At sufficient concentrations, antibiotics effectively eradicate many bacterial infections. However, during therapy, bacteria are unavoidably exposed to lower antibiotic concentrations, and sub-MIC exposure can result in a wide variety of other effects, including the induction of virulence, which can complicate therapy, or horizontal gene transfer (HGT), which can accelerate the spread of resistance genes.
KEYWORDS: antibiotic resistance, antibiotics, drug design, secretion, signal peptidase
ABSTRACT
At sufficient concentrations, antibiotics effectively eradicate many bacterial infections. However, during therapy, bacteria are unavoidably exposed to lower antibiotic concentrations, and sub-MIC exposure can result in a wide variety of other effects, including the induction of virulence, which can complicate therapy, or horizontal gene transfer (HGT), which can accelerate the spread of resistance genes. Bacterial type I signal peptidase (SPase) is an essential protein that acts at the final step of the general secretory pathway. This pathway is required for the secretion of many proteins, including many required for virulence, and the arylomycins are a class of natural product antibiotics that target SPase. Here, we investigated the consequences of exposing Escherichia coli cultures to sub-MIC levels of an arylomycin. Using multidimensional protein identification technology mass spectrometry, we found that arylomycin treatment inhibits the proper extracytoplasmic localization of many proteins, both those that appear to be SPase substrates and several that do not. The identified proteins are involved in a broad range of extracytoplasmic processes and include a number of virulence factors. The effects of arylomycin on several processes required for virulence were then individually examined, and we found that, at even sub-MIC levels, the arylomycins potently inhibit flagellation, motility, biofilm formation, and the dissemination of antibiotic resistance via HGT. Thus, we conclude that the arylomycins represent promising novel therapeutics with the potential to eradicate infections while simultaneously reducing virulence and the dissemination of resistance.
INTRODUCTION
Antibiotics have revolutionized health care, curing infections that were once both common and life threatening. At sufficient concentrations (at or above the MIC), antibiotics kill or halt the growth of bacteria by inhibiting essential targets. However, at sub-MIC levels, antibiotics also have significant, and often unpredictable, effects, including the exacerbation of virulence (1–4) and the induction of horizontal gene transfer (HGT) (5), which facilitates the interspecies and intraspecies spread of virulence and antibiotic resistance genes. For example, at sub-MIC levels, tetracycline, linezolid, vancomycin, colistin, and macrolides can induce virulence (6–10), while ciprofloxacin, tetracycline, and β-lactams can induce HGT (11–13). Because any therapy unavoidably exposes both pathogens and commensal bacteria to a gradient of drug concentrations, including concentrations below the MIC (1, 14), antibiotic therapy can actually exacerbate virulence (15, 16) and induce the dissemination of virulence and resistance genes (17–22).
Although the complications associated with sub-MIC levels of antibiotics are well documented, the underlying mechanisms are generally unclear. For example, while a variety of ribosome inhibitors induce biofilm formation, others reduce it (1, 2, 4), and, in the case of tobramycin, biofilm formation is induced in Pseudomonas aeruginosa bacteria regardless of whether the bacteria are sensitive or resistant to its growth-inhibitory effects (23). While these idiosyncratic effects may result from the induction of stress responses (3, 12, 18, 24), they have also led to the suggestion that some antibiotics may have natural roles as signaling molecules (25).
Due to their roles in pathogenicity and the dissemination of antibiotic resistance, it has been suggested that virulence and HGT should be targeted for therapeutic development (26–31). However, neither virulence nor HGT is essential for viability, and thus, unlike the essential proteins targeted by antibiotics, proteins involved in virulence and HGT are generally only poorly conserved. As a result, any inhibitors would be unlikely to have a spectrum of activity broad enough for development as therapeutics. In contrast, the identification of antibiotics that have essential targets but that also potently and predictably reduce virulence and/or HGT at sub-MIC levels would represent a novel and potentially more effective therapeutic modality.
Protein secretion allows bacteria to interact with their environment and is thus a critical aspect of their physiology and pathogenicity. As most virulence factors are secreted, they are typically synthesized as preproteins with type I N-terminal signal peptides (or leader sequences) that target them for the general secretory pathway. The essential step of releasing preproteins after they translocate across the cytoplasmic membrane is mediated by type I signal peptidase (SPase). SPase is thus essential, and it has long been appreciated as a promising target for conventional broad-spectrum antibiotic development (32). The arylomycins are a family of natural product antibiotics that inhibit SPase (33, 34) (Fig. 1), and they are currently under development in the pharmaceutical industry as broad-spectrum therapeutics (35–37). While the mechanism of arylomycin killing is the accumulation of unprocessed preproteins in the cytoplasmic membrane, which compromises the membrane’s integrity (38), the inhibition of SPase obviously also prevents the proper localization of extracytoplasmic proteins, and, as a result, sub-MIC levels of an arylomycin could in theory reduce virulence and possibly other processes that rely on extracytoplasmic proteins (39).
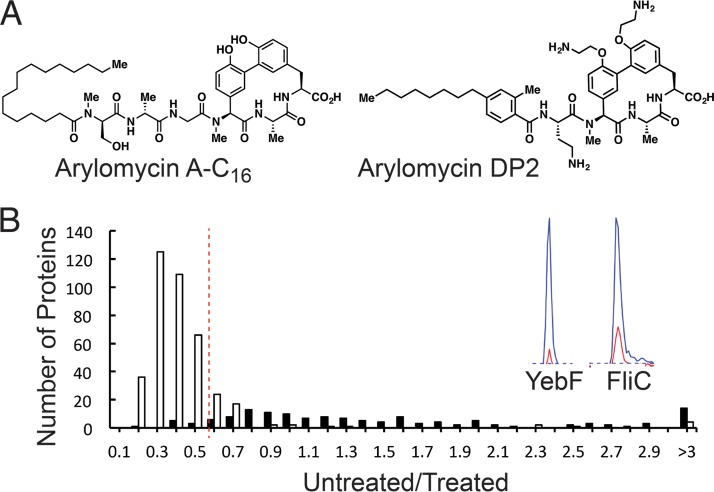
FIG 1.
(A) Arylomycin A-C16 and arylomycin DP2. (B) Multidimensional protein identification technology (MudPIT) reveals a differential response to arylomycin treatment for proteins encoded without (white) and with (black) a signal peptide at 2 μg/ml (see Fig. S1 for other treatment regimens), with representative chromatograms (YebF and FliC) shown. The dotted line represents the median ratio plus two median absolute deviations for proteins encoded without a signal peptide. Proteins with median ratios above this cutoff for all three treatment regimens were considered SPase dependent.
Here, we evaluate the sub-MIC effects of arylomycins on Escherichia coli cells using a proteomics approach, followed by in vitro studies. The data show that sub-MIC arylomycin treatment inhibits the proper localization of many known or suspected virulence factors, including those that are not themselves SPase substrates but the proper localization of which appears to depend on other proteins that are SPase substrates. We then show that sub-MIC arylomycin treatment, apparently as a result of this protein mislocalization, inhibits flagella formation, biofilm formation, motility, and HGT of a clinically important mobile genetic element.
RESULTS
Sub-MIC levels of an arylomycin inhibit the proper localization of many proteins.
The potential antivirulence activities of the arylomycins obviously depend on the substrate repertoire of SPase. To characterize the substrate repertoire of E. coli SPase, we used mass spectrometry to identify the proteins whose extracytoplasmic localization is inhibited by addition of an arylomycin to the growth medium. These studies were conducted with arylomycin A-C16, an extensively characterized analog of the natural variant arylomycin A2 (Fig. 1) (38, 40–46). Because E. coli is not naturally sensitive to the arylomycins, including arylomycin A-C16, we employed a mutant strain of E. coli MG1655 that carries a sensitizing mutation in the gene encoding SPase [LepB(P84S); strain PAS0232 (45); arylomycin A-C16 MIC, 4 μg/ml]. The P84S mutation does not affect fitness, and a serine at this position is common in the native genomes of other bacteria that are sensitive to arylomycin A-C16 (45).
