Buruli ulcer (BU), caused by Mycobacterium ulcerans, is a neglected tropical skin and soft tissue infection that is associated with disability and social stigma. The mainstay of BU treatment is an 8-week course of rifampin (RIF) at 10 mg/kg of body weight and 150 mg/kg streptomycin (STR).
KEYWORDS: Buruli ulcer, Mycobacterium ulcerans, clarithromycin, high-dose rifamycins, rifampin, rifapentine
ABSTRACT
Buruli ulcer (BU), caused by Mycobacterium ulcerans, is a neglected tropical skin and soft tissue infection that is associated with disability and social stigma. The mainstay of BU treatment is an 8-week course of rifampin (RIF) at 10 mg/kg of body weight and 150 mg/kg streptomycin (STR). Recently, the injectable STR has been shown to be replaceable with oral clarithromycin (CLR) for smaller lesions for the last 4 weeks of treatment. A shorter, all-oral, highly efficient regimen for BU is needed, as the long treatment duration and indirect costs currently burden patients and health systems. Increasing the dose of RIF or replacing it with the more potent rifamycin drug rifapentine (RPT) could provide such a regimen. Here, we performed a dose-ranging experiment of RIF and RPT in combination with CLR over 4 weeks of treatment in a mouse model of M. ulcerans disease. A clear dose-dependent effect of RIF on both clinical and microbiological outcomes was found, with no ceiling effect observed with tested doses up to 40 mg/kg. RPT-containing regimens were more effective on M. ulcerans. All RPT-containing regimens achieved culture negativity after only 4 weeks, while only the regimen with the highest RIF dose (40 mg/kg) did so. We conclude that there is dose-dependent efficacy of both RIF and RPT and that a ceiling effect is not reached with the current standard regimen used in the clinic. A regimen based on higher rifamycin doses than are currently being evaluated against tuberculosis in clinical trials could shorten and improve therapy of Buruli ulcer.
INTRODUCTION
Buruli ulcer (BU) is a neglected tropical disease caused by Mycobacterium ulcerans. This pathogen is phylogenetically related to M. tuberculosis and M. leprae and is believed to have evolved from a common ancestor, M. marinum (1). BU presents as skin and soft tissue lesions, potentially leading to scarring, contracture, physical impairment, and psychosocial exclusion of patients due to disfiguring wounds (2). In 2015, BU was reported from 33 countries mainly in sub-Saharan West Africa, where it predominantly occurs in rural communities (3). BU treatment formerly consisted of wide surgical excision and skin grafting (4). Following a phase of antibiotic regimen discovery and development in a murine model of BU and a subsequent clinical trial, the World Health Organization (WHO) recommended treatment with rifampin (RIF) at 10 mg/kg of body weight orally and streptomycin (STR) at 15 mg/kg by intramuscular injection, both administered daily for 8 weeks. Despite good clinical outcomes with this regimen (5, 6), injectable streptomycin causes ototoxicity in 25% to 30% of patients, nephrotoxicity, and risk of infection by contaminated needles, and daily injections confer great discomfort mainly in young Buruli ulcer patients that could lead to reduced tolerability and adherence (7). Streptomycin can be replaced by clarithromycin (CLR), which has a largely bacteriostatic (8) effect on M. ulcerans, for the last 4 weeks of the 8-week regimen for early, limited lesions (9). An ongoing trial (Clinicaltrials.gov identifier NCT01659437) is investigating an all-oral 8-week RIF+CLR regimen with the standard dose of 10 mg/kg RIF. Still, despite free treatment in most countries, the indirect costs of hospitalization and loss of income burden patients and families (10, 11). A shortened, highly effective, all-oral regimen is urgently needed to improve care for this neglected tropical disease.
