Abstract
Absorbable hemostats such as microporous polysaccharide hemospheres (MPH) are used to manage hemostasis and prevent complications in total knee arthroplasty (TKA). We aimed to determine safety and effectiveness of MPH use in TKA. Records were reviewed for blood loss, hematomas, and infections. No differences existed regarding demographics, superficial infections (P = 0.933) or hematomas (P = 0.393). Positive correlation existed between hematoma and superficial infection (P = 0.009). Blood loss was greater in the treatment group (P = 0.014). MPH demonstrated inferior bleeding control and had no effect on complications. Our results suggest application of this agent may be unnecessary.
Keywords: Microporous polysaccharide hemospheres, Arista®AH, Total knee arthroplasty, Complications, Hematoma, Hemostasis
1. Introduction
Total Knee Arthroplasty (TKA) is amongst the most common operations performed in the field of orthopedic surgery; in large part it has been reliable and successful.1 However, there are continued efforts to improve results by limiting postoperative wound complications such as hematoma formation and infection.2 Consideration of these problems makes intraoperative control of blood loss and obtaining final hemostasis during wound closure important.
Arista®AH (C. R. Bard, Inc. – Davol, Warwick, RI) is an absorbable topical hemostat which harnesses hydrophilic microporous polysaccharide hemosphere (MPH) technology to assist in blood loss control.3 The product is derived from purified plant starch and is supplied as a powdered substance with a variety of applicators. Hemostatic activity is produced as it forms a molecular lattice when encountering blood. Liquid components are absorbed by polysaccharide hemospheres which concentrates solid components of blood including red blood cells, platelets, and proteins involved in the coagulation cascade. Aggregation of solid constituents not only forms a mechanical barrier to bleeding but also approximates natural coagulation products allowing for efficient thrombus formation. The product enhances biological clotting on contact by creating a hemostatic plug.4
Use of this technology has been studied in various specialties including cardiothoracic surgery, urology, general surgery, otolaryngology, as well as plastic surgery.5, 6, 7, 8, 9, 10, 11, 12, 13 Arista®AH has been FDA approved for use in orthopedic surgery for several years now. A randomized clinical study compared Arista®AH to absorbable gelatin sponge used with or without thrombin across general, cardiac, and orthopedic procedures. Arista®AH was found to be an effective agent in obtaining hemostasis and was able to do so faster than the comparison group. Zhang et al. demonstrated a reduction in blood loss in total knee arthroplasty with use of the hemostatic agent, to our knowledge this is the only other study investigating the use of microporous polysaccharide hemosphere in this setting.14 Conflicting evidence on efficacy does exist which brings MPH technology into question and may only support its use in low level bleeding.10
Arista®AH has several advantages making it an attractive surgical adjunct. The sterile product is stored at room temperature and has a long shelf life making it readily accessible. Ease of application is also a benefit. Containers holding one, three, or 5-g dosages are preassembled with a short applicator to spread dry product over a surface area.4 It is a high volume, low mass, and low-density substance allowing for greater coverage when compared to absorbable bovine thrombin-gelatin hemostatic matrix (FloSeal Hemostatic Matrix, Baxter).15 Extended applicator options are available which allow for placement in hard to reach areas or more focal regions of bleeding. Microporous polysaccharide hemospheres are biocompatible and absorb within 24–48 h as compared to other absorbable hemostats which take 2–10 weeks.3,4,16
Warnings exist against use of Arista®AH in areas of confine due to its swelling activity.3 Microporous polysaccharide hemospheres can expand to five times their original volume immediately upon contact with fluid. Specific precautions in orthopedics exist as no studies have determined the effect of Arista®AH on the interaction between bone surfaces and implants attached with adhesives.3
The ability of microporous polysaccharide hemospheres to reduce blood loss has been established in some specialties however the absorbable hemostat has been called into question in other areas of the literature.4,10, 11, 12 Continued evaluation of safety with this technology in orthopedic surgery is paramount where implant related wound complications and infections remain a significant concern.17 An indirect evaluation of cost effectiveness should also be considered in our current healthcare environment.
The purpose of this study was to evaluate microporous polysaccharide hemosphere effectiveness and to investigate any influence on rates of hematoma formation and wound infection in primary total knee arthroplasty.
