrations in the kidney exceeded plasma concentrations by 20- to 25-fold (7), which is comparable to the kidney levels of radioactivity with both i.v. and oral dosing in the current study. Most of the [14C]SCY-078 radioactivity was recovered over 48 h after a dose, and at least 80% of the administered dose was excreted in feces after i.v. and oral dosing. Based on these results, SCY-078 should be suitable by both oral and i.v. administration in patients with invasive infections that require prolonged courses of therapy or by the oral route for those with recurrent vulvovaginal infections.
Penetration of antifungal drugs into target tissues at an adequate fungicidal concentration is essential for treating serious fungal infections (13). The echinocandins anidulafungin, caspofungin, and micafungin, the only approved glucan synthesis inhibitors, distribute into most tissues (14, 15) and, similar to SCY-078, distribute into kidney, liver, lung, and spleen (16–18). However, echinocandins as a class exhibit poor oral bioavailability and consequently are approved for i.v. infusion only, thereby limiting these agents to parenteral administration.
Based on current data, SCY-078 represents a potential advance over existing antifungal agents, with its potency against wild-type and antifungal drug-resistant organisms, excellent distribution in tissues associated with fungal infections, and PK profile consistent with once-daily oral and i.v. administration. These attributes may be especially beneficial to patients requiring oral therapy for recurrent infections or prolonged courses of treatment.
ACKNOWLEDGMENTS
S.W. was the primary author and lead investigator. E.S. was responsible for the in-life phase and QWBA analyses. K.B.-E. and D.A. provided guidance on the program and review of the data. Richard Perry provided editorial assistance in the finalization and submission of the manuscript.
The studies described in this article were funded by SCYNEXIS.
We were employees of our affiliated organizations at the time of the research. Richard Perry is a paid consultant to SCYNEXIS.
REFERENCES
- 1.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. 2013. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother 57:1065–1068. doi: 10.1128/AAC.01588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiménez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. 2014. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. 2013. Activity of MK-3118, a new oral glucan synthase inhibitor, tested against Candida spp. by two international methods (CLSI and EUCAST). J Antimicrob Chemother 68:858–863. doi: 10.1093/jac/dks466. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. 2017. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother 61:e00161-17. doi: 10.1128/AAC.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scorneaux B, Angulo D, Borroto-Esoda K, Ghannoum M, Peel M, Wring S. 2017. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother 61:e01961-16. doi: 10.1128/AAC.01961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepak AJ, Marchillo K, Andes DR. 2015. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother 59:1265–1272. doi: 10.1128/AAC.04445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wring SA, Randolph R, Park S, Abruzzo G, Chen Q, Flattery A, Garrett G, Peel M, Outcalt R, Powell K, Trucksis M, Angulo D, Borroto-Esoda K. 2017. Preclinical pharmacokinetics and pharmacodynamic target of SCY-078, a first-in-class orally active antifungal glucan synthesis inhibitor, in murine models of disseminated candidiasis. Antimicrob Agents Chemother 61:e02068-16. doi: 10.1128/AAC.02068-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borroto-Esoda K, Barat S, Angulo D, Holden K, Warn P. 2017. SCY-078 demonstrates significant antifungal activity in a murine model of invasive aspergillosis. Open Forum Infect Dis 4:S472. doi: 10.1093/ofid/ofx163.1207. [DOI] [Google Scholar]
- 9.Pappas PG, Pullman J, Thompson G, Powderly W, Spec A, Tobin E, Vazquez J, Wring S, Angulo D, Helou S, Investigators of the Mycoses Study Group. 2017. A prospective, phase 2, multicentre, open-label, randomized, comparative study to estimate the safety, tolerability, pharmacokinetics, and efficacy of oral SCY-078 vs standard-of-care following initial intravenous echinocandin therapy in the treatment of invasive candidiasis (including candidaemia) in hospitalized non-neutropenic adults, abstr OS0846. Abstr 27th Eur Congr Chemother Microbiol Infect Dis. [Google Scholar]
- 10.Angulo D, Helou S, Wring S. 2017. Pharmacokinetics and pharmacodynamics in patients from a phase 2, multicenter, open-label, randomized, comparative study of oral SCY-078 vs. standard-of-care following initial intravenous echinocandin therapy in the treatment of invasive candidiasis (including candidemia) in hospitalized non-neutropenic adults, abstr AAID03. Abstr 2017 ASM Microbe. [Google Scholar]
- 11.Roman M, Hernandez C, Blanco D, Obrychi G, Helou S, Angulo D. 2017. SCY-078 phase 2 study in moderate to severe vulvovaginal candidiasis (VVC), abstr P1742. Abstr 27th Eur Congr Chemother Microbiol Infect Dis. [Google Scholar]
- 12.Wring S, Murphy G, Atiee G, Corr C, Hyman M, Willett M, Angulo D. 2018. Clinical pharmacokinetics and drug-drug interaction potential for coadministered SCY-078, an oral fungicidal glucan synthase inhibitor, and tacrolimus. Clin Pharmacol Drug Dev doi: 10.1002/cpdd.588. [DOI] [PubMed] [Google Scholar]
- 13.Felton T, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar-Zapata D, Petraitiene R, Petraitis V. 2015. Echinocandins: the expanding antifungal armamentarium. Clin Infect Dis 61:S604–S611. doi: 10.1093/cid/civ814. [DOI] [PubMed] [Google Scholar]
- 15.Kofla G, Ruhnke M. 2011. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur J Med Res 16:159–166. doi: 10.1186/2047-783X-16-4-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JA, Xu X, Winchell GA, Deutsch PJ, Pearson PG, Migoya EM, Mistry GC, Xi L, Miller A, Sandhu P, Singh R, deLuna F, Dilzer SC, Lasseter KC. 2004. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob Agents Chemother 48:815–823. doi: 10.1128/AAC.48.3.815-823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damle B, Stogniew M, Dowell J. 2008. Pharmacokinetics and tissue distribution of anidulafungin in rats. Antimicrob Agents Chemother 52:2673–2676. doi: 10.1128/AAC.01596-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groll AH, Mickiene D, Petraitis V, Petraitiene R, Ibrahim KH, Piscitelli SC, Bekersky I, Walsh TJ. 2001. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob Agents Chemother 45:3322–3327. doi: 10.1128/AAC.45.12.3322-3327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]