Introduction
An intraerythrocytic protozoon of genus Babesia causes human babesiosis, earlier known as “Nantucket fever”. The incidence of babesiosis is steadily increasing across the Northeast and upper Midwest United States [1]. Most infections are asymptomatic, although older and immunocompromised persons can develop severe and fatal disease. In addition, the clinical presentation can vary substantially posing diagnostic challenges for the clinician. Splenic infarction, a known complication with plasmodium infection, has only rarely been described with human babesiosis [2]. Here we describe a case of severe babesiosis leading to infarction of the spleen.
Case report
A 60-year-old man with hypertension, diabetes mellitus and hyperlipidemia presented to the emergency department with complaints of intermittent high-grade fever, chills and rigors for 7–10 days. He had seen a physician 4 days before the presentation and was treated with ciprofloxacin but he remained febrile and developed malaise, anorexia and diffuse abdominal pain. He denied nausea, vomiting, change in bowel habits, rash, chest pain, cough or dyspnea. He had moved to Connecticut, US, from India 8-months prior and denied any recent travel, exposure to pets or tick bites. The initial physical examination revealed fever (39.4 °C), tachycardia (heart rate 120/min), tachypnea (respiratory rate 24/min) and normal blood pressure. Icterus was noted and there was no thrush, oral ulcer, skin rash, joint swelling or cardiac murmur. The pulmonary, cardiovascular and abdominal examinations were unremarkable except for the above findings. Laboratory results included a total leukocyte count of 6300 cells/mm3, hemoglobin level of 10.2 g/dL, platelet count of 36,000/mm3, aspartate aminotransferase level of 124 U/L, alanine aminotransferase level of 65 U/L, alkaline phosphatase level of 126 U/L, total bilirubin 5.7 mg/dL and direct bilirubin of 1.6 mg/dL. HIV antibody was negative, urinalysis was negative for pyuria and two sets of blood cultures were negative for any growth. Electrocardiogram and chest radiographs were normal.
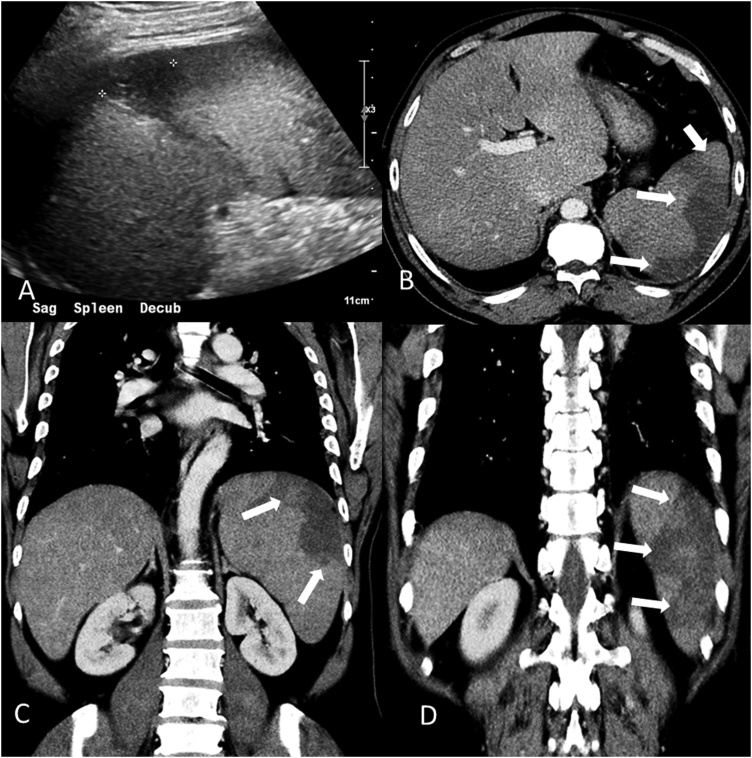
Due to his non-specific systemic symptoms and elevated aminotransferases, ultrasound and contrast enhanced CT scan of the abdomen were done which showed hepatosplenomegaly and features suggestive of acute splenic infarction (Fig. 1). The patient was admitted to the intensive care unit for further management. The follow up laboratory studies revealed worsening anemia (hemoglobin 7.3 g/dL), an elevated reticulocyte count (corrected 4.8%) and lactate dehydrogenase (1443 U/L) and a negative direct Coombs test. Parasite smear showed intraerythrocytic ring forms suggestive of Babesia microti infection with a parasite load of 11% and was negative for plasmodium. Treatment with azithromycin and atovaquone was started. Doxycycline therapy was added for possible co-infection with Borrelia species or Anaplasma species. On day 3 of hospitalization the patient developed left sided upper abdominal pain. Ultrasonography of the abdomen was done which showed a large, wedge shaped hypoechoic area in the mid-splenic area, along with multiple peripherally distributed hypoechoic areas most consistent with infarction. The spleen capsule was intact and there was no free fluid. The Western blot tests for reactivity to Babesia microti were positive for both IgM (>1:320) and IgG (1:256). The Lyme serum enzyme immunoassay was positive but Western blot testing did not confirm infection. Western blot analysis for reactivity to Anaplasma phagocytophilum IgM and IgG were negative. Doxycycline was discontinued after confirmation of the absence of co-infection. The parasitemia improved gradually with disappearance of parasites from the blood smear on day 6. The patient improved symptomatically with normalization of liver function testing and he was discharged after 14 days of hospitalization with a hemoglobin level of 12.2 g/dL.
