Abstract
Background
Circulating estrogen (E2) levels are high throughout pregnancy and increase towards term, however its local tissue specific actions vary across gestation. For example, myometrial E2 regulated uterotonic action is disabled until term, whereas it's proliferative function is maintained in the breast. We have identified gestationally regulated splicing events, mediated by hnRNPG and modulated by E2 that generate alternatively spliced estrogen receptor alpha (ERα) variants (ERΔ7 and ERα46) in the myometrium. These variants allow for differential, gestationally regulated, modulation of the uterotonic action of E2.
Methods
Human myometrium isolated from preterm and term non-laboring and laboring pregnant women were analyzed for ERα isoforms and splice factor levels. Lentiviral mediated shRNA knockdown of hnRNPG and overexpression of ERΔ7 were performed in human myometrial (hTERT-HM) cells. Functional 3D collagen contraction assays were executed.
Findings
ERΔ7 acts as a dominant negative repressor of the uterotonic action of ERα66 and ERα46 isoforms through the regulation of the myometrial gap junction protein GJA1. Elimination of hnRNPG inhibits the generation of ERΔ7 while overexpression of ERΔ7 inhibited GJA1 expression. Moreover in vivo human myometrial hnRNPG levels decline at term in an E2 dependent manner resulting in a withdrawal of ERΔ7 levels and its tocolytic action at term.
Interpretation
Our findings implicate the unique role of ERΔ7 as a modulator of myometrial quiescence and define the mechanism of ERΔ7 generation, through hormonally regulated splicing events.
Fund
This study was supported by NIH OPRU U01 supplement (HD047905), University of Pittsburgh and Wayne State University Perinatal Research Initiative (USA).
Keywords: Estrogen receptor alpha, Alternative splicing, hnRNPG, Myometrium, Parturition, GJA1
Research in context.
Evidence before this study
Preterm birth (<37 wk. gestation) accounts for 10% of births in the United States and is the leading cause of neonatal death. Until we understand the molecular, physiological and biochemical underpinnings related to gestational length, the development of a successful tocolytic that can either reverse or prevent the onset of precocious uterine contractions during pregnancy will remain unattainable. Our current understanding is that the uterotonic state of the myometrium at term is dependent on increased estrogen action mediated contractile associated protein expression (e.g. GJA1). Multiple studies have demonstrated the existence of estrogen receptor alpha (ERα) splice variants in various tissues but their function as regulators of uterine contractility remains undefined.
Added value of this study
This study identifies the functional interplay of alternatively spliced myometrial ERα isoforms during pregnancy. Here we show the significance of a dominant negative ERα variant, ERΔ7 that curtails estrogen action prior to term by down-regulating myometrial gap junction connexin-43 (GJA1) expression. We have determined the genesis of ERΔ7 through estrogen mediated regulation of the splice factor hnRNPG.
Implications of all the available evidence
Our findings implicate a previously undiscovered mechanism in the regulation of uterine contractility during pregnancy thereby opening avenues for development of effective therapeutics, at the small molecule level interacting with the alternative splicing of ERα with the ultimate goal of preventing preterm birth.
Alt-text: Unlabelled Box
1. Introduction
Preterm birth (<37 wk. gestation) accounts for 10% of births in the United States and is the leading cause of neonatal death (https://www.cdc.gov/features/prematurebirth). A key to future effective preterm birth prevention is understanding the mechanisms involved in the maintenance of myometrial quiescence during pregnancy and its transition to a contractile state with the onset of labor. Several studies indicate the importance of a functional progesterone (P4) withdrawal locally in the myometrial compartment, independent of circulating levels of P4, as a critical factor in the upregulation of ERα and therefore E2 mediated uterotonic events such as increased contractile associated protein (CAP) gene expression and the onset of uterine contractions at term [[1], [2], [3]]. However the gestationally regulated modulation of the uterotonic action of circulating E2 locally in myometrial tissue during pregnancy remains unanswered. Despite circulating E2 levels being high throughout pregnancy and increasing towards term [4], the consequences of E2 signaling differ significantly in a tissue dependent manner. During pregnancy, breast tissue continues to differentiate in an E2 dependent manner across gestation [5] whereas endometrial E2 action is largely required early in gestation for implantation [6]. In contrast the uterotonic consequences of myometrial E2 must be suppressed across gestation until term, maintaining the pregnant myometrium in a quiescent state, thereby ensuring an appropriate gestational length [7]. Given the high levels of E2 during pregnancy, we can safely preclude E2 signaling at the ligand level as the limiting factor in regulating gestational E2 action in a tissue specific manner. Therefore, in this study we examine the hypothesis that, gestationally regulated modification at the level of the ER, allows for tailored adaptation and differential modulation of local tissue specific E2 responsiveness. In humans, E2 effects are mediated by two forms of the ER: ERα and ERβ, each with different ligand binding affinities and tissue distributions [8,9]. The uterotonic action of E2 in the pregnant myometrium is largely mediated in an ERα dependent manner (SFig. 1) [7,10], however this current study is the first to examine the genesis and function of the alternative ERα isoforms mediated by hormone dependent alternative splicing events in the human myometrial cell as it pertains to the uterotonic action of E2 and the maintenance of uterine quiescence during pregnancy.
The human ERα locus is complex, containing 8 exons and multiple promoter regions [11] and undergoes alternative splicing resulting in multiple splice variants [12]. Indeed we see that the expansion of the proteome by alternative splicing permits a single gene, such as ERα, to be functionally flexible. Three major ERα splice variants have been identified in the human myometrium (SFig. 2 A–C), ERα66 is the full-length ERα isoform, ERα46 isoform is the product of an exon 1 skip, lacks the N-terminal transactivation domain (173 N-terminal amino acids) and has been demonstrated to modify transactivation mediated by the full-length isoform ERα66 [12,13] as well as acting as a transactivating ER when expressed in the absence of ERα66 [13,14]. Human ERα46 expression has been reported in multiple tissue types such as osteoblasts [15], macrophages [16], vascular endothelial cells [17], and breast tumor tissues [18]. ERΔ7, a 51kD protein, is the product of an exon 7 skip, causing a frameshift in exon 8 with a precocious truncation resulting with in the loss of the ligand binding domain (LBD) and activation function 2 (AF2) domains. In humans, ERΔ7 mRNA has been detected in the brain, breast and uterus as well as endocrine tumors. [[19], [20], [21], [22], [23]] Importantly ERΔ7 has been previously demonstrated to bind the DNA binding domain (DBD) and heterodimerize with other ERα isoforms acting in a dominant negative manner limiting ERα action [24].
In this study, we demonstrate that alternatively spliced myometrial ERΔ7 acts as a dominant negative repressor of myometrial ERα uterotonic function through the suppression of the gap junction protein GJA1. Through lentiviral driven over-expression and knockdown experiments, we establish that ERΔ7 markedly suppresses GJA1 expression and uterine myocyte contractility. In the absence of ERΔ7, ERα66/ERα46 isoforms resumes its transactivating ability and upregulates GJA1 expression, allowing for enhanced uterine contractile responsiveness at term. Among a large number of factors implicated in splicing events are the SR (Serine/Arginine Rich) and heterogeneous nuclear ribonucleoproteins (hnRNP) proteins [25]. Previous analysis has described the spatial regulation of the ubiquitous transacting splice factors SF2/ASF (SRSF1) and hnRNPA1 in the pregnant human myometrium [26]. Our current study demonstrates for the first time a novel role for the splice factor hnRNPG in the pregnant myometrium. hnRNPG is required for the exclusion of exon 7 in the derivation of the dominant negative myometrial ERΔ7 isoform and is negatively regulated in an E2 dependent manner. As circulating E2 levels rise towards term, both hnRNPG and consequently ERΔ7 levels decline allowing for the de-repression of ERα66/ERα46 isoforms specifically in the myometrium promoting enhanced uterotonic action of E2 at term and the onset of labor. This analysis is the first to define the tocolytic consequences of ERα alternative splicing in the human myometrial cell, illustrating its potential importance in generating a gestationally regulated, tissue specific E2 responsiveness in the pregnant human myometrium.