Briefly, a culture of E. coli PAS0232 was grown to the mid-exponential phase and treated for one hour with 0, 0.5, 1, or 2 μg/ml of arylomycin A-C16, which had little effect on growth (Fig. S1). Periplasmic and outer membrane proteins were then extracted and analyzed via multidimensional protein identification technology (MudPIT) mass spectrometry (47), wherein proteins were quantified through differential isotopic labeling of tryptic peptides by reductive dimethylation (ReDiMe) with heavy and light formaldehyde. Comparison of protein abundances, given as ReDiMe ratios between the arylomycin-treated samples and no-arylomycin (dimethyl sulfoxide [DMSO]) controls, revealed that proteins encoded with or without a signal peptide responded differently to arylomycin treatment (Fig. 1 and Table S1). The ReDiMe ratios determined for proteins encoded without signal peptides loosely approximate a normal distribution centered between 0.3 and 0.5, depending on the treatment regimen. Some unavoidable cell lysis during growth and/or protein extraction likely explains why these proteins were detected at higher levels in arylomycin-treated samples. However, a subset of proteins clearly fell outside this distribution. To more rigorously define this subset of proteins, which were detected at less increased or actually decreased levels in arylomycin-treated samples, we designated two median absolute deviations above the median of all proteins encoded without a signal peptide as a minimum cutoff (ratios of 0.57, 0.57, and 0.74 for the samples treated with 2, 1, and 0.5 μg/ml of arylomycin A-C16, respectively). With these criteria, we identified 98 proteins whose extracytoplasmic localization is inhibited by the arylomycin (Table S2). Only one of the 98 proteins, YfcD, is not predicted to be extracytoplasmic, and it only barely satisfied the selection criteria. Of the 98 proteins, 86 are encoded with a type I N-terminal signal peptide, based on their UniProt annotations (48) (Table S2), suggesting that the approach effectively identified SPase substrates. These proteins include the known or suspected virulence factors YhcN, YdeI, YgiW, HdeB, MdoG, PotD, SlyB, PtrA, PstS, OmpX, OmpF, OmpC, PliG, Ivy, and Eco.
Surprisingly, five lipoproteins—Lpp, FlgH, LoiP, BamC, and SlyB—with predicted type II signal peptides, which are not recognized by SPase, were among the proteins identified. Even more surprisingly, the remaining seven proteins are each produced without a signal peptide of any type, with six, FlgG, FliC, FlgE, FlgL, FlgF, and FlgD being components of the flagellum. Analysis of mRNA levels of fliC revealed that treatment with arylomycin A-C16 did not reduce transcription (Table S3). While we cannot rule out the possibility that some of the observed effects result from alterations in transcription or translation, the data with fliC, the fact that the vast majority of the identified proteins had type I signal peptides, and the known specificity of the arylomycins for SPase, suggests that most of the induced mislocalization observed results from the inhibition of SPase. Regardless of the underlying mechanism, it is clear that sub-MIC arylomycin treatment results in the reduced export of many proteins, including that of many virulence factors.
Sub-MIC levels of arylomycins inhibit assembly of the flagellum.
Our data demonstrate that arylomycin treatment inhibits the proper localization of multiple components of the flagellum, which in turn suggests that arylomycin treatment would inhibit the formation of these surface appendages. To verify the physiological relevance of the proteomic data, we used electron microscopy to qualitatively gauge the extent of flagellation of E. coli PAS0232 cells grown on semisolid medium in the absence or presence of arylomycin A-C16 (Fig. 2). Cells grown without arylomycin were hyperflagellated, while those grown with 0.5 μg/ml arylomycin A-C16 (8-fold below the MIC) possessed virtually no flagella. Thus, the inhibition of SPase by even sub-MIC levels of an arylomycin inhibits flagellum formation and likely also inhibits any process that requires flagella.
FIG 2.
Electron microscopy visualization of E. coli PAS0232 cells grown in the absence (A) or presence (B) of 0.5 μg/ml arylomycin A-C16.
Sub-MIC levels of arylomycins inhibit biofilm formation.
As sub-MIC treatment with an arylomycin inhibited the proper localization of several proteins implicated in biofilm formation (including components of the flagellum [49]), we explored sub-MIC effects on biofilm formation using a standard crystal violet assay. With E. coli PAS0232, we found that arylomycin A-C16 almost completely prevents biofilm formation at concentrations as low as one-sixteenth its MIC (Fig. 3A). To confirm that the effects on biofilm formation resulted from the inhibition of SPase, we repeated the assays using wild-type E. coli MG1655, the parental strain of PAS0232. Despite this strain not being sensitive to arylomycin A-C16 (MIC, >128 μg/ml), biofilm formation was again inhibited, but in this case, consistent with the effects being mediated by SPase inhibition, it required the addition of ∼32-fold more arylomycin A-C16 (8 μg/ml) (Fig. 3B). Using analogous conditions, ampicillin, kanamycin, and ciprofloxacin reduced biofilm formation, but significantly less so than did the arylomycin (Fig. 3A).
FIG 3.
(A) Percent biofilm growth relative to that of the untreated control for E. coli PAS0232 cells grown in the presence of arylomycin A-C16, ampicillin, ciprofloxacin, or tobramycin. White bars represent growth with each antibiotic added at one-eighth the MIC, and black bars represent growth at one-sixteenth the MIC. (B) Percent biofilm growth relative to that of the untreated control for wild-type (WT) E. coli cells grown in the presence of arylomycin A-C16. (C) Swimming (black) and swarming (gray) motility of E. coli PAS0232 as a function of added arylomycin A-C16. (D) The effect of arylomycin DP2, ampicillin, ciprofloxacin, or tobramycin on uropathogenic E. coli (UPEC) UTI89 average swarming motility at one-eighth their respective MIC (data indicate percent swarming relative to that of the untreated control). All data are shown as averages with one standard deviation.
Sub-MIC levels of arylomycins inhibit motility.
Motility is critical for virulence, and, depending on the viscosity of the growth medium, E. coli exhibits two types of motility, swimming and swarming, both of which are dependent on the assembly of functional flagella. Thus, we examined the effect of arylomycin A-C16 on the swimming and swarming of E. coli PAS0232 cells using standard plate assays with semisolid growth medium. Arylomycin A-C16 treatment was found to inhibit both swimming and swarming motility (Fig. 3C and Fig. S3), with swarming inhibited to a greater extent than swimming, which is consistent with its requirement for a greater number of flagella (50). Remarkably, a significant decrease in swarming motility was observed even at concentrations 160-fold lower than the MIC.
Given the pronounced effect on the motility of E. coli PAS0232 cells, we were interested in examining uropathogenic E. coli (UPEC) cells, for which motility is critical for pathogenicity (51). We found that arylomycin A-C16 had no effect on the motility of the UPEC clinical isolate UTI89 (52) at concentrations up to 128 μg/ml (data not shown). To test if a more potent SPase inhibitor might affect motility, we synthesized the more recently reported arylomycin derivative, arylomycin DP2 (Fig. 1), which has activity against wild-type E. coli, likely due, at least in part, to increased outer membrane permeability (36, 53). We found that arylomycin DP2 inhibits growth of UTI89 cells at an MIC of 2 μg/ml, but dramatically reduces swarming motility at 0.25 μg/ml (Fig. 3D). The potent inhibition of motility by arylomycin DP2, but not by arylomycin A-C16. is again consistent with the effects being mediated by SPase inhibition and not by any nonspecific physicochemical properties of the arylomycin scaffold. In contrast to arylomycin DP2, at the analogous concentrations relative to their MICs, neither kanamycin nor ciprofloxacin nor ampicillin had a significant effect on motility of UTI89 cells (Fig. 3D).