2. Methodology
Institutional Review Board approval was obtained for this case control retrospective chart review. A well-established private practice was used to identify patients from May of 2013 to January of 2017. Billing queries detected unilateral primary total knee arthroplasty cases performed with and without intraoperative use of microporous polysaccharide hemospheres. Treatment and control groups were established by intraoperative use of Arista®AH. No patient selection criteria were used as the absorbable hemostat had not been implemented for patients enlisted from 2013 to 2014. This was followed by a period of continuous use of the agent on all patients from 2015 to 2017.
Inclusion required unilateral primary total knee arthroplasty with posterior cruciate retaining or posterior cruciate sacrificing implants performed at a single institution by a single surgeon. Diagnoses included osteoarthritis, rheumatoid arthritis, and post-traumatic arthritis. Subjects were age greater than 18 years old, used warfarin for postoperative venous thromboembolism (VTE) prophylaxis, and had follow up of at least 90 days. Exclusion criteria included any revision total knee arthroplasty, prostheses with more constraint than posterior stabilized components, use of topical hemostatic agent besides Arista®AH, body mass index (BMI) greater than 45, history of liver disease or coagulopathy, NSAID use other than aspirin (81 or 325 mg) during the period of pharmacologic VTE prophylaxis, perioperative use of antiplatelet medications not discontinued within five days of surgery, postoperative anticoagulation with agents other than warfarin, or postoperative anticoagulation stopped before or continued after a one month follow up appointment.
Primary outcome measures included postoperative blood loss as well as number of hematomas and infections encountered within a 90-day period. Secondary outcome measures included correlations between hematomas or infections with patient age, BMI, and return trips to the operating room for further intervention. Transfusion rates between groups were also evaluated.
Expected surgical blood loss was determined by agreement between surgeon and anesthesiologist. Postoperative blood loss was quantified by hemoglobin levels obtained at 24 and 48 h after surgery. These levels were referenced to a preoperative hemoglobin level obtained within one month of the surgical date. Superficial infection was defined as surgical wound drainage after seven postoperative days necessitating treatment with oral antibiotics or any documented cellulitis of the operative extremity treated with oral antibiotics. Deep periprosthetic joint infection was defined by Musculoskeletal Infection Society criteria from Parvizi et al., in 2011.17
2.1. Technique
Patients obtained pre-operative clearance for surgery by a primary care provider or cardiologist detailing medical history and laboratory screening within approximately 30 days of operation. All surgeries were performed by a single surgeon with experience of over 20 years. Patients underwent general or spinal anesthesia with regional femoral nerve block. A weight-based dose of preoperative intravenous antibiotics was administered within an hour of incision and discontinued 24 h postoperatively. A nonsterile tourniquet was applied and inflated after extremity exsanguination. Standardized exposure was used with a midline incision and medial parapatellar arthrotomy. The majority of dissection was performed with electrocautery. An intramedullary femoral bone plug was used to limit canal bleeding postoperatively. All patients received cemented femoral, tibial, and patellar resurfacing components. Arthrotomy closure was performed with interrupted absorbable heavy braided suture. In the treatment group between 2015 and 2017, a single 3-g dose of Arista®AH was applied over the closed arthrotomy, retinaculum, and deep surface of the skin flaps. The subcutaneous layer was closed with braided absorbable suture and final skin closure with staples. In the non-treatment group between 2013 and 2014, Arista®AH was not used and the remainder of the procedure and perioperative protocol remained the same. Tourniquet deflation occurred after complete wound closure and application of a sterile compressive dressing.
Warfarin was used without any bridging agent for VTE prophylaxis; 5 mg was given the night before surgery and dosed daily thereafter for one month. Dosage was adjusted based on regularly drawn protime/INR levels with the goal of achieving an INR between 2.0 and 3.0. Hemoglobin levels were obtained the morning of the first two postoperative days. The threshold for transfusion with packed red blood cells was a hemoglobin level less than 7.0 g/dL per institutional protocol. All patients were allowed to weight bear as tolerated and standard physical therapy services were utilized during the admission and after discharge. Subjects were instructed to wear thigh high compression stockings for two weeks after surgery and change the dressing daily for wound inspection. Follow up occurred at 2, 4, 8, and 12 weeks postoperatively. Staples were routinely removed at the two-week appointment.
