Intravenous colistimethate sodium (CMS) is used to treat infections with multiresistant Gram-negative bacteria. Optimal dosing in patients undergoing continuous renal replacement therapy (CRRT) is unclear.
KEYWORDS: colistimethate sodim, colistin, continuous renal replacement therapy, hemodiafiltration, population pharmacokinetics
ABSTRACT
Intravenous colistimethate sodium (CMS) is used to treat infections with multiresistant Gram-negative bacteria. Optimal dosing in patients undergoing continuous renal replacement therapy (CRRT) is unclear. In a prospective study, we determined CMS and colistin pharmacokinetics in 10 critically ill patients requiring CRRT (8 underwent continuous venovenous hemodialysis [CVVHD]; median blood flow, 100 ml/min). Intensive sampling was performed on treatment days 1, 3, and 5 after an intravenous CMS loading dose of 9 million international units (MU) (6 MU if body weight was <60 kg) with a consecutive 3-MU (respectively, 2 MU) maintenance dose at 8 h. CMS and colistin concentrations were determined by liquid chromatography with mass spectroscopy. A model-based population pharmacokinetic analysis incorporating CRRT settings was applied to the observations. Sequential model building indicated a monocompartmental distribution for both CMS and colistin, with interindividual variability in both volume and clearance. Hematocrit was shown to affect the efficacy of drug transfer across the filter. CRRT clearance accounted for, on average, 41% of total CMS and 28% of total colistin clearance, confirming enhanced elimination of colistin compared to normal renal function. Target colistin steady-state trough concentrations of at least 2.5 mg/liter were achieved in all patients receiving 3 MU at 8 h. In conclusion, a loading dose of 9 MU followed after 8 h by a maintenance dose of 3 MU every 8 h independent of body weight is expected to achieve therapeutic colistin concentrations in patients undergoing CVVHD using low blood flows. Colistin therapeutic drug monitoring might help to further ensure optimal dosing in individual patients. (This study has been registered at ClinicalTrials.gov under identifier NCT02081560.)
INTRODUCTION
Colistin is a polymyxin antibiotic with concentration-dependent bactericidal properties against a variety of Gram-negative species, including Pseudomonas aeruginosa, Klebsiella spp., Enterobacter spp., and Acinetobacter spp. Colistimethate sodium (CMS) is the inactive prodrug of colistin, bearing methanesulfonate residues linked to its five amine radicals. It is commercially available as colistin (1) and is usually used to treat multidrug-resistant strains. After intravenous administration, CMS is partially hydrolyzed (30 to 40%) into the active form colistin and to sulfomethylated derivatives, while two-thirds of the prodrug dose is renally excreted unchanged by glomerular filtration (2, 3). Colistin`s bioavailability is therefore limited by what could be considered a type of “renal first-pass effect.” Renal clearance of CMS in healthy volunteers approximates 100 ml/min (4). Conversely, the renal excretion of colistin itself is modest, due to active tubular reabsorption (3). In critically ill patients, Karaiskos and colleagues found the renal clearance of colistin to be not significantly different from zero (5). In patients with renal impairment, a larger fraction of CMS is available for transformation into colistin and both agents accumulate (6, 7), so a significant dosage reduction is needed (8). The two major dose-dependent toxic effects of colistin are nephrotoxicity due to acute tubular necrosis and neurotoxicity (9).
Critically ill patients may develop acute or acute-on-chronic renal failure requiring a period of continuous renal replacement therapy (CRRT). Although pharmacokinetic (PK) studies and dosing recommendations for CMS in critically ill patients with normal or impaired renal function exist, there are only a few systematic studies of the pharmacokinetics of CMS and colistin in patients undergoing CRRT (10–13). Dose recommendations in these patients therefore differ widely, and there is evidence that certain recommendations might lead to underdosing (14). CMS and colistin have molecular weights of 1,750 and 1,155 Da, respectively, meaning that they are small enough to pass through hemodiafiltration high-flux membranes. Protein binding of colistin is dependent on drug concentration and ranges between 10 and 50% in animals (15), which further enables the drug to be eliminated by CRRT. Similar protein binding values have been observed for critically ill patients (16). CMS and colistin are therefore filtered and dialyzed during CRRT (13). In addition, as no tubular reabsorption occurs during CRRT, colistin clearance is—as to be expected—higher than that achieved by a healthy kidney, even though filtration rates applied are usually less than half of a normal glomerular filtration rate (13).
The standard dosage of CMS for a 70-kg patient with normal renal function is 9 million international units (MU) per day, divided into 2 or 3 doses. In the United States, dosages are usually expressed in milligrams of colistin base activity, with a conversion factor of 33 mg for 1 MU, which corresponds to 80 mg of CMS. Previous studies have shown that reduced CMS dosages ranging from 1.8 MU every 48 h to 0.9 MU every 8 h in patients undergoing continuous venovenous hemodiafiltration (CVVHDF) were associated with insufficient steady-state colistin concentrations (Cssav) ranging from 0.7 mg/liter to 1.7 mg/liter (10, 12, 14). These values are below the suggested target Cssav of 2.0 to 2.5 mg/liter for susceptible bacteria with a MIC of <0.5 mg/liter (12, 17–19). MICs for Gram-negative bacteria such as Acinetobacter baumannii and P. aeruginosa are >1 mg/liter (both breakpoints, 2 mg/liter [20]).