Sub-MIC levels of arylomycins inhibit HGT.
Many genes involved in mediating virulence and antibiotic resistance are encoded on mobile genetic elements. HGT of these elements to other bacteria requires type IV secretion systems (T4SSs), which are commonly encoded on the same element and which include components predicted to depend on SPase for proper localization (54). To explore the possibility that arylomycin treatment would inhibit mobility of these elements, we examined transfer of SXT, a clinically important self-transmissible element that encodes resistance to the antibiotics sulfamethoxazole, trimethoprim, chloramphenicol, and streptomycin (55). Components of the SXT T4SS include TraE, TraW, TraU, TraN, TraF, and TraH, all of which are predicted to require SPase for proper localization (54).
We first examined transfer of SXT-mediated chloramphenicol resistance from PAS0232 to a gentamicin-resistant derivative of its parental strain MG1655, RTC0102 (ΔlacZ::Genr) (Table 1). The frequency of HGT was determined by enumeration of colonies resistant to both chloramphenicol and gentamicin after coincubation. In the absence of arylomycin A-C16, the frequency of SXT transfer from PAS0232 donor cells was indistinguishable from that of a kanamycin-resistant derivative of E. coli MG1655 (PAS002 ΔlacZ::Kanr), indicating, as expected, that the sensitizing mutation itself had no effect on conjugation. However, the addition of arylomycin A-C16 resulted in a significant and dose-dependent decrease in SXT transfer from PAS0232. For example, at 0.25 μg/ml (16-fold below the MIC), we observed an ∼20-fold reduction in chloramphenicol resistant conjugates (Fig. 4A). The total number of chloramphenicol-resistant conjugates was even lower at higher arylomycin concentrations, although this was accompanied by a reduction in the number of viable donor cells (Fig. 4B). In contrast, when the sensitized PAS0232 strain was used as the recipient, arylomycin A-C16 addition had no effect on SXT transfer at concentrations up to 1 μg/ml (4-fold below the MIC, the highest concentration examined; data not shown). We then examined the effects of arylomycin A-C16 on transfer of chloramphenicol resistance between the kanamycin-resistant wild-type donor (PAS002) and RTC0102 acceptor cells and found that it was again inhibited in a dose-dependent manner, but, as with biofilm formation, the same levels of inhibition with the arylomycin-resistant strain required ∼32-fold more arylomycin A-C16 (Fig. 4A), again consistent with the effects resulting from the inhibition of SPase. Finally, when we examined transfer from donor PAS0232 cells overexpressing either SPase or a catalytically inactive SPase mutant [LepB(S90A) (56)] (Table 1), transfer in the presence of the arylomycin A-C16 was partially rescued with overexpression of the wild-type SPase, but not with the catalytically inactive mutant (Fig. 4C), again demonstrating that the observed effects are due to the inhibition of SPase. In contrast to arylomycin treatment, we found that treatment with spectinomycin had little effect on the frequency of HGT, while HGT was increased by the addition of rifampin, ciprofloxacin, or gentamicin (Fig. 4D to F).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain, integrating conjugative element, or plasmid | Relevant characteristic(s)a | Reference and/or source |
|---|---|---|
| Strains | ||
| MG1655 | 90 | |
| UTI89 | Clinical UPEC isolate | 52 |
| PAS002 | ΔlacZ::Kanr + SXT element | This work |
| PAS0232 | LepB(P84S)::Kanr | 45 |
| PAS0233 | LepB(P84S)::Kanr + SXT element | This work |
| PAS0168 | ΔlacZ::Kanr + pTetBHR2 | This work |
| PAS0169 | ΔlacZ::Kanr + pPAS037 | This work |
| PAS0184 | ΔlacZ::Kanr + pPAS041 | This work |
| RTC0002 | ΔpolB::Sptr | 90 |
| PAS0314 | RpoB(D516G) Rifr | This work |
| RTC0110 | GyrA(S83L) Cipr | 90 |
| RTC0102 | ΔaraA::Genr | This work |
| Integrating conjugative element | ||
| SXT | Strr, Chlr, Sulr, Tmpr | 55 |
| Plasmids | ||
| pTETBHR2 | Ampr, aTc-inducible | This work |
| pPAS0237 | Ampr, aTc-inducible LepB expression | This work |
| pPAS041 | Ampr, aTc-inducible LepB(S90A) expression | This work |
Kanr, kanamycin resistance; Sptr, spectinomycin resistance; Rifr, rifampin resistance; Cipr, ciprofloxacin resistance; Genr, gentamicin resistance; Strr, streptomycin resistance; Chlr, chloramphenicol resistance; Sulr, sulfonamide resistance; Tmpr, trimethoprim resistance; Ampr, ampicillin resistance; UPEC, uropathogenic E. coli.
FIG 4.
(A) Arylomycin A-C16 inhibits SXT transfer from the E. coli MG1655-derived strains, PAS0232 (black) and PAS0002 (gray). (B) Viability of PAS0232 (black) and PAS0002 (gray) donor cells as a function of added arylomycin A-C16. (C) Expression of WT SPase from plasmid pPAS0237 (gray) induced by addition of anhydrotetracycline (aTc) (128 ng/ml) partially restores conjugation efficiency in the presence of arylomycin A-C16 (32 μg/ml). Conjugation efficiency is not restored upon expression of a catalytically inactive mutant (S90A) (white) nor that of an empty plasmid (black). (D) Effect of four different concentrations of arylomycin A-C16, gentamicin, spectinomycin, rifampin, or ciprofloxacin (0×, 0.25×, 1×, and 2× MIC; represented by black, 70% gray, 35% gray, and 7.5% gray, respectively) on SXT conjugates per viable donor, (E) conjugation efficiency, and (F) donor cell viability. For panels D to F, recipient cells were resistant to the antibiotic tested. Data in all panels are presented as averages with one standard deviation. Asterisks denote a significant difference, determined via the Student's t test. *, P < 0.05; **, P < 0.02; ***, P < 0.01.
DISCUSSION
Exposing bacteria to sub-MIC levels of an antibiotic during therapy is unavoidable and can complicate therapy by increasing virulence and the dissemination of virulence and antibiotic resistance genes via HGT. Such potentially problematic effects have been observed with virtually every class of traditional antibiotic. The arylomycins are a novel class of antibiotic that inhibit SPase, have been extensively studied in our lab (38, 40–46, 53), and are under development in the pharmaceutical industry (35, 37, 57). While their mechanism of antibacterial action is the loss of cytoplasmic membrane integrity that results from the accumulation of unprocessed preproteins, the inhibition of SPase also prevents the proper extracytoplasmic localization of the corresponding mature proteins. Thus, we speculated that, at even sub-MIC levels, the arylomycins might reduce virulence and other processes mediated by extracytoplasmic proteins.
Using a mass spectrometry (MS)-based proteomic approach, we identified 98 proteins whose extracytoplasmic localization was inhibited by sub-MIC levels of an arylomycin. A total of 86 of the identified proteins are encoded with a type I N-terminal signal peptide and are thus likely SPase substrates. A total of 34 of these proteins have not been validated as SPase substrates. The most prominent function associated with this apparent repertoire of SPase substrates is the transport of metabolites, including monosaccharides and polysaccharides, amino acids, and lipids, as well as other housekeeping functions, such as cell wall and outer membrane biogenesis and extracytoplasmic protein folding.