2.2. Statistical analyses
Continuous variables were summarized with mean and standard deviation, between groups these variables were analyzed with independent sample t-tests. Variables that exhibited significant skewness were analyzed with the non-parametric Mann-Whitney U test. Categorical variables were summarized with frequencies and percentages for each group. Between group differences were assessed using Chi-square and Fisher's exact tests if appropriate (observed values ≤ 5). Statistical significance was determined by α ≤ 0.05. SPSS software was utilized.
3. Results
A total of 206 cases were identified with billing codes, 147 met inclusion/exclusion criteria, ninety-three within the treatment (Arista®AH) group and fifty-four in the control group. Five patients were lost to insufficient follow up (2.4%). Demographically the groups had no significances in age, body mass index, gender, or laterality which is summarized in Table 1. Overall, mean age and BMI were 66.1 yr ± 9.4 and 32.7 kg/m2 +/− 5.9 respectively; females represented 61.2% of the population. The majority of implants were DePuy Synthes™ cruciate retaining components, summarized in Table 2. Spinal anesthesia with a regional femoral nerve block (RFNB) was the primary means of anesthetic as shown in Table 3.
Table 1.
Demographics.
| Variable | Treatment Group (n = 93) | Non-Treatment Group (n = 54) | P-Value |
|---|---|---|---|
| Age (year) | 66.8±9.4 | 65.1±9.3 | 0.290 |
| BMI (kg/m2) | 33.2±6.1 | 31.7±5.6 | 0.134 |
| Male | 41 (44.1%) | 16 (29.6%) | 0.083 |
| Female | 52 (55.9%) | 38 (70.4%) | |
| Right | 52 (55.9%) | 35 (64.8%) | 0.290 |
| Left | 41 (44.1%) | 19 (35.2%) |
BMI = Body Mass Index.
Table 2.
Implants.
| Implants | Treatment Group (n = 93) | Non-Treatment Group (n = 54) | P-Value |
|---|---|---|---|
| Cruciate Retaining | 84 (90.3%) | 48 (88.9%) | 0.268 |
| Cruciate Sacrificing | 9 (9.7%) | 6 (11.1%) | |
| DePuy Synthes™ | 90 (96.8%) | 49 (90.7%) | |
| Smith and Nephew™ | 3 (3.2%) | 3 (5.6%) | |
| Stryker® | 0 (0) | 2 (3.7%) |
Table 3.
Anesthesia.
| Type of Anesthesia | Treatment Group (n = 93) | Non-Treatment Group (n = 54) | P-Value |
|---|---|---|---|
| General Endotracheal with RFNB | 5 (5.4%) | 5 (9.3%) | 0.499 |
| Spinal with RFNB | 88 (94.6%) | 49 (90.7%) |
RFNB = Regional Femoral Nerve Block.
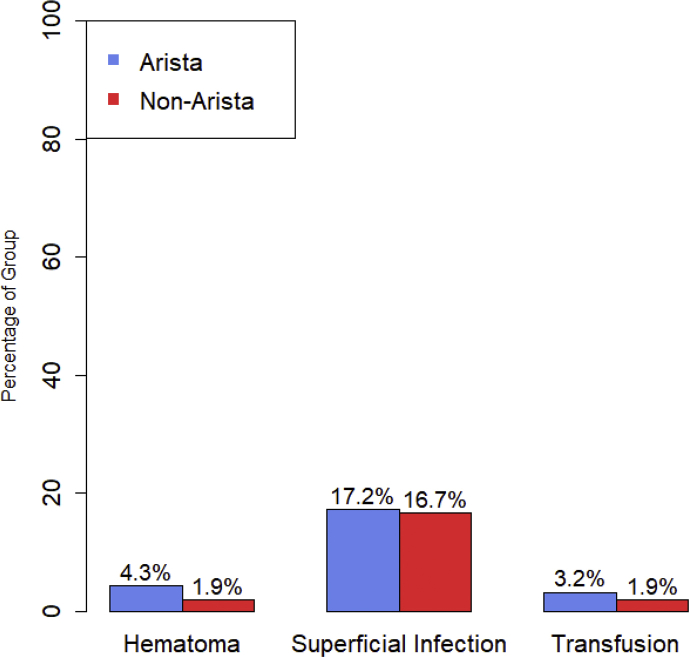
Superficial infection rates were 17.2% in the treatment group and 16.7% in the non-treatment group, no significant intergroup difference existed (P = 0.933). Overall there were five hematomas, four of which occurred in the treatment group and one occurred in the non-treatment group, this was not statistically significant (P = 0.393). See Fig. 1. No deep infections or need for further operative intervention occurred in either group, accordingly no variance was found for these parameters. Summarized in Table 4. A positive correlation existed between hematoma formation and superficial infection (R = 0.215, P = 0.009). No correlation was found between age and hematoma (P = 0.687), age and superficial infection (P = 0.281), BMI and superficial infection (P = 0.054), BMI and hematoma (P = 0.715). Summarized in Table 5.