Based on those studies, some authors suggest that a higher dosage is required to achieve therapeutic colistin concentrations in patients undergoing CVVHDF. A recent study of 8 patients undergoing CVVHDF (dialysate fluid rate, 1 to 1.5 liters/h) showed that the median peak colistin concentrations after a loading dose of 9 MU was 1.55 mg/liter, and during maintenance therapy with 4.5 MU every 12 h a Cssav of 1.72 mg/liter was achieved (13).
These data indicate significant extracorporal clearance by CRRT and support the suggestion for higher dosages of CMS in critically ill patients undergoing CVVHDF, in order to avoid underdosing and subsequent therapeutic failure. Recent studies propose a CMS loading dose of 6 to 12 MU in critically ill patients in order to achieve effective colistin plasma concentrations from the first treatment day (13, 16, 19, 21). A loading dose of 9 MU has been shown to be well tolerated without significant renal or neurotoxicity in a study including 22 patients with normal renal function (21).
The main objective of our study was therefore to characterize the population pharmacokinetics of intravenous CMS and colistin in patients undergoing CRRT and receiving the standard dosage of CMS, predicted to achieve therapeutic concentrations in this situation according to current knowledge. Secondary study objectives were to assess clinical outcomes and drug-related adverse events.
RESULTS
Eleven patients aged between 29 and 70 years were included in the study. One patient was subsequently excluded due to bleeding and multiple blood transfusions that led to incorrect colistin plasma concentrations. This left 10 patients for whom data were available for analysis. Patients 5 and 6 underwent CVVHDF; all other patients underwent CVVHD. Further patient characteristics, including the CMS dosages administered and the settings of CRRT, are shown in Table 1. The loading dose was sampled in 3 patients, whereas in the remaining patients CMS was started or continued with a maintenance dose when colistin treatment was already initiated at inclusion of the patient.
TABLE 1.
Patient description: age, body weight, sex, loading dose, maintenance dose, number of maintenance doses administered, average blood flow, and effluent rate through CRRT devicea
| Patient ID or statistical parameter | Age (yr) | Wt (kg) | Sex | Diagnoses and indication for colistin | Colistin MIC (mg/liter) | Ht | Alb (g/liter) | Dload (MU) | Dmaint (MU) | Dmaint tot (MU) | CRRT | Qblood (ml/min) | Qeffl (liters/h) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||||||||
| 1 | 60 | 65 | M | Acute lymphoblastic leukemia, pneumonitis, P. aeruginosa bacteremia | <4 | NA | NA | 9 | 3 | 18 | CVVHD | 100 | 2,180 | Infection controlled |
| 2 | 51 | 73 | M | Sepsis with multiresistant P. aeruginosa, multiorgan failure | NA | NA | NA | 9 | 3 | 7 | CVVHD | 100 | 2,000 | Died (multiorgan failure) |
| 3 | 29 | 82 | M | Polytrauma, P. aeruginosa pneumonia and septic shock | 0.75–1 | 0.24 | 22 | 9 | 3 | 15 | CVVHD | 108 | 2,793 | Infection controlled |
| 4 | 44 | 60 | M | Lung transplant, mediastinal abscess, acute respiratory failure (P. aeruginosa VAP) | 0.38 | 0.33 | 20 | 2 | 2 | 14 | CVVHD | 122 | 3,071 | Infection controlled |
| 5 | 34 | 60 | M | Cystic fibrosis, liver cirrhosis, P. aeruginosa pneumonia and septic shock | 0.047–0.5 | 0.27 | 28 | 2 | 2 | 24 | CVVHDF | 120 | 2,479 | Died (pulmonary hemorrhage) |
| 6 | 47 | 66 | M | HIV, pulmonary hypertension, PCP pneumonia, acute respiratory distress syndrome, P. aeruginosa and Citrobacter freundii VAP | 10.125 | 0.23 | 21 | 9 | 3 | 12 | CVVHDF | 127 | 2,473 | Died (respiratory failure) |
| 7 | 67 | 60 | M | Cholangitis, septic shock (ESBL-producing Escherichia coli), acute respiratory distress syndrome | NA | 0.26 | 22 | 6 | 2 | 6 | CVVHD | 118 | 2,250 | Died (cholangitis) |
| 8 | 70 | 85 | M | Pancreas carcinoma, postoperative septic shock, P. aeruginosa VAP | 1.5 | NA | NA | 3 | 3 | 19 | CVVHD | 100 | 2,278 | Died (multiorgan failure) |
| 10 | 44 | 46 | F | Lung transplant, P. aeruginosa tracheobronchitis | 0.125 | 0.23 | 20 | 2 | 2 | 22 | CVVHD | 80 | 1,601 | Died (cause unknown) after infection cleared |
| 11 | 68 | 60 | F | Necrotizing pancreatitis, ventilator-associated pneumonia, severe sepsis | NA | 0.25 | 24 | 9 | 3 | 10 | CVVHD | 100 | 2,652 | Died (multiorgan failure) |
| Summary statistics | ||||||||||||||
| Mean | 50.4 | 65.7 | 108 | 2,378 | ||||||||||
| SD | 16.0 | 11.6 | 14 | 416 | ||||||||||
| Median | 49.0 | 62.5 | 104 | 2,376 | ||||||||||
| Min | 24.0 | 46.0 | 80 | 1,601 | ||||||||||
| Max | 70.0 | 85.0 | 127 | 3,071 | ||||||||||
| Gmean | 47.8 | 64.8 | 107 | 2,343 | ||||||||||
| CV (%) | 34 | 18 | 13 | 18 |
ID, identifier; Alb, albumin; CV, coefficient of variation; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; Dload, CMS loading dose; Dmaint, CMS maintenance dose; Dmaint tot, CMS total number of maintenance doses; Min, minimum; Max, maximum; Gmean, geometric mean; Ht, hematocrit; NA, not available; Qblood, blood flow; Qeffl, effluent flow rate; VAP, ventilator-associated pneumonia; M, male; F, female, ESBL, extended-spectrum β-lactamase. Ranges for MICs indicate results of different isolates tested over time.