Consistent with its antivirulence potential, a variety of known or suspected virulence factors were among the proteins identified as likely SPase substrates that showed reduced extracytoplasmic localization when treated with sub-MIC levels of the arylomycin. These include YhcN, YdeI, YgiW, HdeB, MdoG, PotD, SlyB, PtrA, PstS, OmpX, OmpF, OmpC, PliG, Ivy, and Eco. YhcN, YdeI, YgiW, and HdeB have been shown to contribute to survival in acidic environments (58, 59), such as the gastrointestinal (GI) tract. YdeI, MdoG, and PotD have been implicated in biofilm formation (58, 60, 61). The outer membrane lipoprotein SlyB and the protease PtrA likely function in hemolytic toxin release and proteolysis of host peptides, respectively (62, 63). PstS is an essential component of a phosphate transport system, the impairment of which has been shown to result in decreased virulence of different pathogenic strains of E. coli (64). Deletion of OmpX in E. coli, and OmpF and OmpC homologues in Salmonella enterica serovar Typhimurium reduced virulence (65–67). Finally, PliG, Ivy, and Eco each inhibit various host proteases (68–70).
Surprisingly, included among the 98 proteins whose extracytoplasmic localization was inhibited by sub-MIC arylomycin exposure were five lipoproteins, Lpp, FlgH, LoiP, BamC, and SlyB, that function in the construction and maintenance of the cell envelope and in proteolytic homeostasis (62, 71), but that, based on their N-terminal sequence, are not substrates of SPase, but rather of type II signal peptidase. The effect of arylomycin treatment on the export of these lipoproteins must be indirect and likely results from mislocalization of the SPase substrate LolA, the periplasmic lipoprotein transporter, and/or in the case of BamC, mislocalization of BamA, a component of the β-barrel assembly machinery (BAM) complex (72). Both BamC and BamA were identified as SPase substrates.
Finally, the proper localization of six components of the flagellum not encoded with signal peptides, FlgG, FliC, FlgE, FlgL, FlgF, and FlgD, was also inhibited by sub-MIC arylomycin. Flagellum assembly is a semi-stepwise process (73, 74), and while secretion of these components is independent of the general secretory pathway and therefore independent of SPase, their assembly depends on FlgI, the P-ring protein component of the flagellum, which was among the SPase substrates we identified. Thus, the mislocalization of these components likely results from the arylomycin-induced mislocalization of FlgI. When combined with those for the above-mentioned lipoproteins, these results suggest that the effects of SPase inhibition include not only the reduced export of canonical substrates, including many virulence factors, but also the mislocalization of many additional proteins, which are not themselves SPase substrates, but which instead depend on SPase substrates for proper localization.
Regardless of whether the effects are direct or indirect consequences of the inhibition of SPase, our results demonstrate that arylomycins, at even sub-MIC levels, inhibit the proper localization of many proteins involved in virulence and other extracytoplasmic processes. To begin to explore the consequences of this mislocalization, we showed that treatment of E. coli cells with sub-MIC levels of an arylomycin inhibits the formation of flagella. Likely, at least in part as a consequence of inhibited flagella formation, sub-MIC levels of an arylomycin were also found to potently inhibit biofilm formation. This could have an important impact on therapy, as biofilms contribute to the virulence of many infections and also commonly complicate the treatment of infections associated with indwelling devices (75). Similarly, we showed that sub-MIC arylomycin exposure inhibited the motility of E. coli PAS0232 and of a clinical isolate of UPEC. Motility is especially critical for the pathogenicity of UPEC, which is the major cause of one of the most problematic infections in the industrialized world (76), urinary tract infections (77). Interestingly, UPEC strains compromised in their ability to form flagella show severe attenuation in urinary tract ascension, host dissemination, and infectivity (51). It is likely that other processes required for virulence, such as adhesion, the avoidance of host defense mechanisms, and cell invasion, similarly require the extracellular localization of proteins that would be directly or indirectly inhibited by arylomycin treatment.
The inhibition of SXT transfer via HGT is especially interesting, as HGT is a major mechanism for the spread of genes that mediate virulence and antibiotic resistance, with the latter threatening to undermine the utility of the current clinical arsenal of antibiotics. (19–22). Clearly the transfer of DNA per se does not require SPase activity, and the inhibition of HGT likely results from the inhibited assembly of the required T4SS, which possesses multiple components that are predicted to rely on SPase for localization (54). Similarly, components of nearly all specialized secretion systems rely on SPase for proper localization (39) and thus, the inhibition of their functions is likely to be included in the activity of an arylomycin therapeutic.
To the best of our knowledge, this is the first demonstration of an antibiotic that, at even sub-MIC levels, inhibits a broad range of virulence and other extracytoplasmic processes. From a practical perspective, it is especially notable that the effects are mediated by the inhibition of an essential and thus highly conserved protein—just as the arylomycins are proving to have a broad spectrum of antibacterial activity (42, 45, 46, 53), they are likely to inhibit virulence and HGT in a broad range of pathogens. Finally, among the proteins whose proper localization was inhibited by arylomycin treatment were several proteins that themselves have been suggested to be promising targets for antibiotic development, including, for example, components of the BAM complex, which is required to insert β-barrel proteins into the outer membrane (78); LptD, which is required for lipopolysaccharide biogenesis (79, 80); and LolA, which functions within the lipoprotein transport system (81). The therapeutic potential of simultaneously inhibiting the activity of all of these targets would be included in an arylomycin therapeutic. Thus, in contrast to traditional antibiotics, which can actually exacerbate virulence and induce the spread of virulence and resistance, an arylomycin therapeutic might potently disarm pathogens and prevent the spread of their genes while eliminating them.
MATERIALS AND METHODS
Growth medium.
Components for growth medium were manufactured by Difco Laboratories (Detroit, MI) or Thermo Fisher Scientific (Waltham, MA); MilliQ purified water was used throughout. Arylomycin A-C16 and DP2 were synthesized as described previously (40, 53). Bacterial strains and conjugal elements are listed in Table 1.
Protein extraction and analysis.
E. coli PAS0232 was grown overnight in LB and used to inoculate LB (110 ml) to an optical density at 600 nm (OD600) of 0.05. Cultures were grown with vigorous shaking at 37°C to an OD600 of 0.6 to 0.65, and afterwards split into two 50-ml cultures. Arylomycin was added to one of the resulting cultures (in DMSO sufficient to yield a final concentration of 0.5, 1, or 2 μg/ml), and an equal volume of DMSO was added to the other. To maximize the chance of detecting both rapid and more delayed responses to arylomycin treatment, cultures were grown for an additional hour with shaking at 37°C before being subjected to periplasmic extraction using the Tris-sucrose solution supplemented with EDTA (TSE) extraction protocol described previously (82). Protein was quantified via Bradford assay using bovine serum albumin (BSA) as a standard, and then precipitated by the addition of 10% wt/vol trichloroacetic acid (TCA). Precipitated protein samples were washed twice with acetone before further analysis. Three independently prepared samples were analyzed for each arylomycin concentration.
Sample preparation and ReDiMe labeling.
Samples were denatured by addition of 8 M urea made in 100 mM triethylammonium bicarbonate (TEAB). DMSO-treated samples were resuspended to give a protein concentration of 1 μg/μl, and arylomycin-treated samples from the same replicate were resuspended in an equal volume. Reduction was carried out by addition of Tris(2-carboxyethyl) phosphine hydrochloride (TCEP; Sigma) to a final concentration of 5 mM, followed by addition of iodoacetamide to a final concentration of 10 mM. The urea concentration was reduced to 2 M through addition of 100 mM TEAB, and trypsin was added at a 100:1 (wt/wt) substrate to enzyme ratio. Samples were incubated at 37°C overnight. ReDiMe labeling was performed the next day, as previously described (83). Briefly, peptides (100 μl containing ∼25 μg DMSO-treated sample with corresponding volume of arylomycin-treated sample) were treated with 4 μl of 4% (vol/vol) of heavy (13CD2O; Sigma) and light formaldehyde, respectively. Sodium cyanoborohydride (4 μl, 0.6 M) was added to each sample, followed by a 1-h incubation at room temperature. Reactions were quenched by addition of 16 μl of 1% ammonium hydroxide and 8 μl of 5% formic acid.
