A patient received continuous infusion of cefazolin 10 g then 8 g daily for an external ventricular drainage-related methicillin-susceptible Staphylococcus aureus (MSSA) ventriculitis. Median free concentrations in the cerebrospinal fluid were 11.9 and 6.1 mg/liter after 10- and 8-g doses, respectively.
KEYWORDS: cefazolin, cerebrospinal fluid, healthcare-associated infection, meningitis, pharmacokinetics
ABSTRACT
A patient received continuous infusion of cefazolin 10 g then 8 g daily for an external ventricular drainage-related methicillin-susceptible Staphylococcus aureus (MSSA) ventriculitis. Median free concentrations in the cerebrospinal fluid were 11.9 and 6.1 mg/liter after 10- and 8-g doses, respectively. Free concentrations in the cerebrospinal fluid were always above the MIC usually displayed by methicillin-susceptible Staphylococcus aureus (MSSA) isolates. These results support the use of high-dose cefazolin to achieve sufficient meningeal concentrations.
INTRODUCTION
Recent studies showed that cefazolin is at least as effective as oxacillin or nafcillin for the treatment of severe methicillin-susceptible Staphylococcus aureus (MSSA) infections (1, 2). External ventricular drainage (EVD)-related ventriculitis and meningitis are mostly caused by Gram-positive cocci and particularly staphylococci (3). Owing to the poor diffusion of oxacillin and nafcillin into the meningeal compartment, cefotaxime, fosfomycin, and rifampin are currently used in this indication (4). Despite in vitro activity, the use of cefazolin for MSSA-related meningitis is limited because of its supposed weak meningeal diffusion due to a high level of protein binding (∼85%) associated with moderate lipophilicity (5). Regarding the antistaphylococcal activity of this first-generation cephalosporin, data about the use of cefazolin in EVD-related ventriculitis or meningitis are required.
We herein describe the unique case of a patient treated by continuous intravenous infusion of cefazolin at high dose (8 to 10 g daily) for an EVD-related MSSA ventriculitis.
A 42-year-old patient (weight, 67 kg) with no medical history was hospitalized for a subarachnoid hemorrhage requiring an EVD. At day 7, neurological impairment led to the diagnosis of ventriculitis: glycorrhachia was <1 mmol/liter and cerebrospinal fluid (CSF) lactate was 8 mmol/liter. An empirical combination of intravenous cefotaxime and linezolid was introduced. Two days later, as the CSF grew MSSA, the antimicrobial therapy relied on a continuous intravenous infusion of cefazolin 10 g plus levofloxacin 750 mg daily. The profile of antibiotic susceptibility was determined by the Vitek* 2 automated system (bioMérieux, France): MIC was 0.5 mg/liter for oxacillin and 1.5 mg/liter for cefotaxime. Moreover, this strain was resistant to co-trimoxazole and rifampin. CSF collected at the time of the antibiotic switch (i.e., after 2 days of antimicrobial therapy) was still positive for S. aureus. Thereafter, CSF was collected every 48 h until sterilization was achieved, which occurred 48 h after initiation of cefazolin. Then, the EVD was changed and was removed 13 days later after a progressive weaning. All the bacteriological cultures performed afterward remained sterile until the end of treatment, 6 weeks later. Glomerular filtration rate estimated by Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) progressively increased from 60 ml/min per 1.73 m2 at cefazolin initiation to 106 ml/min per 1.73 m2 after 6 weeks.
CSF and blood samples were simultaneously collected from day 2 after cefazolin initiation to day 21. Plasma samples were obtained by centrifugation, and ultrafiltrates were obtained after CSF or plasma centrifugation at 37°C into a Millipore UFC503024 Amicon Ultra-0.5 (Millipore Merck, Cork, Ireland) for 20 min at 14,000 × g. Cefazolin concentrations were measured in CSF, plasma, and ultrafiltrates by a liquid-chromatography mass-spectrometry-validated assay. The limit of quantitation was 2 mg/liter in CSF, plasma, and ultrafiltrates. Clinical and biological data were collected during patient hospitalization. Written informed consent was obtained from the patient for this publication.
