We performed bedaquiline broth microdilution susceptibility testing using Clinical and Laboratory Standards Institute (CLSI) guidelines on 104 nonduplicate isolates of Mycobacterium abscessus complex [M. abscessus subsp. abscessus (76); M. abscessus subsp.
KEYWORDS: Mycobacterium abscessus complex, bedaquiline, nontuberculous mycobacteria, susceptibility
ABSTRACT
We performed bedaquiline broth microdilution susceptibility testing using Clinical and Laboratory Standards Institute (CLSI) guidelines on 104 nonduplicate isolates of Mycobacterium abscessus complex [M. abscessus subsp. abscessus (76); M. abscessus subsp. massiliense (10); M. abscessus subsp. bolletii (2); and M. abscessus subsp. abscessus-M. abscessus subsp. massiliense hybrid, i.e., M. abscessus subsp. abscessus by rpoB gene and M. abscessus subsp. massiliense by erm(41) gene (16)]. All isolates from patients not known to have been on bedaquiline prior had MIC values of ≤0.25 μg/ml. The bedaquiline MIC50 value for all 76 isolates of M. abscessus subsp. abscessus and 16 isolates of M. abscessus subsp. abscessus-M. abscessus subsp. massiliense hybrid was 0.06 μg/ml. The MIC50 and MIC90 values for 10 isolates of M. abscessus subsp. massiliense were 0.12 μg/ml. Only two isolates of M. abscessus subsp. bolletii were tested with bedaquiline MICs of 0.06 μg/ml. Our study suggests that oral bedaquiline may have potential use in the treatment of disease caused by the M. abscessus complex. Combination therapy with other agents (imipenem, cefoxitin, amikacin, and/or tigecycline) is recommended.
INTRODUCTION
Bedaquiline, previously TMC207 or R207910, trade name Sirturo (Janssen Therapeutics, Inc.), is a diarylquinoline which strongly inhibits the mycobacterial enzyme complex ATP synthase, interfering with energy production and homeostasis in the cell (1–7). Bedaquiline was the first FDA-approved drug in 40 years for the treatment of pulmonary multidrug-resistant (MDR) Mycobacterium tuberculosis (8). It has been previously noted that bedaquiline also exhibits antimycobacterial activity against several species of nontuberculous mycobacteria (NTM), including Mycobacterium avium complex (MAC) (6, 9) and the Mycobacterium abscessus complex (10–12).
Recent reports suggest that, at least in many geographic areas in the United States, the prevalence of NTM surpasses that of Mycobacterium tuberculosis (13). The incidence of rapidly growing mycobacteria (RGM), predominantly the M. abscessus complex, also appears to be increasing, especially in pulmonary disease (14, 15). NTM are ubiquitous in the environment (especially in municipal water supplies), although the reservoir for M. abscessus is largely unknown. NTM have been associated with health care infections, outbreaks, and pseudo-outbreaks globally (16–18). It should also be noted that although Mycobacterium tuberculosis is a reportable disease in the United States, in most states, NTM are not required to be reported; thus, the prevalence of NTM, including the M. abscessus complex, may be underestimated.
Members of the M. abscessus complex are the most difficult to treat RGM due to their resistance to multiple drugs and are responsible for respiratory disease in older patients with bronchiectasis and younger patients with cystic fibrosis. Members of this complex are encountered in skin and soft tissue infections following trauma and/or surgery, disseminated disease, and other often fatal infections. Thus, new antimicrobials for the treatment of RGM, including the M. abscessus complex (especially oral agents), are desperately needed.