CMS and colistin population pharmacokinetic profiles.
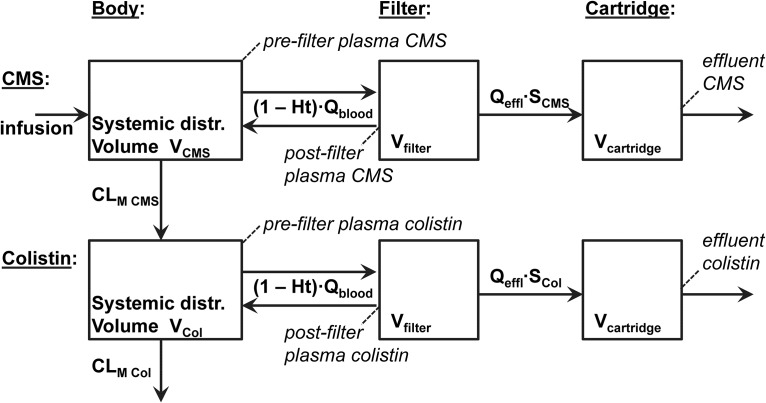
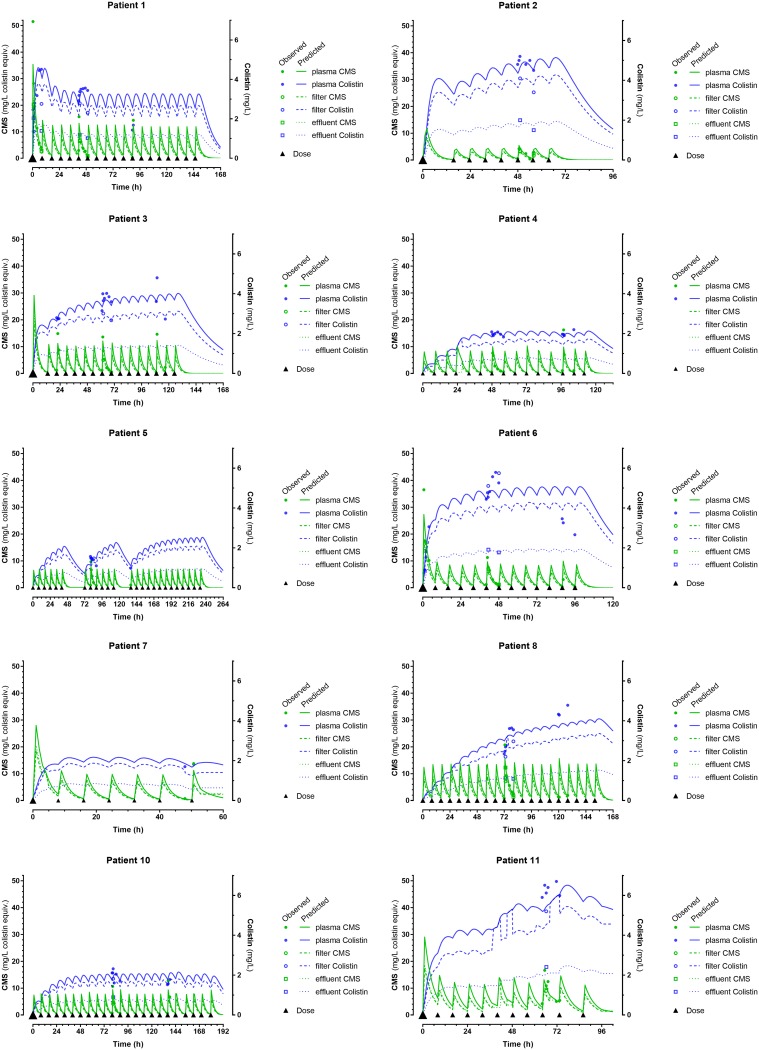
A satisfactory description of observations was obtained by fitting the 6-compartment model described with NONMEM (Fig. 1). Individual curve fittings of patients’ measured CMS and colistin concentrations are shown in Fig. 2 (see Fig. S1 in the supplemental material for a logarithmic display). Sequential model building indicated that a model assuming interindividual variability in terms of volume and clearance parameters (VCMS, VCol, CLM CMS, and CLM Col) fitted the data better than a model assuming constant population values for these parameters. Conversely, the results did not show evidence for interindividual differences in sieving coefficients (SCMS and Scol). We found no indication that age, body weight, sex, or albumin had a role to play as a covariate. Conversely, the inclusion of hematocrit (Ht) as a covariate affecting the efficacy of blood transfer (Qblood) in and out of the filter improved the model significantly. Diagnostic plots in arithmetic and logarithmic form (Fig.S2 and S3, respectively) visualized model validation.
FIG 1.
Six-compartment pharmacokinetic model describing the disposition of CMS and colistin during CRRT and showing the sampling points (in italics). See text for parameter definitions.
FIG 2.
Individual curve fitting of patients’ observations (solid circles) in arithmetic scale.