2. Materials and methods
2.1. Acquisition of human myometrium
Human myometrial tissues were biopsied from pregnant women undergoing elective cesarean hysterectomy. Informed consent was obtained in writing from each woman prior to surgery using protocols approved by the Institutional Review Board of the Wayne State University. Lower uterine segments were collected from three groups of subjects: 1) pregnant non-laboring women who underwent scheduled cesarean hysterectomy between 32 and 34 wk. of gestation (pre-term non-laboring; PTNL), 2) pregnant women who underwent cesarean section prior to the onset of labor at term (term non-laboring; TNL) between 39 and 42 wk. of gestation and 3) pregnant women in active labor at term (39–42 wk) undergoing elective cesarean section (term laboring; TL). The tissues were flash frozen in liquid nitrogen and stored at −80 °C for subsequent protein and mRNA analysis.
2.2. hTERT-HM cell culture and shRNA lentivirus infection
Telomerase-immortalized human myometrial cells (hTERT-HM) derived from premenopausal non-pregnant uterine tissue were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), 1% antibiotic/antimycotic (ThermoFisher Scientific, Waltham, MA, USA) at 37 °C in 5% CO2.
3 different shRNAs targeting hnRNPG were initially screened for their knockdown efficiency in HEK293T cells and clone V3LHS_645229 (antisense TTTTGTTTCTTTGAACTGGGAT; Open Biosystems) was selected. For ESR1 targeting shRNA clone V2LHS_239351 (antisense AGTAAATTATCAGTAAGTC; Open Biosystems) was selected for making lentiviral particles. Briefly, lentiviral particles (2 × 108 TU/mL) were isolated after co-transfection of shRNA plasmids along with Trans-Lentiviral shRNA Packaging mix (Open Biosystems, Lafayette, CO, USA) in HEK293T cells. Lentiviral supernatants after 48 and 72 h of culture were pooled and concentrated using Lenti-X concentrator (Takara Bio USA, Inc). Viral titers were determined by measuring the co-expressed GFP after infection of HEK 293 T cells.
hTERT-HM cells (50–75 × 104) were seeded in a six-well plate 24 h before transduction. Cells (60–70% confluency) were infected with lentiviral shRNAs targeting either ESR1 or hnRNPG along with polybrene (6 μg/ml; Sigma-Aldrich, St. Louis, MO) in phenol red-free DMEM/F12 complete growth medium (10% charcoal-stripped FBS and 1% antibiotic/antimycotic solution). Cells infected with non-silencing pGIPZ lentiviral shRNA served as controls. Media was replenished every 48 h and the cells were harvested for mRNA and protein analysis 96 and 120 h after transduction.
2.3. siRNA transfection
hTERT-HM cells (50–75 × 104) were reverse transfected on a six-well plate using Lipofectamine 2000 (ThermoFisher scientific). Briefly, 5 nM of scrambled (#4390846) or ERα siRNA (#s4824) from (ThermoFisher scientific) and 6 μl of Lipofectamine 2000 was used per well of a six-well dish to transfect cells in phenol red-free DMEM/F12 complete growth medium (10% charcoal-stripped FBS and 1% antibiotic/antimycotic solution). Cells transfected with scrambled siRNA served as controls. Cells were harvested for protein analysis 72 h after transfection.
2.4. hTERT-HMTet3G cell culture and ERΔ7 overexpression
Lentiviral producing rtTA (Tet-On 3G) were generated after transfection of the pLVX-EF1a-Tet3G plasmid using the viral packaging mix (Clontech, Mountain View, CA, USA) in HEK293T cells. hTERT-HM cells were first infected with Tet-On 3G lentiviral particles to produce sublines that constitutively expresses the reverse tetracycline receptor complex. Stably transduced hTERT-HMTet3G cells were generated after 3 wk. of selection with G418 and were found to be phenotypically similar to the parental hTERT-HM cell line (i.e. gene expression, smooth muscle cells morphology, and proliferation rate).
Full length of ERΔ7 cDNA was subcloned into mammalian, lentiviral expression vector (pLVX-TREG-mCherry) using In-Fusion cloning (Takara Bio USA, Inc). Lentivirus expressing ERΔ7 were generated after transfecting pLVX-TREG-mCherry-ERΔ7 plasmid in conjunction with the viral packaging mix in HEK293T cells. Lentiviral supernatants after 48 and 72 h of culture were pooled and concentrated using Lenti-X concentrator. Titer was determined using the Lenti-X qRT-PCR titration kit (Takara Bio USA, Inc).
hTERT-HMTet3G cells were infected with ERΔ7 lentiviral particles (1.5 × 1010 ± 2.4 × 109 copies/mL) along with polybrene (6 μg/ml) in phenol red-free DMEM/F12 media containing 10% charcoal-stripped FBS and 1% antibiotic-antimycotic. Doxycycline (Dox; 500 ng/ml) was added after 4 h of lentiviral infection to overexpress ERΔ7, which is controlled by a doxycycline responsive promoter regulated by binding constitutively expressed rtTA. Media was replenished after 24 h and the cells were treated with E2 (100 nM) for 24 h. Cells were harvested for protein analysis 48 h after transduction. No Dox treatment served as control.
2.5. ERΔ7 overexpression in HEK293T cells
HEK293T cells (4.5 × 105) were seeded in a six-well plate 24 h before transfection. Cells were co-transfected with recombinant ERΔ7 plasmid (pLVX-TREG-mCherry-ERΔ7; 2 μg) along with transactivator rtTA plasmid (pLVX-EF1a-Tet3G; 2 μg) and 6 μl of Lipofectamine 2000. Dox (500 ng/ml) was added and 48 h later the cells were harvested for protein analysis. No Dox treatment served as control.
2.6. Immunohistochemistry and immunofluorescence
Human myometrial tissue was fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5-μm sections and collected on superfrost slides (Fisher Scientific). Parafiin slide sections were deparaffinized and rehydrated through a xylene/alcohol series followed by antigen retrieval by microwaving on full power for 5 min in 10 mM citrate buffer (pH 6.0). Tissue sections were blocked in 5% normal donkey serum and incubated in primary antibodies ERβ (1:200, ab3577; Abcam, Cambridge, MA, USA) or ERα (1:100, sc-543; Santa Cruz Biotechnology, Dallas, Texas, USA) overnight at 4 °C. Sections were washed and incubated with goat anti-rabbit biotinylated secondary antibody (1:500) using the Vectastain ABC Elite Kit (Peroxidase Goat IgG). Sections were stained using the Vector Red HRP Detection Kit (Vector Laboratories, Burlingame, CA) following the manufacturers protocol.
To examine the levels of GJA1, hTERT-HMTet3G cells (2.5 × 105) were infected with ERΔ7 lentiviral particles and cultured for 24 h. Cells were trypsinized and cultured in multichamber Lab-Tek plates (Nalgene Nunc International, Rochester, NY, USA) containing phenol-red free DMEM/F12 supplemented with 2% charcoal-stripped FBS, 1% antibiotic-antimycotic, Dox (500 ng/mL) and E2 (100 nM) for 48 h. Cells were fixed in 4% paraformaldehyde, washed in PBS and blocked with 5% normal donkey serum for 30 min. Cells were washed and incubated with primary antibody overnight at 4 °C for GJA1 (1:200, C6219; Sigma-Aldrich). The cells were washed and incubated with FITC conjugated donkey anti-rabbit secondary antibody (1:500, Jackson Laboratory) for 1 h, nuclear stained using DAPI and mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). No Dox treatment served as control. The images were visualized and digital images acquired using fluorescence microscopy (Olympus IX51 inverted microscope, Center Valley, PA, USA).
2.7. Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from frozen human uterine tissues and hTERT-HM cells was extracted using an RNeasy Mini Kit (Qiagen). cDNA was synthesized from 1 μg RNA using the High Capacity cDNA Reverse Transcription Kit (ThermoFisher scientific). Real-time qPCR was performed on the CFX384 Touch Real-Time System (BioRad, Hercules, CA, USA) using a SYBR Green PCR Master Mix (ThermoFisher scientific). For each reaction, 25 ng of cDNA and a final primer concentration of 300 nM was used. cDNA samples were assayed for each treatment in triplicate and relative quantification of mRNA levels (fold change) normalized to Rplp0 was calculated using the 2-ΔΔCT method. The primer sequences are designated in detail in Supplementary Table 1.
2.8. Whole cell extract
Cells were lysed in RIPA buffer (150 mM NaCl, 1% TritonX-100, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, 50 mM Tris [pH 8.0], and protease and phosphatase inhibitors). The samples were incubated at 4 °C for 10 min and then centrifuged at 10,600 ×g for 10 min at 4 °C. The supernatant was retained as the whole cell extract.