RIF is a rifamycin antibiotic and the mainstay oral drug in the treatment of M. ulcerans. The currently recommended dose of 10 mg/kg has been adopted from regimens to treat tuberculosis (TB), where this dose was chosen mainly for economic reasons and in fear of toxicity associated with high-dose intermittent regimens when the drug was first introduced in the 1970s (12). Recently, interest in using higher doses of RIF as a means to shorten the duration of treatment for TB has intensified. Trials have demonstrated that treatment with up to 35 mg/kg RIF appears to be safe and offers a nonlinear increase in exposure to the drug that is associated with more rapid sputum culture conversion (13, 14). Further studies showed that high-dose RIF-containing regimens improve and shorten the duration of TB therapy (15–17). Rifapentine (RPT) is another rifamycin antibiotic that is active against M. tuberculosis and M. ulcerans. It is slightly more active in vitro against M. ulcerans, with an MIC of 0.125 μg/ml, than RIF (0.25 μg/ml) and is effective in vivo in mice at a daily dose of 10 mg/kg (18). The longer half-life of RPT also makes it an attractive alternative to RIF. Higher daily doses of RPT are more active in murine models of TB (19). Furthermore, RPT doses up to 20 mg/kg were safe in humans (20) and shown to increase the rate of sputum culture conversion in TB patients in an exposure-dependent manner that may lead to shorter treatment durations for TB (21, 22). In Buruli ulcer, a regimen of RPT 10 mg/kg/day plus CLR was shown to be more effective than RIF+STR in mice (23). Also, combining RIF at 10 to 40 mg/kg/day with clofazimine was recently shown to reduce treatment duration in the Buruli ulcer footpad model (24). We hypothesize that regimens containing high-dose RIF or RPT will be a next pivotal step in improving Buruli ulcer chemotherapy by increasing efficacy and reducing duration.
To evaluate an all-oral regimen with high-dose RIF or RPT in combination with the standard macrolide used today, CLR, we performed dose-ranging experiments with escalating doses of both drugs in combination with CLR in the mouse footpad model of BU. We identified high-dose rifamycin-containing regimens that are more bactericidal than the same all-oral regimen with RIF at the standard dose. These regimens can be evaluated in clinical trials in hopes of unburdening patients from long-term treatment, reducing indirect costs, and improving adherence.
RESULTS
Infection.
The successful implantation of viable bacteria was confirmed in footpads by a mean (±SD) CFU count of 4.09 log10 (± 0.23) CFU on the day after infection. In untreated animals, clinical pathology, as assessed by swelling grade, stayed at a level of 2 during the first 2 weeks of the treatment phase and improved to 1.25 in week 4 (Fig. 1). Relative light unit (RLU) counts also declined over time in untreated mice but less so than in treated animals (Fig. 2).
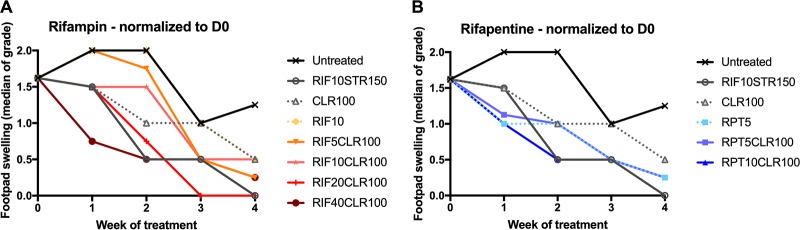
FIG 1.
Footpad swelling grade of infected mouse footpads in response to treatment with high-dose rifamycins and clarithromycin. Treatment was initiated 6 weeks after infection, when swelling approached swelling grade 2. Swelling grade 0 corresponds to no clinically visible pathology. Swelling grade 1 infers redness of the footpad, grade 2 edematous swelling of the footpad, and grade 3 ascending swelling of the leg and impeding necrosis. Data points represent medians per treatment group. Data were normalized to day 0 (beginning of treatment) by subtracting from the median swelling grade of all mice at D0 and assuming the total median as group mean for that time point. All regimens reduced swelling grade compared with untreated mice. There was a visible dose-dependent effect; with escalating doses of rifampin (A) and rifapentine (B), swelling grade was reduced more drastically. Minimum swelling grade values of 0.23 and 0.15 were recorded at week 4 for the highest-dose regimens RIF40CLR100 and RPT20CLR100, respectively. Numbers after drugs indicate doses in mg/kg. D, day; RIF, rifampin; STR, streptomycin; CLR, clarithromycin; RPT, rifapentine.
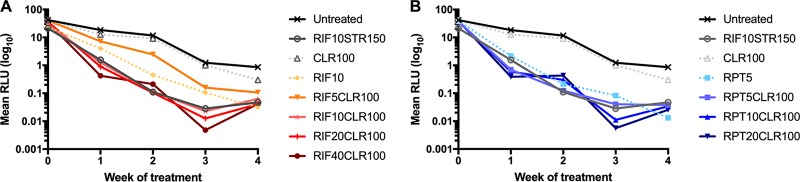
FIG 2.