Mass spectrometry.
Mass spectrometry was performed using a Thermo Elite mass spectrometer, following previously described protocols (84). Peptides were pressure loaded onto an in-house-made 250-μm desalting column, which was connected to a 100-μm (inner diameter) fused silica capillary column with a 5-μm tip that contained 10 cm of C18 resin (Aqua 5 μm; Phenomenex) and 3 cm of SCX resin (Luna 5 μm; Phenomenex). Peptides were eluted using a five-step multidimensional liquid chromatography-mass spectrometry (LC-MS) (47) protocol consisting of increasing salt bumps of 25%, 50%, 80%, and 100% 500 mM ammonium acetate, followed by an increasing gradient of acetonitrile and 0.1% formic acid. Data were collected in data-dependent acquisition mode over a range from m/z 400 to 1,800. Each full scan was followed by up to 30 fragmentation events. Dynamic exclusion was enabled (repeat count of 1 and exclusion duration of 20 s). The data were searched using the ProLuCID algorithm against an E. coli K-12 reverse-concatenated FASTA database that was assembled from the UniProt database (48) (downloaded 9 March 2016). ProLuCID searches specified static modification of cysteine residues (m/z +57.0215; iodoacetamide alkylation) and required peptides to contain at least one tryptic terminus. Each data set was independently searched with light and heavy parameter files with static modifications on lysine and N termini selected (+28.0313 m/z and +28.0313 m/z, respectively, for the light peptide search, and +34.06312 m/z and +34.06312 m/z, respectively, for the heavy peptide search). The resulting matched tandem mass spectrometry (MS-MS) spectra were assembled into protein identifications, then filtered using DTASelect (85) (version 2.0.47). Peptides were restricted to a specified false-positive rate of ≤1%. ReDiMe ratios were quantified using in-house software, as previously described (86). Peptides detected as singletons, where only the heavy or light isotopically labeled peptide was detected and sequenced, but which passed all other filtering parameters, were given a standard ratio of 20, which is the maximum ReDiMe ratio reported herein.
Determination of SPase dependence.
Proteins with fewer than two quantified peptides in more than one replicate for any treatment regimen were excluded, and the remaining proteins were grouped based on the presence or absence of a signal peptide, as annotated in the UniProt database (48) (Fig. S2 and Table S1). The median ReDiMe ratio of proteins lacking signal peptides was calculated separately for each arylomycin treatment regimen and used to estimate the typical response to arylomycin for proteins not dependent on SPase. Proteins that exhibited a ratio of at least two median absolute deviations above these values for all treatment regimens, independent on the presence of a signal peptide, were considered to be dependent on SPase activity (Table S2). The coefficient of variance was then calculated for each of these proteins. Proteins that had >60% variance were examined manually. Peptides containing missed tryptic cleavage sites were removed and the median ratios were recalculated, after which proteins failing to meet these criteria were excluded.
Strain and plasmid construction.
Strains bearing conjugal plasmids and elements (Table 1) were constructed by mixing equal volumes of log-phase cultures of donor and recipient cells, incubating overnight, and plating serial dilutions of the mixtures onto LB agar plates with the appropriate antibiotics. To generate donor strains with wild-type arylomycin resistance, the plasmids/elements were conjugated into recipient cells harboring a kanamycin resistance cassette in place of the nonessential lactose metabolism gene lacZ. To examine the effects of higher levels of SPase inhibition, plasmids/elements were transferred into a previously described E. coli strain, LepB(P84S)::Kmr, in which a specific Pro residue, Pro84, that confers arylomycin resistance was replaced by Ser and the mutation marked with a kanamycin resistance cassette (45). For conjugation experiments testing the effects of other antibiotics, an MG1655 strain harboring a gentamicin resistance cassette in place of the nonessential arabinose metabolism gene araA was generated using the gentamicin resistance cassette from pBBR1-MCS5 (87). Additionally, a rifampin-resistant MG1655 mutant strain harboring a spontaneous RpoB(D516G) mutation was isolated by plating an overnight culture on solid medium containing rifampin at 2-fold above its MIC. The plasmid for examining SPase overexpression was constructed from the pBBR1-MCS4 backbone and put SPase expression solely under the control of the anhydrotetracycline-inducible promoter pLtetO-1.
Motility assays.
Swarming plates (LB, 0.6% agar, 0.5% dextrose) were prepared as follows: medium was autoclaved and cooled to 55°C, then dextrose and appropriate antibiotics were added, and plates were allowed to solidify for 10 min in a biosafety cabinet before inoculation in the center of the plate with 2 μl of saturated overnight culture of the appropriate E. coli strain. Plates were wrapped in parafilm, incubated at 37°C for 24 h, and then imaged using a Bio-Rad Universal Hood II/ChemiDoc XRS and white light epi-illumination. The total swarming area was defined by visual inspection and quantified using ImageJ. The procedure for swimming plates (LB, 0.3% agar) was essentially the same, with the exception that, following inoculation, plates were incubated first at room temperature for 5 h, then at 37°C for 18 h prior to imaging.
Electron microscopy.
Cultures grown on swarm plates, with or without 0.5 μg/ml arylomycin, were resuspended in phosphate-buffered saline (PBS), diluted to an OD600 of 0.2, and fixed with 4% paraformaldehyde + 1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. A suspension of cells was placed on polylysine-coated 12-mm coverslips and allowed to attach for 30 min. The cells were then buffer washed in 0.1 M cacodylate and postfixed in cacodylate-buffered 2% osmium tetroxide. After extensive water washes followed by dehydration in a graded ethanol series, the coverslips were processed through hexamethyldisilazane (HMDS) and mounted onto scanning electron microscopy (SEM) stubs with carbon tape. The stubs with attached coverslips were sputter coated with iridium (EMS 150T S model) for subsequent examination and documentation on a Hitachi S-4800 scanning electron microscope (Hitachi High Technologies America, Inc., Pleasanton, CA).
Biofilm growth and quantification.
Saturated bacterial cultures were grown overnight at 37°C and diluted to a final OD600 of 0.1 in polystyrene round-bottom 96-well plates (Greiner Bio-One) containing a concentration gradient of appropriate antibiotics in LB. Cultures (200 μl each) were then grown at 30°C without agitation for 24 h, after which plates were washed twice with water and stained for 15 min with 0.1% crystal violet (225 μl). Plates were then washed with water to remove unbound crystal violet and allowed to dry. To each well, 225 μl 95% ethanol was added, and the plates were incubated at room temperature for 15 min without agitation. The resulting solubilized crystal violet was then transferred to a new 96-well plate, and OD570 was measured using an Infinite 200 Pro (Tecan) plate reader. Average OD570 readings from wells containing LB only were calculated for each plate and subtracted from data points from the same plate to account for background absorption. Replicate wells (24) for each experiment were used (biological triplicate average values resulting from 8 technical replicates each were used for statistical analysis).
Conjugation assays.