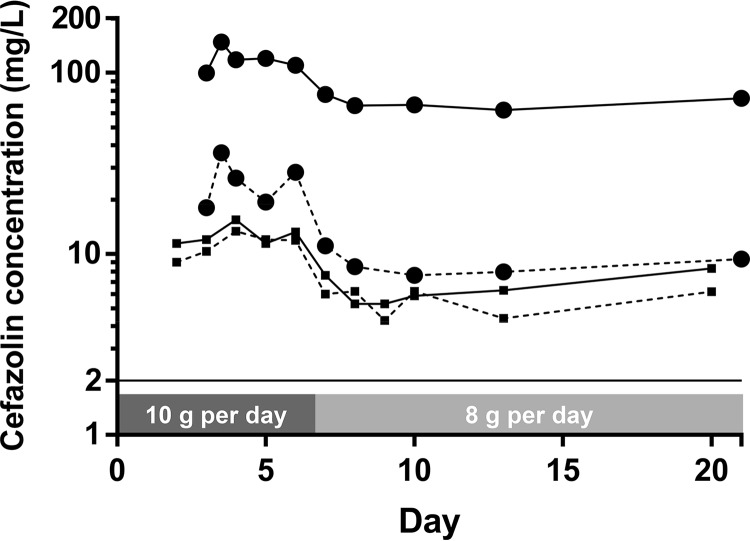
Median total plasma concentrations at steady state (Css) were 118 mg/liter with the 10-g dose and 66.5 mg/liter with the 8-g dose (Fig. 1). The patient was switched to a lower dose because of elevated plasma Css after a 10-g daily dose. These results are presented in Table 1. Median free fraction was 22% after a 10-g dose but only 13% after an 8-g dose. Conversely, diffusion of the free fraction through the blood-brain barrier showed significant intraindividual variability, and CSF/plasma free Css (fCss) ratio decreased with the dose, from 73% after 8 g to 54% after 10 g. fCss in CSF was 11.9 mg/liter after a 10-g dose and 6.1 after an 8-g dose. Cefazolin, as a β-lactam, displays time-dependent activity. Hence, its efficacy relies on the percentage of time with a free drug concentration above the MIC of the bacteria (% fT>MIC) over a dosing interval. Considering that MSSA strains usually display MICs of ≤2 mg/liter according to the epidemiological cutoff values of the European Committee on Antimicrobial Susceptibility Testing, the fCss in CSF in this study was above the MIC throughout the treatment, as usually targeted (100% fT>MIC) (6).
FIG 1.
Pharmacokinetics of cefazolin in cerebrospinal fluid (squares) and plasma (circles) after a 10-g dose followed by an 8-g dose. Total concentrations are represented by continuous lines and unbound by dashed lines.
TABLE 1.
Cefazolin concentrations in plasma and cerebrospinal fluid after 8 or 10 g daily administered in continuous infusion
| Parameter | Cefazolin concentration (median [interquartile range]) after: |
|||||
|---|---|---|---|---|---|---|
| 10 g/day |
8 g/day |
|||||
| Plasma (n = 5) | CSF (n = 5) | CSF/plasma ratio (%) | Plasma (n = 5) | CSF (n = 6) | CSF/plasma ratio (%) | |
| Total concentration (mg/liter) | 118 (10) | 12 (1.8) | 12.0 (0.9) | 66.5 (6.6) | 6.1 (1.8) | 10.0 (1.3) |
| Free concentration (mg/liter) | 26.2 (8.9) | 11.9 (1.7) | 54.1 (9.97) | 8.5 (1.4) | 6.1 (1.4) | 73.1 (26.3) |
| Free fraction (%) | 22.2 (6.4) | 86.4 (4.0) | 12.9 (0.1) | 79.7 (24.3) | ||
In this case of a patient treated with continuous infusion of cefazolin for an EVD-related ventriculitis, the targeted fCss in the CSF above MIC was achieved for MSSA strains, which usually display MICs of ≤2 mg/liter (100% fT>MIC), provided that a high dose (at least 8 g/day administered by continuous infusion) was used. During EVD-related ventriculitis and meningitis, the degree of meningeal inflammation is variable and known to have a large impact on antibiotic penetration (7, 8). This case highlights the important intraindividual variability of cefazolin plasma concentrations (particularly free-fraction concentration in critically ill patients) and the variability of CSF diffusion in case of infection with inconstant inflammation. In addition, it demonstrates the lack of linearity of the dose-concentration relationship in critically ill patients (9). Two previous studies reported high cefazolin CSF concentrations in patients not treated for meningitis and experiencing neurological adverse events. (10, 11). Of note, to the best of our knowledge, cefazolin pharmacokinetics in patients treated for meningeal infection has not been reported. Of course, our results need to be confirmed by a larger pharmacokinetics study. Moreover, a specific clinical investigation should address whether a cefazolin-based treatment of MSSA meningitis is suitable, because the success of this approach may depend on the inoculum effect, even if cefazolin meningeal diffusion is sufficient (12).