Mean relative light unit (RLU) counts from infected mouse footpads in response to treatment with escalating doses of rifamycins. Mice were infected with an autoluminescent strain of M. ulcerans that emits light when metabolically active. The mean per group RLU values are displayed, compared with day 0 when the mean (±SD) RLU was 35.09 (±6.89). As observed in previous experiments, RLU counts stagnate at 7- to 9-weeks postinfection (i.e., here, 3 weeks of treatment) when a static bacterial growth phase is reached, as seen in untreated mice. Treatment with rifamycins radically reduced RLU counts compared with untreated and CLR-only-treated mice. RIF, rifampin; STR, streptomycin; CLR, clarithromycin; RPT, rifapentine.
Response to treatment.
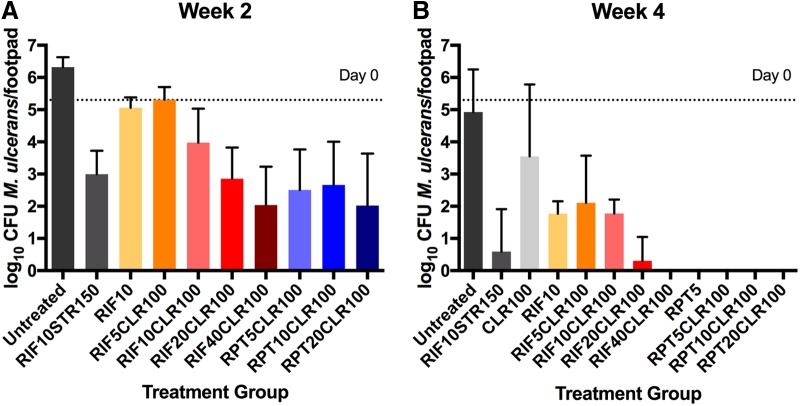
Other than 100 mg/kg CLR (CLR100), all treatment regimens reduced the number of RLUs measured from footpads. Treatments 5 mg/kg RIF + 100 mg/kg CLR (RIF5CLR100) and 10 mg/kg RIF (RIF10) were less effective than the other combination treatments which all had very similar effects on RLU counts. However, the swelling grade was affected in a more dose-dependent manner. At week 2, the median swelling of untreated mice was 2 with 6.31 (± 0.31) log10 CFU. The positive-control regimen 10 mg/kg RIF + 150 mg/kg STR (R10S150) led to rapid clinical improvement, as shown by decreased swelling grade (median 0.5), which is in agreement with previous studies (25). Monotherapy with CLR100 performed worst in all parameters, as expected. All test regimens resulted in a statistically significant (P < 0.0015 to P < 0.0001) reduction in CFU by week 2 compared with untreated mice, except RIF10 and RIF5CLR100 (Fig. 3; Table S1). Animals treated with CLR100 and 5 mg/kg RPT (RPT5) were not sacrificed for CFU evaluation at week 2. RIF-containing combination regimens efficiently reduced footpad CFU counts by week 2 in a dose-dependent fashion (Fig. 3). RPT-containing regimens had a larger effect in terms of swelling grade and CFU reduction than RIF-containing regimens (Fig. 3). All groups receiving rifapentine or rifampin at ≥20 mg/kg had mean CFU counts as low or lower than the rifampin-streptomycin control. Although no dose-response effect was observed for RPT, the mean CFU count in the RPT5CLR100-treated group includes one footpad with no detectable CFU which was likely due to a nonproductive infection, implying unsuccessful implantation of a sufficient quantity of bacteria to produce disease. This assumption is supported by the observation that a very low RLU count of 1.22 was detected from this mouse at day 0 (D0), the start of treatment, while all other mice in that group had an RLU above 20. Excluding this one footpad, the mean CFU count is 2.98 ± 0.96. After 4 weeks of treatment, the swelling grades were reduced to minimum medians of 0.25 and 0.125 in the high-dose 40 mg/kg RIF + 100 mg/kg CLR (R40CLR100) and 20 mg/kg RPT + 100 mg/kg CLR (P20CLR100) groups, respectively. In terms of CFU reduction, all treatment groups, except CLR100, resulted in a statistically significant reduction of CFU compared with untreated mice (P = 0.030 to P = 0.001). All high-dose RIF- and RPT-containing regimens performed better than standard R10CLR100 (P = 0.001) and no worse than the RS control. Low-dose RIF5CLR100 performed less well, especially if 2 footpads with no detectable CFU were excluded as possibly never being productively infected (in which case the mean CFU count was 2.64 ± 1.09). Footpads from the R40CLR100 group and all RPT-containing regimens were culture negative after 4 weeks of treatment (i.e., no colonies could be observed after plating the undiluted footpad homogenate). The CFU counts in RIF10 and R10CLR100 groups after 4 weeks of treatment were both 1.77 (±0.39 or 0.44). Thus, the addition of CLR to RIF10 seemed to have no effect on the outcome in this experiment.
