The objective of this study was to determine the phenotypic patterns of antibiotic resistance and the epidemiology of drug-resistant Campylobacter spp. from a low-resource setting.
KEYWORDS: Campylobacter, Iquitos, MAL-ED, antibiotic resistance, diarrhea
ABSTRACT
The objective of this study was to determine the phenotypic patterns of antibiotic resistance and the epidemiology of drug-resistant Campylobacter spp. from a low-resource setting. A birth cohort of 303 patients was followed until 5 years of age. Stool samples from asymptomatic children (n = 10,008) and those with diarrhea (n = 3,175) were cultured for Campylobacter. Disk diffusion for ciprofloxacin (CIP), nalidixic acid (NAL), erythromycin (ERY), azithromycin (AZM), tetracycline (TE), gentamicin (GM), ampicillin (AMP), amoxicillin and clavulanic acid (AMC), ceftriaxone (CRO), chloramphenicol (C), and trimethoprim-sulfamethoxazole (TMS) was determined. Antibiotic resistances in Campylobacter jejuni and non-C. jejuni isolates from surveillance and diarrhea samples were compared, and the association between personal macrolide exposure and subsequent occurrence of a macrolide-resistant Campylobacter spp. was assessed. Of 917 Campylobacter isolates, 77.4% of C. jejuni isolates and 79.8% of non-C. jejuni isolates were resistant to ciprofloxacin, while 4.9% of C. jejuni isolates and 24.8% of non-C. jejuni isolates were not susceptible to azithromycin. Of the 303 children, 33.1% had been diagnosed with a Campylobacter strain nonsusceptible to both azithromycin and ciprofloxacin. Personal macrolide exposure did not affect the risk of macrolide-resistant Campylobacter. Amoxicillin and clavulanic acid (94.0%) was one of the antibiotics with the highest rates of susceptibility. There is a high incidence of quinolone- and macrolide-resistant Campylobacter infections in infants under 24 months of age. Given the lack of association between personal exposure to macrolides and a subsequent Campylobacter infection resistant to macrolides, there is a need to evaluate the source of multidrug-resistant (MDR) Campylobacter. This study provides compelling evidence to propose amoxicillin/clavulanic acid as a treatment for campylobacteriosis.
INTRODUCTION
Campylobacter is a globally disseminated Gram-negative zoonotic bacterium that is the main cause of gastrointestinal disease in children and adults. The principal transmission pathways of Campylobacter include fecal contamination of undercooked meat, water, and other poultry by-products (1). In industrialized nations, Campylobacter jejuni is the most common species of Campylobacter isolated in patients with diarrheal disease, followed by Campylobacter coli (2–5). In low-income settings, the epidemiology of campylobacteriosis may be distinct, and recent studies suggest that other Campylobacter species such as C. hyointestinalis and C. concisus are a major cause of diarrheal disease in pediatric populations were the disease is endemic (6–8).
In recent years, C. jejuni and C. coli resistance to antibiotics has increased throughout the world (9–12). Specifically, high levels of resistance to fluoroquinolones and macrolides in C. jejuni and C. coli isolates, as well as emerging resistance to aminoglycosides, have been reported in human and animal isolates (11–14). Although Campylobacter-associated diarrhea is generally a self-limiting disease and antibiotic treatment is not commonly advised (15), for patients with severe symptoms, dysentery, and compromised immunological systems and pregnant women, treatment is warranted (16). Fluoroquinolones, specifically, ciprofloxacin, were once considered the main treatment option (16). However, due to high levels of resistance to this drug, macrolides, namely, azithromycin, are the currently recommended first line of treatment for regular citizens and deployed military personnel as stated by the Infectious Disease Society of America, American College of Gastroenterology, and the U.S. military (16–21). Nonetheless, the recent emergence of macrolide resistance is beginning to threaten this treatment option (11, 22, 23).
Published statistics on antibiotic resistance in Campylobacter isolates are limited by the number of isolates analyzed. The vast majority of data comes from studies involving a few dozen isolates, and only a few studies report data on more than one hundred isolates, which includes data from countries such as Thailand (24, 25), South Korea (26), China (27), Japan (28), Tanzania (29), Peru (12, 30), Sweden (31, 32), Finland (33), and Poland (34, 35).
Two global multicenter studies identified Campylobacter as a pathogen with one of the highest attributable burdens of pathogenic diarrhea among children under 2 or 5 years of age (36, 37). Specifically, the results from the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health (MAL-ED) cohort in Iquitos, Peru, show that the highest incidence of diarrhea in children under 12 months of age is attributed to Campylobacter infections. Among children under 2 years of age, Campylobacter is the second leading diarrhea-causing pathogen (36). In this same setting in a separate cohort of children 0 to 5 years of age, symptomatic and asymptomatic Campylobacter infections were associated with reduced weight over a 3-month period, and severe symptomatic infections were associated with reduced linear growth (38). A reanalysis of the Global Enteric Multicenter Study (GEMS) case-control study found Campylobacter among the top 6 pathogens with the highest attributable burden to diarrhea. Thus, there is compelling evidence of disease burden attributable to Campylobacter infections in pediatric populations of low-resource settings. However, neither of these studies have reported (to date) antibiotic resistance patterns of the Campylobacter isolates obtained.
The majority of human observational studies reporting antibiotic resistance in Campylobacter have been conducted in high-income settings, and only a limited number of longitudinal studies have assessed antibiotic resistance in Campylobacter species isolates from children. Therefore, there is a need to present evidence on the burden of antibiotic-resistant Campylobacter on pediatric populations. Characterizing the patterns and epidemiology of antibiotic-resistant Campylobacter in a low-resource tropical area is also of critical importance for guiding clinical management, as routine antimicrobial resistance (AMR) testing is not done in most settings where the disease is endemic, as well as for guiding clinical antibiotic stewardship and regulating veterinary antibiotic usage in both low- and high-income settings. As 18% of culture-confirmed cases of Campylobacter within the United States are associated with international travel, the importance of characterizing drug-resistant Campylobacter infections in developing areas of the world (39) is relevant to U.S. and European populations as well as the populations from which the data are derived. Finally, we evaluate the effect of azithromycin and erythromycin administration for therapeutic purposes on the risk of acquisition of macrolide-resistant Campylobacter strains.