RESULTS
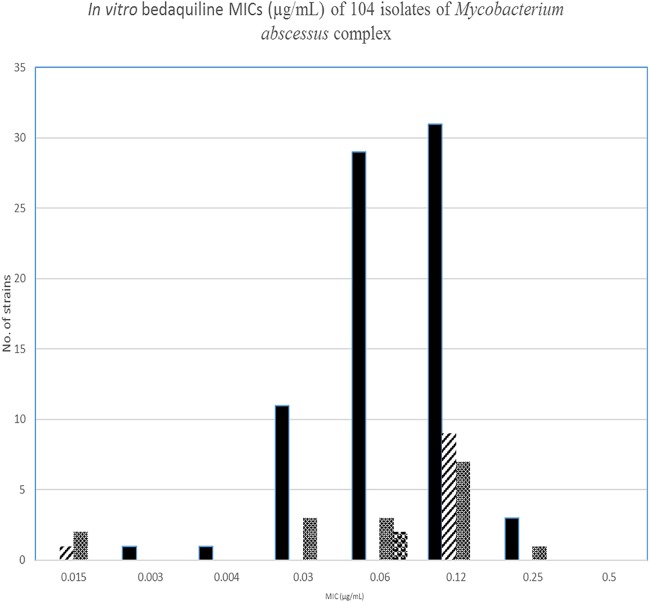
Bedaquiline MICs were determined for 104 nonduplicate isolates of the M. abscessus complex from 94 respiratory cultures (approximately 90%) and 10 isolates from wounds and disseminated disease. Bedaquiline MICs ranged from 0.008 to 0.5 μg/ml, with an MIC50 of 0.06 and MIC90 value of 0.12 μg/ml for 76 isolates of M. abscessus subsp. abscessus. The MIC range for bedaquiline with 10 isolates of M. abscessus subsp. massiliense was 0.015 to 0.12 μg/ml, with MIC50 and MIC90 values of 0.12 μg/ml. Sixteen isolates of an M. abscessus subsp. abscessus-M. abscessus subsp. massiliense hybrid had a bedaquiline MIC range of 0.03 to 0.25 μg/ml, with an MIC50 of 0.06 μg/ml and MIC90 of 0.12 μg/ml. For two isolates of M. abscessus subsp. bolletii, the MICs were 0.06 μg/ml (Fig. 1 and Table 1).
FIG 1.
In vitro bedaquiline MICs (μg/ml) of 104 isolates of Mycobacterium abscessus complex. The epidemiological cutoff value (ECV) for nontuberculous mycobacteria has not yet been addressed by the Clinical and Laboratory Standards Institute (CLSI). However, using the software designed for fungal and some bacterial isolates, the 95% subset ECV for these isolates of the M. abscessus complex was 0.25 μg/ml and 0.5 μg/ml for the M. abscessus subsp. abscessus and M. abscessus subsp. massiliense (including the hybrid isolates), respectively.
TABLE 1.
MIC ranges, MIC50 values, and MIC90 values of 104 isolates of the Mycobacterium abscessus complex against bedaquiline
| Species (no. of isolates) | Antimicrobial | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|---|
| M. abscessus subsp. abscessus (76) | Bedaquiline | 0.004 to 0.25 | 0.06 | 0.12 |
| Amikacin | ≤1 to >64 | 16 | 32 | |
| Cefoxitin | 16 to 64 | 64 | 64 | |
| Ciprofloxacin | 2 to >4 | >4 | >4 | |
| Clarithromycin | ≤2 to >8 | ≥8 | ≥8 | |
| Doxycycline | >16 | >16 | >16 | |
| Imipenem | 4 to >64 | 16 | 32 | |
| Linezolid | ≤1 to >32 | 8 | 16 | |
| Minocycline | >8 | >8 | >8 | |
| Moxifloxacin | 2 to >8 | 8 | >8 | |
| Tigecycline | 0.06 to 1 | 0.25 | 0.5 | |
| Trimethoprim-sulfamethoxazole | 0.5/4.75 to 8/152 | 4/76 | 8/152 | |
| M. abscessus subsp. massiliense (10) | Bedaquiline | 0.015 to 0.12 | 0.12 | 0.12 |
| Amikacin | 4 to 32 | 8 | 16 | |
| Cefoxitin | 32 to 64 | 64 | 64 | |
| Ciprofloxacin | 4 to 8 | 4 | 8 | |
| Clarithromycin | ≤2 | ≤2 | ≤2 | |
| Doxycycline | >16 | >16 | >16 | |
| Imipenem | 4 to 16 | 8 | 16 | |