FIG 3.
Microbiological outcome after 2 (A) and 4 (B) weeks of treatment with rifamycin-containing regimens. Mice were infected with 4.56 log10 CFUs of M. ulcerans into hind footpads. After 6 weeks of incubation, treatment was initiated (D0). At this time point, the CFU mean (±SD) equaled 5.77 (±0.60). Groups of mice (n = 5) were sacrificed at week 2 and week 4, and footpads were dissected, minced, and plated on 7H11 agar for colony counting and CFU analysis. There was a dose-dependent reduction in CFU with elevating rifamycin doses. No ceiling effect was observed for RIF. At week 4, all rifapentine-containing regimens were culture negative, as was RIF40CLR100. Numbers after drugs indicate doses in mg/kg. RIF, rifampin; STR, streptomycin; CLR, clarithromycin; RPT, rifapentine.
The previously WHO-recommended treatment of R10S150 resulted in the highest healing rate, as evidenced by the finding that 80% of treated animals exhibited a swelling grade of 0 upon completion of treatment. The swelling grade was reduced to 0 in 60% and 50% of animals receiving R20CLR100 and P20CLR100, respectively.
DISCUSSION
Drug development for Buruli ulcer is greatly hampered by little economic interest for this neglected tropical disease. Repurposing and refining existing antibiotic regimens is a viable, rapid, and relatively cost-effective measure to provide better care for BU patients. Here, we reevaluated the dosage of RIF in the RC regimen for BU where the combination of 40 mg/kg RIF with 100 mg/kg CLR, as well as all RPT-containing regimens, were most efficacious in reducing BU disease, as measured by swelling grade, and hastening the time to culture negativity in the mouse footpads. These findings are timely and important because high-dose RIF- and RPT-containing regimens are currently being evaluated in phase 3 clinical trials for the ability to shorten the duration of TB treatment after promising results in phase 2 trials (17, 21). Our results indicate that similar dose optimization should be explored to enhance the efficiency of all-oral regimens for BU.
Shorter treatment would benefit patients by reducing indirect costs and barriers to treatment and increasing adherence (10, 11, 26). The results for monotherapy with standard doses of RIF, RPT, and CLR were similar to previous studies (8). With RIF, a clear dose-response relationship was demonstrated, with both clinical (swelling grade) and microbiological (CFU) outcomes. There appeared to be no ceiling effect with the doses tested. In contrast, for RPT, no dose-response effect was evident at week 2, and the CFU counts were reduced to 0 in all groups receiving RPT at week 4 of treatment. It is possible that the pharmacological ceiling was reached. However, this would be surprising given the increasing dose-response relationship up to RPT doses of 160 mg/kg that we previously observed in a mouse model of TB (27). Despite a 3-day interval between the last dose of drug and sacrificing animals for microbiological analysis of footpads, we cannot exclude RPT drug carryover, owing to its long half-life and possible elevated tissue concentration (18). In terms of RLU, no clear difference between rifampin and rifapentine was seen (Fig. 2).
A dose-response relationship for RPT may have been evident if we had held mice beyond the end of treatment to determine if relapse occurred. While demonstrating cure without relapse is considered to be the gold standard outcome for preclinical TB efficacy models (28), culture negativity and relapse-free follow-up rates are debated as outcomes of choice in the antimicrobial evaluation for M. ulcerans infection models (18). The hallmark of M. ulcerans infection is the presence of mycolactone, the analgesic, necrosis-causing, and immune-suppressive toxin (29, 30). The reduction of the bacterial burden to a critical threshold at which the host immune response gains foothold enough to clear the remainder of bacteria may be sufficient to successfully treat the infection. If so, then comparing regimens on the basis of bactericidal activity alone, and not a relapse endpoint, would be reasonable. Clinically, relapse after a successful antimicrobial therapy of M. ulcerans infection is exceedingly rare. For example, Klis et al. have shown that defaulters from the 8-week regimen often proceeded to heal their lesions despite the early discontinuation of antimicrobial therapy (31). We hypothesize that more mice from culture-negative as well as low-CFU-treatment groups would have progressed to swelling grade 0 during follow-up. Future experiments following this initial dose-ranging, proof-of-principle study will include longer follow-up durations and additional secondary outcomes, such as histopathological analysis of treated footpads.