Fig. 1.
Ultrasonography image (A) demonstrating hypo-echoic peripheral area (asterisk) in the enlarged spleen. Contrast enhanced CT Abdomen: axial (B) and coronal (C-D), demonstrating enlarged spleen (14 cm in craniocaudal dimension) with several peripheral hypo-dense regions (arrows) compatible with splenic infarction.
Discussion
Human babesiosis caused by hemoprotozoa of the genus Babesia is an emerging tick-borne infectious disease. Primarily recognized as pathogens affecting wild and domestic animals, over the last few decades it has been increasingly recognized as a cause of significant human disease. Though more than 100 species of Babesia are well known, most of the human infections in United States are caused by the parasite Babesia microti. In eastern North America, the primary reservoir for B. microti is the white-footed mouse and the primary tick vector of this species is I. scapularis. In endemic areas, up to two-thirds of these mice have been found to be parasitic with B. microti along with Borrelia burgdorferi and Anaplasma phagocytopholum. Ticks may subsequently become infected with these parasites during the blood meal. Human babesiosis is commonly caused by direct tick inoculation, although contaminated blood transfusion and rarely transplacental transmission have been reported [1,3].
Immunocompetent patients with babesiosis typically develop only low-level parasitemia and clinically manifest with non-specific flu-like symptoms, which may resolve spontaneously over a few weeks. However, patients with HIV infection, advanced age, asplenia, splenomegaly or other factors associated with immunosuppression are at high mortality risk [4,5]. The first human case of babesiosis was identified in an asplenic person who died of renal failure during the second week of illness, emphasizing the importance of a healthy spleen to fight the infection [6]. It is well known that the host immune response is required to clear parasite infected red blood cells. In animal models IFN-γ in synergy with TNF-α activates macrophages to produce nitric oxide and kill the parasite [7,8]. The effects of these inflammatory mediators is countered by anti-inflammatory cytokines, mainly IL-4 and IL-10, which help to restrict the inflammatory process to the spleen and prevent systemic overflow (Fig. 1).
Severe parasitemia is associated with a stronger inflammatory reaction that can damage the spleen in part by interrupting the microcirculation, potentially manifesting as splenic infarction. This mechanism may account for splenic infarction seen in high parasitemia states such as in our patient, although there are reports of splenic infarction occurring in low parasitemia states as well [2]. In such circumstances a proposed mechanism may be red cell lysis leading to formation of microthrombi. It should also be noted that subclinical splenomegaly due to splenic erythrophagocytosis has been found in many cases. In a Syrian hamster animal model B. microti infection caused multifocal coagulative necrosis of the spleen [9]. In some case reports, autopsy revealed the parasite within the spleen, although there was no evidence of thrombosed or embolized splenic vessels [8]. With the increasing incidence of babesiosis infection the incidence of splenic infarction or rupture may be expected to increase as well. The prognosis of cases complicated by splenic infarction remains uncertain and it is unclear whether more extensive infarction or non-functionality of the spleen is associated with worse outcomes, though mortality up to 42% has been reported in asplenic patients [10].