| Linezolid | 2 to 16 | 8 | 16 | |
| Minocycline | 4 to >8 | >8 | >8 | |
| Moxifloxacin | 2 to >8 | 8 | >8 | |
| Tigecycline | 0.06 to 0.25 | 0.25 | 0.25 | |
| Trimethoprim-sulfamethoxazole | 4/76 to 8/152 | 8/152 | 8/152 | |
| M. abscessus subsp. abscessus-M. abscessus subsp. massiliense (hybrid) (16) | Bedaquiline | 0.03 to 0.25 | 0.06 | 0.12 |
| Amikacin | 4 to 32 | 16 | 16 | |
| Cefoxitin | 32 to 64 | 64 | 64 | |
| Ciprofloxacin | 2 to >4 | >4 | >4 | |
| Clarithromycin | ≤2 | ≤2 | ≤2 | |
| Doxycycline | >16 | >16 | >16 | |
| Imipenem | 8 to 64 | 16 | 32 | |
| Linezolid | 2 to 16 | 4 | 16 | |
| Minocycline | 4 to >8 | >8 | >8 | |
| Moxifloxacin | 4 to >8 | 8 | >8 | |
| Tigecycline | 0.06 to 0.5 | 0.25 | 0.5 | |
| Trimethoprim-sulfamethoxazole | 4/76 to 8/152 | 8/152 | 8/152 | |
| M. abscessus subsp. abscessus-M. abscessus subsp. bolletii (2) | Bedaquiline | 0.06 | ||
| Amikacin | 8 to 16 | |||
| Cefoxitin | 32 | |||
| Ciprofloxacin | >4 | |||
| Clarithromycin | >8 | |||
| Doxycycline | >16 | |||
| Imipenem | 8 to 16 | |||
| Linezolid | 8 to 16 | |||
| Minocycline | >8 | |||
| Moxifloxacin | 8 to >8 | |||
| Tigecycline | 0.25 | |||
| Trimethoprim-sulfamethoxazole | 4/76 to 8/152 |
To our knowledge, none of the patients were treated with bedaquiline or clofazimine prior to our testing. MICs of the comparator antimicrobials were within expected range for each of the subspecies. Two clinical isolates of M. abscessus subsp. abscessus had mutational resistance to amikacin and one isolate of the M. abscessus subsp. abscessus-M. abscessus subsp. massiliense had clarithromycin mutational resistance. One isolate of M. abscessus subsp. abscessus had unusual susceptibility to trimethoprim-sulfamethoxazole (TMP-SMX).
There are currently no CLSI or manufacturer guidelines for testing bedaquiline against NTM, and because our laboratory is not a biosafety level 3 facility, this prohibited testing Mycobacterium tuberculosis (the only species for which the manufacturer provided guidelines for MICs) against the agent. Quality control for M. peregrinum ATCC 700686 was within the acceptable ranges for all comparator antimicrobials. Thirteen replicates of this strain of M. peregrinum had bedaquiline MICs from 0.008 to 0.015 μg/ml. Bedaquiline MICs ranged from 0.12 to 0.25 μg/ml for M. abscessus subsp. abscessus ATCC 19977T for 11 replicates, and 15 replicates of M. smegmatis ATCC 19240 had MICs of 0.004 to 0.015 μg/ml (see Table 2).
TABLE 2.