Integrated approaches to control neglected tropical diseases are increasingly advocated. Thus, it is noteworthy that high-dose, short-course regimens with rifampin are being investigated as potential therapeutic agents for lymphatic filariasis and onchocerciasis (32) in addition to TB, offering potential synergies in the research and implementation of new, more efficient regimens in areas of coendemicity.
We successfully employed a mouse model of M. ulcerans disease and achieved comparable clinical and microbiological results in the control groups as in previous experiments with the MU1059AL strain (33, 34). The decline in swelling grade and CFU in untreated mice can be attributed to a transition into a stationary phase at about 7- to 9-weeks postinfection. Although the autoluminescent strain used in this experiment was previously reported to be fully virulent, we cannot exclude the possibility that it is slightly less virulent than its wild-type parent, rendering it more prone to spontaneous clearance from the footpads (34).
In summary, high-dose RIF and RPT in combination with CLR performed well in a mouse model of M. ulcerans disease and warrant further investigation. These regimens can be administered orally; the elevated doses appear safe in humans when administered for 2 months or less (13, 14, 20). As BU has already been treated with RIF and clarithromycin for several years, logistics and handling knowledge is already in place with health care providers and a dose adjustment could be easily implemented if shown effective in humans. One potential issue is that higher doses of rifamycins are likely to have a greater inductive effect on CLR metabolism in patients and could lead to subtherapeutic CLR exposures. The overall role of CLR in BU therapy is unclear. We observed a reduction in swelling (Fig. 1) and an additive effect on RIF alone (Fig. 3) in terms of CFU outcomes, but CLR monotherapy seems to be of only limited efficacy. Macrolides have inherent anti-inflammatory activity that may contribute to healing in BU treatment. We are currently evaluating other potential companion drugs for high-dose rifamycins, such as other macrolides, clofazimine, oxazolidinones, and newly available, highly potent antimycobacterial agents developed for TB treatment. We will proceed to test whether they can cure M. ulcerans as short-course regimens in mice and, ultimately, patients.
MATERIALS AND METHODS
Ethical clearance.
Animal experiments described in this study were conducted in strict adherence with the Animal Welfare Act and Public Health Service Policy. Experiments were performed at the Johns Hopkins University which is accredited by the private Association for the Assessment and Accreditation of Laboratory Animal Care International. All procedures involving mice were approved by the Johns Hopkins University Animal Care and Use Committee.
Bacteria.
M. ulcerans 1059 is an isolate originating from a clinical specimen from a patient in Ghana. An autoluminescent version of this isolate, Mu1059AL, was generated in our laboratory, as previously described (33, 34). The MICs for the Mu1059AL strain are 0.06 μg/ml for RIF, 0.50 μg/ml for STR and 0.13 μg/ml for CLR, as described earlier (34); the MIC for Mu1059AL for RPT was not determined. Bacteria are passaged in our lab in mouse footpads, and frozen footpad homogenate suspensions were stored and used for infection of mice in this study. To quantify CFUs in the inoculum, serial 10-fold dilutions of the thawed stock footpad homogenate were plated onto 7H11 agar. CFUs from footpads of animals harvested during the experiment were assessed as discussed below. All bacteria were cultured on Middlebrook 7H11 selective agar (Becton, Dickinson, Sparks, MD) at 32°C.
Antibiotics.
RIF and STR were purchased from Sigma (St. Louis, MO, USA). RPT and CLR were kindly provided by Sanofi (Bridgewater, NJ, USA) and Abbott (Abbott Park, IL, USA), respectively. RIF, RPT, and STR were dissolved in distilled water, while CLR was suspended in distilled water with 0.05% agarose. The doses for RIF were 5, 10, 20, and 40 mg/kg; the doses for RPT were 5, 10, and 20 mg/kg; the dose for STR was 150 mg/kg; and the dose for CLR was 100 mg/kg. The doses for the rifamycins produce exposures similar to the average plasma area under the concentration time-curve (AUC) observed in humans at doses up to 35 mg/kg for RIF and 20 mg/kg for RPT (17, 35). The doses for STR and CLR produce plasma AUC values similar to human doses of 15 mg/kg and 7.5 mg/kg, respectively (36–39).
Infection and treatment.