Donor and recipient strains were recovered from glycerol stocks by growth on LB agar plates containing the appropriate antibiotics to ensure retention of plasmids/elements and then streaked onto LB agar plates without antibiotic for 18 to 24 h. Unless otherwise noted, antibiotics were used at the following concentrations: rifampin, 16 μg/ml; gentamicin, 15 μg/ml; spectinomycin, 100 μg/ml; and ciprofloxacin, 60 ng/ml. Colonies from antibiotic-free plates were suspended in LB to an OD600 of 1.0 and diluted into 3 ml LB without antibiotic to an OD600 of 0.05. Cultures were incubated with shaking at 275 rpm for approximately 100 min, until the OD600 reached 0.4 to 0.6, at which time equal volumes of donor and recipient cells were mixed, and 200 μl aliquots were immediately added to flat bottom 96-well plates containing 2 μl of the antibiotic of interest at 100-fold the desired final concentration. The 96-well mating plates were incubated at the appropriate temperature for 4 h, after which the mating cultures were serially diluted and plated onto selective medium to enumerate viable donors, recipients, and conjugates. Donor and recipient cells were selected based on their respective chromosomally encoded antibiotic resistance phenotypes (kanamycin resistance), while conjugates were selected on media containing a combination of recipient and plasmid/integrating element-specific antibiotics. All growth and conjugation steps were conducted at 37°C. Conjugation experiments testing the effect of SPase overexpression were performed similarly, except that colony suspensions were inoculated into medium containing 128 ng/ml anhydrotetracycline (aTc) to induce SPase expression prior to mating. For experiments testing the effects of antibiotics other than arylomycin A-C16, recipient cells harbored resistance genes to the particular antibiotic being tested. Conjugation frequencies are reported as conjugates per donor cell.
Determination of antibiotic sensitivities.
Antibiotics were 2-fold serially diluted in 100 μl of medium in 96-well plates. Bacteria from freshly streaked LB agar plates were used to prepare an inoculum, following standard Clinical and Laboratory Standards Institute (CLSI) guidelines for broth microdilution susceptibility testing (88). The MICs were defined as the lowest concentrations of antibiotic to prevent visible growth after 24 h of incubation at 37°C.
RNA isolation and quantitative PCR.
E. coli PAS0232 cells were grown in LB to an OD600 of 0.6 to 0.65, after which arylomycin A-C16 was added to a final concentration of 2 μg/ml. Aliquots removed immediately before arylomycin addition and after 2, 5, 10, 15, and 30 min of incubation with antibiotic were pelleted and resuspended in RNAsnap solution (18 mM EDTA, 0.025% SDS, 1% 2-mercaptoethanol, and 95% formamide) (89), vortexed, and heated at 95°C for 10 min. Samples were then centrifuged, and the supernatant was added to precipitating solution (75 mM NaOAc, 75% ethanol) for overnight incubation at −80°C. The following day, samples were pelleted to remove supernatant and washed with 75% ethanol. After pelleting again and removing ethanol, samples were air dried to remove residual ethanol. Pellets were resuspended in Tris-EDTA buffer for quantification and storage, and as much as 5 μg was used for DNase (Roche) digestion, followed by purification using the RNA Clean and Concentrate-5 kit (Zymo). Total RNA was converted to cDNA using the Superscript III First Strand synthesis kit (Invitrogen). The cDNA was subjected to reverse transcription-quantitative PCR (qRT-PCR) using the Power SYBR green master mix (Thermo Fisher), and the following 5′ to 3′ gene-specific primer pairs were used in a CFX Connect real-time PCR detection system (Bio-Rad): fliC-FP, CATTTGCCGTTGCTCCAGTC; fliC-RP, CCTTTCTACGGAAGCAGCCA; and gapA-FP, GCTCGTAAACACATCACCGC; gapA-RP, TAACTTTAGCCAGCGGAGCC). Relative quantification of each gene in the arylomycin-treated samples compared to that in the untreated control was done using the standard curve method and normalization relative to the housekeeping gene gapA.
Data availability.
The raw data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary Material
ACKNOWLEDGMENT
Funding was provided by the National Institutes of Health (grant AI081126 to F.E.R., grant CA231991 to B.F.C., and grant CA211526 to M.M.D.) and the National Science Foundation (graduate research fellowship DGE-1346837 to D.S.P.).
We thank Malcolm R. Wood and the TSRI Core Microscopy Facility for assistance in the preparation of Fig. 2.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01253-18.
REFERENCES
- 1.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan JB. 2011. Antibiotic-induced biofilm formation. Int J Artif Organs 34:737–751. doi: 10.5301/ijao.5000027. [DOI] [PubMed] [Google Scholar]
- 3.Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol 9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Stoitsova SR, Paunova-Krasteva TS, Borisova DB. 2016. Modulation of biofilm growth by sub-inhibitory amounts of antibacterial substances, Ch 19 In Dhanasekaran D, Thajuddin N (ed), Microbial biofilms–importance and application. IntechOpen; London, UK. [Google Scholar]
- 5.Blázquez J, Couce A, Rodríguez-Beltrán J, Rodríguez-Rojas A. 2012. Antimicrobials as promoters of genetic variation. Curr Opin Microbiol 15:561–569. doi: 10.1016/j.mib.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Deneve C, Bouttier S, Dupuy B, Barbut F, Collignon A, Janoir C. 2009. Effects of subinhibitory concentrations of antibiotics on colonization factor expression by moxifloxacin-susceptible and moxifloxacin-resistant Clostridium difficile strains. Antimicrob Agents Chemother 53:5155–5162. doi: 10.1128/AAC.00532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond LJ, Smith DG, Poxton IR. 2003. Effects of sub-MIC concentrations of antibiotics on growth of and toxin production by Clostridium difficile. J Med Microbiol 52:1033–1038. doi: 10.1099/jmm.0.05387-0. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Shi Y, Zhang D, Wei J, Surette MG, Duan K. 2008. Modulation of secreted virulence factor genes by subinhibitory concentrations of antibiotics in Pseudomonas aeruginosa. J Microbiol 46:441–447. doi: 10.1007/s12275-008-0054-x. [DOI] [PubMed] [Google Scholar]
- 9.Joo HS, Chan JL, Cheung GY, Otto M. 2010. Subinhibitory concentrations of protein synthesis-inhibiting antibiotics promote increased expression of the agr virulence regulator and production of phenol-soluble modulin cytolysins in community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:4942–4944. doi: 10.1128/AAC.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummins J, Reen FJ, Baysse C, Mooij MJ, O'Gara F. 2009. Subinhibitory concentrations of the cationic antimicrobial peptide colistin induce the Pseudomonas quinolone signal in Pseudomonas aeruginosa. Microbiology 155:2826–2837. doi: 10.1099/mic.0.025643-0. [DOI] [PubMed] [Google Scholar]
- 11.Barr V, Barr K, Millar MR, Lacey RW. 1986. Beta-lactam antibiotics increase the frequency of plasmid transfer in Staphylococcus aureus. J Antimicrob Chemother 17:409–413. doi: 10.1093/jac/17.4.409. [DOI] [PubMed] [Google Scholar]
- 12.Beaber JW, Hochhut B, Waldor MK. 2004. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 427:72–74. doi: 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- 13.Stevens AM, Shoemaker NB, Li LY, Salyers AA. 1993. Tetracycline regulation of genes on Bacteroides conjugative transposons. J Bacteriol 175:6134–6141. doi: 10.1128/jb.175.19.6134-6141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baquero F, Negri MC, Morosini MI, Blazquez J. 1998. Antibiotic-selective environments. Clin Infect Dis 27:S5–11. doi: 10.1086/514916. [DOI] [PubMed] [Google Scholar]