MICs and MIC ranges of three mycobacterial reference strains tested against bedaquiline
| Reference strain | MIC range (μg/ml) | No. of strains at an MIC (μg/ml) of: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.002 | 0.004 | 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | ||
| Mycobacterium abscessus subsp. abscessus ATCC 19977T | 0.12–0.25 | 8 | 3 | |||||||
| Mycobacterium peregrinum ATCC 700686 | 0.008–0.015 | 2 | 11 | |||||||
| Mycobacterium smegmatis ATCC 19420 | 0.004–0.015 | 1 | 11 | 3 | ||||||
DISCUSSION
For the M. abscessus complex, new additions to the current treatment armamentarium are critically needed. Our current study demonstrates that bedaquiline MICs, at least with untreated patients, are within clinically achievable ranges and, thus, may provide a promising treatment option for the M. abscessus complex. Moreover, this novel diarylquinoline would be the first potentially useful oral agent for this complex since the introduction of the macrolides more than two decades ago.
Previous studies have shown that bedaquiline resistance in M. intracellulare is associated with a nonsynonymous mutation in the atpE gene or in one of multiple nonsynonymous mutations of the efflux repressor gene mmpT5 (19, 20). It is also known that the amino acid sequence is highly conserved in some species of NTM, including members of MAC (8). Whole-genome sequencing has indicated that the locus associated with low-level bedaquiline resistance in M. tuberculosis (Rv0678) has no ortholog in the MAC (21, 22). Recent studies by Alexander and colleagues have shown that low-level bedaquiline resistance in MAC (i.e., 2- to 8-fold increase in MICs) is associated with the efflux repressor gene mmpT5 nonsynonymous mutations, while bedaquiline-resistant MAC strains with high-level resistance contain atpE mutations. Although nonsynonymous mutations were commonly seen in the repressor gene mmpT5 for the efflux operon mmpL5-mmpS5 among relapse isolates of MAC, nonsynonymous mutations in the ATP synthase subunit E (atpE) were only observed in two cases (21).
A 2017 study (of 685 NTM, presumed untreated strains) in China reported 218 isolates of M. abscessus subsp. abscessus and 163 isolates of M. abscessus subsp. massiliense with bedaquiline MIC50 and MIC90 values of 0.13 μg/ml and >16 μg/ml (23), respectively. The explanation for the high MICs is not clear, as the highest MICs in our current study were only 0.25 μg/ml. Sequencing of the atpE genes revealed the highest frequency of genetic polymorphisms in M. abscessus subsp. massiliense (21/163, 12.8%) compared with 21/218 (9.6%) in M. abscessus subsp. abscessus. An analysis of nucleotide substitutions showed that all polymorphisms represented synonymous changes. Fewer than 10 isolates were reported to be resistant to bedaquiline, although no breakpoint for bedaquiline against isolates of NTM, including with the M. abscessus complex, has been established. No sequencing studies of an efflux repressor gene were reported in this study (23).
Our MICs were comparable to those obtained in a study performed in France; Dupont and colleagues tested 32 clinical (including cystic fibrosis and non-cystic-fibrosis patients) isolates of the M. abscessus complex, including 11 M. abscessus subsp. abscessus, 12 M. abscessus subsp. massiliense, and 9 M. abscessus subsp. bolletii. Bedaquiline MICs ranged from 0.031 to 0.062 μg/ml for M. abscessus subsp. abscessus, from 0.062 to 0.125 μg/ml for M. abscessus subsp. massiliense, and from 0.031 to 0.125 μg/ml for M. abscessus subsp. bolletii (24). MIC50 values were 0.06 μg/ml for M. abscessus subsp. abscessus and M. abscessus subsp. massiliense compared with 0.06 μg/ml for the former and 0.12 μg/ml for the latter subspecies in our study.
Although Alexander and colleagues identified one nonsynonymous atpE gene mutation associated with a 50-fold increase in bedaquiline MICs in a bedaquiline-treated strain of M. intracellulare, Pang and colleagues in China found no nonsynonymous mutations in any of the NTM species that they sequenced, including isolates of M. intracellulare, the M. abscessus complex, M. fortuitum, M. avium, and M. kansasii (21, 23).