The primary pharmacokinetic estimates corresponding to our final model, namely, metabolic clearance, distribution volumes, and sieving coefficients for CMS and colistin, are given in Table 2, along with the maximum likelihood individual parameters corresponding to each study patient. In accordance with its physicochemical characteristics, CMS had a higher sieving coefficient and a smaller volume of distribution than colistin (Table 2).
TABLE 2.
Population averages and coefficients of variation, followed by maximum likelihood individual estimates of primary pharmacokinetic parameters: metabolic clearances, distribution volumes, and sieving coefficients for CMS and colistina
| Estimate | CMS |
Colistin |
||||
|---|---|---|---|---|---|---|
|
CLM CMS (liters/h) |
VCMS (liters) |
SCMS |
CLM col (liters/h) |
Vcol (liters) |
Scol | |
| Pop avg | 2.31 | 12.1 | 1.05 | 1.93 | 70.1 | 0.454 |
| SE | 0.412 | 1.42 | 0.0667 | 0.444 | 15.5 | 0.0181 |
| Pop CV (%) | 52 | 36 | 67 | 50 | ||
| SE | 0.162 | 0.106 | 0.218 | 0.127 | ||
| Patient estimates | ||||||
| 1 | 2.34 | 12.2 | 1.050 | 3.18 | 35.5 | 0.454 |
| 2 | 7.33 | 28.3 | 1.050 | 2.50 | 69.0 | 0.454 |
| 3 | 2.41 | 11.7 | 1.050 | 1.81 | 84.8 | 0.454 |
| 4 | 1.98 | 8.9 | 1.050 | 2.01 | 85.4 | 0.454 |
| 5 | 2.41 | 11.9 | 1.050 | 2.14 | 73.3 | 0.454 |
| 6 | 3.12 | 14.8 | 1.050 | 1.54 | 67.6 | 0.454 |
| 7 | 1.62 | 8.0 | 1.050 | 2.49 | 70.8 | 0.454 |
| 8 | 1.46 | 9.6 | 1.050 | 1.38 | 123.6 | 0.454 |
| 10 | 2.79 | 10.1 | 1.050 | 4.09 | 73.1 | 0.454 |
| 11 | 1.13 | 13.4 | 1.050 | 0.54 | 39.0 | 0.454 |
| Mean | 2.66 | 12.9 | 1.050 | 2.17 | 72.2 | 0.454 |
| SD | 1.75 | 5.8 | 0.99 | 24.6 | ||
| Median | 2.37 | 11.8 | 2.07 | 72.0 | ||
| Min | 1.13 | 8.0 | 0.54 | 35.5 | ||
| Max | 7.33 | 28.3 | 4.09 | 123.6 | ||
| Gmean | 2.32 | 12.1 | 1.93 | 68.3 | ||
| CV (%) | 75 | 48 | 51 | 36 | ||
Residual error is described by a mixed exponential and additive error model, with, respectively, a coefficient of variation of 22.2% and a standard deviation (SD) of 0.459 mg/liter. CLM CMS, metabolic clearance of CMS; VCMS, volume of distribution of CMS; SCMS, CMS sieving coefficient; CLM Col, metabolic clearance of colistin; VCol, volume of distribution of colistin; SCol, colistin sieving coefficient; Pop, population.
The primary pharmacokinetic parameters enabled the calculation of derived parameter values such as cumulative areas under the curve, fractions excreted through CRRT, total clearances, and steady-state trough concentrations of both CMS and colistin (Table 3). CMS had a greater clearance (both total and through CVVHD) than colistin, with consequent longer colistin half-life and higher steady-state trough concentrations. CMS was more efficiently removed by CVVHD than colistin, as reflected by CRRT clearance accounting for, on average, 41% of the total CMS and 28% of the total colistin clearance, respectively (Table 3).
TABLE 3.
| Patient ID or statistical parameter | CMS |
Colistin |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AUCCMS (h·mg/liter) |
Fe CMS |
CLtot CMS (liters/h) |
CLCVVHD CMS (liters/h) |
t1/2 CMS (h) |
CSS min CMS (mg/liter) |
Cssav CMS (mg/liter) |
AUCCol (h·mg/liter) |
Fe col |
CLtot col (liters/h) |
CLCVVHD col (liters/h) |
t1/2 Col (h) |
CSS min col (mg/liter) |
Cssav Col (mg/liter) |
|
| 1* | 865 | 0.393 | 3.850 | 1.513 | 2.20 | 1.26 | 5.15 | 506 | 0.203 | 3.993 | 0.811 | 6.16 | 2.58 | 3.01 |
| 2* | 181 | 0.163 | 8.766 | 1.429 | 2.24 | 0.58 | 2.26 | 404 | 0.230 | 3.283 | 0.755 | 14.57 | 4.63 | 5.05 |
| 3* | 658 | 0.434 | 4.265 | 1.851 | 1.91 | 0.86 | 4.65 | 548 | 0.348 | 2.899 | 1.009 | 20.29 | 3.69 | 3.87 |
| 4 | (406) | 0.484 | 3.840 | 1.859 | 1.61 | (0.34) | (3.44) | (251) | 0.338 | 3.204 | 1.083 | 18.48 | (1.91) | (2.13) |
| 5 | (621) | 0.424 | 4.183 | 1.774b | 1.98 | (0.62) | (3.16) | (480) | 0.299 | 3.124 | 0.934b | 16.26 | (2.28) | (2.44) |
| 6* | 479 | 0.361 | 4.889 | 1.765b | 2.10 | 0.83 | 4.05 | 590 | 0.370 | 2.534 | 0.938b | 18.47 | 4.64 | 5.00 |
| 7 | (312) | 0.459 | 2.996 | 1.375 | 1.85 | (0.58) | (4.41) | (163) | 0.163 | 3.110 | 0.507 | 15.79 | (1.87) | (2.30) |
| 8* | 1,044 | 0.511 | 2.990 | 1.528 | 2.24 | 1.60 | 6.63 | 607 | 0.329 | 2.511 | 0.826 | 34.12 | 3.92 | 3.85 |
| 10 | (608) | 0.290 | 3.933 | 1.141 | 1.78 | (0.51) | (3.36) | (361) | 0.128 | 4.702 | 0.602 | 10.78 | (1.75) | (1.99) |
| 11* | 765 | 0.574 | 2.651 | 1.522 | 3.50 | 2.41 | 7.47 | 741 | 0.430 | 1.164 | 0.501 | 23.21 | 4.00 | 7.24 |
| Mean | 665 | 0.409 | 4.236 | 1.576 | 2.14 | 1.26 | 5.03 | 566 | 0.284 | 3.052 | 0.796 | 17.81 | 3.91 | 4.67 |
| SD | 304 | 0.117 | 1.731 | 0.222 | 0.52 | 0.67 | 1.86 | 112 | 0.098 | 0.933 | 0.194 | 7.48 | 0.76 | 1.48 |