2.9. Subcellular fractionation
Cytoplasmic and nuclear protein extracts were prepared from frozen human uterine tissues and hTERT-HM cells. In brief, myometrial tissue was pulverized in liquid nitrogen and homogenized (IKA homogenizer) in ice-cold NE1 buffer [10 mM Hepes pH 7.5, 10 mM MgCl2, 5 mM KCl, 0.1% Triton X-100 and protease/phosphatase inhibitor mixture (#88669; ThermoFisher scientific)]. hTERT-HM cell lysates were prepared by passing 10 times through a 23-gauge needle in NE1 buffer. The homogenate was centrifuged at 3000 ×g for 6 min at 4 °C, and the supernatant was retained as the cytoplasmic fraction. The pellet was washed with NE1 buffer and resuspended in ice- cold NE2 buffer (25% glycerol, 20 mM Hepes pH 7.9, 500 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA pH 8.0 containing protease/phosphatase inhibitor). The samples were incubated at 4 °C in an Eppendorf thermomixer with vigorous shaking (15 s/1400 rpm every 5 min) for 1 h and then centrifuged at 10,600 ×g for 10 min at 4 °C. The supernatant was retained as the nuclear protein extract.
2.10. Western blot analysis
Equivalent amounts of protein determined by Bicinchoninic acid protein assay kit were resolved by NuPAGE 4–12% Bis-Tris gel (ThermoFisher scientific) electrophoresis and blotted to Hybond-P PVDF membranes (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Blots were blocked with 5% nonfat dry milk in Tris-saline buffer (pH 7.4) containing 0.1% Tween-20, and then probed with the following primary antibodies: anti- ERα HC-20 (1:200; sc-543; carboxy terminus), anti-ERα G-20 (1:250; sc-544; hinge region), anti-ERα H-184 (1:250; sc-7207; amino terminus) anti-hnRNPG (RBMX) (1:500; sc-48,796) from Santa Cruz Biotechnology, anti-ERα D8H8 (1:1000; #8644; carboxy terminus), anti-ERα D6R2W (1:1000; #13258; amino-terminus) from Cell Signaling Technology, anti-ERα (1:100; ab16660; carboxy terminus) from Abcam and GJA1 (1:1000; C6219) from Sigma-Aldrich. Immunoreactivity was detected using HRP- conjugated secondary antibody, and the bands were visualized using an ECL detection system (ThermoFisher Scientific). The membranes were probed with anti-NCOA3 (for nuclear protein; 1:5000, #PA1-845; ThermoFisher Scientific) or anti-GAPDH (for cytoplasmic protein; 1:5000, #5174S Cell Signaling Technology, Danvers, MA, USA) to quantify the relative protein expression level. Images of the bands were scanned and analyzed using ImageJ software (NIH, Bethesda, MD, USA).
2.11. Collagen based cell-contractility assay
hTERT-HMTet-3G cells (2.5 × 105) cultured overnight in phenol red free DMEM/F12 complete growth medium were infected with ERΔ7 lentiviral particles in the presence or absence of Dox (500 ng/ml). After 48 h, cells were trypsinized and seeded into collagen gel and contraction assay was carried out following the manufacturer's protocol (Cell Biolabs Inc. San Diego, CA, USA). In brief, collagen lattice was prepared by mixing 2 parts of cell suspension and 8 parts of cold collagen gel solution to achieve 1.5 × 105 cells/well. 0.5 mL of the cell-collagen mixture was added per well in a 24-well plate and incubated for 1 h at 37 °C to allow gelling. 1.0 mL of phenol-red free DMEM/F12 containing 2% charcoal-stripped FBS, 1% antibiotic-antimycotic, supplemented with or without Dox (500 ng/mL) and E2 (100 nM) was added over the cell-collagen matrix. The gels were gently released after 20 h and the area of the lattices were measured periodically from 4 to 36 h. Images of the floating gels were captured and digitized using a flatbed scanner (Hewlett Packard) and the mean gel area (cm2) was measured using IMAGEJ software. No Dox treatment served as control.
2.12. Steroid hormone treatment
hTERT-HM cells (2.5 × 105) cultured overnight in phenol red free DMEM/F12 complete growth medium were treated with vehicle (ethanol), progesterone (100 nM; P4) or estradiol (100 nM; E2) for 24 h. The cells were harvested and nuclear extracts were prepared for hnRNPG and ERΔ7 analysis.
3. Data analyses
Values are expressed as mean ± SE. Statistical comparisons were done using the Student t-test, or using one-way ANOVA followed by the Newman-Keuls multiple-comparison test. P ≤ 0.05 was considered to indicate statistical significance.
4. Results
4.1. ERα is the functional estrogen isoform found in human pregnant uterine myometrium
Utilizing antibodies specific for ERα and ERβ immunohistochemical analysis revealed ERα was present in both myometrial and endometrial compartments of uterine samples obtained from pregnant women undergoing c-section at 36 weeks, while ERβ was isolated solely to the endometrial compartment (Fig. S1).
4.2. Alternative splicing of ERα generates ERΔ7 and ERα46 in pregnant human myometrium
The structure of the three human ERα isoforms found to be expressed in the human myometrium in their mRNA and protein forms respectively is outlined in Fig. S2. RT-PCR utilizing forward primers specific to the 5′ UTR associated with promoter F and a reverse primer in exon 2 of ERα confirmed the presence of two bands observed at 830 bp and 308 bp. The 308 bp band was consistent with the exon 1 skip of ERα46, the 830 bp represents ERα66/ERΔ7 (Fig. S2C). Similarly, RT-PCR performed utilizing a forward primer in exon 4 and a reverse primer in exon 8 of ERα produced a 681 bp and a 497 bp band. The 497 bp band lacking exon 7 represents ERΔ7 (Fig. S2D) while the 681 bp band represents ERα66/ERα (primer sequences in Supplementary Table 1).