RESULTS
A total of 303 children were enrolled between 2009 and 2012 and followed until 5 years of age. From these, 242 children (79.9%) had tested positive for Campylobacter spp. by culture (8). Between March 2010 and February 2016, 10,008 surveillance fecal samples, 3,174 diarrhea samples, and 22 samples of undetermined status were submitted for stool culture (N = 13,204). Nine-hundred seventeen Campylobacter species isolates were cultured: 664 were from surveillance (asymptomatic) samples and 252 were from diarrheal samples, translating into an isolation rate of 6.6% among surveillance fecal samples and 7.9% among diarrhea samples. One Campylobacter strain was isolated from a sample of undetermined status. C. jejuni was identified in 596 samples (65.0%), of which 169 (28.4.0%) correspond to diarrheal samples. Non-C. jejuni isolates were identified in 321 samples (35.0%), of which 83 (25.9%) corresponded to diarrheal samples (Table 1). Throughout the follow-up period, 26.6% of children had only 1 isolate of Campylobacter cultured, and 53.3% of children had between 2 and 4 isolates identified.
TABLE 1.
Types of fecal samples associated with Campylobacter species
| Speciesa | % (n) of samples |
Total (n) | |
|---|---|---|---|
| Diarrhea | Surveillance | ||
| C. jejuni | 28.4 (169) | 71.5 (426) | 596b |
| Non-C. jejuni | 25.9 (83) | 74.1 (238) | 321 |
In 917 stool samples from which Campylobacter was isolated, non-jejuni isolates accounted for 35% of the total number of Campylobacter isolates.
One C. jejuni isolate was not determined as diarrhea or surveillance.
The prevalence of phenotypic resistance for all antibiotics tested is presented in Table 2. The most effective oral antibiotic was amoxicillin and clavulanic acid. The highest levels of resistance were recorded for ciprofloxacin: 77.4% of C. jejuni isolates and 79.8% of non-C. jejuni isolates. Azithromycin resistance was detected in only 4.9% of C. jejuni isolates and in 24.8% of non-C. jejuni isolates. All azithromycin-resistant isolates were also ciprofloxacin resistant with the exception of 1 C. jejuni and 1 non-C. jejuni isolate. Other striking patterns include tetracycline resistance in 55.8% of C. jejuni isolates and in 49.0% of non-C. jejuni isolates, as well as gentamicin resistance in 15.8% of non-C. jejuni isolates. No significant longitudinal trends in antibiotic resistance and multidrug resistance were observed throughout the 5-year study period. Multidrug resistance (defined as phenotypic nonsusceptibility to 3 or more classes of antibiotics) was observed in 56.8% (335/590) of C. jejuni isolates and 59.1% (176/298) of non-C. jejuni isolates. Concomitant phenotypic resistance to ciprofloxacin and azithromycin was observed in 24.5% (75/298) of non-C. jejuni isolates and in 4.8% (28/588) of C. jejuni isolates. Concomitant resistance to ciprofloxacin, azithromycin, and gentamicin was observed in 13.8% (41/298) of non-C. jejuni isolates and in 0.8% (5/596) of C. jejuni isolates (Fig. 1).
TABLE 2.
Phenotypic antibiotic susceptibility of Campylobacter jejuni and non-Campylobacter jejuni isolates
| Antibiotic |
C. jejuni isolates |
Non-C. jejuni isolates |
||||||
|---|---|---|---|---|---|---|---|---|
| % (n)a |
Total (N) | % (n) |
Total (N) | |||||
| R | I | S | R | I | S | |||
| CIP | 77.4 (455) | 1.7 (10) | 20.9 (123) | 588 | 79.8 (237) | 1.1 (3) | 19.2 (57) | 297 |
| NAL | 64.9 (383) | 35.1 (207) | 590 | 80.1 (237) | 19.9 (59) | 296 | ||
| ERY | 5.3 (31) | 0.2 (1) | 94.6 (556) | 588 | 25.2 (75) | 74.8 (223) | 298 | |
| AZM | 4.9 (29) | 0.2 (1) | 94.9 (558) | 588 | 24.8 (74) | 75.2 (224) | 298 | |
| TE | 55.8 (328) | 44.2 (250) | 588 | 49 (146) | 51 (152) | 298 | ||
| AMP | 46.8 (276) | 3.9 (23) | 49.3 (291) | 590 | 50.7 (151) | 8.7 (26) | 40.6 (121) | 298 |
| AMC | 0.7 (4) | 0.3 (2) | 98.9 (584) | 590 | 1 (3) | 5.0 (15) | 94.0 (280) | 298 |
| C | 0.2 (1) | 99.8 (589) | 590 | 0.3 (1) | 0.3 (1) | 99.3 (296) | 298 | |
| CRO | 44.7 (263) | 28.7 (169) | 26.7 (157) | 589 | 55.0 (164) | 22.1 (66) | 22.8 (68) | 298 |
| GM | 1 (6) | 0.2 (1) | 98.8 (581) | 589 | 15.8 (47) | 0.3 (1) | 83.9 (250) | 298 |
| TMS | 85.2 (501) | 1.7 (10) | 13.1 (77) | 588 | 80.9 (241) | 3.4 (10) | 15.8 (47) | 298 |
R, resistant; I, intermediate; S, susceptible.
FIG 1.