- 15.Goneau LW, Hannan TJ, MacPhee RA, Schwartz DJ, Macklaim JM, Gloor GB, Razvi H, Reid G, Hultgren SJ, Burton JP. 2015. Subinhibitory antibiotic therapy alters recurrent urinary tract infection pathogenesis through modulation of bacterial virulence and host immunity. mBio 6:e00356-15. doi: 10.1128/mBio.00356-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song T, Duperthuy M, Wai SN. 2016. Sub-optimal treatment of bacterial biofilms. Antibiotics (Basel) 5:23. doi: 10.3390/antibiotics5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis 181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 19.Hocquet D, Llanes C, Thouverez M, Kulasekara HD, Bertrand X, Plesiat P, Mazel D, Miller SI. 2012. Evidence for induction of integron-based antibiotic resistance by the SOS response in a clinical setting. PLoS Pathog 8:e1002778. doi: 10.1371/journal.ppat.1002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huddleston JR. 2014. Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes. Infect Drug Resist 7:167–176. doi: 10.2147/IDR.S48820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schjorring S, Krogfelt KA. 2011. Assessment of bacterial antibiotic resistance transfer in the gut. Int J Microbiol 2011:312956. doi: 10.1155/2011/312956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binnewies TT, Motro Y, Hallin PF, Lund O, Dunn D, La T, Hampson DJ, Bellgard M, Wassenaar TM, Ussery DW. 2006. Ten years of bacterial genome sequencing: comparative-genomics-based discoveries. Funct Integr Genomics 6:165–185. doi: 10.1007/s10142-006-0027-2. [DOI] [PubMed] [Google Scholar]
- 23.Elliott D, Burns JL, Hoffman LR. 2010. Exploratory study of the prevalence and clinical significance of tobramycin-mediated biofilm induction in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother 54:3024–3026. doi: 10.1128/AAC.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penades JR. 2005. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol 56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 25.Yim G, Wang HH, Davies J. 2007. Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–1200. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams JJ, Hergenrother PJ. 2008. Exposing plasmids as the Achilles’ heel of drug-resistant bacteria. Curr Opin Chem Biol 12:389–399. doi: 10.1016/j.cbpa.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen RC, Popat R, Diggle SP, Brown SP. 2014. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol 12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 28.Sintim HO, Smith JA, Wang J, Nakayama S, Yan L. 2010. Paradigm shift in discovering next-generation anti-infective agents: targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Future Med Chem 2:1005–1035. doi: 10.4155/fmc.10.185. [DOI] [PubMed] [Google Scholar]
- 29.Anthouard R, DiRita VJ. 2015. Chemical biology applied to the study of bacterial pathogens. Infect Immun 83:456–469. doi: 10.1128/IAI.02021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brument S, Sivignon A, Dumych TI, Moreau N, Roos G, Guerardel Y, Chalopin T, Deniaud D, Bilyy RO, Darfeuille-Michaud A, Bouckaert J, Gouin SG. 2013. Thiazolylaminomannosides as potent antiadhesives of type 1 piliated Escherichia coli isolated from Crohn's disease patients. J Med Chem 56:5395–5406. doi: 10.1021/jm400723n. [DOI] [PubMed] [Google Scholar]
- 31.Greene SE, Pinkner JS, Chorell E, Dodson KW, Shaffer CL, Conover MS, Livny J, Hadjifrangiskou M, Almqvist F, Hultgren SJ. 2014. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5:e02038. doi: 10.1128/mBio.02038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smitha Rao CV, Anné J. 2011. Bacterial type I signal peptidases as antibiotic targets. Future Microbiol 6:1279–1296. doi: 10.2217/fmb.11.109. [DOI] [PubMed] [Google Scholar]
- 33.Schimana J, Gebhardt K, Höltzel A, Schmid DG, Sussmuth R, Muller J, Pukall R, Fiedler HP. 2002. Arylomycins A and B, new biaryl-bridged lipopeptide antibiotics produced by Streptomyces sp. Tu 6075. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot 55:565–570. doi: 10.7164/antibiotics.55.565. [DOI] [PubMed] [Google Scholar]
- 34.Höltzel A, Schmid DG, Nicholson GJ, Stevanovic S, Schimana J, Gebhardt K, Fiedler HP, Jung G. 2002. Arylomycins A and B, new biaryl-bridged lipopeptide antibiotics produced by Streptomyces sp. Tü 6075. II. Structure elucidation. J Antibiot 55:571–577. doi: 10.7164/antibiotics.55.571. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Smith PA, Roberts TC, Higuchi RI, Paraselli P, Koehler MFT, Schwarz JB, Crawford JJ, Ly CQ, Hu H, Yu Z November 2016. Macrocyclic broad spectrum antibiotics. Patent PCT/CN2016/106597.
- 36.Roberts TC, Smith PA, Higuchi RI, Paraselli P, Bergeron P, Koehler MFT, Hu H, Schwarz JB, Ly C, Crawford J, Hostetler MJ May 2015. Macrocyclic broad spectrum antibiotics. Patent PCT/US2015/031631.
- 37.Smith PA, Koehler MFT, Girgis HS, Yan D, Chen Y, Chen Y, Crawford JJ, Durk MR, Higuchi RI, Kang J, Murray J, Paraselli P, Park S, Phung W, Quinn JG, Roberts TC, Rouge L, Schwarz JB, Skippington E, Wai J, Xu M, Yu Z, Zhang H, Tan MW, Heise CE. 2018. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature 561:189–194. doi: 10.1038/s41586-018-0483-6. [DOI] [PubMed] [Google Scholar]
- 38.Smith PA, Romesberg FE. 2012. Mechanism of action of the arylomycin antibiotics and effects of signal peptidase I inhibition. Antimicrob Agents Chemother 56:5054–5060. doi: 10.1128/AAC.00785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh SI, Craney A, Romesberg FE. 2016. Not just an antibiotic target: exploring the role of type I signal peptidase in bacterial virulence. Bioorg Med Chem 24:6370–6378. doi: 10.1016/j.bmc.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts TC, Smith PA, Cirz RT, Romesberg FE. 2007. Structural and initial biological analysis of synthetic arylomycin A2. J Am Chem Soc 129:15830–15838. doi: 10.1021/ja073340u. [DOI] [PubMed] [Google Scholar]
- 41.Powers ME, Smith PA, Roberts TC, Fowler BJ, King CC, Trauger SA, Siuzdak G, Romesberg FE. 2011. Type I signal peptidase and protein secretion in S. epidermidis. J Bacteriol 193:340–348. doi: 10.1128/JB.01052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts TC, Schallenberger MA, Liu J, Smith PA, Romesberg FE. 2011. Initial efforts toward the optimization of arylomycins for antibiotic activity. J Med Chem 54:4954–4963. doi: 10.1021/jm1016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schallenberger MA, Niessen S, Shao C, Fowler BJ, Romesberg FE. 2012. Type I signal peptidase and protein secretion in Staphylococcus aureus. J Bacteriol 194:2677–2686. doi: 10.1128/JB.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith PA, Powers ME, Roberts TC, Romesberg FE. 2011. In vitro activities of arylomycin natural-product antibiotics against Staphylococcus epidermidis and other coagulase-negative staphylococci. Antimicrob Agents Chemother 55:1130–1134. doi: 10.1128/AAC.01459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith PA, Roberts TC, Romesberg FE. 2010. Broad spectrum antibiotic activity of the arylomycin natural products is masked by natural target mutations. Chem Biol 17:1223–1231. doi: 10.1016/j.chembiol.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steed DB, Liu J, Wasbrough E, Miller L, Halasohoris S, Miller J, Somerville B, Hershfield JR, Romesberg FE. 2015. Origins of Yersinia pestis sensitivity to the arylomycin antibiotics and the inhibition of type I signal peptidase. Antimicrob Agents Chemother 59:3887–3898. doi: 10.1128/AAC.00181-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jessani N, Niessen S, Wei BQing. Q, Nicolau M, Humphrey M, Ji Y, Han W, Noh D-Y, Yates JR III, Jeffrey SS, Cravatt BF. 2005. A streamlined platform for high-content functional proteomics of primary human specimens. Nat Meth 2:691–697. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- 48.The UniProt Consortium. 2017. UniProt: the universal protein knowledgebase. Nucleic Acids Res 45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guttenplan SB, Kearns DB. 2013. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev 37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kearns DB. 2010. A field guide to bacterial swarming motility. Nat Rev Microbiol 8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane MC, Alteri CJ, Smith SN, Mobley HL. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A 104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters DS, Romesberg FE, Baran PS. 2018. Scalable access to arylomycins via C-H functionalization logic. J Am Chem Soc 140:2072–2075. doi: 10.1021/jacs.8b00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawley TD, Klimke WA, Gubbins MJ, Frost LS. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol Lett 224:1–15. doi: 10.1016/S0378-1097(03)00430-0. [DOI] [PubMed] [Google Scholar]