4.3. Differential expression of uterine ERα isoforms in term and preterm laboring women
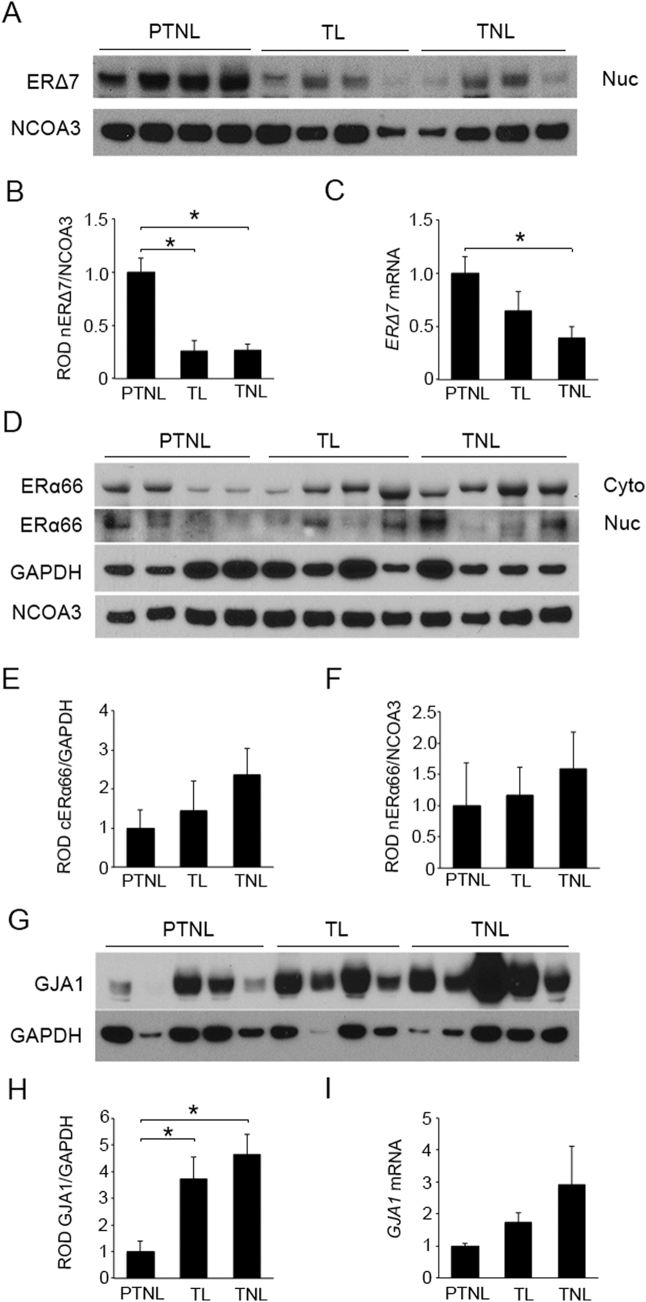
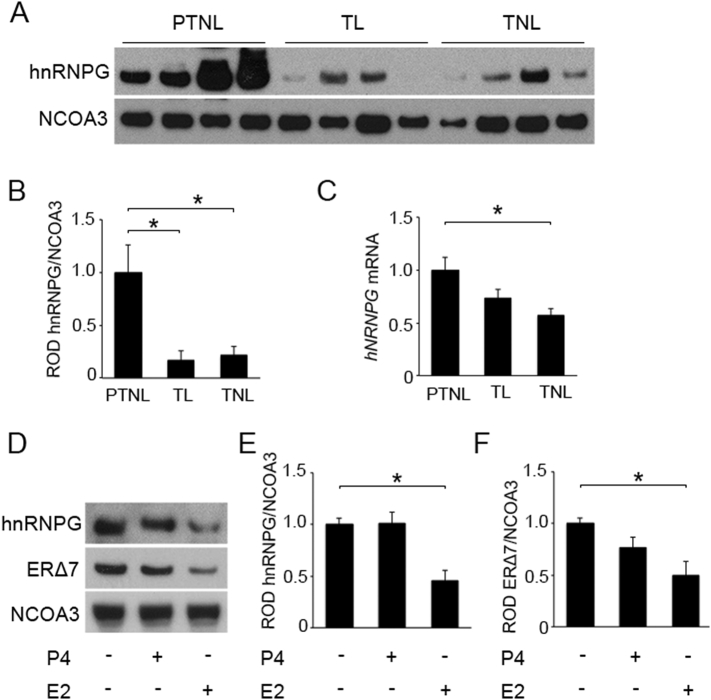
To examine the temporal expression of ERα isoforms in the human myometrium during late gestation, we determined their expression in the nuclear and cytoplasmic compartments of uterine myometrial tissue isolated from term (≥ 37 weeks gestation) non-laboring (TNL), term laboring (TL) and preterm non-laboring (PTNL) women (32–34 weeks gestation). Nuclear ERΔ7 (utilizing ERα G-20 and ERα D6R2W antibodies which are directed to N-terminus) was highly expressed at earlier gestational time points as observed in the PTNL group and was found to decrease by 4 fold as the pregnancy progressed to term (TL and TNL) (Fig. 1A and B). A 2.5 fold decline in ERΔ7 mRNA levels was also detected in TNL myometrium compared to PTNL (Fig. 1C). Utilizing HC20 and D8H8 antibodies (directed against human ERα c-terminus), we detected ERα66 in both myometrial cytoplasmic and nuclear compartments. However, no relative changes in the compartmentalization or expression levels of ERα66 were associated with increasing gestational age or the onset of labor (Fig. 1D–F). Several attempts to detect ERα46 (46 kD band) in the pregnant myometrial protein extracts with commercially available antibodies were unsuccessful.
Fig. 1.
Expression of ERα isoforms and GJA1 in pregnant human myometrium. Representative western blot (A) and densitometric analysis (B) demonstrate ERΔ7 protein in nuclear (Nuc) extracts significantly declined in term laboring (TL) and term non-laboring (TNL) myometrium compared to myometrium from preterm non-laboring (PTNL) women. (C) ERΔ7 mRNA levels are also down-regulated in the TNL samples as compared to PTNL. Representative western blot (D) and densitometric analysis demonstrated no significant change in ERα66 levels in cytoplasmic (Cyto) (E) and nuclear (F) fractions of TL and TNL myometrium as compared to PTNL. Representative western blot (G) and densitometric analysis (H) demonstrated cytoplasmic GJA1 expression levels were significantly upregulated in the TL and TNL as compared to PTNL myometrium. An increasing trend in GJA1 mRNA levels (I) was also observed towards term. GAPDH and NCOA3 are cytoplasmic and nuclear loading controls. Gene expression was normalized to Rplp0. *p < 0.05 using one-way ANOVA, followed by the Newman-Keuls multiple-comparison test; N = 20 per group. Data shown are mean ± SEM.
4.4. Uterine myometrial GJA1 expression increases at term
We next determined whether a decline in ERΔ7 isoform, correlates with an increase in local E2 regulated uterotonic signaling by measuring the levels of the CAP protein GJA1 in myometrial tissues isolated at TNL, TL and PTNL. As can be observed in Fig. 1G–I, both GJA1 mRNA and proteins levels were elevated up to 4 fold towards term compared to tissues obtained prior to term.
4.5. ERα isoforms regulate GJA1 expression in uterine myocytes in vitro
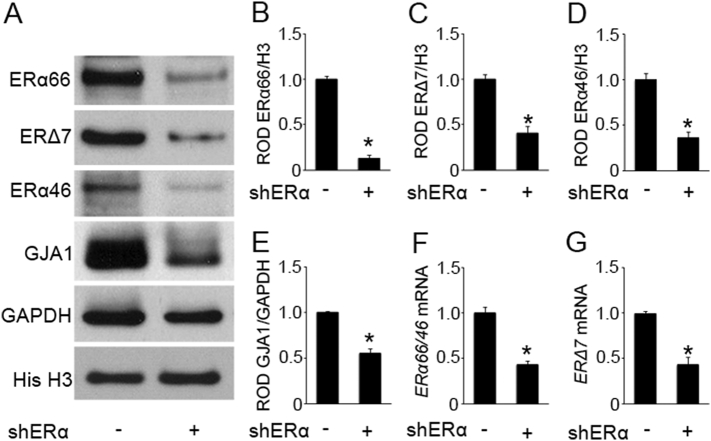
Ablation of all ERα isoforms utilizing a lentivirus-mediated shRNA targeting ESR1 at the 3′UTR (common to all isoforms of ERα) and analyzing GJA1 expression in the hTERT-HM uterine myocyte allowed us to determine the role of ERα in regulating GJA1. Western blotting and Q-PCR demonstrate a significant knockdown of basal ERα66, ERα46 and ERΔ7 levels in comparison to cells infected with non-silencing pGIPZ shRNA lentiviral control (Fig. 2A–D, F and G). The knockdown of ERα consequently led to a substantial decline in GJA1 levels in uterine myocytes (Fig. 2A and E). Furthermore, utilizing siRNA-targeting exon 7 of ESR1, specific knockdown of ERα46 and ERα66 in hTERT-HM uterine myocytes resulted in decreased GJA1 expression (Fig. S3).
Fig. 2.
ERα regulation of GJA1 in hTERT-HM cells. RNA and protein extracts were isolated after 96 h of infection with ERα shRNA lentivirus that targets the 3′UTR of ERα mRNA. Representative western blot (A) and densitometric analysis demonstrate a decline in the nuclear ERα66 (B), ERΔ7 isoforms (C) and ERα46 isoforms (D) and the cytoplasmic GJA1 (E) in the hTERT-HM cells upon lentivirus-mediated knockdown of ERα compared to non-silencing pGIPZ shRNA lentiviral control. A significant decline in ERα66/ERα46 (F) and ERΔ7 isoform (G) mRNA levels were also observed in the hTERT-HM cells upon lentivirus-mediated knockdown of ERα compared to non-silencing pGIPZ shRNA lentiviral control. GAPDH and Histone H3 are cytoplasmic and nuclear loading controls. Gene expression was normalized to Rplp0. *p < 0.05 by two-tailed Student's unpaired t-test, each experiment was performed in triplicate with n = 3 per group. Data shown are mean ± SEM. See also Fig. S3.