Female BALB/c mice (n = 110), aged 4 to 6 weeks, were purchased from Charles River (Wilmington, MA, USA) and allowed to acclimatize for 5 days upon arrival at the facility. Food and water were provided ad libitum. Mice were infected with approximately 4.56 log10 CFU of Mu1059AL in 0.03 ml PBS into both hind footpads via subcutaneous injection. Five untreated mice were sacrificed the following day to confirm the number of implanted CFU. Animals were regularly checked for signs of general illness and progression of footpad infection. The swelling grade was used to evaluate M. ulcerans-induced footpad pathology, as previously described (38). A swelling grade of 0 corresponds to a normal footpad, grade 1 to a noninflammatory but swollen footpad, grade 2 to inflammatory swelling with edema and redness, and grade 3 to swelling of the entire hindfoot. At week 6 postinfection (day 0), animals were randomized into treatment groups, and treatment was initiated. Drugs were administered once daily, 5 days per week. STR was administered by subcutaneous injection. All other drugs were administered in 0.2 ml doses via esophageal gavage. Sacrifices for CFU counts were always performed 3 days after the last dose of antibiotic was administered to minimize a drug carryover effect.
Treatment outcome monitoring.
After treatment commenced, the swelling grade and RLU emitted from autoluminescent bacteria in the footpads were measured weekly, as previously described (34), while mice were anesthetized via intraperitoneal injection of 87.5/12.5 mg/kg ketamine/xylazine (Zoetis, Kalamazoo, MI). At day 0, week 2, and week 4, 5 mice per group were sacrificed to assess CFU counts. Mouse footpad tissue was carefully harvested from both hind footpads with the exception of the well-defined control groups RIF10, RIF10CLR100, and RIF10STR150 where only one footpad was analyzed per mouse. Tissue was finely minced with scissors before being suspended in 2.0 ml PBS. The suspensions were vortexed for homogenization, and quantitative bacterial cultures were performed as described above. Half of the footpad homogenate was plated and the lower limit of detection was 2 CFU/footpad.
Data analysis.
CFU counts were log transformed. To compare mean CFU between treatment groups, the Student’s t test and analysis of variance (ANOVA) were used. The value of α was set at 0.05. Data were analyzed and graphs were computed using GraphPad Prism version 7.0a (GraphPad Software, Inc., San Diego, CA).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (R01-AI113266). T.F.O. was supported by a personal grant from the Junior Scientific Masterclass at the University of Groningen, The Netherlands.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01478-18.
REFERENCES
- 1.Doig KD, Holt KE, Fyfe JAM, Lavender CJ, Eddyani M, Portaels F, Yeboah-Manu D, Pluschke G, Seemann T, Stinear TP. 2012. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genomics 13:258. doi: 10.1186/1471-2164-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Zeeuw J, Omansen TF, Douwstra M, Barogui YT, Agossadou C, Sopoh GE, Phillips RO, Johnson C, Abass KM, Saunderson P, Dijkstra PU, van der Werf TS, Stienstra Y, Stientstra Y. 2014. Persisting social participation restrictions among former Buruli ulcer patients in Ghana and Benin. PLoS Negl Trop Dis 8:e3303. doi: 10.1371/journal.pntd.0003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2018. Buruli ulcer (Mycobacterium ulcerans infection). WHO, Geneva, Switzerland: http://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection). Accessed 1 November 2018. [Google Scholar]
- 4.van der Werf TS, Stienstra Y, Johnson RC, Phillips R, Adjei O, Fleischer B, Wansbrough-Jones MH, Johnson PDR, Portaels F, van der Graaf WTA, Asiedu K. 2005. Mycobacterium ulcerans disease. Bull World Health Organ 83:785–791. [PMC free article] [PubMed] [Google Scholar]