| Median | 712 | 0.429 | 3.892 | 1.525 | 2.04 | 1.06 | 4.90 | 569 | 0.314 | 3.117 | 0.804 | 17.37 | 3.96 | 4.43 |
| Min | 181 | 0.163 | 2.651 | 1.141 | 1.61 | 0.58 | 2.26 | 404 | 0.128 | 1.164 | 0.501 | 6.16 | 2.58 | 3.01 |
| Max | 1,044 | 0.574 | 8.766 | 1.859 | 3.50 | 2.41 | 7.47 | 741 | 0.430 | 4.702 | 1.083 | 34.12 | 4.64 | 7.24 |
| Gmean | 583 | 0.390 | 4.003 | 1.559 | 2.09 | 1.12 | 4.70 | 557 | 0.266 | 2.894 | 0.771 | 16.35 | 3.84 | 4.49 |
| CV (%) | 52 | 30 | 43 | 14 | 25 | 60 | 40 | 20 | 37 | 32 | 25 | 46 | 20 | 33 |
Parameters included cumulative areas under curve (AUC), fractions excreted through CRRT (Fe), total clearances (CLtot), and trough concentrations at steady-state (Css min) and average concentrations at steady-state (Cssav) of CMS and colistin. For dose-dependent parameters (AUC and CSS min), means, SD, medians, and ranges were calculated only with data from patients receiving a maintenance regimen of 3 MU every 8 h (indicated by asterisks) excluding the values in parentheses.
CLCVVHDF.
A target colistin Cssav of 2.5 mg/liter was achieved in all patients who received a maintenance dose of 3 MU but in none of the patients who received a maintenance dose of 2 MU every 8 h (Table 3).
Adverse events.
Overall, no alarming drug accumulation was observed over the five treatment days (Fig. 2; one exception was possibly patient 8) and no adverse events attributable to CMS or colistin were recorded.
Patient outcomes.
Infection control (as evidenced by a reduction in inflammatory markers such as C-reactive protein and leukocyte count) was achieved in three patients and microbial cure in one patient. However, 7 of the 10 included patients experienced a fatal outcome of their severe illness. Renal function improved in 4 patients (moderate renal impairment, no renal replacement therapy), and 1 patient remained on intermittent hemodialysis.
DISCUSSION
In a population pharmacokinetic study of intravenous CMS in patients undergoing CRRT, we found that a compartmental model incorporating CRRT settings best described the disposition of CMS and colistin. This population analysis revealed a fair degree of interindividual variability in volume and clearance parameters (VCMS, VCol, CLM CMS, and CLM Col), as is often found for therapeutic drugs undergoing metabolic biotransformations. This variability was not significantly explained by age, body weight, sex, or serum albumin concentration, as these covariates did not improve model performance. However, hematocrit improved the model significantly, a finding consistent with its direct impact on drug transfer from the patient’s body to the CRRT filter cartridge. It is also remarkable that the model did not indicate between-patient variability in the sieving coefficients of CMS and colistin. This is not surprising for CMS, a highly hydrophilic prodrug that is poorly bound to plasma proteins. As our patients had little heterogeneity regarding their circulating albumin concentrations (Table 1), no effect of this covariate on colistin sieving could be observed, even though colistin is more extensively protein bound than CMS. We propose that our findings will enable future optimal CMS dosing in patients undergoing CRRT to achieve adequate antibacterial efficacy, to avoid the emergence of colistin-resistant organisms (22) and to minimize toxicity.
In their population pharmacokinetic study of CMS in critically ill patients not undergoing CRRT, Karaiskos and colleagues similarly found that a model with several compartments (5 in total) best described their data (5).
In patients with normal renal function, renal clearance accounts for two-thirds of CMS clearance and almost none of colistin clearance (3, 4). In comparison, in the present study, CRRT clearance accounted for 41% of CMS clearance and 28% of colistin clearance. In a study performed by Karaiskos et al., CVVHDF clearances of CMS and colistin were estimated at 60% and 62%, respectively (13). The lower clearance found in our study may be related to the lower blood flows (100 to 127 ml/min, compared to 100 to 180 ml/min). The significant colistin clearance during CRRT is attributable to the absence of tubular reabsorption. In other words, while CMS clearance is reduced in renal impairment managed with CRRT, colistin clearance is increased. Consequently, higher doses of intravenous CMS are required, as already noted in some current guidelines, such as the Sanford Guide to Antimicrobial Therapy, which recommends a daily CMS dose of 13 MU (442-mg colistin base) (23), but not in the Food and Drug Administration-approved drug label (8).