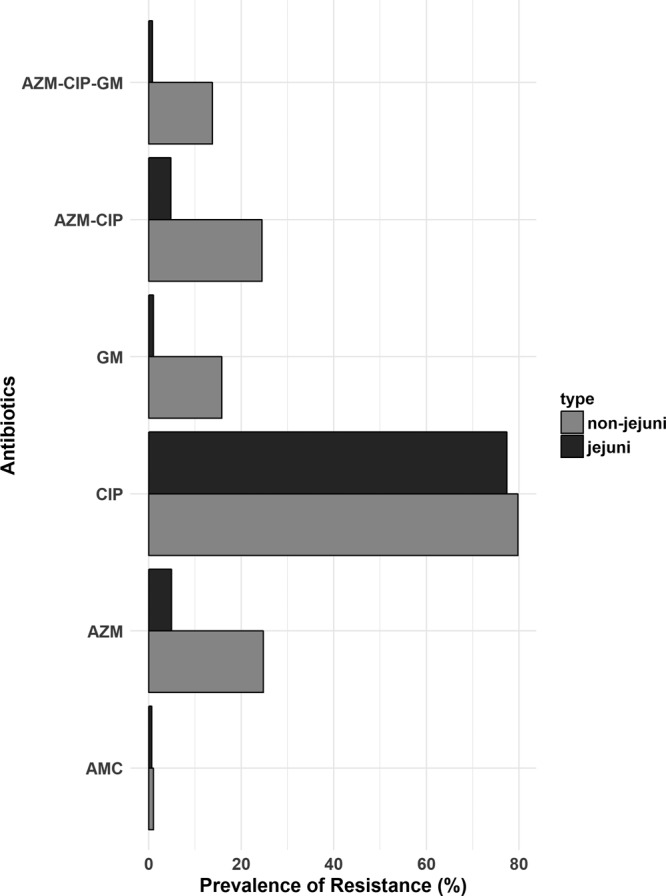
Azithromycin, gentamicin, ciprofloxacin, and amoxicillin-clavulanic acid phenotypic multidrug resistance in Campylobacter spp.
Of the 303 children enrolled and followed up, a total of 80 (33.1%) were found to have a Campylobacter strain nonsusceptible to both azithromycin and ciprofloxacin. Additionally, 26 (8.6%) had a diarrhea episode due to an azithromycin-resistant isolate, of which all strains where also resistant to ciprofloxacin, with the exception of one. Ciprofloxacin resistance was far more common than azithromycin resistance: 232 (76.6%) children ever diagnosed with a resistant ciprofloxacin isolate and 41.6% of children had a diarrhea episode due to a ciprofloxacin-resistant Campylobacter spp.
The mean age at which a child presented with the first diarrhea episode due to a ciprofloxacin-resistant isolate was 12 months, and the mean age was 18 months for the first diarrhea episode due to an azithromycin-resistant isolate (P < 0.001). Among the 29 diarrhea episodes caused by azithromycin-nonsusceptible isolates, 10 were diagnosed during the 12 months, and all but two were diagnosed before the child was 36 months of age. Within the first 12 months of life, 57 (18.8%) children had already experienced diarrhea caused by a ciprofloxacin-resistant isolate. This increased to 107 (35.3%) by 24 months and to 122 (39.9%) by 36 months.
Of the 303 children, on average, a child was on antibiotics for 5.6% of the days during their first 5 years of life, of which, macrolides (azithromycin or erythromycin) account for 1.1% of this time period. The median number of courses of macrolides a child received was 3 (interquartile range [IQR], 1 to 5 courses), which translates to an average of 12 days (IQR, 4 to 21 days). A child’s risk of being culture positive for a macrolide-resistant Campylobacter was not statistically different between children with high macrolide exposure and those with low macrolide exposure. Similar results were found for the time to first isolation of a macrolide-resistant Campylobacter. The cumulative effect of macrolide intake early in life was not statistically associated with the odds of acquisition of a macrolide-resistant isolate.
DISCUSSION
Of 917 Campylobacter isolates obtained from children enrolled in the MAL-ED cohort of Peru, amoxicillin and clavulanic acid was the antibiotic with the highest rate of susceptibility. Resistance to ciprofloxacin was expressed in 77.4% of C. jejuni isolates and in 79.8% of non-C. jejuni isolates, while resistance to azithromycin was found in 4.9% of C. jejuni isolates and in 24.8% of non-C. jejuni isolates.
Previous studies assessing antibiotic resistance in human gastroenteritis associated with Campylobacter have found various levels of resistance. Studies in the United Kingdom, South Korea, Israel, and Peru have demonstrated lower levels of fluoroquinolone resistance, ranging from 24% to 65% (2, 12, 26, 40). However, observational studies from China (87%) and Japan (90%) report higher estimates of ciprofloxacin resistance (41, 42). Data from the National Antimicrobial Resistance Monitoring System (NARMS) of the United States shows that in 2015, 25.3% of C. jejuni isolates and 39.8% of C. coli isolates were resistant to ciprofloxacin. Macrolide resistance is even more wide ranging. We report an overall resistance of 24.8% for non-C. jejuni isolates, a higher prevalence than was reported in a nearby region in 2010 (10.0%) (12). However, the C. jejuni resistance to macrolides (4.9%) in our study is lower than what was previously reported in the same region (14.9%) (12). Worldwide, macrolide resistance in human Campylobacter species isolates varies between 0.8% in South Korea, 2.2% in the United Kingdom, 12.5% in Thailand, 21.8% in China, and 22.2% in India (10, 26, 40–42). Additionally, cases of Campylobacter-associated travelers’ diarrhea in U.S. military troops show as low as 2% nonsusceptibility to azithromycin (43). NARMS data showed azithromycin resistance in 2.7% of C. jejuni isolates and in 12.7% of C. coli isolates. According to the 2015 NARMS report, between 2011 and 2015, erythromycin resistance in human-associated C. coli isolates increased from 2.7% to 12.7%.
Antibiotic resistance of Campylobacter isolates from animal sources, most importantly, poultry, also appear to be country dependent. A study conducted in China reported that 73.2% of C. coli isolates were erythromycin resistant, and a study from Spain similarly reported 73.0% resistance (44, 45). Reports from Latin America are limited. Sierra-Arguello et al. reported an overall 2% resistance to erythromycin (46). Given that azithromycin is the currently prescribed treatment for campylobacteriosis (16, 19, 21), our reported levels of resistance to macrolides in a pediatric population is worrisome but not unique.