- 55.Beaber JW, Hochhut B, Waldor MK. 2002. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J Bacteriol 184:4259–4269. doi: 10.1128/JB.184.15.4259-4269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung M, Dalbey RE. 1992. Identification of potential active-site residues in the Escherichia coli leader peptidase. J Biol Chem 267:13154–13159. [PubMed] [Google Scholar]
- 57.Morisaki JH, Smith PA, Date SV, Kajihara KK, Truong CL, Modrusan Z, Yan D, Kang J, Xu M, Shah IM, Mintzer R, Kofoed EM, Cheung TK, Arnott D, Koehler MF, Heise CE, Brown EJ, Tan MW, Hazenbos WL. 2016. A putative bacterial ABC transporter circumvents the essentiality of signal peptidase. mBio 7:e00412-16. doi: 10.1128/mBio.00412-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Hiibel SR, Reardon KF, Wood TK. 2010. Identification of stress-related proteins in Escherichia coli using the pollutant cis-dichloroethylene. J Appl Microbiol 108:2088–2102. doi: 10.1111/j.1365-2672.2009.04611.x. [DOI] [PubMed] [Google Scholar]
- 59.Kern R, Malki A, Abdallah J, Tagourti J, Richarme G. 2007. Escherichia coli HdeB is an acid stress chaperone. J Bacteriol 189:603–610. doi: 10.1128/JB.01522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanoulle X, Rollet E, Clantin B, Landrieu I, Odberg-Ferragut C, Lippens G, Bohin JP, Villeret V. 2004. Structural analysis of Escherichia coli OpgG, a protein required for the biosynthesis of osmoregulated periplasmic glucans. J Mol Biol 342:195–205. doi: 10.1016/j.jmb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Zhang Y, Liu J, Liu H. 2013. PotD protein stimulates biofilm formation by Escherichia coli. Biotechnol Lett 35:1099–1106. doi: 10.1007/s10529-013-1184-8. [DOI] [PubMed] [Google Scholar]
- 62.Ludwig A, Tengel C, Bauer S, Bubert A, Benz R, Mollenkopf HJ, Goebel W. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol Gen Genet 249:474–486. doi: 10.1007/BF00290573. [DOI] [PubMed] [Google Scholar]
- 63.Cornista J, Ikeuchi S, Haruki M, Kohara A, Takano K, Morikawa M, Kanaya S. 2004. Cleavage of various peptides with pitrilysin from Escherichia coli: kinetic analyses using beta-endorphin and its derivatives. Biosci Biotechnol Biochem 68:2128–2137. doi: 10.1271/bbb.68.2128. [DOI] [PubMed] [Google Scholar]
- 64.Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R 3rd, Dubreuil JD, Harel J. 2005. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 73:4138–4145. doi: 10.1128/IAI.73.7.4138-4145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng X, Liu X, Zhang L, Hou B, Li B, Tan C, Li Z, Zhou R, Li S. 2016. Virulence characteristics of extraintestinal pathogenic Escherichia coli deletion of gene encoding the outer membrane protein X. J Vet Med Sci 78:1261–1267. doi: 10.1292/jvms.16-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogt J, Schulz GE. 1999. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 7:1301–1309. doi: 10.1016/S0969-2126(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 67.Chatfield SN, Dorman CJ, Hayward C, Dougan G. 1991. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect Immun 59:449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGrath ME, Hines WM, Sakanari JA, Fletterick RJ, Craik CS. 1991. The sequence and reactive site of ecotin. A general inhibitor of pancreatic serine proteases from Escherichia coli. J Biol Chem 266:6620–6625. [PubMed] [Google Scholar]
- 69.Vanderkelen L, Van Herreweghe JM, Vanoirbeek KG, Baggerman G, Myrnes B, Declerck PJ, Nilsen IW, Michiels CW, Callewaert L. 2011. Identification of a bacterial inhibitor against g-type lysozyme. Cell Mol Life Sci 68:1053–1064. doi: 10.1007/s00018-010-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monchois V, Abergel C, Sturgis J, Jeudy S, Claverie JM. 2001. Escherichia coli ykfE ORFan gene encodes a potent inhibitor of C-type lysozyme. J Biol Chem 276:18437–18441. doi: 10.1074/jbc.M010297200. [DOI] [PubMed] [Google Scholar]
- 71.Lutticke C, Hauske P, Lewandrowski U, Sickmann A, Kaiser M, Ehrmann M. 2012. E. coli LoiP (YggG), a metalloprotease hydrolyzing Phe-Phe bonds. Mol Biosyst 8:1775–1782. doi: 10.1039/c2mb05506f. [DOI] [PubMed] [Google Scholar]
- 72.Tokuda H. 2009. Biogenesis of outer membranes in gram-negative bacteria. Biosci Biotechnol Biochem 73:465–473. doi: 10.1271/bbb.80778. [DOI] [PubMed] [Google Scholar]
- 73.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S. 1992. Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol 226:433–446. doi: 10.1016/0022-2836(92)90958-M. [DOI] [PubMed] [Google Scholar]
- 75.Lebeaux D, Ghigo JM, Beloin C. 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foxman B. 2003. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 77.Hooton TM, Stamm WE. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 11:551–581. doi: 10.1016/S0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 78.Hagan CL, Wzorek JS, Kahne D. 2015. Inhibition of the beta-barrel assembly machine by a peptide that binds BamD. Proc Natl Acad Sci U S A 112:2011–2016. doi: 10.1073/pnas.1415955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RL, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Kach A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. 2010. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 80.Zhang G, Meredith TC, Kahne D. 2013. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr Opin Microbiol 16:779–785. doi: 10.1016/j.mib.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLeod SM, Fleming PR, MacCormack K, McLaughlin RE, Whiteaker JD, Narita S, Mori M, Tokuda H, Miller AA. 2015. Small-molecule inhibitors of gram-negative lipoprotein trafficking discovered by phenotypic screening. J Bacteriol 197:1075–1082. doi: 10.1128/JB.02352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quan S, Hiniker A, Collet JF, Bardwell JC. 2013. Isolation of bacteria envelope proteins. Methods Mol Biol 966:359–366. doi: 10.1007/978-1-62703-245-2_22. [DOI] [PubMed] [Google Scholar]
- 83.Inloes JM, Hsu KL, Dix MM, Viader A, Masuda K, Takei T, Wood MR, Cravatt BF. 2014. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc Natl Acad Sci U S A 111:14924–14929. doi: 10.1073/pnas.1413706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parker CG, Galmozzi A, Wang Y, Correia BE, Sasaki K, Joslyn CM, Kim AS, Cavallaro CL, Lawrence RM, Johnson SR, Narvaiza I, Saez E, Cravatt BF. 2017. Ligand and target discovery by fragment-based screening in human cells. Cell 168:527–541 e529. doi: 10.1016/j.cell.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tabb DL, McDonald WH, Yates JR 3rd. 2002. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MB, Bachovchin DA, Mowen K, Baker D, Cravatt BF. 2010. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 88.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 89.Stead MB, Agrawal A, Bowden KE, Nasir R, Mohanty BK, Meagher RB, Kushner SR. 2012. RNA snap™: a rapid, quantitative and inexpensive method for isolating total RNA from bacteria. Nucleic Acids Res 40:e156. doi: 10.1093/nar/gks680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cirz RT, Chin JK, Andes DR, de Crecy-Lagard V, Craig WA, Romesberg FE. 2005. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol 3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.