Several preclinical infection models have demonstrated variable antimycobacterial activity of bedaquiline. In a 2014 study in France, Lerat and colleagues showed that bedaquiline did not affect the bacillary load of M. abscessus ATCC 19977T, except for a statistically insignificant decrease in the bacillary load of approximately 1.5 log10 in the lungs and spleen of nude mice at one month. They also showed that there was no increase in the bedaquiline MICs of the type strain of M. abscessus following treatment, although MICs were performed by macrodilution in brain heart infusion broth and agar (bedaquiline MICs, 0.06 and 0.5 μg/ml, respectively) rather than the CLSI-recommended broth microdilution in cation-adjusted Mueller-Hinton broth (25). Despite the broth and methodology used in their study, the broth MICs were similar to our findings among clinical isolates in the current study (25). Nude mouse model studies in 2015 from the United States have also shown that the use of bedaquiline monotherapy, which may be associated with high bedaquiline MICs, did not prevent death (10). However, this study differed as the investigators used a clinical strain of M. abscessus. The bedaquiline MIC was reported as 1 μg/ml using alamarBlue added to broth microdilution wells.
The same US investigators evaluated bedaquiline activity against M. abscessus-infected gamma interferon knockout (GKO), granulocyte-macrophage colony-stimulating factor (GM-CSF) knockout, and severe combined immunodeficiency (SCID) nude mice (10). The investigators demonstrated that M. abscessus infection of SCID, nude and GM-CSF−/− mice resulted in a sustained high level of infection, with lung pathology similar to that of human disease. They also showed statistically significant decreases in mycobacterial load in spleen and liver following 5 days of bedaquiline treatment (10). Following 8 days of bedaquiline treatment, there was a decrease in mycobacterial load in the lung, spleen, and liver (10).
A later small study performed outside the United States (and not using the current CLSI antimicrobial susceptibility testing [AST] guidelines), showed bedaquiline MICs of 0.062 and 0.25 μg/ml for two reference strains of M. abscessus (11). The same investigators also studied other RGM species (1 to 2 isolates each) of M. smegmatis, M. phlei, M. cosmeticum, M. mucogenicum, M. peregrinum, M. parafortuitum, M. fortuitum, M. mageritense, M. wolinskyi, M. chelonae, and M. franklinii, with bedaquiline MICs ranging from 0.007 to 0.062 μg/ml. Additionally, one of each of three reference strains of RGM (Mycobacterium flavescens, Mycobacterium duvalii, and Mycobacterium neoaurum) had bedaquiline MICs of ≥2 μg/ml, and a new finding was a mutation in the atpE gene in M. flavescens associated with bedaquiline resistance (11).
The 2017 study by Dupont et al. used a zebrafish preclinical mode to show that bedaquiline caused a rapid depletion of ATP in isolates of M. abscessus-infected zebrafish (24). This action was consistent with the drug targeting the FoF1 ATP synthase. Single point mutations were introduced into genes encoding the drug target to construct genetically isogenic mutant strains using a multicopy plasmid carrying atpE and the mutation. Bedaquiline pressure was achieved by growing the M. abscessus isolates in a concentration 4 to 8 times the MIC, which allowed the selection of double homologous recombination events. Subsequent curing of the plasmid lead to the development of an isogenic strain of M. abscessus differing from the parent strain by one single nucleotide polymorphism (SNP) in atpE. Amino acid substitutions led to high resistance in bedaquiline. The authors speculated this was due to the structured interference of the mutations with the binding of bedaquiline (24).
Additionally, Dupont and colleagues selected spontaneous resistant mutants exhibiting low levels of bedaquiline resistance. They sequenced atpE, atpG, and pepQ as well as three genes encoding TetR repressors (MAB_4384, MAB_4312, and MAB_4709c) of MmpL5-like proteins. However, no SNPs were found in any of these genes. The authors concluded that although their results precluded MAB_4382c as an efflux pump in bedaquiline resistance in M. abscessus, further studies are needed to determine if other MmpL members could play a role in the development of low-level bedaquiline resistance in M. abscessus (24).