The rise in fluoroquinolone and macrolide resistance has been attributed to antibiotic administration for growth promotion in poultry and hog production (9, 47, 48). However, Campylobacter antibiotic resistance in animal hosts has not been well assessed in this region. Poultry production and commercialization in Iquitos includes market vendors from concentrated animal operation facilities as well as from small backyard production within households. Slaughtering is generally performed at home or within the live markets, yet there is little evidence that characterizes the risk of Campylobacter contamination and infection within the poultry industry in this region. The use of antibiotics as growth promoters is not regulated in Peru, and over-the-counter access to antibiotics from local vendors is common. Therefore, further studies that characterize Campylobacter antibiotic resistance patterns in poultry and other animal hosts are required to evaluate the zoonotic component of Campylobacter epidemiology in this area. An incredibly common infection could potentially end up untreatable if effective control interventions involving antibiotic stewardship in both humans and animals are not promptly executed.
Another concern regarding the acquisition of widespread drug resistance is the recent reports of standard periodic dosing in early childhood to decrease mortality (49). The apparent volume of distribution is very large, 25 to 30 liter/kg, and slow release from intracellular compartments extends the time for which the drug is available at subinhibitory concentrations (50). The agent is principally excreted unchanged in the feces, with an elimination half-life of 2 to 4 days. Prolonged subtherapeutic concentrations and the active agent elimination route into the gastrointestinal tract both favor the selection of drug resistance to a greater extent than the more routine use of antibiotics. Our analysis shows that intermittent azithromycin use for therapeutic purposes is unlikely to have a population-level effect on the emergence of Campylobacter resistance to macrolides. Therefore, we stress the need to explore macrolide use for animal production purposes and its effect on the emergence of Campylobacter resistance. The loss of azithromycin as a widespread feasible option for the treatment of campylobacteriosis, as is happening here, has meaningful consequences given the prevalence of both symptomatic disease and enteropathy associated with this infection.
Gentamicin resistance is a novel phenomenon in Campylobacter isolates (14). We report an overall prevalence of 15.8% for non-C. jejuni isolates and 1.0% resistance for C. jejuni isolates. This is a lower estimate than the 28.8% reported in human diarrheal samples from China yet higher than reports from South Korea (6.6%) (26, 42). In Belgium, gentamicin resistance in poultry isolates has increased from almost being nonexistent in 2004 to approximately 20% by 2009 (11). Multiple phosphotransferase (aph) genes are commonly associated with aminoglycoside resistance, and many of them have been identified as transferable (13, 14, 51).
Multidrug resistance (MDR) was observed in 56.8% (335/590) of C. jejuni isolates and 59.1% (176/298) of non-C. jejuni isolates. This is striking given that the only previous evidence of such higher rates came from poultry isolates in China, where 81.1% of C. jejuni isolates and 47.7% of C. coli isolates were resistant to 3 or more antibiotics (44). It is important to consider the definition of MDR when comparing overall susceptibility trends across the globe. Whole-genome sequencing explorations have identified a transferable multidrug resistance genomic island (MDRGI) which contains antibiotic resistance determinants for quinolone and macrolides, as well as tetracycline and aminoglycosides (52). Thus, future studies exploring molecular resistance determinants in this species should aim to identify the prevalence of this MDRGI. Clinically relevant MDR can be considered to be toward both ciprofloxacin and azithromycin. This was observed in 4.8% of C. jejuni and 24.5% of non-C. jejuni isolates, a highly significant percentage considering that this study was conducted in a pediatric population in a remote tropical setting.
Surprisingly, amoxicillin and clavulanic acid expressed the lowest proportion of resistance, although this has been noted in earlier studies from Spain (53). Therefore, we propose that this antibiotic should be considered a treatment option for Campylobacter-associated gastroenteritis when isolates are nonsusceptible against fluoroquinolones and macrolides.
We acknowledge that disc diffusion breakpoints for antibiotics other than erythromycin, ciprofloxacin, and tetracycline have not been standardized for Campylobacter. More commonly utilized are the MIC breakpoints; yet, these were not used in this study. Second, we did not explore the presence and diversity of antibiotic resistance genes in these samples. Quinolone resistance has been attributed to target mutations in the quinolone resistance-determining region (QRDR) (54–56), as well as to the presence of the cmeABC efflux pump conferring intrinsic resistance to fluoroquinolones (57–59). The reported mechanisms of macrolide resistance include target mutations of the 23S rRNA genes (23, 55, 60), target mutations in ribosomal proteins (55, 61), and ribosomal methylation encoded by the erm genes, of which the erm(B) gene is associated with high-level resistance, mostly in C. coli (23, 55, 62, 63). In Peru, a gyrA mutation associated with quinolone resistance and a 23S rRNA mutation associated with macrolide resistance were detected within a limited sample of Campylobacter isolates from a pediatric cohort in Lima (30, 64).
A large proportion of diarrhea episodes associated with resistant Campylobacter against current treatment recommendations indicates that this multidrug-resistant campylobacteriosis is highly endemic in the Peruvian Amazon. To our knowledge, no other cohort studies report the susceptibility patterns of Campylobacter infections in a pediatric population in low-resource settings, and this is one of the few studies available reporting statistics on Campylobacter antibiotic resistance in more than 800 isolates cultured from children. Future studies that evaluate the molecular determinants of antibiotic resistance in thoroughly characterized Campylobacter species isolates will shed light on the origins and dynamics of this infection in the Peruvian Amazon.
Conclusions.
Campylobacter isolates from children under 5 years of age in the Peruvian Amazon show a higher prevalence of phenotypic resistance than other regional and country estimates. As expected, non-C. jejuni isolates showed higher levels of azithromycin resistance than C. jejuni isolates. Although both C. jejuni and non-C. jejuni isolates showed a high prevalence of ciprofloxacin resistance, this phenotypic trait is more common among C. jejuni isolates. We report for the first time in the region moderate to increased levels of gentamicin resistance, especially among non-C. jejuni isolates. When considering treatment options for gastroenteritis associated with Campylobacter, amoxicillin and clavulanic acid should be considered.