- 5.Etuaful S, Carbonnelle B, Grosset J, Lucas S, Horsfield C, Phillips R, Evans M, Ofori-Adjei D, Klustse E, Owusu-Boateng J, Amedofu GK, Awuah P, Ampadu E, Amofah G, Asiedu K, Wansbrough-Jones M. 2005. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother 49:3182–3186. doi: 10.1128/AAC.49.8.3182-3186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization, Global Buruli Ulcer Initiative. 2004. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. 2014. Long term streptomycin toxicity in the treatment of Buruli ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis 8:e2739. doi: 10.1371/journal.pntd.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. 2011. Activities of rifampin, rifapentine and clarithromycin alone and in combination against Mycobacterium ulcerans disease in mice. PLoS Negl Trop Dis 5:e933. doi: 10.1371/journal.pntd.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, Tuah W, Awua-Boateng NY, Ampadu EO, Siegmund V, Schouten JP, Adjei O, Bretzel G, van der Werf TS. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664–672. doi: 10.1016/S0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 10.Amoakoh HB, Aikins M. 2013. Household cost of out-patient treatment of Buruli ulcer in Ghana: a case study of Obom in Ga South Municipality. BMC Health Serv Res 13:507. doi: 10.1186/1472-6963-13-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters Grietens K, Um Boock A, Peeters H, Hausmann-Muela S, Toomer E, Muela Ribera J. 2008. “It is me who endures but my family that suffers”: social isolation as a consequence of the household cost burden of Buruli ulcer free of charge hospital treatment. PLoS Negl Trop Dis 2:e321. doi: 10.1371/journal.pntd.0000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, Gillespie SH, Boeree MJ. 2011. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 52:e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 13.Jindani A, Borgulya G, de Patiño IW, Gonzales T, de Fernandes RA, Shrestha B, Atwine D, Bonnet M, Burgos M, Dubash F, Patel N, Checkley AM, Harrison TS, Mitchison D. 2016. A randomised phase II trial to evaluate the toxicity of high-dose rifampicin to treat pulmonary tuberculosis. Int J Tuberc Lung Dis 20:832–838. doi: 10.5588/ijtld.15.0577. [DOI] [PubMed] [Google Scholar]
- 14.Boeree MJ, Diacon AH, Dawson R, Narunsky K, Bois Du J, Venter A, Phillips PPJ, Gillespie SH, McHugh TD, Hoelscher M, Heinrich N, Rehal S, van Soolingen D, van Ingen J, Magis-Escurra C, Burger D, Plemper van Balen G, Aarnoutse RE. 2015. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 191:1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A. 2015. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 6:641. doi: 10.3389/fmicb.2015.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milstein M, Lecca L, Peloquin C, Mitchison D, Seung K, Pagano M, Coleman D, Osso E, Coit J, Vargas Vasquez DE, Sanchez Garavito E, Calderon R, Contreras C, Davies G, Mitnick CD. 2016. Evaluation of high-dose rifampin in patients with new, smear-positive tuberculosis (HIRIF): study protocol for a randomized controlled trial. BMC Infect Dis 16:453. doi: 10.1186/s12879-016-1790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M, PanACEA consortium. 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida DV, Converse PJ, Li S-Y, Tyagi S, Nuermberger EL, Grosset JH. 2013. Bactericidal activity does not predict sterilizing activity: the case of rifapentine in the murine model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 7:e2085. doi: 10.1371/journal.pntd.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 56:4331–4340. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, Fuchs EJ, Melia MT, Burman WJ, Dorman SE. 2012. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin Pharmacol Ther 91:881–888. doi: 10.1038/clpt.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorman SE, Savic RM, Goldberg S, Stout JE, Schluger N, Muzanyi G, Johnson JL, Nahid P, Hecker EJ, Heilig CM, Bozeman L, Feng P-JI, Moro RN, MacKenzie W, Dooley KE, Nuermberger EL, Vernon A, Weiner M. 2015. Daily rifapentine for treatment of pulmonary tuberculosis. a randomized, dose-ranging trial. Am J Respir Crit Care Med 191:333–343. doi: 10.1164/rccm.201410-1843OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jindani A, Harrison TS, Nunn AJ, Phillips PPJ, Churchyard GJ, Charalambous S, Hatherill M, Geldenhuys H, McIlleron HM, Zvada SP, Mungofa S, Shah NA, Zizhou S, Magweta L, Shepherd J, Nyirenda S, van Dijk JH, Clouting HE, Coleman D, Bateson ALE, McHugh TD, Butcher PD, Mitchison DA, RIFAQUIN Trial Team. 2014. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 371:1599–1608. doi: 10.1056/NEJMoa1314210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauffour A, Robert J, Veziris N, Aubry A, Jarlier V. 2016. Sterilizing activity of fully oral intermittent regimens against Mycobacterium ulcerans infection in mice. PLoS Negl Trop Dis 10:e0005066. doi: 10.1371/journal.pntd.0005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Converse PJ, Almeida DV, Tasneen R, Saini V, Tyagi S, Ammerman NC, Li S-Y, Anders NM, Rudek MA, Grosset JH, Nuermberger EL. 2018. Shorter-course treatment for Mycobacterium ulcerans disease with high-dose rifamycins and clofazimine in a mouse model of Buruli ulcer. PLoS Negl Trop Dis 12:e0006728. doi: 10.1371/journal.pntd.0006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Converse PJ, Tyagi S, Xing Y, Li S-Y, Kishi Y, Adamson J, Nuermberger EL, Grosset JH. 2015. Efficacy of rifampin plus clofazimine in a murine model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 9:e0003823. doi: 10.1371/journal.pntd.0003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velink A, Woolley RJ, Phillips RO, Abass KM, van der Werf TS, Agumah E, de Zeeuw J, Klis S, Stienstra Y. 2016. Former Buruli ulcer patients’ experiences and wishes may serve as a guide to further improve Buruli ulcer management. PLoS Negl Trop Dis 10:e0005261. doi: 10.1371/journal.pntd.0005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal I, Zhang M, Grosset J, Nuermberger E. 2008. Is it possible to cure TB in weeks instead of months?, abstr 19. Abstr. 1st Int. Workshop Clin Pharmacol Tuberculosis Drugs, Toronto, Canada. [Google Scholar]
- 28.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. 2015. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 211:S83–S95. doi: 10.1093/infdis/jiv183. [DOI] [PubMed] [Google Scholar]
- 29.George KM, Pascopella L, Welty DM, Small PL. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect Immun 68:877–883. doi: 10.1128/IAI.68.2.877-883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marion E, Song O-R, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, Guérineau NC, Saint André J-P, Gersbach P, Altmann K-H, Stinear TP, Comoglio Y, Sandoz G, Preisser L, Delneste Y, Yeramian E, Marsollier L, Brodin P. 2014. Mycobacterial toxin induces analgesia in Buruli ulcer by targeting the angiotensin pathways. Cell 157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 31.Klis S, Kingma RA, Tuah W, van der Werf TS, Stienstra Y. 2016. Clinical outcomes of Ghanaian Buruli ulcer patients who defaulted from antimicrobial therapy. Trop Med Int Health 21:1191–1196. doi: 10.1111/tmi.12745. [DOI] [PubMed] [Google Scholar]
- 32.Aljayyoussi G, Tyrer HE, Ford L, Sjoberg H, Pionnier N, Waterhouse D, Davies J, Gamble J, Metugene H, Cook DAN, Steven A, Sharma R, Guimaraes AF, Clare RH, Cassidy A, Johnston KL, Myhill L, Hayward L, Wanji S, Turner JD, Taylor MJ, Ward SA. 2017. Short-course, high-dose rifampicin achieves Wolbachia depletion predictive of curative outcomes in preclinical models of lymphatic filariasis and onchocerciasis. Sci Rep 7:210. doi: 10.1038/s41598-017-00322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Li S-Y, Nuermberger EL. 2012. Autoluminescent Mycobacterium tuberculosis for rapid, real-time, non-invasive assessment of drug and vaccine efficacy. PLoS One 7:e29774. doi: 10.1371/journal.pone.0029774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Li S-Y, Converse PJ, Grosset JH, Nuermberger EL. 2013. Rapid, serial, non-invasive assessment of drug efficacy in mice with autoluminescent Mycobacterium ulcerans infection. PLoS Negl Trop Dis 7:e2598. doi: 10.1371/journal.pntd.0002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savic RM, Lu Y, Bliven-Sizemore E, Weiner M, Nuermberger E, Burman W, Dorman SE, Dooley KE. 2014. Population pharmacokinetics of rifapentine and desacetyl rifapentine in healthy volunteers: nonlinearities in clearance and bioavailability. Antimicrob Agents Chemother 58:3035–3042. doi: 10.1128/AAC.01918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grosset JH. 1994. Assessment of new therapies for infection due to the Mycobacterium avium complex: appropriate use of in vitro and in vivo testing. Clin Infect Dis 18:S233–S236. doi: 10.1093/clinids/18.Supplement_3.S233. [DOI] [PubMed] [Google Scholar]
- 37.Grosset J, Ji B. 1998. Experimental chemotherapy of mycobacterial diseases, p 51–97. In Mycobacteria. Springer US, Boston, MA. [Google Scholar]
- 38.Dega H, Bentoucha A, Robert J, Jarlier V, Grosset J. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother 46:3193–3196. doi: 10.1128/AAC.46.10.3193-3196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tessier PR, Kim M-K, Zhou W, Xuan D, Li C, Ye M, Nightingale CH, Nicolau DP. 2002. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob Agents Chemother 46:1425–1434. doi: 10.1128/AAC.46.5.1425-1434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.