4.6. Gap junction associated protein, GJA1 is the downstream target of ERΔ7 action
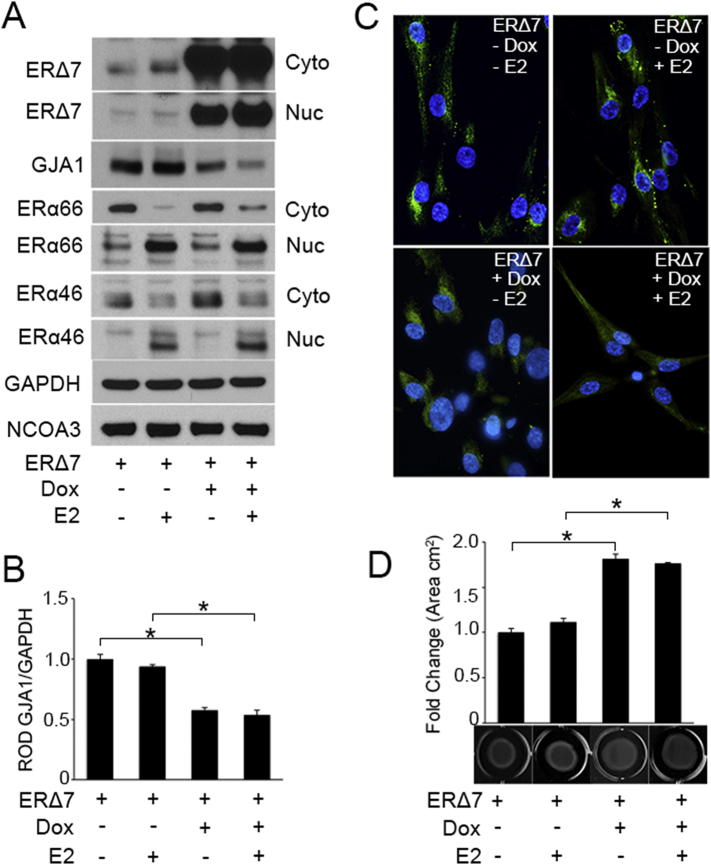
RNA interference knock down of ERΔ7 alone is impossible due to shared sequence homology with ERα66 and ERα46, therefore we overexpressed ERΔ7 in hTERT-HMTet3G cells to determine the capacity of ERΔ7 to regulate GJA1 in uterine myocytes utilizing a doxycycline (Dox) inducible recombinant lentivirus. Western blot analysis revealed increased cytoplasmic and nuclear localization of ERΔ7 in hTERT-HMTet3G cells (Fig. 3A) and HEK293T cells (Fig. S4B) following addition of Dox (500 ng/ml). ERΔ7 over-expression caused a marked decline in GJA1 expression (Fig. 3A and B) while ERα66 and ERα46 expression levels remained unchanged when compared to control cells (no Dox). Furthermore, Dox induced ERΔ7 over-expression resulted in a dose-dependent down-regulation of GJA1 expression (Fig. S4A). 100 nM E2 promoted increased nuclear translocation of ERα66 and ERα46 isoforms from the cytoplasmic compartment (Fig. 3A), however ERΔ7 translocation to the nucleus was found to be E2 independent and GJA1 levels upon ERΔ7 overexpression were also unaffected by E2 (Fig. 3A and B).
Fig. 3.
ERΔ7 over-expression inhibits GJA1 expression and disrupts the uterine myocyte contractile ability. ERΔ7 was transiently over-expressed in hTERT-HMTet3G cells using a doxycycline (Dox; 500 ng/ml) inducible recombinant lentivirus for 48 h in the presence or absence of E2 (100 nM). Representative western blot (A) and densitometric analysis (B) demonstrate a decrease in the protein level of GJA1 upon ERΔ7 overexpression in the presence and absence of E2. Western blot analysis (A) also demonstrates E2 mediated nuclear translocation of ERα66 and ERα46, though no changes in ERα66 and ERα46 levels were found to be associated with ERΔ7 overexpression. Immunocytochemical analysis (C) of hTERT-HMTet3G cells indicates GJA1 was largely limited to the perinuclear area and cell membrane in control and E2 treated uterine myocytes and was diminished upon ERΔ7 overexpression (Dox) in the presence and absence of E2. (D) Collagen gel contraction analysis reveals reduced contractile responsiveness in ERΔ7 overexpressing hTERT-HMTet3G cells both in the absence or presence of E2 (100 nM) compared to control, as a result of decreased GJA1 levels. GAPDH and NCOA3 are cytoplasmic and nuclear loading controls. A representative gel with mean gel area (cm2) is given for each group (n = 4), each experiment was performed in triplicate. *p < 0.05 by two-tailed Student's unpaired t-test. Data shown are mean ± SEM. See also Fig. S4A.
4.7. ERΔ7 regulates uterine myocyte contractile responses
Dox induced ERΔ7 overexpression resulted in diminished localization of GJA1 to the cell membrane, when compared to no-Dox treated control cells where there was extensive immunostaining for GJA1 largely isolated to the cell membrane at cell-to cell contact sites (Fig. 3C). As a test of ERΔ7 functional relevance as a regulator of CAP protein expression we performed collagen gel contraction assays, to validate the negative regulation of GJA1 by ERΔ7 in uterine myocytes. hTERT-HMTet3G cells overexpressing ERΔ7 and control (no-Dox treated) cells were embedded in a three-dimensional collagen gel matrix. The matrix was gently released after 24 h of culture in the continuous presence or absence of Dox. As shown in Fig. 3D, the spontaneous contraction of the gel matrix was significantly reduced in cells over-expressing ERΔ7 compared with the control. The addition of E2 (100 nM) did not modify myometrial contractility in ERΔ7 overexpressing cells.
4.8. hnRNPG regulates alternate splicing of ERα Exon7
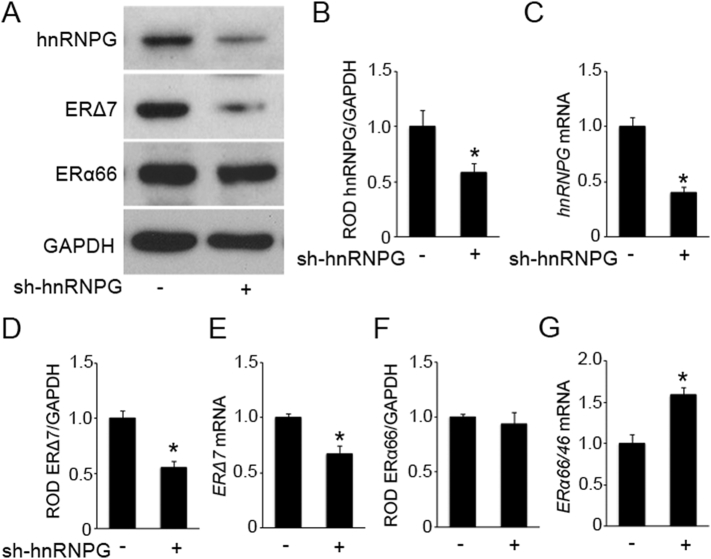
A recent study observed multiple potential exon splice inhibitor binding sites for splice factor hnRNPG (AAGU, CC(A/C) on Exon 7 of ERα [20]. In order to elucidate hnRNPGs regulation of ERΔ7 alternative splicing in the human myometrium through these exon splice inhibitor (ESI) binding sites, we knocked down hnRNPG using shRNA lentivirus in hTERT-HM cells. Western blotting and Q-PCR demonstrate a significant knockdown of basal hnRNPG levels in comparison to cells infected with non-silencing pGIPZ shRNA lentiviral control (Fig. 4A–C). The knockdown of hnRNPG led to a significant reduction in both ERΔ7 mRNA and protein levels in uterine myocytes (Fig. 4A, D and E). In contrast ERα66 protein levels were comparable to the control (Fig. 4A and F), however a concomitant increase in endogenous ERα66/ERα46 transcript levels was observed (Fig. 4G).
Fig. 4.
Lentiviral shRNA knockdown of hnRNPG leads to ERΔ7 Loss in hTERT-HM cells. RNA and protein total lysates were isolated after hTERT-HM cells were infected with hnRNPG shRNA lentivirus. Representative western blot (A) and densitometric analysis demonstrating a decrease in hnRNPG (B) and ERΔ7 isoform (D) and no change in ERα66 (F) protein levels in hTERT-HM cells upon lentivirus-mediated knockdown of hnRNPG compared to non-silencing pGIPZ shRNA lentiviral control. Q-PCR analysis also demonstrates a knockdown of hnRNPG (C), ERΔ7 (E) and an increase in ERα66/ERα46 (G) mRNA levels upon lentivirus-mediated knockdown of hnRNPG compared to non-silencing pGIPZ shRNA lentiviral control. GAPDH is the loading control. Gene expression was normalized to Rplp0. *p < 0.05 by two-tailed Student's unpaired t-test, each experiment was performed in triplicate with n = 3 per group. Data shown are mean ± SEM.