MATERIALS AND METHODS
Setting and study design.
The MAL-ED study is a multisite, prospective, community-based cohort study. Between 2009 and 2012, 303 newborns were enrolled within the first 17 days of life and were followed up until 5 years of age. The study site in Peru consisted of 3 communities 15 km southeast of Iquitos, the largest city of the Peruvian Amazon. Details of enrollment and surveillance procedures have been published previously (65). Surveillance stool samples were collected monthly, and diarrheal samples were collected within 48 h of a reported episode. A diarrheal episode was defined as three or more loose stools within 24 h or one dysenteric stool. Subjects were visited 2 times weekly and a continual symptom history was recorded, which included data on antibiotic use, generating a continual daily history for the period of study. Further data and sample collection procedures have also been described previously (66).
Laboratory procedures.
Diarrheal and surveillance fecal samples were placed in Cary Blair transport medium and were processed within 12 h (8). Stools were inoculated on Campylobacter agar base supplemented with Blaser’s supplement (Becton Dickinson, Sparks, MD) containing vancomycin, cephalothin, trimethoprim, polymyxin, and amphotericin B. Plates were incubated for 48 h at 42°C at 5% O2, 10% CO2, and 85% N2. If no growth was observed, agar plates were held at least 72 h to confirm this finding. Gram-negative colonies demonstrating typical Campylobacter morphology were assessed using oxidase and catalase tests as well as Gram staining. Colonies with typical Campylobacter species morphology as well as oxidase and catalase activity were further assessed using the hippurate hydrolysis test to distinguish Campylobacter jejuni from non-C. jejuni (38). Campylobacter species were identified as C. jejuni if positive for hippurate hydrolysis and non-C. jejuni if negative for hippurate hydrolysis. We referred to isolates as C. jejuni or non-C. jejuni throughout the paper due to uncertainty as to whether all non-jejuni Campylobacter truly represent C. coli.
Phenotypic antimicrobial susceptibility patterns were assessed using standard disc diffusion (Kirby-Bauer) methods. Resistance to the following antibiotics was tested: ciprofloxacin (CIP), nalidixic acid (NAL), erythromycin (ERY), azithromycin (AZM), tetracycline (TE), gentamicin (GM), ampicillin (AMP), amoxicillin and clavulanic acid (AMC), ceftriaxone (CRO), chloramphenicol (C), and trimethoprim-sulfamethoxazole (TMS). Zone diameter breakpoints (millimeters) for Campylobacter spp. validated by the Clinical and Laboratory Standards Institute (CLSI document M45) were applied to assess ciprofloxacin, erythromycin, azithromycin, and tetracycline resistance. CLSI zone diameter breakpoints (millimeters) for Enterobacteriaceae were used for the remaining antibiotics for which there are no established breakpoints for Campylobacter spp. Table S1 in the supplemental material displays all zone diameter breakpoints.
Data management and analysis.
CLSI standards were used to categorize isolates as susceptible, resistant, or intermediate. The proportion nonsusceptible to each antibiotic was tabulated for both C. jejuni and non-C. jejuni isolates when resistant and intermediate categories were combined. Multidrug resistance (MDR) was defined as an isolate expressing phenotypic nonsusceptibility to three or more classes of antibiotics (67). Cephalosporins (CRO) were not included in this classification given the intrinsic resistance of Campylobacter spp. Pearson’s chi-square was used to test the differences in resistance to all antibiotics between C. jejuni and non-C. jejuni isolates, as well as between surveillance and diarrhea samples.
Macrolide exposure was analyzed as continuous time-varied exposure as well as binary exposure. High macrolide exposure was defined as higher than the population’s median number of courses of antibiotics (68). Risk ratios for the association between daily macrolide (AZM and ERY) exposure and the isolation of macrolide-resistant Campylobacter were modeled using log-binomial regression fitted with generalized linear models and robust variance estimation. Differences in the times to first detection of a macrolide-resistant Campylobacter isolate between those with high and low macrolide exposure were assessed by comparing cumulative incidence curves and performing log-rank tests of statistical significance. Finally, the effects of the cumulative exposure of macrolide use early in life (number of days with macrolide intake before 6 months and 12 months of age) and the odds of acquiring a macrolide-resistant Campylobacter later in life (after 12 and 18 months of age) were assessed by fitting logistic regression models. Data manipulation and statistical analysis were performed with STATA 14 (Stata Corp., College Station, TX) and R (version 3.3.2).
Supplementary Material
ACKNOWLEDGMENTS
The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) project was carried out as a collaborative project supported by the Bill & Melinda Gates Foundation (47075), the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center. M.N.K. was additionally supported by the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases of the Johns Hopkins School of Medicine (10POS2015). F.S. was supported by FONDECYT-CONCYTEC (grant contract number 246-2015-FONDECYT), and the National Institutes of Health Fogarty Global Health Fellows Consortium comprised of Johns Hopkins University, the University of North Carolina, Morehouse University, and Tulane University (grant no. D43TW009340).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01911-18.