Fig. 1.
ComplicationsColumn
chart of complications by treatment group.
Table 4.
Complications.
| Complications | Treatment Group (n = 93) | Non-Treatment Group (n = 54) | P-Value |
|---|---|---|---|
| Hematoma | 4 (4.3%) | 1 (1.9%) | 0.393 |
| Superficial Infection | 16 (17.2%) | 9 (16.7%) | 0.933 |
| Deep Infection | 0 | 0 | – |
| Return Trip to Operating Room | 0 | 0 | – |
Table 5.
Correlations.
| Variables | Pearson Correlation | P-Value |
|---|---|---|
| Hematoma and Superficial Infection | 0.215 | 0.009 |
| Age and Hematoma | 0.034 | 0.687 |
| Age and Superficial Infection | −0.090 | 0.281 |
| BMI and Hematoma | 0.030 | 0.715 |
| BMI and Superficial Infection | 0.159 | 0.054 |
BMI = Body Mass Index.
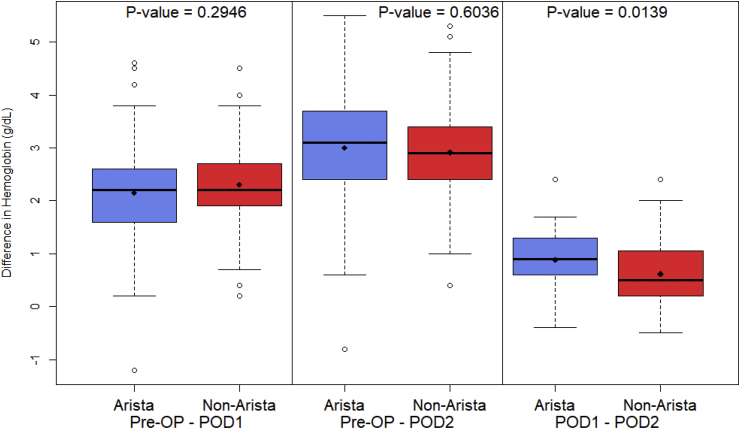
Expected surgical blood loss was significantly lower in the non-treatment group (74.5 mL ± 71.9) than in the treatment group (101.5 mL ± 57.9), (P = 0.0004). A significant difference also existed between groups regarding average hemoglobin decrease from postoperative day one to postoperative day two. The average decrease in hemoglobin during this timeframe in the non-treatment group was (0.62 g/dL ± 0.65) and in the treatment group (0.89 g/dL ± 0.52), (P = 0.014). There was no significant difference in average hemoglobin decrease from preoperative values to postoperative day one between non-treatment (2.31 g/dL ± 0.86) and treatment groups (2.15 g/dL ± 0.89), (P = 0.295). No significant difference was found in average hemoglobin decrease from preoperative values to postoperative day two between non-treatment (2.91 g/dL ± 0.95) and treatment groups (3.0 g/dL ± 1.03), (P = 0.604). There was no difference in transfusion rates between the treatment group 3.2% than in the non-treatment group 1.9% (P = 0.844). Summarized in Fig. 2 and Table 6.