Given the temporal decline in myometrial ERΔ7 levels at term, we next examined hnRNPG levels in the pregnant myometrium. Western blot and Q-PCR analysis revealed a 5 and 2 fold respective decline in both hnRNPG protein and mRNA levels in the uterine myometrial tissues isolated from women at term compared to prior to term (Fig. 5A–C). To determine the effects of steroid hormones on hnRNPG expression, we treated uterine myocytes with physiological levels of P4 (100 nM) and E2 (100 nM) and analyzed for hnRNPG and its downstream target ERΔ7. Western blots demonstrate a 2 fold downregulation of both hnRNPG and ERΔ7 as a result of E2 exposure (Fig. 5D–F) whereas P4 had no effect.
Fig. 5.
hnRNPG expression declines in myometrium from women at term. Representative western blot (A) and densitometric analysis (B) demonstrates a significant decline in hnRNPG protein levels in nuclear extracts of term laboring (TL) and term non-laboring (TNL) myometrium compared to myometrium from preterm non-laboring (PTNL) women. Q-PCR analysis (C) also demonstrates a significant decline in hnRNPG TNL myometrium compared to myometrium from PTNL women. Representative western blot (D) and densitometric analysis demonstrating a significant decline in hnRNPG (E) and ERΔ7 (F) protein levels upon estradiol (E2) treatment in the hTERT-HM cell. hTERT-HM cells were treated with vehicle, progesterone (100 nM; P4) or E2 (100 nM) for 24 h. Nuclear extracts were prepared and analyzed for hnRNPG and ERΔ7 proteins. NCOA3 is used as nuclear loading control. Gene expression was normalized to Rplp0. *p < 0.05 using one-way ANOVA, followed by the Newman-Keuls multiple-comparison test; each experiment was performed in triplicate with n = 4 per group. Data shown are mean ± SEM.
5. Discussion
E2 levels steadily rise as human gestation advances, however the cause of myometrial contractile refractoriness, prior to the onset of labor despite this increasing estrogenic stimulus has remained a mystery. In this current study we demonstrate that an alternately spliced ERα variant, ERΔ7, limits local myometrial E2 action by suppressing E2 contractile responsiveness at earlier gestational timepoints during pregnancy. We also establish the mechanism of myometrial ERΔ7 regulation through alternative splicing events and define a critical role for ERΔ7 in the maintenance of uterine quiescence through regulation of the gap junction protein GJA1. In vitro, ERΔ7 overexpression blocks myometrial cell E2 dependent GJA1 transcriptional activity and contractile ability, through its dominant negative action on ERα66/ERα46. Our findings also identify the splice factor, hnRNPG as critical for the gestationally regulated generation of the ERΔ7 variant. Elimination of myometrial hnRNPG results in the loss of ERΔ7 and an increase in ERα66/46 transcript levels. Furthermore, we demonstrate in the pregnant human myometrial compartment both ERΔ7 and hnRNPG are significantly down regulated as term approaches, moreover hnRNPG is regulated in a negative manner by increasing E2 levels. Therefore we propose, as term approaches highly elevated E2 levels eliminate hnRNPG, resulting in the suppression of local myometrial ERΔ7 expression. These events allow for enhanced local estrogenic responsiveness driven by de-repressed ERα66 action resulting in increased GJA1 and intensified uterine myocyte contractile ability allowing for the onset of labor at term. In this model ERΔ7 maintains the myometrium in a quiescent state across gestation. As our analysis in Fig. 3D demonstrates, ERΔ7 over expression prevents uterine myocyte contractility. The loss of hnRNPG and related decline in ERΔ7 in term non laboring myometrial tissues allows for enhanced contractile responsiveness. However we propose for term myometrial cells, which express lower levels of ERΔ7, that there is still a requirement for a uterotonic trigger to initiate a contractile response in vivo. Therefore though ERΔ7 levels help to maintain the myometrial cell in quiescent non-contractile state, we suspect in vivo its decline alone will not trigger a uterine contraction.
Genomic estrogen signaling is mediated via interaction with ERα and ERβ. Within the pregnant human myometrium, in agreement with other reports [7,27], we identified ERα in the pregnant myometrial compartment whereas ERβ was isolated to the endometrial compartment (Fig. S1). Additionally, ERβ KO mice though sub-fertile appear to have normal pregnancies and parturition [10], thus the uterotonic action of E2 in the human myometrium is thought to be mediated by ERα signaling.
Though others have identified the presence of ERα in the pregnant myometrium, this study is the first comprehensive examination of ERα isoform distribution and function as it pertains to E2 action in the pregnant human myometrium. Other prior studies have reported changes in myometrial ERα expression levels are associated with the onset of labor [7,27], though the protein levels did not change [28,29]. These studies limited their investigation to ERα66 mRNA and whole cell protein extracts [7,[27], [28], [29]]. In this current study, we address for the first time the genesis and function of the multiple isoforms of ERα at both the nuclear and cytoplasmic protein and mRNA level that are regulated differentially in a gestational and compartment specific manner within the lower uterine segment of the pregnant human myometrium.
In the pregnant human myometrium we have identified that though nuclear ERα66 levels do not increase towards term (Fig. 1D and F), ERΔ7 levels decline up to 4 fold (Fig. 1A–C). ERΔ7, is the product of ESR1 mRNA alternative splicing, where exon 7 is skipped. Previous studies demonstrate that ERΔ7 functions as a dominant negative repressor of ERα66 transactivating properties [24,30,31]. Furthermore ERα66 has been demonstrated to form heterodimers with ERΔ7 with the same efficiency it forms homodimers, in a ligand independent manner. Increasing the expression of ERΔ7 resulted in the progressive inhibition of the E2-dependent transcriptional activation on ERE-driven promoters by ERα66 to below basal activity levels. This study also reported that the transcriptional inhibition promoted by the presence of ERΔ7 is due to its inhibitory effect on the binding of ERα66 to EREs [24]. As ligand binding is not a prerequisite for receptor dimerization, ERΔ7 forms inactive heterodimers with ERα66 rendering it unable to bind to the ERE [24,32]. In our study we demonstrate that ERΔ7, although devoid of LBD, retains the ability to localize to the nuclear compartment without binding ligand (Figs. 3A and S4B). We observed an increasing trend in cytoplasmic ERα66 protein expression (Fig. 1D and E) but no change in the nuclear compartment at term (Fig. 1D and F). Nuclear levels of myometrial ERα66 were low in comparison to the cytoplasmic fraction (Fig. 1E and F), which we believe is due to a rapid turnover of nuclear ERα through the ubiquitin-proteasome pathway as demonstrated by others [27,[33], [34], [35]]. As our mRNA and protein data unequivocally demonstrate a decline in myometrial ERΔ7 levels at term, we aimed to discern the functional role of ERΔ7 in the pregnant myometrium. Utilizing GJA1, as our myometrial target for increased estrogenic activity, we examined the action of ERΔ7 in the context of the human uterine myocyte. GJA1 forms gap junctions that enhance myometrial contractility by increasing myometrial cell coupling and mediating intercellular communications, allowing for enhanced coordinated synchronous myometrial contractions necessary for the onset of labor [[36], [37], [38]]. E2 administration increases GJA1 protein expression and gap junction communication in human myometrial cells [39,40]. Furthermore it has been reported in other tissues that inhibition of ERα66 reduced, while its overexpression increased GJA1 expression [41,42]. Our study however, demonstrates for the first time the role of alternate ERα isoforms in GJA1 regulation in the human myometrial cell. In our hTERT-HM myometrial cell, ERα shRNA knockdown through targeting the 3′UTR that is common to all 3 ERα isoforms, we demonstrate a decrease in ERα66, ERα46 and ERΔ7 levels that results in a significant decline in GJA1 levels, indicating ERα plays an important role in regulating myometrial GJA1 expression levels (Fig. 2A–G). Importantly however overexpression of ERΔ7 alone in the presence of ERα66 decreased GJA1 levels in the absence or presence of E2 (Fig. 3A and B), thereby identifying a novel role of ERΔ7 in the negative regulation of GJA1. Immunocytochemical analysis revealed myometrial cell GJA1 was largely concentrated at the cell membrane of the control hTERT-HMTet3G cells, but was decreased and dispersed upon ERΔ7 overexpression (Fig. 3C). Furthermore, ERΔ7 overexpression inhibited contractility of myometrial cells embedded in a collagen matrix (Fig. 3D) further demonstrating the tocolytic potential of myometrial ERΔ7 through the suppression of GJA1 expression. Similarly in the human myometrium we observed an inverse relationship between GJA1 (Fig. 1G and H) and ERΔ7 (Fig. 1A and B). Taken together these data suggest at term, existing levels of ERα66 upregulate GJA1 expression in the absence of the dominant negative activity of ERΔ7, which we propose acts to repress ERα66 estrogenic action in the myometrium prior to term.