A previous preliminary clinical study by Philley, et al. (26) indicated that bedaquiline had potential in vivo activity in a small number (four) of patients with refractory M. abscessus and MAC lung disease. Three of four patients with M. abscessus (all M. abscessus subsp. abscessus) showed a decrease in semiquantitative sputum counts after 5 months of treatment with bedaquiline (in combination with companion agents, including amikacin, imipenem, cefoxitin, and/or tigecycline), with one patient converting from 4+ baseline growth to negative at that time interval (26).
Based upon previous experience with mycobacterial (NTM) clinical trials, we would not add a single drug to a failing regimen, as microbiologic relapses after culture conversion/clinical improvement was common (26) and a subsequent study of the M. intracellulare isolates showed all to have developed mutational resistance (21). In the previous study by Alexander et al., the isolates with mutational resistance involving the Mmp efflux system only showed minimal (1 dilution) susceptibility differences (21). Hence, we recommend that bedaquiline not be administered as monotherapy or as the addition of a single agent to a failing regimen. Despite the Lerat et al. findings in the mouse model, composite data from the 2015 clinical study, the Pang in vitro study in China, the US animal models, and the current in vitro study in the United States suggest that bedaquiline may be a promising addition to therapeutic regimens for the M. abscessus complex. More in vitro studies are necessary to establish optimal test methods, quality control, and MIC breakpoints for bedaquiline with NTM, including the M. abscessus complex. Further genomic studies may also confirm additional mechanisms of resistance of bedaquiline which will help to elucidate more information about this unique antimicrobial. Moreover, larger clinical trials are needed to confirm the efficacy of bedaquiline in the management of disease due to the M. abscessus complex (23, 25, 26).
The results of this current study are similar to another study performed in China by Li and coworkers in 2018 using a broth microdilution method. Specific details of the AST method were not given, but for 191/197 strains, MICs were ≤0.25 μg/ml (12). Importantly, these investigators showed that for 4/6 isolates with MICs of ≥0.5 μg/ml, transcription of the M. abscessus mmpL5 gene was elevated. This is consistent with the suggestion that low-level bedaquiline resistance may be due to MmpSL-mediated drug efflux.
As has been noted previously, the unique target and mechanism of resistance for bedaquiline, along with the apparent lack of cross-resistance with other antimicrobial agents except for clofazimine, make the potential use of this agent in combination with other agents, including amikacin, imipenem, cefoxitin, and/or tigecycline, for the treatment of NTM, including the M. abscessus complex, promising (27, 28).
MATERIALS AND METHODS
We performed broth microdilution and determined MICs of 104 clinical isolates of the M. abscessus complex (including M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, M. abscessus subsp. bolletii, and M. abscessus subsp. abscessus-M. abscessus subsp. massiliense hybrid) submitted to the Mycobacteria/Nocardia Laboratory at the University of Texas Health Science Center at Tyler from 2014 to 2018. No duplicate isolates were tested.
Isolates were identified by rpoB and erm(41) gene sequences (29, 30). The hybrid group was identified as M. abscessus subsp. abscessus by the rpoB gene and M. abscessus subsp. massiliense by sequencing of the erm(41) gene.
Isolates were tested by broth microdilution antimicrobial susceptibility testing (AST) in cation-adjusted Mueller-Hinton broth using customized frozen microtiter panels from Thermo Fisher (previously Trek Diagnostics, Cleveland, OH) with doubling dilution concentration (0.0005 to 4 μg/ml) following the Clinical and Laboratory Standards Institute (CLSI) recommended procedure for NTM, although there are no specific guidelines for bedaquiline susceptibility testing (31). MICs were read using a mirrored light box after incubation at 3 to 5 days at 30°C when sufficient growth was evident 55 isolates were read at 3 days, 37% were read at 4 days, and only 9% were read at 5 days. Comparator antimicrobials amikacin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline, imipenem, linezolid, minocycline, moxifloxacin, tigecycline, and trimethoprim-sulfamethoxazole were also tested using the commercially available lyophilized panels. CLSI breakpoints for susceptibility of RGM were followed except for tigecycline for which there are no breakpoints established (31).