REFERENCES
- 1.Hafez HM, Hauck R. 2015. Zoonoses with public health relevance in poultry In Sing A. (ed), Zoonoses–infections affecting humans and animals. Springer, Dordrecht, Netherlands. [Google Scholar]
- 2.Abulreesh HH, Paget TA, Goulder R. 2006. Campylobacter in waterfowl and aquatic environments: incidence and methods for detection. Environ Sci Technol 40:7122–7131. doi: 10.1021/es060327l. [DOI] [PubMed] [Google Scholar]
- 3.Reed RP, Friedland IR, Wegerhoff FO, Khoosal M. 1996. Campylobacter bacteremia in children. Pediatr Infect Dis J 15:345–348. doi: 10.1097/00006454-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Scallan E, Crim SM, Runkle A, Henao OL, Mahon BE, Hoekstra RM, Griffin PM. 2015. Bacterial enteric infections among older adults in the United States: Foodborne Diseases Active Surveillance Network, 1996–2012. Foodborne Pathog Dis 12:492–499. doi: 10.1089/fpd.2014.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.091101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francois R, Yori PP, Rouhani S, Siguas Salas M, Paredes Olortegui M, Rengifo Trigoso D, Pisanic N, Burga R, Meza R, Meza Sanchez G, Gregory MJ, Houpt ER, Platts-Mills JA, Kosek MN. 2018. The other campylobacters: not innocent bystanders in endemic diarrhea and dysentery in children in low-income settings. PLoS Negl Trop Dis 12:e0006200. doi: 10.1371/journal.pntd.0006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man SM. 2011. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 8.Platts-Mills JA, Liu J, Gratz J, Mduma E, Amour C, Swai N, Taniuchi M, Begum S, Penataro Yori P, Tilley DH, Lee G, Shen Z, Whary MT, Fox JG, McGrath M, Kosek M, Haque R, Houpt ER. 2014. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J Clin Microbiol 52:1074–1080. doi: 10.1128/JCM.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieczorek K, Osek J. 2015. A five-year study on prevalence and antimicrobial resistance of Campylobacter from poultry carcasses in Poland. Food Microbiol 49:161–165. doi: 10.1016/j.fm.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh R, Uppal B, Aggarwal P, Chakravarti A, Jha AK. 2013. Increasing antimicrobial resistance of Campylobacter jejuni isolated from paediatric diarrhea cases in a tertiary care hospital of New Delhi, India. J Clin Diagn Res 7:247–249. doi: 10.7860/JCDR/2013/5267.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattheus W, Botteldoorn N, Heylen K, Pochet B, Dierick K. 2012. Trend analysis of antimicrobial resistance in Campylobacter jejuni and Campylobacter coli isolated from Belgian pork and poultry meat products using surveillance data of 2004–2009. Foodborne Pathog Dis 9:465–472. doi: 10.1089/fpd.2011.1042. [DOI] [PubMed] [Google Scholar]
- 12.Pollett S, Rocha C, Zerpa R, Patiño L, Valencia A, Camiña M, Guevara J, Lopez M, Chuquiray N, Salazar-Lindo E, Calampa C, Casapia M, Meza R, Bernal M, Tilley D, Gregory M, Maves R, Hall E, Jones F, Arriola CS, Rosenbaum M, Perez J, Kasper M. 2012. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect Dis 912:193. doi: 10.1186/1471-2334-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Mukherjee S, Hoffmann M, Kotewicz ML, Young S, Abbott J, Luo Y, Davidson MK, Allard M, McDermott P, Zhao S. 2013. Whole-genome sequencing of gentamicin-resistant Campylobacter coli isolated from U.S. retail meats reveals novel plasmid-mediated aminoglycoside resistance genes. Antimicrob Agents Chemother 57:5398–5405. doi: 10.1128/AAC.00669-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao S, Mukherjee S, Chen Y, Li C, Young S, Warren M, Abbott J, Friedman S, Kabera C, Karlsson M, McDermott PF. 2015. Novel gentamicin resistance genes in Campylobacter isolated from humans and retail meats in the USA. J Antimicrob Chemother 70:1314–1321. doi: 10.1093/jac/dkv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. 2007. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 44:696–700. doi: 10.1086/509924. [DOI] [PubMed] [Google Scholar]
- 16.Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis 65:e45–e80. doi: 10.1093/cid/cix669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tribble DR. 2017. Antibiotic therapy for acute watery diarrhea and dysentery. Mil Med 182:17–25. doi: 10.7205/MILMED-D-17-00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddle MS, Martin GJ, Murray CK, Burgess TH, Connor P, Mancuso JD, Schnaubelt ER, Ballard TP, Fraser J, Tribble DR. 2017. Management of acute diarrheal illness during deployment: a deployment health guideline and expert panel report. Mil Med 182:34–52. doi: 10.7205/MILMED-D-17-00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle MS, Tribble D. 2017. Preface: guidelines for the treatment of travelers' diarrhea in deployed military personnel. Mil Med 182:1–3. doi: 10.7205/MILMED-D-17-00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor DN, Hamer DH, Shlim DR. 2017. Medications for the prevention and treatment of travellers' diarrhea. J Travel Med 24:S17–S22. doi: 10.1093/jtm/taw097. [DOI] [PubMed] [Google Scholar]
- 21.Riddle MS, DuPont HL, Connor BA. 2016. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 111:602–622. doi: 10.1038/ajg.2016.126. [DOI] [PubMed] [Google Scholar]
- 22.Post A, Martiny D, van Waterschoot N, Hallin M, Maniewski U, Bottieau E, Van Esbroeck M, Vlieghe E, Ombelet S, Vandenberg O, Jacobs J. 2017. Antibiotic susceptibility profiles among Campylobacter isolates obtained from international travelers between 2007 and 2014. Eur J Clin Microbiol Infect Dis 36:2101–2107. doi: 10.1007/s10096-017-3032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolinger H, Kathariou S. 2017. The current state of macrolide resistance in Campylobacter spp.: trends and impacts of resistance mechanisms. Appl Environ Microbiol 83:e00416-17. doi: 10.1128/AEM.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham NT, Ushijima H, Trinh QD, Khamrin P, Komine-Aizawa S, Okitsu S, Maneekarn N, Hayakawa S. 2015. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from adult hospitalized patients with diarrhea in Thailand. Clin Lab 61:1809–1812. [DOI] [PubMed] [Google Scholar]