Fig. 2.
Postoperative Blood LossBoxplot
of the change in Hemoglobin (g/dL) by treatment group, comparing Pre-OP, POD1, and POD2 hemoglobin levels. The p-values are from independent two-sample t
-test.
Table 6.
Blood loss and transfusion rates.
| Variable | Treatment Group (n = 93) | Non-Treatment Group (n = 54) | P-Value |
|---|---|---|---|
| Surgical Blood Loss (mL) | 101.5±57.9 | 74.5±71.9 | 0.0004 |
| Preoperative Hgb – POD1 Hgb (g/dL) | 2.15±0.89 | 2.31±0.86 | 0.295 |
| Preoperative Hgb – POD2 Hgb (g/dL) | 3.0±1.03 | 2.91±0.95 | 0.604 |
| POD1 Hgb – POD2 Hgb (g/dL) | 0.89±0.52 | 0.62±0.65 | 0.014 |
| Transfusion Rate | 3.2% | 1.9% | 0.844 |
Hgb = Hemoglobin; POD = Postoperative Day.
4. Discussion
Our study demonstrates inferior bleeding control with use of Arista®AH in total knee arthroplasty, no significant effect was seen regarding wound complications. More expected surgical blood loss and more postoperative blood loss occurred in the treatment group between postoperative day one and two. These findings may be related to the mechanism of action of MPH which deprives the local bleeding environment of solid coagulation constituents. Coagulation factors aggregate as they are absorbed by the polysaccharide hemospheres. This process theoretically creates a mechanical hemostatic plug which may be beneficial in a focal area but is less effective in a large and more diffuse field of bleeding. The agent was placed superficial to the capsule in our technique as no studies are available describing the effect of Arista®AH on the interaction between bone surfaces and implants attached with adhesives. Although few hematomas occurred overall, 80% were within the treatment group. Reason for this finding may be related to the initial immense swelling observed as polysaccharide hemospheres encounter bodily fluid. This swelling is another known component of the mechanism of action for MPH technology. The product is thought to form a gelatinous fibrin clot and reabsorb within 48 h.3,4 However, this timeframe allows for maintenance of a potential space between the arthrotomy and skin flaps of the closed incision creating undue tension during initial phases of healing. The swelling could have implications in areas where skin flaps are somewhat tenuous. This is supported by the works of Offodile et al. and Miller et al. where distal tip skin flap necrosis and wound healing problems were seen with use of microporous polysaccharide hemospheres.11,12 One of the most important risk factors for seroma development is a delay in wound healing.8 Additionally, our results demonstrate postoperative hematomas have a significant positive correlation with superficial wound infection. This emphasizes the importance of preventing these fluid collections. The rate of superficial infection in our study was elevated but similar in both groups. We attribute this to our low threshold to treat any drainage or erythematous wound after seven postoperative days with oral antibiotics.
Novel innovative techniques and devices are frequently applied to total knee arthroplasty with aspirations of improving outcomes and limiting complications. Traditional methods of obtaining hemostasis include application of direct pressure, suture-ligature, and electrocautery. Newer methods may utilize evolving technology toward this objective. Although agents such as topical fibrin spray and intravenous tranexamic acid have proven to reduce blood loss in total knee replacement, other options exist.18 Topical hemostats, sealants, and adhesives have been in use for quite some time as part of an arsenal available to surgeons obtaining hemostasis. These agents provide a frontier for advancements in procedures across many specialties. The need to monitor safety and effectiveness of such innovations is imperative.