These preliminary findings show that cefazolin may be an alternative to cefotaxime, fosfomycin, or rifampin in EVD ventriculitis or meningitis. Indeed, its meningeal diffusion appeared to be sufficient. However, in critically ill patients, inter- and intraindividual variations in plasma and CSF cefazolin concentrations mandate close monitoring of these concentrations.
ACKNOWLEDGMENTS
K.L. received, during the past 3 years, lecture fees from Medtronic, congress registration fees from Sanofi Aventis, and travel fees from MSD France, Novex Pharma, and Gilead Sciences.
We have no conflicts of interest to declare.
REFERENCES
- 1.McDanel JS, Roghmann M-C, Perencevich EN, Ohl ME, Goto M, Livorsi DJ, Jones M, Albertson JP, Nair R, O'Shea AMJ, Schweizer ML. 2017. Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: a nationwide cohort study. Clin Infect Dis 65:100–106. doi: 10.1093/cid/cix287. [DOI] [PubMed] [Google Scholar]
- 2.Pollett S, Baxi SM, Rutherford GW, Doernberg SB, Bacchetti P, Chambers HF. 2016. Cefazolin versus nafcillin for methicillin-sensitive Staphylococcus aureus bloodstream infection in a California tertiary medical center. Antimicrob Agents Chemother 60:4684–4689. doi: 10.1128/AAC.00243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korinek A-M, Reina M, Boch AL, Rivera AO, De Bels D, Puybasset L. 2005. Prevention of external ventricular drain-related ventriculitis. Acta Neurochir (Wien) 147:39–45. doi: 10.1007/s00701-004-0416-z. [DOI] [PubMed] [Google Scholar]
- 4.Beer R, Lackner P, Pfausler B, Schmutzhard E. 2008. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol 255:1617–1624. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 5.Radouane A, Péhourcq F, Tramu G, Creppy EE, Bannwarth B. 1996. Influence of lipophilicity on the diffusion of cephalosporins into the cerebrospinal fluid. Fundam Clin Pharmacol 10:309–313. doi: 10.1111/j.1472-8206.1996.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 6.Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J. 2018. MIC-based dose adjustment: facts and fables. J Antimicrob Chemother 73:564–568. doi: 10.1093/jac/dkx427. [DOI] [PubMed] [Google Scholar]
- 7.Varatharaj A, Galea I. 2017. The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Nau R, Sörgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumta N, Roberts JA, Lipman J, Cotta MO. 2018. Antibiotic distribution into cerebrospinal fluid: can dosing safely account for drug and disease factors in the treatment of ventriculostomy-associated infections? Clin Pharmacokinet 57:439–454. doi: 10.1007/s40262-017-0588-3. [DOI] [PubMed] [Google Scholar]
- 10.Moore TD, Bechtel TP, Ayers LW. 1981. Effect of multidose therapy on cerebrospinal fluid penetration of cefazolin. Am J Hosp Pharm 38:1496–1499. [PubMed] [Google Scholar]
- 11.Bechtel TP, Slaughter RL, Moore TD. 1980. Seizures associated with high cerebrospinal fluid concentrations of cefazolin. Am J Hosp Pharm 37:271–273. [PubMed] [Google Scholar]
- 12.Saeki M, Shinagawa M, Yakuwa Y, Nirasawa S, Sato Y, Yanagihara N, Takahashi S. 2018. Inoculum effect of high concentrations of methicillin-susceptible Staphylococcus aureus on the efficacy of cefazolin and other beta-lactams. J Infect Chemother 24:212–215. doi: 10.1016/j.jiac.2017.10.021. [DOI] [PubMed] [Google Scholar]