- 25.Serichantalergs O, Dalsgaard A, Bodhidatta L, Krasaesub S, Pitarangsi C, Srijan A, Mason CJ. 2007. Emerging fluoroquinolone and macrolide resistance of Campylobacter jejuni and Campylobacter coli isolates and their serotypes in Thai children from 1991 to 2000. Epidemiol Infect 135:1299–1306. doi: 10.1017/S0950268807008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin E, Oh Y, Kim M, Jung J, Lee Y. 2013. Antimicrobial resistance patterns and corresponding multilocus sequence types of the Campylobacter jejuni isolates from human diarrheal samples. Microb Drug Resist 19:110–116. doi: 10.1089/mdr.2012.0099. [DOI] [PubMed] [Google Scholar]
- 27.Hou FQ, Sun XT, Wang GQ. 2012. Clinical manifestations of Campylobacter jejuni infection in adolescents and adults, and change in antibiotic resistance of the pathogen over the past 16 years. Scand J Infect Dis 44:439–443. doi: 10.3109/00365548.2011.652163. [DOI] [PubMed] [Google Scholar]
- 28.Bakeli G, Sato K, Kumita W, Saito R, Ono E, Chida T, Okamura N, Saito R, Ono E. 2008. Antimicrobial susceptibility and mechanism of quinolone resistance in Campylobacter jejuni strains isolated from diarrheal patients in a hospital in Tokyo. J Infect Chemother 14:342–348. doi: 10.1007/s10156-008-0631-2. [DOI] [PubMed] [Google Scholar]
- 29.Komba EV, Mdegela RH, Msoffe PL, Nielsen LN, Ingmer H. 2015. Prevalence, antimicrobial resistance and risk factors for thermophilic Campylobacter infections in symptomatic and asymptomatic humans in Tanzania. Zoonoses Public Health 62:557–568. doi: 10.1111/zph.12185. [DOI] [PubMed] [Google Scholar]
- 30.Lluque A, Riveros M, Prada A, Ochoa TJ, Ruiz J. 2017. Virulence and antimicrobial resistance in Campylobacter spp. from a Peruvian pediatric cohort. Scientifica (Cairo) 2017:7848926. doi: 10.1155/2017/7848926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjogren E, Lindblom GB, Kaijser B. 1997. Norfloxacin resistance in Campylobacter jejuni and Campylobacter coli isolates from Swedish patients. J Antimicrob Chemother 40:257–261. doi: 10.1093/jac/40.2.257. [DOI] [PubMed] [Google Scholar]
- 32.Maesaar M, Kramarenko T, Meremae K, Sogel J, Lillenberg M, Hakkinen L, Ivanova M, Kovalenko K, Horman A, Hanninen ML, Roasto M. 2016. Antimicrobial resistance profiles of Campylobacter spp. isolated from broiler chicken meat of Estonian, Latvian and Lithuanian origin at Estonian retail level and from patients with severe enteric infections in Estonia. Zoonoses Public Health 63:89–96. doi: 10.1111/zph.12208. [DOI] [PubMed] [Google Scholar]
- 33.Hakanen AJ, Lehtopolku M, Siitonen A, Huovinen P, Kotilainen P. 2003. Multidrug resistance in Campylobacter jejuni strains collected from Finnish patients during 1995–2000. J Antimicrob Chemother 52:1035–1039. doi: 10.1093/jac/dkg489. [DOI] [PubMed] [Google Scholar]
- 34.Rozynek E, Dzierzanowska-Fangrat K, Korsak D, Konieczny P, Wardak S, Szych J, Jarosz M, Dzierzanowska D. 2008. Comparison of antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from humans and chicken carcasses in Poland. J Food Prot 71:602–607. doi: 10.4315/0362-028X-71.3.602. [DOI] [PubMed] [Google Scholar]
- 35.Szczepanska B, Andrzejewska M, Spica D, Klawe JJ. 2017. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiol 17:80. doi: 10.1186/s12866-017-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IFN, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AAAM, Mason CJ, Zaidi AKM, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Global Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque ASG, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. The Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee G, Pan W, Penataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, Oberhelman R, Burga R, Chavez CB, Kosek M. 2013. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis 7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricotta EE, Palmer A, Wymore K, Clogher P, Oosmanally N, Robinson T, Lathrop S, Karr J, Hatch J, Dunn J, Ryan P, Blythe D. 2014. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am J Public Health 104:e108–e114. doi: 10.2105/AJPH.2013.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockdale AJ, Beeching NJ, Anson J, Beadsworth MB. 2016. Emergence of extensive fluoroquinolone resistance in Campylobacter gastroenteritis in Liverpool, UK. J Infect 72:398–400. doi: 10.1016/j.jinf.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Pham NT, Thongprachum A, Tran DN, Nishimura S, Shimizu-Onda Y, Trinh QD, Khamrin P, Ukarapol N, Kongsricharoern T, Komine-Aizawa S, Okitsu S, Maneekarn N, Hayakawa S, Ushijima H. 2016. Antibiotic resistance of Campylobacter jejuni and C. coli isolated from children with diarrhea in Thailand and Japan. Jpn J Infect Dis 69:77–79. doi: 10.7883/yoken.JJID.2014.582. [DOI] [PubMed] [Google Scholar]
- 42.Pan H, Ge Y, Xu H, Zhang J, Kuang D, Yang X, Su X, Huang Z, Shi X, Xu X, Meng J. 2016. Molecular characterization, antimicrobial resistance and Caco-2 cell invasion potential of Campylobacter jejuni/coli from young children with diarrhea. Pediatr Infect Dis J 35:330–334. doi: 10.1097/INF.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 43.Mason CJ, Sornsakrin S, Seidman JC, Srijan A, Serichantalergs O, Thongsen N, Ellis MW, Ngauy V, Swierczewski BE, Bodhidatta L. 2017. Antibiotic resistance in Campylobacter and other diarrheal pathogens isolated from U.S. military personnel deployed to Thailand in 2002–2004: a case-control study. Trop Dis Travel Med Vaccines 3:13. doi: 10.1186/s40794-017-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Dong Y, Deng F, Liu D, Yao H, Zhang Q, Shen J, Liu Z, Gao Y, Wu C, Shen Z. 2016. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008–14. J Antimicrob Chemother 71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- 45.Torralbo A, Borge C, Garcia-Bocanegra I, Meric G, Perea A, Carbonero A. 2015. Higher resistance of Campylobacter coli compared to Campylobacter jejuni at chicken slaughterhouse. Comp Immunol Microbiol Infect Dis 39:47–52. doi: 10.1016/j.cimid.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Sierra-Arguello YM, Perdoncini G, Morgan RB, Salle CT, Moraes HL, Gomes MJ, do Nascimento VP. 2016. Fluoroquinolone and macrolide resistance in Campylobacter jejuni isolated from broiler slaughterhouses in southern Brazil. Avian Pathol 45:66–72. doi: 10.1080/03079457.2015.1120272. [DOI] [PubMed] [Google Scholar]