A target colistin Cssav of 2.5 mg/liter for susceptible bacteria with a MIC of <0.5 mg/liter (16) was achieved in all patients who received a maintenance dose of 3 MU but in none of the patients who received a maintenance dose of 2 MU every 8 h (Table 3). In the study by Karaiskos and colleagues in which 4.5 MU was administered every 12 h, 24 h after receiving a 9-MU loading dose, only half of the subjects achieved steady-state colistin concentrations (6 h after a dose) of 1.72 mg/liter or higher (13). A reason for this discrepancy is the higher CVVHDF clearance in the above-mentioned study. A further explanation might be that the 6-h postdose concentration as measured by Karaiskos and colleagues does not reflect the peak concentration attained before the next CMS dose as accurately as the predose sample, which was analyzed in our study (8 h postdose).
In a study by Nation and colleagues, which included data from 9 patients undergoing CRRT (average blood flow, 160 ml/min; 9-MU CMS loading dose and 13-MU daily maintenance dose) (19), CMS clearance due to CRRT was 1.57 liters/h, which was identical to our finding. Colistin CRRT clearance, however, was higher (2.68 liters/h, compared to 0.796 liter/h in our study). In the above-mentioned study by Karaiskos and colleagues of 8 patients undergoing CRRT (blood flow, 100 to 180 ml/min; 9-MU CMS loading dose and 9-MU daily maintenance dose), CMS and colistin CRRT clearances were 1.17 liters/h and 2.09 liters/h, respectively (13). Taken together, these findings suggest that CMS clearance may be filter limited, while colistin CRRT clearance depends on blood flow. Two other studies both using 9-MU daily maintenance doses and a blood flow of 120 ml/min (comparable to the present study) in seven and four patients undergoing CRRT (11, 24) showed colistin CRRT clearances of about 0.7 liter/h, which compared to our findings.
For daily clinical practice, we therefore suggest starting CMS in patients that are undergoing CVVHD using low blood flows with a loading dose of 9 MU and a maintenance dose of 3 MU every 8 h (independent of body weight), so that therapeutic colistin concentrations have a high likelihood of being achieved. As Cssav depends only on clearance (and not volume of distribution), the maintenance dose does not need to be weight adjusted.
The present study has some limitations. Although the study is prospective in design, patients were included based on their clinical need for intravenous CMS and concurrent CRRT. This resulted in a modest sample size and inability to perform PK sampling after a loading dose in the setting of CRRT in every case. Despite satisfactory global diagnostics (Fig. S2 and S3 in the supplemental material), isolated misfit points were observed, most likely resulting from technical issues such as inaccuracies in dose preparation, sample collection, or CVVHD efficacy due to filter clogging or clotting. A further limitation is the missing data regarding hematocrit. However, the available data showed a small spread, so we are confident that using the average value for the group as a whole did not significantly affect the results.
In conclusion, CMS and colistin disposition after intravenous application is complex and CRRT substantially increases the clearance of colistin compared to normal renal function. A loading dose of 9 MU and a maintenance dosage of 3 MU every 8 h independent of body weight are expected to quickly achieve and maintain therapeutic colistin concentrations in patients undergoing CRRT with low blood flows. We suggest starting the maintenance dosage 8 h after administering the loading dose. Furthermore, this dosage was not associated with adverse events. Therapeutic drug monitoring of CMS and colistin might further ensure optimal dosing, particularly in problematic situations such as comorbid hepatic insufficiency or inefficient CRRT.
MATERIALS AND METHODS
The study was performed in accordance with the Declaration of Helsinki and its amendments, International Conference on Harmonization (ICH) good clinical practice (GCP) guidelines and applicable national laws and regulations. Ethical approval for the study was granted by the internal review boards of the participating centers (ZH 2012-0451, CER-VD 197/14, and EKNZ 2014-213), and permission to conduct the study was given by the national authority (Swissmedic; 2013DR1132). The study was registered at ClinicalTrials.gov (NCT02081560). All patients enrolled in the study gave their written, informed consent if able to do so. When patients were not able to give consent themselves, the protocol for studies in adult patients unable to give consent was followed, in accordance with the internal review boards’ permission.
Study design.
The study was an investigator-driven, multicenter, prospective, nonrandomized, open-label pharmacokinetic (PK) investigation of CMS in critically ill patients requiring CVVHDF. The participating centers were University Hospital Zürich, University Hospital Lausanne, and University Hospital Basel.
Study patients.
Patients were eligible for enrollment if they fulfilled the following criteria: male or female aged 18 years or older, hospitalized on the intensive care unit (ICU) with a clinical necessity for both, treatment with CMS (for a proven severe infection with multidrug-resistant Gram-negative bacteria susceptible to colistin with no standard first-line treatment available), and continuous venovenous renal replacement therapy.
Study drug.
Colistin (colistimethate) was delivered in dry-powder form together with an ampoule of 3 ml of isotonic saline solvent (1). One bottle of colistin contains 1 million units (MU) of sodium colistimethate. Colistin was dispensed from the local hospital pharmacy and stored on the intensive care units. CMS maintenance dosing was set to 3 MU (=240 mg) every 8 h (total 9 MU/day = 720 mg/day) after administration of a loading dose of 9 MU. In patients with a low body weight (≤60 kg), the dose was reduced to a loading dose of 6 MU and to a maintenance dose of 2 MU every 8 h. The CMS solution was to be administered intravenously over 30 min. For pharmacokinetic calculations, CMS doses in milligrams and concentrations in milligrams per liter were converted into colistin equivalents using a conversion factor of 1,155/1,750 = 0.66.