Treatment of babesiosis remains challenging. Current guidelines recommend combination therapy, which could be azithromycin and atovaquone for mild infection or clindamycin and quinine for severe infection for total of 7–10 days. There is still debate regarding the best regimen for severe infection though azithromycin and atovaquone may be better tolerated and more efficacious [[11], [12], [13]]. Plasmapheresis is another potential option for persistent high-grade parasitemia (>10%) with significant hemolysis, or renal, hepatic, or pulmonary compromise [14]. In immunosuppressed patients with independent risk factors for a poor prognosis such as asplenia, HIV and cytopenia there is a significant risk for delayed parasitic clearance and relapse [5]. In these circumstances a prolonged treatment course and monitoring for parasitic clearance using PCR-based testing may be warranted [15].
While it is possible that there was another etiology for our patient’s splenic infarction, he had no evidence of an immunocompromised state or conditions such as endocarditis, vascular disease or malignancy, which could account for his developing splenic infarction. Moreover, the patient’s symptoms improved quickly and parasitemia cleared after starting appropriate treatment, suggesting babesiosis was the likely etiology.
Conclusion
Our case illustrates that babesiosis should be considered in the differential diagnosis of patients presenting with non-specific symptoms and splenic infarction, particularly in endemic areas, as prompt and early treatment is crucial to prevent life-threatening complications such as spontaneous splenic rupture. Of note, our case also illustrates that, in contrast to other complications of babesiosis, splenic infarction can occur despite the lack of an immunocompromised state. While often under-recognized, early recognition and treatment of babesiosis can greatly reduce morbidity and mortality.
Conflict of interest
There is no conflict of interest.
Funding
No source of funding.
Consent
Patient’s consent was obtained for the publication.
References
- 1.Vannier E., Krause P.J. Human babesiosis. N Eng J Med. 2012;366(25):2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 2.Florescu D., Sordillo P.P., Glyptis A., Zlatanic E., Smith B., Polsky B. Splenic infarction in human babesiosis: two cases and discussion. Clin Infect Dis. 2008;46(1):e8–11. doi: 10.1086/524081. [DOI] [PubMed] [Google Scholar]
- 3.Ord R.L., Lobo C.A. Human babesiosis: pathogens, prevalence, diagnosis, and treatment. Curr Clin Microbiol Rep. 2015;2(4):173–181. doi: 10.1007/s40588-015-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatcher J.C., Greenberg P.D., Antique J., Jimenez-Lucho V.E. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32(8):1117–1125. doi: 10.1086/319742. [DOI] [PubMed] [Google Scholar]
- 5.Krause P.J., Gewurz B.E., Hill D., Marty F.M., Vannier E., Foppa I.M. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46(3):370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 6.Skrabalo Z., Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9(1):11–16. [PubMed] [Google Scholar]
- 7.Chen D., Copeman D.B., Burnell J., Hutchinson G.W. Helper T cell and antibody responses to infection of CBA mice with Babesia microti. Parasite Immunol. 2000;22(2):81–88. doi: 10.1046/j.1365-3024.2000.00279.x. [DOI] [PubMed] [Google Scholar]
- 8.Goff W.L., Johnson W.C., Tuo W., Valdez R.A., Parish S.M., Barrington G.M. Age-related innate immune response in calves to Babesia bovis involves IL-12 induction and IL-10 modulation. Ann NY Acad Sci. 2002;969:164–168. doi: 10.1111/j.1749-6632.2002.tb04371.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu R., Yeh M.T., Hyland K.E., Mather T.N. Experimental Babesia microti infection in golden hamsters: immunoglobulin G response and recovery from severe hemolytic anemia. J Parasitol. 1996:728–732. [PubMed] [Google Scholar]
- 10.Hunfeld K.P., Hildebrandt A., Gray J.S. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38(11):1219–1237. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wormser G.P., Dattwyler R.J., Shapiro E.D., Halperin J.J., Steere A.C., Klempner M.S. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 12.Weiss L.M. Babesiosis in humans: a treatment review. Expert Opin Pharmacother. 2002;3(8):1109–1115. doi: 10.1517/14656566.3.8.1109. [DOI] [PubMed] [Google Scholar]
- 13.Krause P.J., Lepore T., Sikand V.K., Jr., Gadbaw J., Burke G., Telford S.R. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343(20):1454–1458. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 14.Saifee N.H., Krause P.J., Wu Y. Apheresis for babesiosis: therapeutic parasite reduction or removal of harmful toxins or both? J Clin Apher. 2016;31(5):454–458. doi: 10.1002/jca.21429. [DOI] [PubMed] [Google Scholar]
- 15.Vannier E., Krause P.J. Update on babesiosis. Interdiscip Perspect Infect Dis. 2009;2009 doi: 10.1155/2009/984568. [DOI] [PMC free article] [PubMed] [Google Scholar]