- 47.Taylor NM, Wales AD, Ridley AM, Davies RH. 2016. Farm level risk factors for fluoroquinolone resistance in E. coli and thermophilic Campylobacter spp. on poultry farms. Avian Pathol 45:559–568. doi: 10.1080/03079457.2016.1185510. [DOI] [PubMed] [Google Scholar]
- 48.Skarp CP, Hanninen ML, Rautelin HI. 2016. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect 22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Keenan JD, Bailey RL, West SK, Arzika AM, Hart J, Weaver J, Kalua K, Mrango Z, Ray KJ, Cook C, Lebas E, O'Brien KS, Emerson PM, Porco TC, Lietman TM, MORDOR Study Group. 2018. Azithromycin to reduce childhood mortality in Sub-Saharan Africa. N Engl J Med 378:1583–1592. doi: 10.1056/NEJMoa1715474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng S, Matzneller P, Zeitlinger M, Schmidt S. 2014. Development of a population pharmacokinetic model characterizing the tissue distribution of azithromycin in healthy subjects. Antimicrob Agents Chemother 58:6675–6684. doi: 10.1128/AAC.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao S, Mukherjee S, Li C, Jones SB, Young S, McDermott PF. 2018. Cloning and expression of novel aminoglycoside phosphotransferase genes from Campylobacter and their role in the resistance to six aminoglycosides. Antimicrob Agents Chemother 62:e01682-17. doi: 10.1128/AAC.01682-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin S, Wang Y, Zhang Q, Chen X, Shen Z, Deng F, Wu C, Shen J. 2012. Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli. Antimicrob Agents Chemother 56:5332–5339. doi: 10.1128/AAC.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez-Garces JL, Cogollos R, Alos JL. 1995. Susceptibilities of fluoroquinolone-resistant strains of Campylobacter jejuni to 11 oral antimicrobial agents. Antimicrob Agents Chemother 39:542–544. doi: 10.1128/AAC.39.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wieczorek K, Osek J. 2013. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res Int 2013:340605. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Payot S, Bolla JM, Corcoran D, Fanning S, Megraud F, Zhang Q. 2006. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes Infect 8:1967–1971. doi: 10.1016/j.micinf.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 56.Changkwanyeun R, Usui M, Kongsoi S, Yokoyama K, Kim H, Suthienkul O, Changkaew K, Nakajima C, Tamura Y, Suzuki Y. 2015. Characterization of Campylobacter jejuni DNA gyrase as the target of quinolones. J Infect Chemother 21:604–609. doi: 10.1016/j.jiac.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Gibreel A, Wetsch NM, Taylor DE. 2007. Contribution of the CmeABC efflux pump to macrolide and tetracycline resistance in Campylobacter jejuni. Antimicrob Agents Chemother 51:3212–3216. doi: 10.1128/AAC.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin J, Michel LO, Zhang Q. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother 46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J, Sahin O, Michel LO, Zhang Q. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun 71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pérez-Boto D, López-Portolés JA, Simón C, Valdezate S, Echeita MA. 2010. Study of the molecular mechanisms involved in high-level macrolide resistance of Spanish Campylobacter jejuni and Campylobacter coli strains. J Antimicrob Chemother 65:2083–2088. doi: 10.1093/jac/dkq268. [DOI] [PubMed] [Google Scholar]
- 61.Cagliero C, Mouline C, Cloeckaert A, Payot S. 2006. Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother 50:3893–3896. doi: 10.1128/AAC.00616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin S, Wang Y, Zhang Q, Zhang M, Deng F, Shen Z, Wu C, Wang S, Zhang J, Shen J. 2014. Report of ribosomal RNA methylase gene erm(B) in multidrug-resistant Campylobacter coli. J Antimicrob Chemother 69:964–968. doi: 10.1093/jac/dkt492. [DOI] [PubMed] [Google Scholar]
- 63.Deng F, Shen J, Zhang M, Wu C, Zhang Q, Wang Y. 2015. Constitutive and inducible expression of the rRNA methylase gene erm(B) in Campylobacter. Antimicrob Agents Chemother 59:6661–6664. doi: 10.1128/AAC.01103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochoa TJ, Chea-Woo E, Baiocchi N, Pecho I, Campos M, Prada A, Valdiviezo G, Lluque A, Lai D, Cleary TG. 2013. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J Pediatr 162:349–356. doi: 10.1016/j.jpeds.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosek M, Guerrant RL, Kang G, Bhutta Z, Yori PP, Gratz J, Gottlieb M, Lang D, Lee G, Haque R, Mason CJ, Ahmed T, Lima A, Petri WA, Houpt E, Olortegui MP, Seidman JC, Mduma E, Samie A, Babji S, Investigators M-EN. 2014. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis 59 (Suppl 4):S239–S247. doi: 10.1093/cid/ciu457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yori PP, Lee G, Olortegui MP, Chavez CB, Flores JT, Vasquez AO, Burga R, Pinedo SR, Asayag CR, Black RE, Caulfield LE, Kosek M. 2014. Santa Clara de Nanay: the MAL-ED cohort in Peru. Clin Infect Dis 59 (Suppl 4):S310–S316. doi: 10.1093/cid/ciu460. [DOI] [PubMed] [Google Scholar]
- 67.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 68.Rogawski ET, Platts-Mills JA, Seidman JC, John S, Mahfuz M, Ulak M, Shrestha SK, Soofi SB, Yori PP, Mduma E, Svensen E, Ahmed T, Lima AA, Bhutta ZA, Kosek MN, Lang DR, Gottlieb M, Zaidi AK, Kang G, Bessong PO, Houpt ER, Guerrant RL. 2017. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 95:49–61. doi: 10.2471/BLT.16.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