There were no manufacturer guidelines for MIC quality control (QC) testing of bedaquiline against NTM. However, we used several reference strains, including Mycobacterium peregrinum ATCC 700686 (replicates tested 13 times) which is currently recommended by the CLSI for AST of RGM, the type strain of M. abscessus ATCC 19977 (replicates tested 11 times), and Mycobacterium smegmatis ATCC 19420 (replicates tested 15 times) for QC of the bedaquiline MIC testing.
ACKNOWLEDGMENTS
We thank the laboratory staff at the University of Texas Health Science Center at Tyler, including Megan Ashcraft, Georgie Bush, Amber McKinney, and Kelly Ritter, for their excellent laboratory assistance in performing susceptibility testing. We thank Sruthi Vasireddy, Elena Iakhiaeva, Terry Smith, Adrian Almodovar, and Ravikiran Vasireddy for molecular identification of the isolates; Katie Shipp and Jacob Mooney for formatting the data and strain maintenance; and Joanne Woodring for her superior manuscript assistance. We appreciate the comments and support by Koné Kaniga of Janssen Pharmaceuticals and the molecular expertise of our colleague David Alexander.
Portions of this work were funded by a grant from Janssen Pharmaceutical Company.
REFERENCES
- 1.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huitric E, Verhasselt P, Koul A, Andries K, Hoffner SE, Andersson DI. 2010. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 54:1022–1028. doi: 10.1128/AAC.01611-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. 2009. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmetti L, Le Du D, Jachym M, Henry B, Martin D, Caumes E, Veziris N, Metivier N, Robert J, Andrejak C, Bernard C, Brossier F, Chadelat K, Dautzenberg B, Jarlier V, Raskine L, Rivoire B, Veziris N, Appere C, Assouline P, Borie R, Boukari L, Caseris M, Caumes E, Douadi Y, Dumoulin J, Duval C, Faucher JF, Gallien S, Godet C, Le Grusse J, Lopes A, Meynard JL, Naccache JM, Philippe B, Richaud C, Saad H, MDR-TB Management Group of the French National Reference Center for Mycobacteria and the Physicians of the French MDR-TB Cohort. 2015. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 60:188–194. doi: 10.1093/cid/ciu786. [DOI] [PubMed] [Google Scholar]
- 5.Palomino JC, Martin A. 2013. TMC207 becomes bedaquiline, a new anti-TB drug. Future Microbiol 8:1071–1080. doi: 10.2217/fmb.13.85. [DOI] [PubMed] [Google Scholar]
- 6.Lounis N, Gevers T, Van Den Berg J, Vranckx L, Andries K. 2009. ATP synthase inhibition of Mycobacterium avium is not bactericidal. Antimicrob Agents Chemother 53:4927–4929. doi: 10.1128/AAC.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox E, Laessig K. 2014. FDA approval of bedaquiline—the benefit-risk balance for drug-resistant tuberculosis. N Engl J Med 371:689–691. doi: 10.1056/NEJMp1314385. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan R. 2013. Bedaquiline: first FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res 3:1–2. doi: 10.4103/2229-516X.112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Elliott BA, Philley JV, Griffith DE, Thakkar F, Wallace RJ Jr. 2017. In vitro susceptibility testing of bedaquiline against Mycobacterium avium complex. Antimicrob Agents Chemother 61:e01798-16. doi: 10.1128/AAC.01798-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obregón-Henao A, Arnett KA, Henao-Tamayo M, Massoudi L, Creissen E, Andries K, Lenaerts AJ, Ordway DJ. 2015. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 59:6904–6912. doi: 10.1128/AAC.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar-Ayala DA, Cnockaert M, André E, Andries K, Gonzalez-Y-Merchand JA, Vandamme P, Palomino JC, Martin A. 2017. In vitro activity of bedaquiline against rapidly growing nontuberculous mycobacteria. J Med Microbiol 66:1140–1143. doi: 10.1099/jmm.0.000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Ye M, Guo Q, Zhang Z, Yang S, Ma W, Yu F, Chu H. 2018. Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 62:e00175-18. doi: 10.1128/AAC.00175-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epron E, Cassidy M, Marshall-Olson A, Hedberg K, Winthrop KL. 2012. Patients with nontuberculous mycobacteria: comparison of updated and previous diagnostic criteria for lung disease. Diagn Microbiol Infect Dis 74:98–100. doi: 10.1016/j.diagmicrobio.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Stout JE, Koh W-J, Yew WW. 2016. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Brown-Elliott BA, Wallace RJ Jr. 2012. Nontuberculous mycobacteria, p 593–608. In Mayhall CG. (ed), Hospital epidemiology and infection control, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 17.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrman J-L, Daffe M, Brosch R, Risler J-L, Gaillard JL. 2009. Non-mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One 4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker AW, Lewis SS, Alexander BD, Chen LF, Wallace RJ Jr, Brown-Elliott BA, Isaacs PJ, Pickett LC, Patel CB, Smith PK, Reynolds JM, Engel J, Wolfe CR, Milano CA, Schroder JN, Davis RD, Hartwig MG, Stout JE, Strittholt N, Maziarz EK, Saullo JH, Hazen KC, Walczak RJ Jr, Vasireddy R, Vasireddy S, McKnight CM, Anderson DJ, Sexton DJ. 2017. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 64:902–911. doi: 10.1093/cid/ciw877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, De Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 20.Huitric E, Verhasselt P, Andries K, Hoffner SE. 2007. In vitro antimycobacterial spectrum of a diarylquinolone ATP synthase inhibitor. Antimicrob Agents Chemother 51:4202–4204. doi: 10.1128/AAC.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander DC, Vasireddy R, Vasireddy S, Philley JV, Brown-Elliott BA, Perry BJ, Griffith DE, Benwill JL, Cameron ADS, Wallace RJ. 2017. Emergence of mmpT5 variants during bedaquiline treatment of Mycobacterium intracellulare lung disease. J Clin Microbiol 55:574–584. doi: 10.1128/JCM.02087-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diacon AH, Pym A, Grobusch M, de los Rios JM, Gotuzzo E, Vasilyeva I, Leimane V, Andries K, Bakare N, De Marez T, Haxaire-Theeuwes M, Lounis N, Meyvisch P, De Paepe E, van Heeswijk RPG, Dannemann B, TMC207-C208 Study Group. 2014. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 371:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 23.Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. 2017. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother 61:e02627-16. doi: 10.1128/AAC.02627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupont C, Viljoen A, Thomas S, Roquet-Banères F, Hermann J-L, Pethe K, Kremer L. 2017. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 61:e01225-17. doi: 10.1128/AAC.01225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerat I, Cambau E, dit Bettoni RR, Gaillard J-L, Jarlier V, Truffot C, Veziris N. 2014. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209:905–912. doi: 10.1093/infdis/jit614. [DOI] [PubMed] [Google Scholar]
- 26.Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, Aksamit TR, Griffith DE. 2015. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 148:499–506. doi: 10.1378/chest.14-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown-Elliott BA, Philley JV. 2017. Rapidly growing mycobacteria, p 703–723. In Schlossberg D. (ed), Tuberculosis and nontuberculous mycobacterial infections, 7th ed ASM Press, Washington, DC. [Google Scholar]
- 28.Yu JLX, Jiang G, Fu Y, Huo F, Ma Y, Wang F, Shang Y, Liang Q, Xue Y, Huang H. 2018. In vitro activity of clofazimine against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob Agents Chemother 62:e00072-18. doi: 10.1128/AAC.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adékambi T, Colson P, Drancourt M. 2003. rpoB-based identification of nonpigmented and late pigmented rapidly growing mycobacteria. J Clin Microbiol 41:5699–5708. doi: 10.1128/JCM.41.12.5699-5708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nash KA, Brown-Elliott BA, Wallace RJ Jr. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes: approved standard, 2nd ed CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]