Mechanical hemostatic agents such as porcine gelatin (Gelfoam®, Pfizer & Pharmacia), bovine collagen (Instat®, Johnson & Johnson, and Avitene™, Bard), as well as oxidized regenerated cellulose (Surgicel®, Johnson & Johnson) are often used in combination with thrombin. These products fall into the same class of hemostatic agents with microporous polysaccharide hemospheres (Arista®AH, Bard, Davol Inc.).19 Mechanical agents create an obstruction to stop bleeding and support natural coagulation making them among the safest and least costly hemostatic commodities.20 However, there is a consistent risk with mechanical agents related to swelling of the materials as they have absorptive properties.21
Polysaccharide hemospheres have been studied more extensively in other surgical specialties. Some investigations have shown successful results and others are less encouraging. Reports investigating use of microporous polysaccharide hemospheres in total hip arthroplasty found it to lower postoperative wound drainage, reduction of hemoglobin, and need for blood transfusion.22,23 These results differed from findings in our study. Bruckner et al. described Arista®AH in the setting of cardiothoracic surgery; they showed decreased time to hemostasis and less postoperative chest tube output without an increase in complications.7 Rajagopal et al. demonstrated polysaccharide hemospheres were safe and efficacious in live donor nephrectomies13 and Gilbert et al. found fewer lymphoceles with use of Arista®AH in prostatectomy and lymphadenectomy although their difference was not statistically significant.9 Antisdel et al. has multiple studies in the field of otolaryngology exhibiting less early postoperative bleeding after endoscopic sinus surgery and lack of any negative effect on postoperative healing in the sinus cavity.5,6 An animal study by Egeli et al. showed significantly lower rates of postoperative seroma after mastectomy with use of polysaccharide hemospheres8 and a separate animal study by Ereth et al. demonstrated lower abdominal wound infection rates with E. coli when comparing Arista®AH to gelatin matrix.24
Several reports emphasize a need for further evaluation of polysaccharide hemosphere technology. Offodile II et al. illustrated use of Arista®AH increasing incidence of distal tip ischemia and necrosis of dorsal skin flaps in a murine model. This study suggested careful use of Arista®AH with vascularly challenged tissue as skin viability can depend on plasmatic imbibition and osmosis. These processes may be impeded by polysaccharide hemospheres which desiccate the local environment to form a hemostatic plug.12 Miller et al. demonstrated polysaccharide hemospheres were associated with prolonged closure and diminished thickness for wound healing in diabetic mice. The study also found MPH altered contractile proteins involved in the wound healing process with several murine models.11 These studies raise concerns for soft tissue complications consistent with the trend noted in the treatment group of our study regarding hematoma formation. Comparison of hemostatic efficacy with Arista®AH and FloSeal Hemostatic Matrix (FloSeal, Baxter) to address parenchymal tears in a porcine model found polysaccharide hemospheres had inferior bleeding control. They suggested Arista®AH may be more suited for low level bleeding which was concordant with our results.10 Although Arista®AH is rapidly absorbed, risks are present with any implant or acellular foreign body as it may provide a surface for bacteria to adhere and produce biofilm associated with more established infection. In addition, thrombi themselves seem to be desirable sites for bacterial colonization.25
There is inherent responsibility to cautiously observe safety and cost efficiency of advancements as they permeate into practice. This should prompt review of the effectiveness and safety for microporous polysaccharide hemospheres used in orthopedics to satisfy a current a void in the literature. There are very few reports regarding use of Arista®AH in orthopedic surgery. The only other study evaluating use of this agent in total knee arthroplasty by Zhang et al. was underpowered and focused primarily on reduction of blood loss rather than associated complications.14
Arista®AH does not seem to influence rates of superficial infection, deep infection, or any postoperative complications requiring further formal operative intervention. In this study, the agent demonstrated inferior bleeding control, potentially related to its performance in more heavy bleeding scenarios as this has been previously called into question.10 We are faced with ever increasing pressures to minimize costs in our current healthcare environment. This presents a challenge of determining which available resources are necessary to achieve an outcome. The approximate cost of a single 3-g dose of Arista®AH is $210.00 at our institution.
Weaknesses of this study include inherent flaws associated with retrospective data and chart review. The study is also underpowered which could account for lack of statistical significance found between groups regarding hematoma formation. A follow up period of 90 days is brief, but we feel this is the most relevant timeframe for the study design and agent of interest. Tranexamic acid was not used for any patients as it had not yet been adopted for routine use by surgeons at our institution during the timeframe of this study. We acknowledge use of warfarin as a primary means of anticoagulation could have contributed to wound complications however this was used in all patients in both groups.