Alternative splicing of mRNA is not unique to the ERα, as approximately 92–94% of all genes are subject to alternative splicing, resulting in diverse gene expression patterns in tissues and cells [43]. The ERα exon 7 sequence contains potential binding sites for splice enhancers such as TRA2B and splice repressors such as hnRNPI and hnRNPG [20]. Our data identifies that the splice factor hnRNPG controls the generation of ERΔ7 in the myometrial cell. We demonstrate that hnRNPG acting as a splicing inhibitor, when eliminated (Fig. 4A–C), decreased exon 7 exclusion at the mRNA level in hTERT-HM cells resulting in decreased ERΔ7 mRNA and protein levels (Fig. 4A, D and E) while ERα66 protein levels remained unchanged (Fig. 4A and F), although an increase was observed at the mRNA level (Fig. 4G). A dramatic decline in hnRNPG was also observed in term myometrium when compared to myometrium isolated from women prior to term at the protein and mRNA level (Fig. 5A–C), compatible with its role as a regulator of ERΔ7, which also declines as term approaches (Fig. 1A–C).
Due to the fluctuations in circulating hormones during pregnancy [44] we next examined whether changes in E2 and P4 levels could play a role in the regulation of myometrial hnRNPG. In previous analysis of cultured human endometrial tissues, E2 mediated an increase in hnRNPG levels [45]. In our analysis E2 at higher physiological concentrations (100 nM) had a significant negative impact on myometrial hnRNPG and consequently ERΔ7 levels (Fig. 5D, E and F). Since hnRNPG generates the ERΔ7 isoform, hormonal regulation of hnRNPG likely also impacts the gene expression pattern of ERΔ7 (Fig. 1A and B) observed in the pregnant myometrium in vivo.
Collectively, our findings suggest that ERΔ7 is a key regulator of GJA1 in the myometrium and blocks its expression throughout gestation prior to term (Fig. 6). Due to the action of the splice repressor hnRNPG, ERΔ7 becomes the dominant isoform in the myometrium and blocks ERα66/ERα46 mediated action. Elevated E2 levels trigger a reversal at term, causing a decline in hnRNPG, that in turn results in the inclusion of exon 7 and a subsequent decrease in ERΔ7 levels. This allows for increased E2 mediated uterotonic action of ERα66/ERα46 resulting in GJA1 upregulation and elevated uterine contractile responsiveness that allow for the transition of the myometrium from its quiescent state to be actively contractile at term. An important limitation of any splice factor knockdown is that it can simultaneously affect multiple proteins. As alternative splicing has the capacity to regulate many of the proteins associated with smooth muscle contractility [46], a systematic survey of proteins regulated by hnRNPG (other than ERΔ7) that are involved in maintaining pregnancy and onset of labor would be advantageous to this analysis. Another important caveat is that we have not examined the effect of ERΔ7 posttranslational modifications such as ubiquitination, phosphorylation, sumoylation etc. that may act to further affect ERΔ7 cellular localization, function and/or stability. Studies are currently in progress to address these concerns.
Fig. 6.
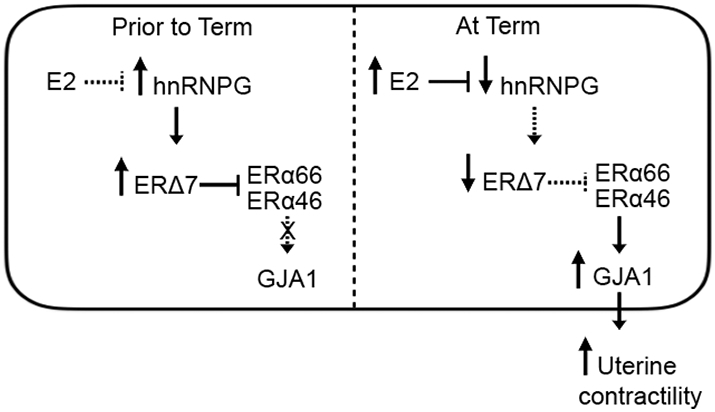
Hypothetical model of ERΔ7 regulation of GJAI in preterm and term labor. During pregnancy, lower E2 levels are permissive for relatively high expression of hnRNPG, which in turn results in increased ERΔ7 isoform generation by promoting exon 7 exclusion. High levels of ERΔ7 block the action of ERα66/ERα46 resulting in down-regulation of GJA1 and maintaining quiescence. Near term increasing levels of circulating E2 result in down-regulation of hnRNPG that manifest in a decline in ERΔ7 levels thus removing the barrier to ERα66/ERα46 transcriptional activity. This results in an upregulation of GJA1 expression leading to increased uterine contractility and labor.
Taken together, our findings implicate a previously undiscovered mechanism in the regulation of uterine contractility during pregnancy thereby opening avenues for development of effective therapeutics, at the small molecule level interacting with the splicing of ERα with the ultimate goal of preventing preterm birth.
Acknowledgments
Acknowledgements
This project was funded partially by NIH OPRU U01 supplement (HD047905), startup funds at the University of Pittsburgh, Department of Cell Biology, and the Wayne State University, School of Medicine, Department of Obstetrics and Gynecology.
Declaration of interests
The authors declare no competing interests.
Author contributions
P.A. conducted the experiments, performed the data analyses and wrote the manuscript. C.K. and J.I. assisted with the experiments and performed the data analyses. S.S.H. and J.C.C. edited the manuscript and provided valuable input for modification of the experimental design. P.J. supervised the project, designed the experiments, wrote and edited the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.038.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hendrix E.M., Mao S.J., Everson W., Larsen W.J. Myometrial connexin 43 trafficking and gap junction assembly at term and in preterm labor. Mol Reprod Dev. 1992;33(1):27–38. doi: 10.1002/mrd.1080330105. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs A.R., Fuchs F., Husslein P., Soloff M.S., Fernstrom M.J. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982;215(4538):1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- 3.Soloff M.S., Alexandrova M., Fernstrom M.J. Oxytocin receptors: triggers for parturition and lactation? Science. 1979;204(4399):1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- 4.Buster J.E., Chang R.J., Preston D.L., Elashoff R.M., Cousins L.M., Abraham G.E. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, delta 5-androstenediol, delta 4-androstenedione, testosterone, and dihydrotestosterone. J Clin Endocrinol Metab. 1979;48(1):139–142. doi: 10.1210/jcem-48-1-139. [DOI] [PubMed] [Google Scholar]
- 5.Macias H., Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasquez Y.M., Demayo F.J. Role of nuclear receptors in blastocyst implantation. Semin Cell Dev Biol. 2013;24(10−12):724–735. doi: 10.1016/j.semcdb.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesiano S., Chan E.C., Fitter J.T., Kwek K., Yeo G., Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor a expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson J.A. An update on estrogen receptors. Semin Perinatol. 2000;24(1):66–69. doi: 10.1016/s0146-0005(00)80059-2. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki S., Fukaya T., Suzuki T., Murakami T., Sasano H., Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999;5(6):559–564. doi: 10.1093/molehr/5.6.559. [DOI] [PubMed] [Google Scholar]
- 10.Krege J.H., Hodgin J.B., Couse J.F., Enmark E., Warner M., Mahler J.F. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuur E.R., McPherson L.A., Yang G.P., Weigel R.J. Genomic structure of the promoters of the human estrogen receptor-alpha gene demonstrate changes in chromatin structure induced by AP2gamma. J Biol Chem. 2001;276(18):15519–15526. doi: 10.1074/jbc.M009001200. [DOI] [PubMed] [Google Scholar]
- 12.Flouriot G., Griffin C., Kenealy M., Sonntag-Buck V., Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-alpha gene are generated by alternative splicing and promoter usage. Mol Endocrinol. 1998;12(12):1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- 13.Flouriot G., Brand H., Denger S., Metivier R., Kos M., Reid G. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19(17):4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penot G., Le Peron C., Merot Y., Grimaud-Fanouillere E., Ferriere F., Boujrad N. The human estrogen receptor-alpha isoform hERalpha46 antagonizes the proliferative influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology. 2005;146(12):5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 15.Denger S., Reid G., Kos M., Flouriot G., Parsch D., Brand H. ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol. 2001;15(12):2064–2077. doi: 10.1210/mend.15.12.0741. [DOI] [PubMed] [Google Scholar]