CRRT procedures.
Continuous venovenous hemodialysis (CVVHD) was performed in all but one patient with Prismaflex ST150 (Gambro AB, Lund, Sweden) using the capillary hemofilter AN69 ST (acrylonitrile-sodium-methyl sulfonate; surface area, 1.5 m2) and infusing citrate as the anticoagulation agent. Continuous venovenous hemodiafiltration (CVVHDF) in a ratio set 1:1 was performed with multiFiltrate (Fresenius Medical Care, Homburg, Germany) using the capillary hemofilter AV 1000 s (polysulfone; surface area, 1.8 m2). Postdilution mode was performed for one patient.
Pharmacokinetic sampling.
Intensive pharmacokinetic sampling over one dosing interval (8 h) was scheduled on treatment day 1 after the CMS loading dose and day 3 (after the 7th dose). Intensive PK sampling involved blood samples taken from the prefilter (filter afferent) line at 0, 0.5, 1, 2, 3, 6, and 8 h after CMS dosing and from the postfilter line at 1 and 8 h after dosing. Dosing and sampling times were precisely recorded, along with the relevant settings of CRRT (blood and hemodiafiltration flow rates). Blood was collected in tubes containing citrate. Plasma was obtained by centrifugation of whole-blood samples within 30 min and then stored at −80°C until analysis to prevent degradation of CMS into colistin ex vivo (25). Peak and trough blood concentrations in the prefilter line were determined on day 5. Patients already on colistin treatment at study entry underwent PK sampling on treatment day 3 (7th dose) and on day 5 if already undergoing CRRT. In patients starting CRRT during established treatment with colistin, the dosage was adapted according to the protocol, the first PK sampling was performed on the third day of CRRT, and the study continued according to the protocol. Patients were monitored daily for any abnormalities in blood biochemistry and hematology values and for signs of adverse drug effects.
Determination of plasma CMS and colistin concentrations.
Plasma CMS and colistin concentrations were determined by high-throughput hydrophilic interaction chromatography coupled to tandem mass spectrometry according to the method described by Mercier and colleagues (26). Briefly, the method was first applied to the native plasma samples to determine colistin concentration; then the samples underwent acidic hydrolysis for 4 h to extensively transform CMS into colistin, the colistin concentration was determined again and the difference was assumed to reflect CMS (including intermediate metabolites partially hydrolyzed). The results for CMS were accordingly expressed in milligrams per liter of colistin. All analyses were performed at the University Hospital Lausanne.
Pharmacokinetic analysis.
A model-based pharmacokinetic analysis was performed using NONMEM (version 7.4, 2017; ICON plc, Dublin, Ireland) with NMTRAN and the PREDPP subroutine ADVAN6. The disposition of CMS and colistin was described using a 6-compartment model with first-order transfer rates describing the metabolic transformation of CMS into colistin, the metabolic elimination of colistin, and the exchanges of both compounds occurring within the CRRT apparatus (Fig. 1). The systemic distributions of CMS and colistin were assumed to be rapid, corresponding to a single-compartment volume each (VCMS and VCol). The fraction of the CMS dose escaping elimination through CRRT was assumed to be completely transformed into colistin through metabolic clearance (CLM CMS); then colistin, in turn, was considered to be eliminated through both CRRT and metabolic clearance (CLM Col). Both compounds were assumed to be transferred into a CRRT filter of fixed volume (Vfilter = 0.2 liter) at the flow rate read on the CRRT device (Qblood), corrected for the patient’s hematocrit (1 − Ht), set to the average Ht value (0.25) when unknown. Nonfiltered amounts of CMS and colistin were driven back into the patient’s circulation with the same blood flow, while filtered amounts passed into the fixed volume filter cartridge (Vcartridge = 0.3 liter), continuously rinsed with the effluent flow rate read on the CRRT device (Qeffl). Sieving coefficients were assumed to characterize the filter permeability for CMS and colistin (SCMS and Scol), multiplying Qeffl to give the respective filtration clearances.
The model, expressed as a set of differential equations, was fitted to the data using first-order conditional estimation with interaction between interpatient and residual variabilities. Classical equations were used for relating model microconstants (k12, k13, k31, k20, k24, k42, k35, k46, k50, and k60) to the estimated parameters, i.e., VCMS, VCol, CLM CMS, CLM Col, SCMS, and Scol, given the known parameters, i.e., Vfilter, Vcartridge, Qblood, Ht, and Qeffl. Interindividual variability in estimated parameters was described using an exponential error model (namely, θj = θ × eηj, where θj is the individual pharmacokinetic parameter of the jth individual, θ is the geometric average population value, and ηj is a random effect normally distributed around a zero mean with a variance of ω2θ). The inclusion of variability was tested on each parameter for improvement of the model, based on the change in objective function (ΔOF) resulting from the addition of a model term (which approximately follows a χ2 distribution and can be regarded as statistically significant at the level P < 0.05 if it exceeds 3.8 for one additional parameter). Residual error was described using a mixture of exponential plus additive distributions; there was no indication for different magnitudes of variability between the types of samples. The influence of covariates such as age, sex, and body weight on distribution volumes and metabolic clearances, and plasma albumin on sieving coefficients, was explored graphically and tested sequentially for inclusion in the model, based on the ΔOF criterion. The following derived parameters were computed numerically from individual parameter estimates obtained by model fitting: cumulative area under plasma concentration curves (AUCCMS and AUCCol), trough concentrations at steady state under maintenance dosage (CSS min CMS and CSS min Col) and total amounts excreted by CRRT (Ae CMS and Ae Col). Taking into account cumulative CMS doses, we estimated the fractions of dose eliminated by CRRT (Fe CMS and Fe Col), the hemofiltration (CLCRRT CMS and CLCRRT Col) and total clearances (CLtot CMS and CLtot Col), and the apparent half-lives deduced from corresponding distribution volumes (t1/2 CMS and t1/2 Col).