There are several strengths of the study including a single surgeon and a single institution setting reducing confounding variables. Two continuous periods were utilized for the control group and treatment group which limits bias as there were no patient selection criteria for use of Arista®AH. Patient demographics in addition to our inclusion and exclusion criteria provide a homogenous and generalizable population. This study is more directly applicable to clinical practice than animal studies investigating this agent in surgical specialties outside of orthopedics. Lastly, to our knowledge this is the first focused review in North American literature detailing use of microporous polysaccharide hemospheres in total knee arthroplasty. Despite limited numbers and lack of power, this is the largest study to date providing insight on application of this absorbable hemostat in the field of orthopedic surgery and represents a valuable set of data.
5. Conclusion
Arista®AH demonstrates inferior bleeding control and has no clinically significant effect on rates of hematoma formation or wound infection in primary total knee arthroplasty. Our results suggest use of the agent may be unnecessary in this surgical setting and better reserved for different applications with low level bleeding. Further investigation on this topic with a larger patient population or in a prospective manner could provide more information on use of microporous polysaccharide hemosphere technology in orthopedics.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
Contributor Information
Scott Gleason, Email: scott.gleason@franciscanalliance.org.
David Mehl, Email: mehlmd@me.com.
William Payne, Email: wkpiii@aol.com.
Steve Parry, Email: slparry81@gmail.com.
Amy Buros Stein, Email: astein@midwestern.edu.
References
- 1.HCUP Fast Stats Healthcare Cost and Utilization Project (HCUP) 2017. Agency for Healthcare Research and Quality, Rockville, MD.https://www.hcup-us.ahrq.gov/faststats/NationalProceduresServlet?year1=2015&characteristic1=0&included1=0&year2=&characteristic2=0&included2=1&expansionInfoState=hide&dataTablesState=hide&definitionsState=hide&exportState=hide [Accessed 4.22.2018] [PubMed] [Google Scholar]
- 2.Simons M.J., Amin N.H., Scuderi G.R. Acute wound complications after total knee arthroplasty: prevention and management. J Am Acad Orthop Surg. 2017;25(8):547–555. doi: 10.5435/JAAOS-D-15-00402. Print. [DOI] [PubMed] [Google Scholar]
- 3.Arista®AH Absorbable Hemostatic Particles Microporous Polysaccharide Hemosphere (MPH) Technology. Medafor, Inc.; 2008. LIT-0057 Rev E. Print. [Google Scholar]
- 4.Wiseman C. Davol Inc. Warwick, RI: Davol Inc.; 2014. Arista®AH Randomized Clinical Study; pp. 1–7. Print. [Google Scholar]
- 5.Antisdel J.L., Matijasec J.L., Ting J.Y., Sindwani R. Microporous polysaccharide hemospheres do not increase synechiae after sinus surgery: randomized controlled study. Am J Rhinol Allergy. 2011;25:268–271. doi: 10.2500/ajra.2011.25.3619. Print. [DOI] [PubMed] [Google Scholar]
- 6.Antisdel J.L., West-Denning J.L., Sindwani R. Vol. 141. Otolaryngology–Head and Neck Surgery; St. Louis, MO: 2009. pp. 353–357. (Effect of Microporous Polysaccharide Hemospheres (MPH) on Bleeding after Endoscopic Sinus Surgery: Randomized Controlled Study). Print. [DOI] [PubMed] [Google Scholar]
- 7.Bruckner B.A., Blau L.N., Rodriguez L. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J Cardiothorac Surg. 2014;9:1–7. doi: 10.1186/s13019-014-0134-4. Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeli T., Sevinc A.I., Bora S. Microporous polysaccharide hemospheres and seroma formation after mastectomy and axillary dissection in rats. J Balkan Med. 2012;29:179–183. doi: 10.5152/balkanmedj.2012.005. Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert D.R., Angell J., Abaza R. Evaluation of absorbable hemostatic powder for prevention of lymphoceles following robotic prostatectomy with lymphadenectomy. J Urol. 2016;98:75–80. doi: 10.1016/j.urology.2016.06.071. Print. [DOI] [PubMed] [Google Scholar]