- 16.Murphy A.J., Guyre P.M., Wira C.R., Pioli P.A. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS One. 2009;4(5) doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L., Haynes M.P., Bender J.R. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100(8):4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chantalat E., Boudou F., Laurell H., Palierne G., Houtman R., Melchers D. The AF-1-deficient estrogen receptor ERalpha46 isoform is frequently expressed in human breast tumors. Breast Cancer Res. 2016;18(1):123. doi: 10.1186/s13058-016-0780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasco M.J., Keyomarsi K., Arcaro K.F., Gierthy J.F. Expression of an estrogen receptor alpha variant protein in cell lines and tumors. Mol Cell Endocrinol. 2000;166(2):156–169. [PubMed] [Google Scholar]
- 20.Hirschfeld M., Ouyang Y.Q., Jaeger M., Erbes T., Orlowska-Volk M., Zur Hausen A. HNRNP G and HTRA2-BETA1 regulate estrogen receptor alpha expression with potential impact on endometrial cancer. BMC Cancer. 2015;15:86. doi: 10.1186/s12885-015-1088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leygue E., Huang A., Murphy L.C., Watson P.H. Prevalence of estrogen receptor variant messenger RNAs in human breast cancer. Cancer Res. 1996;56(19):4324–4327. [PubMed] [Google Scholar]
- 22.Perlman W.R., Matsumoto M., Beltaifa S., Hyde T.M., Saunders R.C., Webster M.J. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134(1):81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Zhang Y., Zhao C., Yu T., Liu Y., Shi W. Reduced alternative splicing of estrogen receptor alpha in the endometrium of women with endometriosis. Oncotarget. 2017;8(66):110176–110186. doi: 10.18632/oncotarget.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia Pedrero J.M., Zuazua P., Martinez-Campa C., Lazo P.S., Ramos S. The naturally occurring variant of estrogen receptor (ER) ERDeltaE7 suppresses estrogen-dependent transcriptional activation by both wild-type ERalpha and ERbeta. Endocrinology. 2003;144(7):2967–2976. doi: 10.1210/en.2002-0027. [DOI] [PubMed] [Google Scholar]
- 25.Busch A., Hertel K.J. Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip Rev RNA. 2012;3(1):1–12. doi: 10.1002/wrna.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard A.J., Sparey C., Robson S.C., Krainer A.R., Europe-Finner G.N. Spatio-temporal expression of the trans-acting splicing factors SF2/ASF and heterogeneous ribonuclear proteins A1/A1B in the myometrium of the pregnant human uterus: a molecular mechanism for regulating regional protein isoform expression in vivo. J Clin Endocrinol Metab. 2000;85(5):1928–1936. doi: 10.1210/jcem.85.5.6537. [DOI] [PubMed] [Google Scholar]
- 27.Welsh T., Johnson M., Yi L., Tan H., Rahman R., Merlino A. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERalpha activates ERK1/2 signaling in term myometrium. J Endocrinol. 2012;212(2):227–238. doi: 10.1530/JOE-11-0358. [DOI] [PubMed] [Google Scholar]
- 28.Rezapour M., Backstrom T., Lindblom B., Ulmsten U. Sex steroid receptors and human parturition. Obstet Gynecol. 1997;89(6):918–924. doi: 10.1016/s0029-7844(97)00116-6. [DOI] [PubMed] [Google Scholar]
- 29.How H., Huang Z.H., Zuo J., Lei Z.M., Spinnato J.A., 2nd, Rao C.V. Myometrial estradiol and progesterone receptor changes in preterm and term pregnancies. Obstet Gynecol. 1995;86(6):936–940. doi: 10.1016/0029-7844(95)00306-C. [DOI] [PubMed] [Google Scholar]
- 30.Fuqua S.A., Fitzgerald S.D., Allred D.C., Elledge R.M., Nawaz Z., McDonnell D.P. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer Res. 1992;52(2):483–486. [PubMed] [Google Scholar]
- 31.Wang H., Zeng X., Khan S.A. Estrogen receptor variants ERdelta5 and ERdelta7 down-regulate wild-type estrogen receptor activity. Mol Cell Endocrinol. 1999;156(1–2):159–168. doi: 10.1016/s0303-7207(99)00125-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Y., Katzenellenbogen B.S., Shapiro D.J. Estrogen receptor mutants which do not bind 17 beta-estradiol dimerize and bind to the estrogen response element in vivo. Mol Endocrinol. 1995;9(4):457–466. doi: 10.1210/mend.9.4.7659089. [DOI] [PubMed] [Google Scholar]
- 33.Chu I., Arnaout A., Loiseau S., Sun J., Seth A., McMahon C. Src promotes estrogen-dependent estrogen receptor alpha proteolysis in human breast cancer. J Clin Invest. 2007;117(8):2205–2215. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laios I., Journe F., Nonclercq D., Vidal D.S., Toillon R.A., Laurent G. Role of the proteasome in the regulation of estrogen receptor alpha turnover and function in MCF-7 breast carcinoma cells. J Steroid Biochem Mol Biol. 2005;94(4):347–359. doi: 10.1016/j.jsbmb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Nawaz Z., Lonard D.M., Dennis A.P., Smith C.L., O'Malley B.W. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A. 1999;96(5):1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balducci J., Risek B., Gilula N.B., Hand A., Egan J.F., Vintzileos A.M. Gap junction formation in human myometrium: a key to preterm labor? Am J Obstet Gynecol. 1993;168(5):1609–1615. doi: 10.1016/s0002-9378(11)90806-0. [DOI] [PubMed] [Google Scholar]
- 37.Chow L., Lye S.J. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170(3):788–795. doi: 10.1016/s0002-9378(94)70284-5. [DOI] [PubMed] [Google Scholar]
- 38.Sakai N., Tabb T., Garfield R.E. Modulation of cell-to-cell coupling between myometrial cells of the human uterus during pregnancy. Am J Obstet Gynecol. 1992;167(2):472–480. doi: 10.1016/s0002-9378(11)91432-x. [DOI] [PubMed] [Google Scholar]
- 39.Di W.L., Lachelin G.C., McGarrigle H.H., Thomas N.S., Becker D.L. Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol Hum Reprod. 2001;7(7):671–679. doi: 10.1093/molehr/7.7.671. [DOI] [PubMed] [Google Scholar]
- 40.Yu W., Dahl G., Werner R. The connexin43 gene is responsive to oestrogen. Proc Biol Sci. 1994;255(1343):125–132. doi: 10.1098/rspb.1994.0018. [DOI] [PubMed] [Google Scholar]
- 41.Das A., Li Q., Laws M.J., Kaya H., Bagchi M.K., Bagchi I.C. Estrogen-induced expression of Fos-related antigen 1 (FRA-1) regulates uterine stromal differentiation and remodeling. J Biol Chem. 2012;287(23):19622–19630. doi: 10.1074/jbc.M111.297663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai C.F., Cheng Y.K., Lu D.Y., Wang S.L., Chang C.N., Chang P.C. Inhibition of estrogen receptor reduces connexin 43 expression in breast cancers. Toxicol Appl Pharmacol. 2018;338:182–190. doi: 10.1016/j.taap.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Hocine S., Singer R.H., Grunwald D. RNA processing and export. Cold Spring Harb Perspect Biol. 2010;2(12):a000752. doi: 10.1101/cshperspect.a000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soldin O.P., Guo T., Weiderpass E., Tractenberg R.E., Hilakivi-Clarke L., Soldin S.J. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84(3):701–710. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao R., Wang X., Weijdegard B., Norstrom A., Fernandez-Rodriguez J., Brannstrom M. Coordinate regulation of heterogeneous nuclear ribonucleoprotein dynamics by steroid hormones in the human fallopian tube and endometrium in vivo and in vitro. Am J Physiol Endocrinol Metab. 2012;302(10):E1269–E1282. doi: 10.1152/ajpendo.00673.2011. [DOI] [PubMed] [Google Scholar]
- 46.Nadal-Ginard B., Smith C.W., Patton J.G., Breitbart R.E. Alternative splicing is an efficient mechanism for the generation of protein diversity: contractile protein genes as a model system. Adv Enzyme Regul. 1991;31:261–286. doi: 10.1016/0065-2571(91)90017-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material