Model validation was based on diagnostic goodness-of-fit plots and on the examination of standard errors and correlation matrix of the estimates. Figures were elaborated with GraphPad Prism (version 7 for Windows, 2017; GraphPad Software, San Diego, CA). The population pharmacokinetic model developed for this study may be accessed on the DDMoRe Model Repository at http://repository.ddmore.foundation//model/DDMODEL00000295.
Supplementary Material
ACKNOWLEDGMENTS
The study was funded by a grant from Forest Laboratories Switzerland.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
Author contributions were as follows: design, all; execution, T.B., N.C., L.D., and N.J.M.; analysis and/or interpretation, all; and drafting, revising, and/or approving submission of manuscript, all.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01957-18.
REFERENCES
- 1.Teva Pharma AG. 2016. Product information. Colistin®. Teva Pharma AG, Basel, Switzerland.
- 2.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregoire N, Aranzana-Climent V, Magreault S, Marchand S, Couet W. 2017. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin Pharmacokinet 56:1441–1460. doi: 10.1007/s40262-017-0561-1. [DOI] [PubMed] [Google Scholar]
- 4.Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther 89:875–879. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- 5.Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, Kostakou E, Baziaka F, Paskalis C, Koutsoukou A, Giamarellou H. 2015. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin NJ, Friedman EA. 1968. The effects of renal impairment, peritoneal dialysis, and hemodialysis on serum sodium colistimethate levels. Ann Intern Med 68:984–994. doi: 10.7326/0003-4819-68-5-984. [DOI] [PubMed] [Google Scholar]
- 8.Par Pharmaceutical. 2017. Coly-Mycin® M parenteral (colistimethate for injection, USP). Par Pharmaceutical, Chestnut Ridge, NY.
- 9.Falagas ME, Kasiakou SK. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Critical Care 10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karvanen M, Plachouras D, Friberg LE, Paramythiotou E, Papadomichelakis E, Karaiskos I, Tsangaris I, Armaganidis A, Cars O, Giamarellou H. 2013. Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 57:668–671. doi: 10.1128/AAC.00985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leporati M, Bua RO, Mariano F, Carignano P, Stella M, Biancone L, Vincenti M. 2014. Determination by LC-MS/MS of colistins A and B in plasma and ultrafiltrate from critically ill patients undergoing continuous venovenous hemodiafiltration. Ther Drug Monit 36:182–191. doi: 10.1097/FTD.0b013e3182a8997c. [DOI] [PubMed] [Google Scholar]
- 12.Markou N, Fousteri M, Markantonis SL, Zidianakis B, Hroni D, Boutzouka E, Baltopoulos G. 2012. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J Antimicrob Chemother 67:2459–2462. doi: 10.1093/jac/dks257. [DOI] [PubMed] [Google Scholar]
- 13.Karaiskos I, Friberg LE, Galani L, Ioannidis K, Katsouda E, Athanassa Z, Paskalis H, Giamarellou H. 2016. Challenge for higher colistin dosage in critically ill patients receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 48:337–341. doi: 10.1016/j.ijantimicag.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J. 2005. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 49:4814–4815. doi: 10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 54:1117–1124. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogue JM, Ortwine JK, Kaye KS. 2017. Clinical considerations for optimal use of the polymyxins: a focus on agent selection and dosing. Clin Microbiol Infect 23:229–233. doi: 10.1016/j.cmi.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. 2016. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, Li J, Silveira FP. 2017. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoint tables for interpretation of MICs and zone diameters, version 8.1. http://www.eucast.org/clinical_breakpoints/.
- 21.Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. 2012. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A III, Forrest A, Bulitta JB, Tsuji BT. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antimicrobial Therapy Inc. 2018. Sanford guide to antimicrobial therapy web edition. Antimicrobial Therapy Inc, Sperryville, VA. [Google Scholar]
- 24.Mariano F, Leporati M, Carignano P, Stella M, Vincenti M, Biancone L. 2015. Efficient removal of colistin A and B in critically ill patients undergoing CVVHDF and sorbent technologies. J Nephrol 28:623–631. doi: 10.1007/s40620-014-0143-3. [DOI] [PubMed] [Google Scholar]
- 25.Dudhani RV, Nation RL, Li J. 2010. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob Chemother 65:1412–1415. doi: 10.1093/jac/dkq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercier T, Tissot F, Gardiol C, Corti N, Wehrli S, Guidi M, Csajka C, Buclin T, Couet W, Marchetti O, Decosterd LA. 2014. High-throughput hydrophilic interaction chromatography coupled to tandem mass spectrometry for the optimized quantification of the anti-Gram-negatives antibiotic colistin A/B and its pro-drug colistimethate. J Chromatogr A 1369:52–63. doi: 10.1016/j.chroma.2014.09.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.