- 10.Lewis K., Atlee H., Mannone A., Lin L., Goppelt A. Efficacy of hemostatic matrix and microporous polysaccharide hemospheres. J Surg Res. 2015;193:825–830. doi: 10.1016/j.jss.2014.08.026. Print. [DOI] [PubMed] [Google Scholar]
- 11.Miller K.J., Cao W., Ibrahim M.M., Levinson H. The effect of microporous polysaccharide hemospheres on wound healing and scarring in wild-type and Db/Db mice. J Adv Skin Wound Care. 2017;30:170–180. doi: 10.1097/01.ASW.0000513149.43488.56. Print. [DOI] [PubMed] [Google Scholar]
- 12.Offodile A.C., II, Chen B., Aherrera A.S., Guo L. Microporous polysaccharide hemospheres potentiate ischemia-induced skin flap necrosis in a murine model. Burlington and Boston, MA. J Plastic Reconstruct Surg. 2017;139:59e–66e. doi: 10.1097/PRS.0000000000002907. Print. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal P., Hakim N. The use of a powdered polysaccharide hemostat (hemostase) in live donor nephrectomies controls bleeding and reduces postoperative complications. Journal of Transplantation Proceedings. 2011;43:424–426. doi: 10.1016/j.transproceed.2011.01.079. Print. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y. Effectiveness of microporous polysaccharide hemospheres in total knee arthroplasty. Chin J Bone Joint Surg. 2009;4:269–272. [Google Scholar]
- 15.Scott J., Liese R., Bruckner B. Bard Inc.; Warwick, RI: 2014. Arista®AH Absorbable Hemostatic Particles Mass, Volume, Density, and Coverage Area Assessment as Compared to Floseal Hemostatic Matrix; pp. 1–7. Print. [Google Scholar]
- 16.Burks S., Spotnitz W. Safety and usability of hemostats, sealants, and adhesives. AORN J. 2014;100:160–176. doi: 10.1016/j.aorn.2014.01.026. Print. [DOI] [PubMed] [Google Scholar]
- 17.Parvizi J., Zmistowski B., Berbari E.F. New definition for periprosthetic joint infection: from the workgroup of the musculoskeletal infection society. Clin Orthop Relat Res. 2011;469(11):2992–2994. doi: 10.1007/s11999-011-2102-9. PMC. 15 Oct. 2017. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molloy D.O., Archbold H.P., Ogonda L., McConway J., Wilson R.K., Beverland D.E. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement. J Bone Joint Surg [Br] 2007;89-B:306–309. doi: 10.1302/0301-620X.89B3.17565. Print. [DOI] [PubMed] [Google Scholar]
- 19.Spotnitz W., Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Journal of Transfusion. 2008;48:1502–1516. doi: 10.1111/j.1537-2995.2008.01703.x. Print. [DOI] [PubMed] [Google Scholar]
- 20.Gabay M., Boucher B. An essential primer for understanding the role of topical hemostats, surgical sealants, and adhesives for maintaining hemostasis. J Pharmacother. 2013;33:935–955. doi: 10.1002/phar.1291. Print. [DOI] [PubMed] [Google Scholar]
- 21.Spotnitz W. Hemostats, sealants, and adhesives: a practical guide for the surgeon. The American Surgeon Journal. 2012;78:1305–1321. Print. [PubMed] [Google Scholar]
- 22.Luo D.F., Chen Y., Gao H., Chen L., Li S. Application of microporous polysaccharide hemospheres in total hip arthroplasty. Chin J Tissue Eng Res. 2012;16:4792–4795. [Google Scholar]
- 23.Liu T.S., Wang Q., Wang W., Liu A., Ma J. Microporous polysaccharide hemospheres reduce blood loss in total hip arthroplasty. Chin J Tissue Eng Res. 2015;19:1872–1877. [Google Scholar]
- 24.Ereth M.H., Yue Dong L., Schrader N.A. Microporous polysaccharide hemospheres do not enhance abdominal infection in a rat model compared with gelatin matrix. J Surg Infect. 2009;10:273–276. doi: 10.1089/sur.2007.033. Print. [DOI] [PubMed] [Google Scholar]
- 25.Mohammad S. Enhanced risk of infection with device-associated thrombi. Am Soc Artif Intern Organs J. 2000:S63–S68. doi: 10.1097/00002480-200011000-00039. Print. [DOI] [PubMed] [Google Scholar]